Abstract
Motor units within human muscles usually exhibit a significant degree of short-term synchronization. Such coincident spiking typically has been attributed to last-order projections that provide common synaptic input across motor neurons. The extent of branched input arising directly from cortical neurons has often been suggested as a critical factor determining the magnitude of short-term synchrony. The purpose of this study, therefore, was to quantify motor unit synchrony in a variety of human muscles differing in the presumed extent of cortical input to their respective motor nuclei. Cross-correlation histograms were generated from the firing times of 551 pairs of motor units in 16 human muscles. Motor unit synchrony tended to be weakest for proximal muscles and strongest for more distal muscles. Previous work in monkeys and humans has shown that the strength of cortical inputs to motor neurons also exhibits a similar proximal-to-distal gradient. However, in the present study, proximal-distal location was not an exclusive predictor of synchrony magnitude. The muscle that exhibited the least synchrony was an elbow flexor, whereas the greatest synchrony was most often found in intrinsic foot muscles. Furthermore, the strength of corticospinal inputs to the abductor hallucis muscle, an intrinsic foot muscle, as assessed through transcranial magnetic stimulation, was weaker than that projecting to the tibialis anterior muscle, even though the abductor hallucis muscle had higher synchrony values compared with the tibialis anterior muscle. We argue, therefore, that factors other than the potency of cortical inputs to motor neurons, such as the number of motor neurons innervating a muscle, significantly affects motor unit synchrony.
Keywords: corticospinal, motoneuron, motor unit, muscle, synchrony
during voluntary contraction, motor units within a muscle usually exhibit coincident spiking (discharges occurring within just a few milliseconds of one another) more often than would be expected due to chance (Datta and Stephens 1990; Datta et al. 1991; Bremner et al. 1991a; Nordstrom et al. 1992; Schmied et al. 1993; Semmler and Nordstrom 1995; Keen and Fuglevand 2004; McIsaac and Fuglevand 2007; Dartnall et al. 2008; Barry et al. 2009). Such short-term synchronization typically has been attributed to last-order projections that provide simultaneous, common synaptic input across motor neurons (Moore et al. 1970, Sears and Stagg 1976; Kirkwood and Sears 1978, 1982; Kirkwood et al. 1982, Farmer et al. 1997). In particular, the extent of branched input arising directly from cortical neurons [i.e., corticomotoneuronal cells (Bernhard et al. 1954; Fetz and Cheney 1980, Porter and Lemon 1993)] has been suggested to be a critical factor influencing the strength of short-term synchronization during voluntary contraction (Adams et al. 1989; Datta et al. 1991; Farmer et al. 1993b; Semmler and Nordstrom 1995; Semmler, 2002). Indeed, in primates (including humans), corticomotoneuronal input to motor nuclei supplying distal muscles appears to be stronger than that supplying more proximal muscles (Phillips and Porter 1964; Clough et al. 1968; Lemon et al. 1986; Palmer and Ashby 1992; Porter and Lemon 1993; Carr et al. 1994; McKiernan et al. 1998, de Noordhout et al. 1999). Likewise, short-term synchrony seems more prevalent in distal muscles compared with proximal muscles (Datta et al. 1991; Farmer et al. 1993b; Marsden et al. 1999; Kim et al. 2001). Such a parallel in the strengths of cortical input and short-term synchrony, therefore, might imply that more potent corticomotoneural input causes greater short-term synchrony. A corollary of this idea is that the magnitude of short-term synchrony might be used as an indirect indicator of the degree of cortical input to motor neurons.
The relationship between short-term synchrony and the presumed extent of corticomotoneuronal input, however, has not been thoroughly investigated. Therefore, the purpose of this study was twofold. First, we sought to quantify the magnitude of short-term motor unit synchrony in several human muscles differing in the presumed extent of corticomotoneuronal input to their respective motor nuclei. While our results confirm the existence of striking variations in the extent short-term synchrony across muscles, with synchrony tending to be higher in more distally located muscles, the greatest synchrony was most often found in the intrinsic muscles of the foot rather than in the hand. Second, to more directly examine the association between short-term synchrony and cortical input, we used transcranical magnetic stimulation (TMS) to estimate the strength of cortical input to motor nuclei innervating two muscles that showed differing amounts of short-term synchrony. Somewhat unexpectedly, the muscle exhibiting greater synchrony appeared to possess weaker corticospinal input. In total, these results suggest that the magnitude of short-term synchrony is shaped, at least in part, by factors other than the strength of corticomotoneuronal input.
METHODS
One hundred and one experiments were performed on fourteen different muscles in thirty-three healthy human volunteers (18 women and 15 men, age: 19–47 yr). The following muscles were studied: third dorsal interosseous, extensor pollicis brevis, extensor carpi ulnaris, brachioradialis, brachialis, lateral head of the biceps brachii, middle head of the deltoid, lumbar multifidus, vastus lateralis, semitendinosus, lateral gastrocnemius, tibialis anterior (TA), extensor hallucis brevis, and abductor hallucis (AH). In addition, previously published data (Keen and Fuglevand 2004; McIsaac and Fuglevand 2007) were included from 8 and 14 experiments on the extensor digitorum (ED) and flexor digitorum superficialis (FDS), respectively. Data from the ED and FDS were restricted to trials when both electrodes were located in a muscle compartment that controlled either the middle or ring finger. The experimental procedures were approved by the Human Investigation Committee of the University of Arizona. All subjects gave their informed consent to participate in the study.
Electromyographic Recording
The experimental methods were essentially the same for all muscles tested. Motor unit action potentials were recorded with two tungsten microelectrodes (Frederick Haer and Co., Bowdoinham, ME) inserted at different locations into one of the target muscles to record the activity of separate motor units on each electrode. In most muscles, weak electrical stimulation was used to verify microelectrode placement in the target muscle based on the evoked motor response. In the large muscles (lateral head of the biceps brachii, middle head of the deltoid, lumbar multifidus, and vastus lateralis), electrode placement was verified when vigorous activity was detected during voluntary contractions of the target muscles. Microelectrodes were connected to differential amplifiers, and the intramuscular electromyographic (EMG) signals were amplified (×1,000), band-pass filtered (0.3–3 kHz, Grass Instruments), displayed on oscilloscopes, and digitally sampled (∼32 kHz/channel, Power 1401, Cambridge Electronics Design,, Cambridge, UK). Two surface electrodes (4-mm diameter, Ag-AgCl) attached to the skin overlying a bony prominence close to the muscle of interest served as reference electrodes for the intramuscular electrodes.
In addition, two other surface electrodes were attached to the skin over the muscle being studied on either side of the two microelectrodes to record surface EMG signals. These signals were differentially amplified (×1,000) band-pass filtered (0.03–1 kHz), and digitally sampled (2 kHz). Surface EMG signals were used to identify individual motor units using spike-triggered averaging (see below) to minimize inadvertent reanalysis of the same motor unit recorded in separate trials within an experiment (McIsaac and Fuglevand 2007).
Synchrony-Assessment Protocol
Subjects performed weak isometric contractions against a restraint to activate the target muscle. The microelectrodes were gently manipulated during the contraction until the action potentials of motor units could be clearly identified on each electrode. Once different motor units were identified on the two electrodes, subjects were instructed to sustain weak isometric contractions such that both units remained discharging tonically at low rates. Forces were not measured because most of the muscles we sampled do not exert joint torque in isolation from that contributed by other muscles. As such, the forces measured externally with a transducer are difficult to resolve into individual muscle contributions. Trials were recorded for 5 min or until the motor unit action potentials could no longer be clearly discriminated. Subjects received visual and auditory feedback on motor unit discharge and 1–2 min of rest between recordings. After each recording, the position of at least one of the microelectrodes (and often both) was adjusted until the action potentials of a presumed different motor unit were identified. This occasionally involved removal of a microelectrode and reinsertion at a new site. Force was not measured in these experiments, but most contractions were estimated to be <5% of the maximal voluntary force.
TMS
Eight additional experiments involving TMS were performed on five healthy volunteers (1 woman and 4 men, age: 32–49 yr). These experiments were performed to assess the strength of corticospinal input to two muscles: the right TA and right AH. The approach we used here was similar to that used by others (Palmer and Ashby 1992, de Noordhout et al. 1999). Single motor unit action potentials were recorded with tungsten microelectrodes inserted percutaneously into the target muscle. Subjects were instructed to perform a weak isometric contraction of the muscle while the microelectrode was manipulated until action potentials of a motor unit could be clearly identified. Subjects then maintained the unit discharging at a low and steady rate for ∼400 s while TMS (Magstim 200, Magstim) was applied at a rate of one pulse every 4 s using a double-cone coil positioned 2 cm posterior of the vertex to activate the leg and foot areas of the motor cortex. Each motor unit was tested at three different TMS intensities (40%, 45%, and 50% of maximum output) in different trials. After the three trials were completed for the motor unit, a new unit was identified for testing by adjusting the microelectrode position. The entire procedure was then repeated for the other muscle. The order of testing of the two muscles was varied across subjects.
Data Analysis
Synchrony.
An approach similar to that which we have previously described (Keen and Fuglevand 2004; Hockensmith et al. 2005; McIsaac and Fuglevand 2007) was used here to quantify synchronous discharge in pairs of motor units. Data analysis was carried out in the same way for all muscles. Briefly, motor unit discrimination was accomplished using a template-matching algorithm (Spike2, Cambridge Electronics Design) based on waveform shape and amplitude. Event channels representing the timing of discharges of accepted action potentials for each motor unit were generated. The discharge times of one unit were plotted relative to the discharge times of a second unit to generate a cross-correlation histogram. Cross-correlations were performed only for motor unit pairs that were recorded on separate electrodes. In situations where more than one unit was detected on a given electrode, multiple cross-correlations could be performed with units detected on the other electrode. However, when temporal overlap sporadically occurred between two units detected on the same electrode, the resulting distorted waveform shape did not match the templates. In such cases, no spike time was identified for either unit. This type of error is not serious for cross-correlation analysis as it simply provides slightly fewer counts in the histogram (Nordstrom et al. 1992). Cross-correlation histograms had 1-ms bin widths and included periods 100 ms both before and after the discharge of the reference unit. A peak in the cross-correlation histogram around time 0 represents the coincident firing of the two units greater than expected due to chance.
The central peak in the histogram was identified using the cumulative sum procedure, which involves progressively summing the differences in the number of counts in each bin of the histogram from the baseline mean bin count (Ellaway 1978). The baseline mean was calculated as the mean count in the first and last 60 ms of the cross-correlogram. A rise in the cusum near time 0 was used to delineate the peak in the cross-correlation histogram. Specifically, peak boundaries were determined as the bins corresponding to 10% and 90% of the difference between the minimum and maximum cumulative sum values (Schmied et al. 1993). A peak in a cross-correlogram was considered to be statistically significant if the z score (representing the number of SDs the mean count in the peak exceeded that in the baseline region) was ≥1.96, associated with P < 0.05.
The magnitudes of the peaks in the cross-correlograms were quantified using two synchronization indexes referred to as common input strength (CIS) and k′. The CIS was calculated as the total number of counts within the boundaries of the peak above the baseline mean divided by the duration of the recording and indicates the rate of extra synchronous impulses above that expected due to chance (Nordstrom et al. 1992). The k′ index was calculated as the ratio of the mean number of counts in the peak to the baseline mean (Ellaway and Murthy 1985). Both these indexes are widely used, and, therefore, reporting both allows for a more complete comparison of our results with other studies.
When cross-correlograms did not exhibit clear peaks, the method described above for identifying the region of the histogram for the calculation of CIS and k′ was not reliable. Therefore, when cross-correlograms did not exhibit statistically significant peaks, CIS and k′ were automatically calculated for an 11-ms region of the cross-correlogram centered at time 0 (Semmler and Nordstrom 1995). All CIS and k′ values, regardless of the method used for calculation, were included in the analysis.
TMS.
The firing probabilities of single motor units in TA and AH to three levels of TMS intensity (40%, 45%, and 50% of maximum output of the stimulator) were evaluated by constructing peristimulus time histograms (PSTHs). Approximately 100 stimuli were delivered at each intensity. PSTHs had 1-ms bin widths and included periods 100 ms both before and 400 ms after the stimulus. The mean and SD of the number of spikes per bin in the 100-ms prestimulus period were used to estimate baseline activity. The bin in the poststimulus period that possessed the greatest number of spike counts and for which the spike count was >2 SD above the baseline mean was identified as the peak firing probability in response to TMS. All contiguous bins on either side of this peak with counts > 2 SD above the baseline mean were considered as part of the peak region of increased firing probability. Firing probability in response to TMS was calculated as the total number of spike counts in the peak region divided by the number of stimuli delivered. The latency to increased firing probability was calculated as the time associated with the first bin in the identified peak region. Peak width was calculated as the total number of bins in the peak region multiplied by bin width.
Spike-triggered averaging.
We have shown previously that we can reliably identify individual motor units based on their surface-detected EMG signatures (Johns and Fuglevand 2005). Therefore, spike-triggered averaging of the surface EMG based on the discharge times of the discriminated motor units was performed to verify that each motor unit pair recorded during the synchrony-assessment protocol or individual motor units during TMS protocol were not inadvertently duplicated across trials. For every discharge of a motor unit, a brief segment (40 ms, 10-ms pretrigger) of the surface EMG signal was sent to averaging algorithm. The resulting spike-triggered average of the surface EMG was used to identify motor units based on visual inspection of waveform shape and amplitude. In cases for which it appeared that the same pair of motor units had been recorded in more than one trial for the synchrony protocol or that the same unit had been recorded across sets of trials for TMS, all parameters (e.g., k′, CIS, and firing probability) were averaged across the duplicate trials.
Statistics.
A Kruskal-Wallis test was performed on the values of k′ and CIS to determine whether short-term synchrony varied across the 16 muscles. The Kruskal-Wallis test is a nonparameteric test based on the sum of the ranks and is used to compare three or more unpaired groups with different sample sizes. For the TMS data, two-way ANOVA, with muscle and stimulus intensity as factors, was applied to firing probability values to determine whether the efficacy by which cortical stimulation provokes motor unit firing differed between TA and AH muscles and across stimulus intensities. Values are reported as means ± SD, with P < 0.05 selected as the level of statistical significance.
RESULTS
Synchrony.
Figure 1 shows example recordings from pairs of motor units detected in an intrinsic hand muscle (A) and from a trunk muscle (B). A total of 499 cross-correlation histograms were generated from such motor unit recordings in 14 different muscles. Based on the spike-triggered average surface EMG, 44 histograms of these were thought to be duplicate trials, leaving a total of 455 different cross-correlation histograms generated from 831 motor units. In 65 trials, >1 unit was discriminated on an electrode, which yielded multiple correlations. Of the 455 cross-correlograms generated, 217 cross-correlograms had significant synchrony peaks with an average peak duration of 11.2 ± 4.7 ms. In addition, data from 29 and 67 motor unit pairs from the ED (Keen and Fuglevand 1994) and FDS (McIsaac and Fuglevand 2007), respectively, are included in the present report to yield a total of 551 cross-correlation histograms. The average number of correlations used to generate the cross-correlograms was 3,774 ± 2,032. The mean firing rate for all of the recorded units was 8.9 ± 2.1 Hz with a mean coefficient of variation in the interspike interval of 0.15 ± 0.03 (see Table 1). Interestingly, the mean firing rate for motor units in upper limb muscles was 10.3 ± 1.6 Hz, which was significantly greater (P < 0.001 by Mann-Whitney U-test) than the mean discharge rates of 7.2 ± 1.3 Hz for multifidus and lower limb motor units. While motor unit synchrony can be modestly affected by differences in discharge rate associated with varying levels of central drive to a particular motor neuron pool (Schmied and Descarreaux 2010), it seems likely that the differences in firing rate seen across muscles in the present study were more probably related to systematic variations in intrinsic motor neuron properties [e.g., afterhyperpolarization duration (Kernell 1965)] than synaptic drive. Nevertheless, we cannot dismiss a small effect that firing rate differences may have on synchrony measures across upper and lower limb muscles.
Fig. 1.
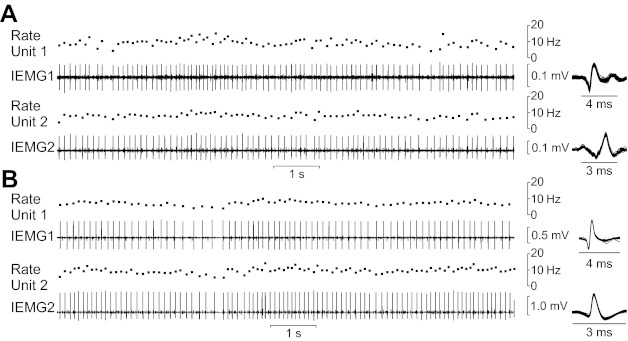
A and B: example recordings of pairs of motor units from a distal muscle [third dorsal interosseus (3DI); A] and from a proximal muscle [lumbar multifidus (M); B]. These 10-s samples were taken from trials each lasting ∼5 min. In A and B, the second and fourth traces show the intramuscular electromyographic (IEMG) signals detected by the two microelectrodes in the target muscle. Shown above each IEMG signal is the associated instantaneous discharge rate of the motor unit detected in the IEMG signal. The insets show all the motor unit action potentials identified for each channel for this 10-s segment overlaid on a brief time scale. The discharge times of one unit relative to the other were then used to generate cross-correlation histograms.
Table 1.
Synchrony and discharge properties for pairs of motor units residing in various muscles
| Muscle | Number of Subjects | Number of Pairs | Percentage of Significant Peaks | k′ | CIS | Peak Duration, ms | Firing Rate, Hz | Coefficient of Variation of the Interspike Interval |
|---|---|---|---|---|---|---|---|---|
| Third dorsal interosseous | 11 | 34 | 70.6 | 2.14 ± 1.09 | 0.64 ± 0.53 | 12.4 ± 6.8 | 9.5 ± 1.8 | 0.17 ± 0.03 |
| Extensor pollicis brevis | 12 | 32 | 75.0 | 1.67 ± 0.38 | 0.47 ± 0.25 | 9.7 ± 3.5 | 10.1 ± 1.4 | 0.16 ± 0.03 |
| Extensor digitorum | 6 | 29 | 75.9 | 1.59 ± 0.31 | 0.67 ± 0.33 | 11.0 ± 3.3 | 11.3 ± 2.2 | 0.19 ± 0.03 |
| Flexor digitorum superficialis | 12 | 67 | 70.1 | 1.55 ± 0.34 | 0.40 ± 0.21 | 8.0 ± 2.5 | 10.5 ± 1.4 | 0.15 ± 0.03 |
| Extensor carpi ulnaris | 8 | 30 | 40.0 | 1.34 ± 0.24 | 0.30 ± 0.22 | 10.4 ± 3.0 | 10.1 ± 1.7 | 0.15 ± 0.02 |
| Brachioradialis | 9 | 30 | 30.0 | 1.23 ± 0.29 | 0.24 ± 0.29 | 10.1 ± 2.0 | 10.2 ± 1.3 | 0.13 ± 0.02 |
| Brachialis | 8 | 30 | 6.7 | 1.12 ± 0.11 | 0.15 ± 0.14 | 9.1 ± 2.7 | 11.0 ± 1.5 | 0.12 ± 0.02 |
| Biceps brachii | 6 | 30 | 16.7 | 1.17 ± 0.15 | 0.17 ± 0.16 | 8.6 ± 2.0 | 10.4 ± 1.3 | 0.13 ± 0.02 |
| Deltoid | 6 | 32 | 62.5 | 1.44 ± 0.23 | 0.43 ± 0.27 | 10.9 ± 2.6 | 10.0 ± 1.3 | 0.15 ± 0.02 |
| Multifidus | 5 | 35 | 17.1 | 1.23 ± 0.27 | 0.08 ± 0.09 | 8.0 ± 4.4 | 7.2 ± 1.3 | 0.14 ± 0.02 |
| Vastus lateralis | 4 | 31 | 12.9 | 1.14 ± 0.21 | 0.05 ± 0.07 | 5.1 ± 1.6 | 7.3 ± 1.8 | 0.13 ± 0.02 |
| Semitendinosus | 5 | 34 | 35.3 | 1.34 ± 0.38 | 0.15 ± 0.20 | 8.1 ± 3.4 | 7.5 ± 1.1 | 0.13 ± 0.03 |
| Lateral gastrocnemius | 8 | 35 | 22.9 | 1.32 ± 0.32 | 0.13 ± 0.16 | 10.1 ± 6.8 | 7.1 ± 1.3 | 0.14 ± 0.02 |
| Tibialis anterior | 5 | 31 | 58.1 | 1.60 ± 0.43 | 0.40 ± 0.32 | 14.2 ± 5.4 | 7.8 ± 0.9 | 0.14 ± 0.02 |
| Extensor hallucis brevis | 12 | 32 | 93.8 | 2.43 ± 0.85 | 0.51 ± 0.32 | 12.3 ± 4.2 | 7.2 ± 0.8 | 0.17 ± 0.03 |
| Abductor hallucis | 10 | 39 | 84.6 | 2.69 ± 1.17 | 0.42 ± 0.23 | 12.3 ± 3.3 | 6.6 ± 1.0 | 0.17 ± 0.02 |
The number of subjects refers to the number of subjects that contributed data from a particular muscle; 20 of 33 subjects contributed data for >1 muscle. For the percentage of significant peaks, the duration of the cross-correlogram peak was measured only for those histograms exhibiting a significant peak.
An average of 33 ± 3 cross-correlation histograms was generated for each muscle. Representative cross-correlograms from each muscle are shown in Figure 2. Cross-correlograms are arranged somatopically in Fig. 2 to represent muscles from the hand to foot from the top to bottom. As shown in these examples, substantial synchrony was found for pairs of motor units located in the distal musculature of the hand and foot. With the exception of the deltoid muscle, the amount of synchrony generally decreased in muscles located more proximally.
Fig. 2.
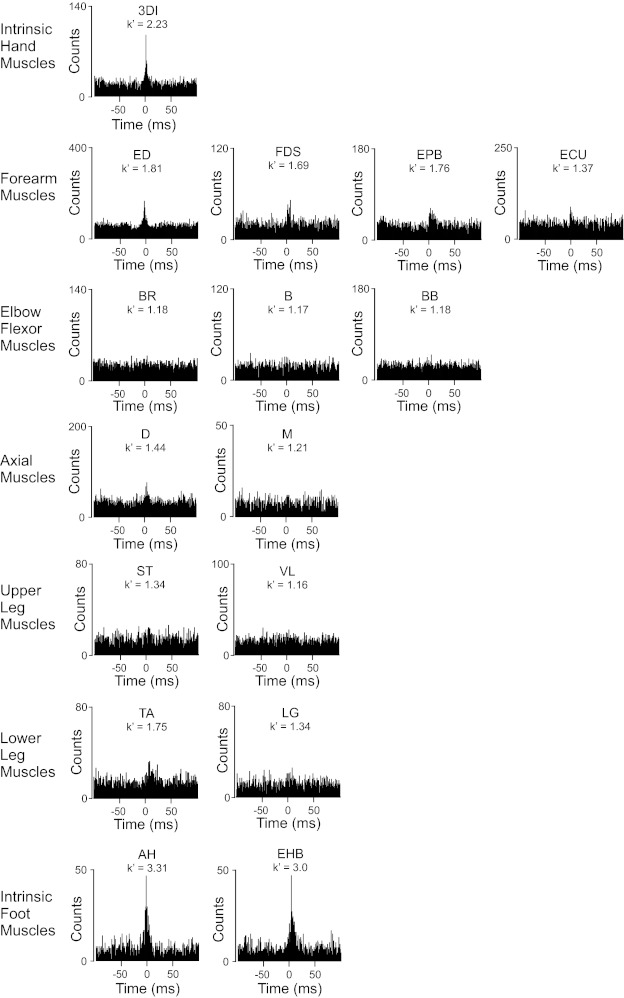
Example cross-correlation histograms for pairs of motor units recorded in 16 different human muscles. A measure of synchrony magnitude (k′) is indicated for each histogram. ED, extensor digitorum; FDS, flexor digitorum superficialis; EPB, extensor pollicis brevis; ECU, extensor carpi ulnaris; BR, brachioradialis; B, brachialis; BB, lateral head of the biceps brachii; D, middle head of the deltoid; VL, vastus lateralis; S, semitendinosus; LG, lateral gastrocnemius; TA, tibialis anterior; AH, abductor hallucis; EHB, extensor hallucis brevis. The example histograms shown for the 3DI and M muscles were derived from the firing times obtained from the recordings shown in Fig. 1.
The average duration of the peaks in the cross-correlograms varied across muscles from 5.1 to 14.2 ms (Table 1). These values are generally in the range of those considered to be associated with last-order synaptic inputs (Kirkwood et al. 1982; Bremner et al. 1991b; Carr et al. 1994; see also Farmer et al. 1997). Nevertheless, we cannot discount a role played by noncommon presynaptic inputs whose activity patterns were themselves correlated.
Kruskal-Wallis tests revealed significant differences across muscles in motor unit synchrony assessed with either k′ (P < 0.001) or CIS (P < 0.001). The levels of motor unit synchrony across all 16 muscles are shown in Fig. 3 using the proportion of the significant peaks in the cross-correlograms, k′, and CIS. While the results were generally similar for the three metrics of synchrony, there were also some differences. For example, the muscles with the largest proportion of significant peaks and k′ values were the intrinsic foot muscles, whereas the muscle with the largest CIS value was an extrinsic hand muscle, the ED. The reasons for such differences are likely related to subtle differences in the information represented by the various synchrony indices. The k′ index, for example, indicates the average count of bins in the peak region above that in the off-peak region. As such, it provides a conceptually simple, average measure of the height of the peak above baseline. CIS, on the other hand, provides a normalized measure of the area of the peak above baseline. Therefore, one can have a relatively large value of CIS when the peak is low but broad, whereas such a profile would yield a small value of k′. On the other hand, a tall narrow peak will yield a large value of k′ but only a modest value of CIS.
Fig. 3.
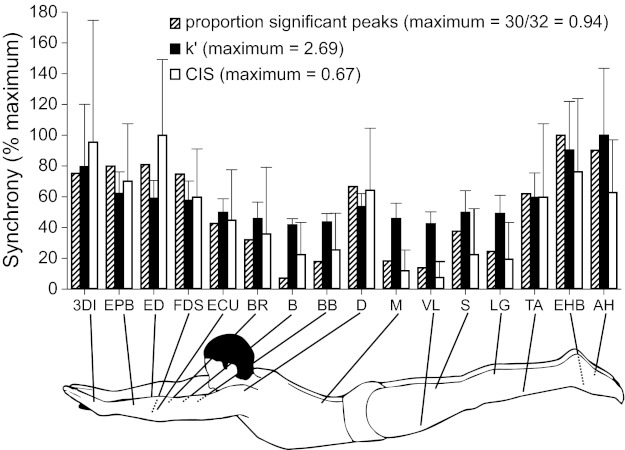
Mean synchrony indexes for motor unit pairs in 16 different muscles. For each synchrony index [proportion of significant peaks, k′, or common input strength (CIS)], mean values were expressed relative to the muscle exhibiting the largest value. The muscles with the largest synchrony values for each index were as follows: EHB (intrinsic foot muscle), AH (intrinsic foot muscle), and ED (extrinsic hand muscle) for the proportion of significant peaks, k′, and CIS, respectively. For k′ and CIS, error bars represent 1 SD. Overall, there was a tendency for distal muscles to exhibit greater synchrony than more proximal muscles. Furthermore, intrinsic foot muscles, whose motor nuclei presumably have less direct cortical input than other muscles examined, tended to exhibit the greatest amount of synchrony. Abbreviations for the muscles are defined in Fig. 2.
Regardless of the metric, as shown in Fig. 3, there appeared to be a general trend for motor unit synchrony to be greatest in the distal musculature and least in muscles located more proximally. The deltoid muscle seemed to be an exception to this trend, as 20 of 32 (i.e., 63%; Table 1) cross-correlograms for the middle head of the deltoid exhibited significant peaks. Figure 4 shows the relation between synchrony (k′) and proximal-distal location of the muscle for all 551 motor unit pairs. We expressed proximal-distal location in two ways. In one way (Fig. 4A), location was based on the approximate number of joints traversed from the torso outward. Thus, trunk muscles were assigned a value = 0, shoulder/hip muscles = 1, elbow and knee muscles = 2, wrist and ankle muscles = 3, extrinsic hand or foot muscles = 4, and intrinsic hand or foot muscles = 5. In the other way (Fig. 4B), location was based on distance from the spinal cord. This was estimated by measuring the physical distance (in cm) from the spinous processes of C6–C7 vertebrae (for an upper limb) and from L3–L4 vertebrae (for a lower limb) to the estimated center of each of the target muscles. These distances were then normalized to a percentage of limb length (C6–C7 to finger tip distance and L3–L4 to tip of large toe distance) and averaged across two subjects (one man and one woman). Despite the extensive variability in synchrony values, there was a significant relation (P < 0.001) between motor unit synchrony and proximal-distal location regardless of the method used to characterize muscle location. Similar results were obtained (not shown) if using CIS or the percentage of significant peaks as indexes of synchrony.
Fig. 4.
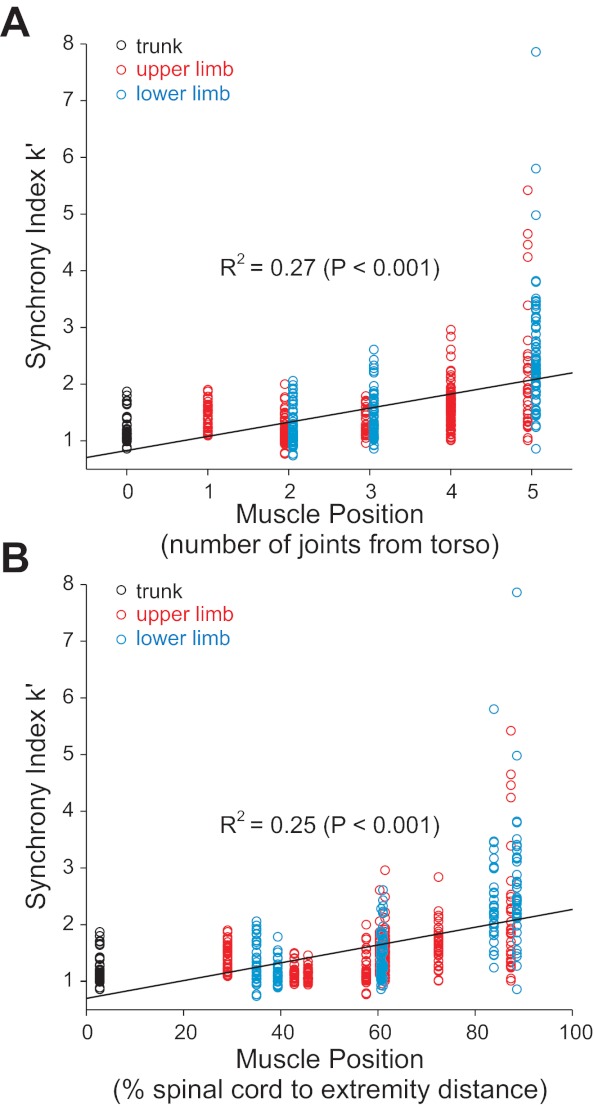
Relation between muscle location and k′ for 551 motor unit pairs. A: proximal-distal location based roughly on the number of joints traversed from the torso outward and was assigned a value of 0 for trunk muscles, 1 for shoulder or hip muscles, 2 for elbow or knee muscles, 3 for wrist or ankle muscles, 4 for extrinsic hand or foot muscles, and 5 for intrinsic hand or foot muscles. B: muscle location based on estimated distance from the spinal cord to the center of muscle. Solid lines indicate significant linear regression (P ≤ 0.001).
Nevertheless, proximal-distal location was not a singular predictor of synchrony. For example, the muscles that exhibited the least amount of synchrony (see Fig. 3) were the brachialis, biceps brachii, and vastus lateralis muscles, with only 6.7%, 16.7%, and 12.9%, respectively, of the cross-correlograms having significant peaks. Post hoc analyses (Dunn's method) showed these muscles to possess significantly (P < 0.05) smaller synchrony values (for both k′ and CIS) than the more proximal deltoid muscle. Interestingly, motor unit synchrony appeared to be greatest in the intrinsic foot muscles for two of the three metrics used. For example, 85% of the cross-correlograms for the AH had significant peaks, and the AH had a mean k′ value of 2.7 ± 1.2, which was significantly greater (post hoc analysis, P < 0.05 by Dunn's method) than, e.g., that in the TA (1.6 ± 1.4), which had 58% of its cross-correlograms with significant peaks. While the mean values of k′ were greater for intrinsic foot muscles (AH and extensor hallucis brevis) than for an intrinsic hand muscle (third dorsal interosseous), these differences were not significant (P > 0.05) upon post hoc comparison.
TMS
Noting the difference in motor unit synchrony between AH and TA muscles, the strength of projections from the motor cortex to motor neurons controlling these two muscles was examined indirectly using TMS. On average, 94 ± 26 and 91 ± 29 stimulation pulses were delivered at each stimulation intensity while 21 and 22 motor units were recorded from the TA and AH, respectively. Figure 5 shows example PSTHs from a single subject for the TA (A) and AH (B) at stimulation strengths of 40%, 45%, and 50% of the maximum output. In general, with increased stimulus intensity, there was an increased probability for the motor unit to spike at relatively fixed latencies from the stimulus. On average, the latency to increased firing probability in the AH (46 ± 7 ms) was significantly (P < 0.001) greater than that of the TA (35 ± 5 ms), as would be expected for the longer conduction distance to AH. Also, as shown in Fig. 5, for the same stimulus intensity, firing probabilities appeared to be larger for the TA than for the AH. The absence of firing probability around time 0 in Fig. 5 was due to the stimulus artifact temporarily preventing identification of motor unit action potentials.
Fig. 5.
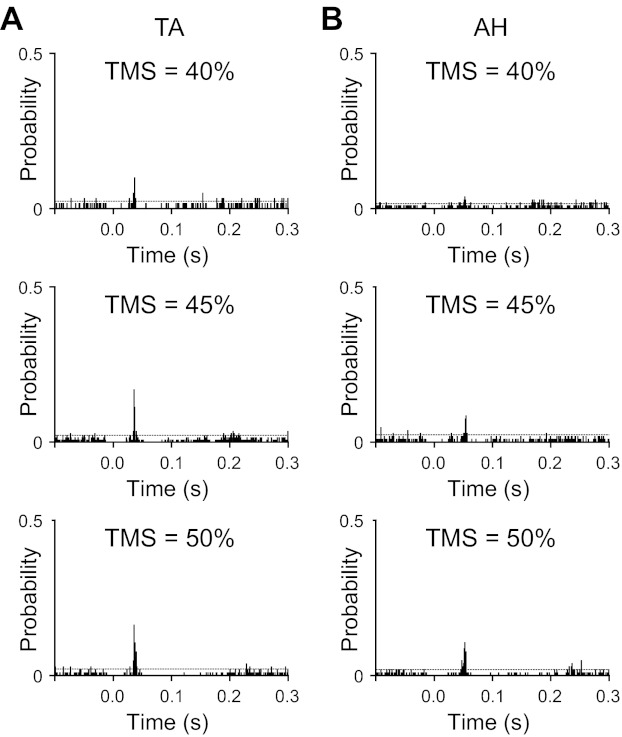
A and B: example transcranial magnetic stimulation (TMS)-evoked peristimulus histograms (PSTHs) generated for a TA muscle (A) and an AH muscle (B) motor unit from one subject. For the TA, the histogram peaks occurred 34 ms after TMS was delivered at all three intensities. For the AH, the histogram peaks had latencies of 50, 50, and 47 ms in response to TMS at intensities of 40%, 45%, and 50% of stimulator output, respectively. For both the TA and AH, the probability for motor units to discharge in response to TMS increased with TMS intensity. In addition, TA motor units had a higher probability to discharge in response to TMS than AH motor units at all three intensities. The dashed horizontal line indicates 2 SD above the prestimulus baseline mean.
As shown in Fig. 6, the average ± SD probability that TMS elicited a short-latency response in TA motor units was 0.20 ± 0.15, 0.34 ± 0.19, and 0.41 ± 0.21 at stimulation intensities of 40%, 45%, and 50%, respectively. This compares with smaller values of 0.11 ± 0.07, 0.24 ± 0.12, and 0.29 ± 0.16 at the same stimulus intensities for the AH. Two-way ANOVA confirmed that there was a significant difference in firing probability for the two muscles (P < 0.004) and that there was a significant effect of stimulus intensity (P < 0.001). There was no significant interaction (P = 0.94) in firing probability between the factors of muscle and stimulus intensity. The average durations of increased firing probability for the TA at stimulus intensities of 40%, 45%, and 50% were 2.9 ± 2.3, 4.4 ± 3.8, and 5.1 ± 2.7 ms, respectively. These were not significantly different (P = 0.81) than the peak durations found at the same intensities for the AH, which were 2.7 ± 1.9, 5.0 ± 2.2, and 5.2 ± 2.8 ms. There was, however, a significant effect of stimulus intensity on the duration of increased firing probability (P < 0.004). Post hoc testing (Holm-Sidak test) indicated a significantly briefer duration of increased firing probability at an intensity of 40% compared with that at 45% (P < 0.006) or at 50% (P < 0.003). The increased duration in firing probability with increased TMS intensity is likely due to additional activation of later corticospinal volleys (I waves) as stimulus strength increased (Di Lazzaro et al. 2001).
Fig. 6.
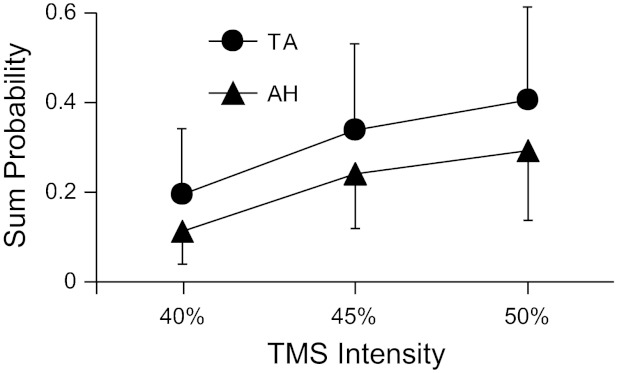
Mean (SD) probability that TMS evoked a short-latency excitatory response in TA (21 units) and AH (22 units) motor units. There was a significant difference in firing probability for the two muscles (P < 0.004) and a significant effect of stimulus intensity (P < 0.001 by two-way ANOVA). Sum probability indicates the total number of counts within the peak region of the PSTH divided by the total number of stimuli.
DISCUSSION
Electrophysiological evidence obtained in monkeys (Phillips and Porter 1964, Clough et al. 1968, Jankowska et al. 1975; Lemon et al. 1986, Porter and Lemon 1993; McKiernan et al. 1998) and humans (Rossini et al. 1985, Benecke et al. 1988, Brouwer and Ashby 1990, Palmer and Ashby 1992, Carr et al. 1994; Kischka et al. 1993; de Noordhout et al. 1999) indicates that motor neurons supplying more distal muscles, particularly intrinsic muscle of the hand, receive greater extents of cortical input compared with more proximally located muscles. Likewise, there is some evidence to suggest that motor units located in more distally located muscles possess higher levels of synchrony than more proximal muscles (Datta et al. 1991; Farmer et al. 1993b; Marsden et al. 1999; Kim et al. 2001). Such a parallel between the strength of synchrony and extent of cortical input, in combination with other evidence (described below), has led to the view that the most significant contributor to motor unit synchronization is direct projections from the motor cortex to motor neurons (Datta et al. 1991; Farmer et al. 1993b; Semmler et al. 2000; Semmler 2002). Here, we thoroughly examined the relationship between motor unit synchrony and muscle location and indeed found a tendency for more distally located muscles to possess higher synchrony. However, proximal-distal location was not an exclusive predictor of the strength of synchrony. Indeed, the muscle that exhibited the least amount of synchrony was an elbow flexor, whereas the greatest synchrony most often occurred in intrinsic foot muscles. Furthermore, the strength of corticospinal inputs to the AH (an intrinsic foot muscle), as assessed through TMS, appeared to be weaker than that projecting to the TA, despite the AH exhibiting an ∼70% higher k′ value of synchrony compared with the TA. Thus, it would appear that factors other than the overall potency of cortical inputs to motor neurons play important roles in shaping the degree of motor unit synchrony. Below, we consider factors that might affect the extent of motor unit synchrony seen across diverse motor nuclei.
Factors Influencing Motor Unit Synchrony
Last-order excitatory inputs branch extensively to provide common inputs to many neurons in a motor nucleus (Mendell and Henneman 1971, Lawrence et al. 1985, Mantel and Lemon 1987, Alstermark et al. 1990). Activity in these branched inputs will cause near simultaneous excitatory postsynaptic potentials (EPSPs) in the recipient motor neurons and thereby slightly increase the probability that these neurons will spike together. In general, the extent of coincident activity, manifested as a narrow central peak in the cross-correlation histogram of the firing times of pairs of motor units, is thought to be primarily related to 1) the proportion of common to noncommon excitatory inputs to motor neurons and 2) the average magnitude of the EPSPs arising from the common synaptic inputs (Sears and Stagg 1976; Kirkwood and Sears 1978; Gustafsson and McCrea 1984; Datta and Stephens 1990; Bremner et al. 1991b; Nordstrom et al. 1992; Farmer et al. 1997; Binder and Powers 2001).
One question then that should be addressed has to do with why cortical inputs are thought to be more evocative of synchrony than other inputs. In part, this idea stems from empirical observations showing that short-term synchrony is minimally affected by deafferentation (Kirkwood et al., 1982, Farmer et al. 1993b) yet is diminished with lesions of descending pathways (Kirkwood et al. 1982; Datta et al., 1991; Farmer et al. 1993a; Marsden et al. 1999). Furthermore, individuals with abnormal bilateral corticospinal projections associated with pathological mirror movements exhibit striking motor unit synchrony across homologous muscles on opposite sides of the body (Farmer et al. 1990, 2004; Mayston et al. 1997, 2001). Collectively, these observations imply that the primary source of synchrony is central rather than peripheral. This may seem surprising given the extensive distribution of muscle spindle primary afferent (Ia) input across a motor nucleus (Mendell and Henneman 1971, Lüscher and Vadar 1989). Such prolific divergence, in theory, should provide considerable common synaptic input, which, in turn, should contribute to significant short-term synchrony.
Peripheral Sources
There are at least two aspects of such peripheral input, however, that might limit its influence on motor unit synchrony during voluntary activity. First, presynaptic inhibition of the spinal terminals of Ia fibers (or other primary afferents) may markedly diminish the efficacy of such input onto their target neurons during voluntary contraction (Schieppati and Crenna 1984; Stein 1995; Seki et al. 2003). Second, it may be that the relative proportion of synaptic inputs arising from peripheral sources that drive motor neurons during voluntary contraction might be quite small compared with central sources, thereby limiting their overall ability to provoke simultaneous discharge across motor neurons. It should also be noted that Ia input to motor neurons supplying distal muscles is relatively weak (Marsden et al. 1976; Lenz et al. 1983; Fritz et al. 1989), yet short-term synchrony tends to be greatest in these muscles. Collectively, therefore, this evidence suggests that synaptic input from peripheral afferents is unlikely to contribute markedly to short-term synchrony during voluntary contraction.
Interneuronal Sources
It is likely that the most abundant source of synaptic input to motor neurons is from spinal interneurons. Little information, however, is presently available about the distribution of synaptic contacts arising from segmental or long propriospinal interneurons to motor neurons. Jankowska and Roberts (1972) estimated that ∼20% of the motor neurons belonging to a motor nucleus received synaptic input from identified individual interneurons. If other interneurons possess similar axon terminal distributions to motor neurons, then this would seem sufficient divergence to underlie widespread common input needed to provoke significant short-term synchrony. Furthermore, recent evidence indicates that many spinal interneurons are robustly active during voluntary activity (Perlmutter et al. 1998; Fetz et al. 2002; Riddle and Baker 2010; Takei and Seki 2010). Therefore, there would not seem to be a compelling argument for discounting spinal interneurons as potential mediators of short-term synchronization.
Brain Stem Sources
In addition to corticospinal inputs, there are descending pathways originating in the brain stem that provide direct synaptic contact with spinal motor neurons (Kuypers 1981). In monkeys, rubrospinal inputs appear to make monosynaptic connections with motor neurons, particularly those supplying the distal musculature (Shapovalov et al. 1971; Belhaj-Saïf et al. 1998). Interestingly, however, the rubrospinal tract in humans seems vestigial and often is not anatomically detectable (Nathan and Smith 1982; Yang et al. 2011). Therefore, it seems unlikely that the rubrospinal tract contributes significantly to motor unit synchrony in human subjects.
Vestibulospinal and reticulospinal pathways also make monosynaptic connections with spinal motor neurons (Kuypers 1981). Vestibular nuclei appear to provide monosynaptic input primarily to motor neurons supplying neck and trunk muscles and extensor muscles of the hindlimb (Wilson and Peterson 1978). Likewise, reticulospinal fibers make direct connections with motor neurons supplying neck and trunk muscles as well as with motor neurons innervating a broad array of limb muscles (Peterson et al. 1979; Davidson et al. 2007, Riddle et al. 2009). Overall, the general connectivity of vestibulo- and recticulospinal inputs to motor neurons would seem to be somewhat more prominent for proximal than distal muscles. This pattern of connectivity is not consistent with the spatial organization of motor unit synchrony observed in the present study.
Differences in EPSP Magnitudes
In addition to the extent of axon arborization, another factor that might predispose cortical inputs as particularly potent contributors to short-term synchronization would be if the average size of their EPSPs tended to be greater than that of other inputs. Unitary EPSPs associated with activity of single Ia afferents recorded in motor neurons supplying a variety of muscles in the anesthetized cat have average amplitudes from ∼90 to 170 μV with overall ranges from ∼20 to 600 μV (Mendell and Henneman 1971; Scott and Mendell 1976; Nelson and Mendell 1978; Kirkwood and Sears 1982). As far as we are aware, only one study (Asanuma et al. 1979) examined EPSPs in spinal motor neurons in response to presumed activation of single motor cortical neurons. In that study, just two such recordings were made in motor neurons of anesthetized monkeys, with unitary EPSPs of 25 and 120 μV. Porter and Hore (1969) and Jankowska et al. (1975) compared minimal EPSPs evoked in response to threshold stimulation of the motor cortex and dorsal root in the same lumbar motor neurons of the monkey. While those responses could not be categorically characterized as unitary, the amplitudes of such minimal EPSPs elicited in response to cortical and Ia afferent stimulation were not different from one another. Based on this somewhat limited evidence, therefore, there is no clear reason to conclude that EPSPs in motor neurons are larger for cortical compared with peripheral inputs.
Temporal Facilitation
Another factor that should be considered related to the efficacy by which cortical inputs might contribute to synchrony has to do with the degree of temporal facilitation (i.e., greater than linear temporal summation) of EPSPs associated with repetitive presynaptic activity. Cortical inputs to spinal motor neurons exhibit marked temporal facilitation of EPSPs (Landgren et al. 1962; Porter and Hore 1969; Porter 1970; Muir and Porter 1973), whereas little such facilitation occurs in response to Ia inputs (Curtis and Eccles 1960; Phillips and Porter 1964; Porter and Hore 1969). However, such temporal facilitation of cortical inputs to motor neurons typically occurs only for interspike intervals less than ∼15 ms [i.e., spike frequencies > 66 Hz (Porter 1970)]. Corticomotoneuronal cells rarely spike at rates above ∼60 Hz (Evarts 1968) except during strong contractions (Cheney and Fetz 1980) or briefly at the outset of a contraction (Cheney and Fetz 1980; Davies et al. 2006). As such, it seems unlikely that EPSP amplification (associated with temporal facilitation) could account for the increased probability of coincident spiking in motor units, as seen in the present study, involving weak contractions sustained for several minutes.
Inhibition
Theoretical and experimental work has shown that common synaptic inhibition can also lead to significant peaks in cross-correlograms (Moore et al. 1970; Türker and Powers 2001; cf. Maltenfort et al. 1998). An important source of inhibition to spinal motor neurons is from Renshaw cells activated by recurrent collaterals of homonymous and synergist motor neurons (Eccles et al. 1961). Indeed, increases in such recurrent inhibition have been shown to increase the magnitude of short-term synchrony in human motor units (Mattei et al. 2003). Interestingly, however, recurrent inhibition seems absent in motor neurons supplying digit muscles of the upper (Horner et al. 1991; Katz et al. 1993) and lower limb (Cullheim and Kellerth 1978; Rossi and Mazzocchio 1991) in both cats and humans. Because the strongest synchrony was observed here for digit muscles of the foot and hand, it seems unlikely, therefore, that inhibition-mediated synchrony (at least that due to recurrent inhibition) could account for the proximal to distal increase in motor unit synchrony seen in the present study.
Corticospinal Sources
In the above sections, several possible contributors to motor unit synchrony, other than that arising from direct corticospinal inputs, were considered. None of these possess a pattern of influence that would seem entirely consistent with the spatial differences in motor unit synchrony observed across muscles. In contrast, the efficacy of corticospinal inputs to motor neurons assessed by the magnitude of 1) compound EPSPs recorded in motor neurons in response to stimulation of motor cortex or the pyramidal tract (Clough et al. 1968; Jankowska et al. 1975; Porter and Lemon 1993), 2) cortical spike-triggered averages of EMG signals (McKiernan et al. 1998), or 3) evoked potentials recorded in human muscle in response to stimulation of the motor cortex (Rossini et al. 1985, Benecke et al. 1988, Brouwer and Ashby 1990, Palmer and Ashby 1992, Kischka et al. 1993; de Noordhout et al. 1999) exhibits a proximal to distal increase similar to that observed for motor unit short-term synchrony arising during voluntary contraction. This correlation in the strengths of cortical input and short-term synchrony, therefore, might be taken to imply that more potent cortical input causes greater short-term synchrony.
There are some considerations, however, that raise questions as to the validity of such an idea. First, on theoretical grounds, there is no clear reason as to why cortical inputs should be more capable of eliciting synchronized discharges among motor units than other inputs. As mentioned above, there is no definitive evidence that individual cortical inputs diverge to contact more members of a motor neuron population than do peripheral or interneuronal inputs. Likewise, there is no indication that unitary EPSPs are larger for cortical inputs compared with other inputs.
In addition, there are aspects of the spatial pattern of short-term synchrony (Fig. 3) that seem incongruent with presumed differences in the extent of cortical input to various motor nuclei. Most striking is the high degree of short-term synchrony found within intrinsic foot muscles. It should be pointed out that control of voluntary contractions in the AH muscle presented a significant challenge in most subjects tested, which, at face value, seems incompatible with AH motor neurons receiving the greatest degree of direct cortical input. Furthermore, the short-term firing probability of AH motor units in response to magnetic stimulation of the cortex was weaker than that for TA motor units (Figs. 5 and 6), suggesting less potent cortical inputs to the AH than TA. In contrast, the elbow flexors rarely exhibited discernable short-term synchrony (Figs. 2 and 3 and Table 1) (see also Farmer et al. 1990), yet voluntary control over the elbow flexion presented no particular cognitive challenge to subjects. Also, direct cortical linkages to elbow flexor motor neurons have been demonstrated in monkeys [based on cortical spike-triggered averages of the EMG (Fourment et al. 1995)] and in humans [based on short-latency EMG responses to TMS (Palmer and Ashby 1992)].
An Alternate Possibility: Motor Neuron Number
It is possible that the many factors described above all play a role in influencing synchrony but in different proportions for different motor neuron pools. As such, it may be the specific combination of factors that gives rise to the observed values of synchronization across muscles, rather than a single factor. It could also be that differences in short-term synchrony across muscles depend on factors in addition to those related to synaptic inputs, such as the size of the target motor neuron population. If one assumes that the degree of ramification is roughly similar for presynaptic axons targeting different motor nuclei, then the number of motor neurons within a nucleus should have a direct influence of the degree of common synaptic input. Consider, for example, a simplified scheme in which each presynaptic neuron gives rise, on average, to 100 terminal branches, and each branch provides one synaptic contact per target neuron. If the target pool possesses only 100 motor neurons, then every motor neuron in this population would receive nearly identical synaptic input. If, on the other hand, the target pool consists of 1000 motor neurons, and each input randomly targets 100 of these neurons, then the overall degree of common synaptic input across this larger population would be diluted to ∼1/10th that of the small pool. According to this simple scheme, therefore, there should exist an inverse relationship between the size of the motor neuron pool and the magnitude of short-term synchrony (reflecting the average degree of common synaptic input).
Unfortunately, there are no direct measures of motor neuron number in human subjects. However, retrograde labeling of motor neurons in nonhuman primates has shown there to be wide variations (10- to 20-fold differences) in the numbers of motor neurons innervating different upper limb muscles (Jenny and Inukai 1983). For example, some intrinsic muscles of the hand are supplied by only ∼50 motor neurons, whereas muscles like the biceps brachii are innervated by >1,000 motor neurons.
Figure 7 shows a plot of the average k′ synchrony index values measured in the present study against estimates of motor neuron number taken from different sources. Where available (namely, for the biceps brachi and extensor carpi ulnaris), direct measures of motor neuron number were taken from the Jenny and Inukai (1983) study. We also used estimates derived from counts of large myelinated axons in human cadavers (Feinstein et al. 1955) for numbers of motor neurons supplying the brachioradialis and TA. For the third dorsal interosseus and lateral gastrocnemius, we used estimates based on axon counts for the first dorsal interosseus and medial gastrocnemius, respectively (Feinstein et al. 1955). For the AH, we previously estimated the number of motor units in this human muscle (Johns and Fuglevand 2011) based on the electrophysiological methods originally developed by McComas and colleagues (1971). Recently, however, a validation of this method carried out in an animal model showed that electrophysiologically based counts underestimate the number of motor units on average by ∼70% (David et al. 2010). Therefore, for the results shown in Fig. 7, we increased our original count of AH motor units by 70%.
Fig. 7.
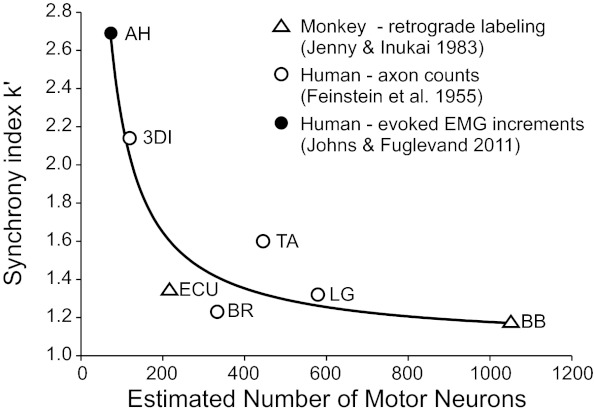
Average value of k′ plotted against estimates of motor neuron number (MNN). Data were fit (R2 = 0.90, P = 0.001) with the following inverse function: k′ = 1.06 + (1/0.0085 × MNN). MNNs were obtained from three different sources: 1) retrograde labeling of motor neurons in the monkey [ECU: 216 and BB: 1,051 (Jenny and Inukai, 1983)], 2) motor axon counts from a human cadaver [3DI: 119 from the first dorsal interosseus, BR: 333, LG: 579 from the medial gastrocnemius, and TA: 445 (Feinstein et al. 1955)], and 3) presumed unitary increments in evoked EMG responses [AH: 73 based on an original estimate of 43 (Johns and Fuglevand 2011) multiplied by 1.7 (David et al. 2010)]. Overall, there was an inverse relation between synchrony magnitude and number of motor neurons innervating a muscle.
Given the many assumptions underlying estimates of motor unit number, the upmost caution should be exercised in interpreting the specific values shown in Fig. 7. Indeed, the results shown in Fig. 7 represent a kind of meta-analysis, based on data taken from different species and methodologies (see different symbols in Fig. 7) and should not necessarily be taken as corroborating evidence for the hypothesis that motor neuron number plays a role in affecting measures of motor unit synchrony. Furthermore, one should consider how well muscles not included in Fig. 7 might fit into this scheme. For example, lumbar multifidus (as shown here) and other paraspinal muscles (Marsden et al. 1999) exhibit weak synchrony. According to the relationship shown in Fig. 7, this might imply that these muscles are supplied by large numbers of motor neurons. As far as we are aware, however, there are no data available regarding motor neuron numbers for these muscles in primates.
While considering the limitations associated with the results shown in Fig. 7, there does nevertheless appear to be a coarse inverse relationship between magnitude of short-term synchrony and number of motor units constituting different muscles. As such, it seems possible that the number of motor neurons innervating a particular muscle could have a significant influence on the magnitude of short-term synchrony. This idea, however, requires thorough validation in future experimental and modeling studies.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-39489 (to A. J. Fuglevand) and National Health and Medical Research Council Grant 349452 (to M. A. Nordstrom).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.K., M.A.N., and A.J.F. conception and design of research; D.A.K., L.-W.C., M.A.N., and A.J.F. performed experiments; D.A.K., L.-W.C., M.A.N., and A.J.F. analyzed data; D.A.K., L.-W.C., M.A.N., and A.J.F. interpreted results of experiments; D.A.K., L.-W.C., and A.J.F. prepared figures; D.A.K. and A.J.F. drafted manuscript; D.A.K., L.-W.C., M.A.N., and A.J.F. edited and revised manuscript; D.A.K., L.-W.C., M.A.N., and A.J.F. approved final version of manuscript.
REFERENCES
- Adams L, Datta AK, Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol 413: 213–231, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Kümmel H, Pinter MJ, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 17. Axonal projection and termination of C3–C4 propriospinal neurones in the C6-Th1 segments. Exp Brain Res 81: 447–461, 1990 [DOI] [PubMed] [Google Scholar]
- Asanuma H, Zarzecki P, Jankowska E, Hongo T, Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res 34: 73–89, 1979 [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Riek S, Carson RG, Enoka RM. Common input to different regions of biceps brachii long head. Exp Brain Res 193: 351–359, 2009 [DOI] [PubMed] [Google Scholar]
- Belhaj-Saïf A, Karrer JH, Cheney PD. Distribution and characteristics of poststimulus effects in proximal and distal forelimb muscles from red nucleus in the monkey. J Neurophysiol 79: 1777–1789, 1998 [DOI] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Göhmann M, Conrad B. Analysis of muscle responses elicited by transcranial stimulation of the cortico-spinal system in man. Electroencephalogr Clin Neurophysiol 69: 412–422, 1988 [DOI] [PubMed] [Google Scholar]
- Bernhard CG, Bohm E. Monosynaptic corticospinal activation of fore limb motoneurones in monkeys (Macaca mulatta). Acta Physiol Scand 31: 104–112, 1954 [DOI] [PubMed] [Google Scholar]
- Binder MD, Powers RK. Relationship between simulated common synaptic input and discharge synchrony in cat spinal motoneurons. J Neurophysiol 86: 2266–2275, 2001 [DOI] [PubMed] [Google Scholar]
- Bremner F, Baker J, Stephens J. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol 432: 381, 1991a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Correlation between the discharges of motor units recorded from the same and from different finger muscles in man. J Physiol 432: 355–380, 1991b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol 76: 509–519, 1990 [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol 475: 217–227, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980 [DOI] [PubMed] [Google Scholar]
- Clough J, Kernell D, Phillips C. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol 198: 145–166, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Kellerth JO. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol 281: 285–299, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D, Eccles J. Synaptic action during and after repetitive stimulation. J Physiol 150: 374, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall TJ, Nordstrom MA, Semmler JG. Motor unit synchronization is increased in biceps brachii after exercise-induced damage to elbow flexor muscles. J Neurophysiol 99: 1008–1019, 2008 [DOI] [PubMed] [Google Scholar]
- Datta A, Farmer S, Stephens J. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol 432: 401, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Stephens J. Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422: 397, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David WS, Goyal N, Henry FP, Baldassari LE, Redmond RW. Validation of an incremental motor unit number estimation technique in rabbits. Muscle Nerve 41: 794–799, 2010 [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RM, Gerstein GL, Baker SN. Measurement of time-dependent changes in the irregularity of neural spiking. J Neurophysiol 96: 906–918, 2006 [DOI] [PubMed] [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gérard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain 122: 1327–1340, 1999 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Meglio M, Cioni B, Tonali P, Rothwell J. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J Physiol 573: 1047–1058, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J, Eccles R, Iggo A. Distribution of recurrent inhibition among motoneurones. J Physiol 159: 479–499, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P, Murthy K. The origins and characteristics of cross-correlated activity between γ-motoneurones in the cat. Exp Physiol 70: 219–232, 1985 [DOI] [PubMed] [Google Scholar]
- Ellaway P. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978 [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. J Physiol 428: 467–484, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol 470: 127–155, 1993a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods 74: 175–187, 1997 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Mayston MJ, Parekh A, James LM, Stephens JA. Abnormal cortex-muscle interactions in subjects with X-linked Kallmann's syndrome and mirror movements. Brain 127: 385–397, 2004 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol 463: 83–105, 1993b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein B, Lindegard B, Nyman E, Wohlfart G. Morphologic studies of motor units in normal human muscles. Acta Anat (Basel) 23: 127–142, 1955 [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol 44: 751, 1980 [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S. Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev 40: 53–65, 2002 [DOI] [PubMed] [Google Scholar]
- Fourment A, Belhaj-Saïf A, Maton B. Functional linkages between motor cortical cells and elbow flexor muscles. Evidence for and characteristics of postspike facilitation. J Neurophysiol 74: 130–141, 1995 [DOI] [PubMed] [Google Scholar]
- Fritz N, Illert M, la Motte de S, Reeh P, Saggau P. Pattern of monosynaptic Ia connections in the cat forelimb. J Physiol 419: 321–351, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol 347: 431–451, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci 25: 4560–4564, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörner M, Illert M, Kümmél H. Absence of recurrent axon collaterals in motoneurones to the extrinsic digit extensor muscles of the cat forelimb. Neurosci Lett 122: 183–186, 1991 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Projections of pyramidal tract cells to alpha-motoneurones innervating hind-limb muscles in the monkey. J Physiol 249: 637–667, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Roberts WJ. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol 222: 623–642, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci 3: 567–575, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns RK, Fuglevand AJ. Profiling individual human motor units for surveillance over multiple days. Soc Neurosci Abstr: 399.15, 2005 [Google Scholar]
- Johns RK, Fuglevand AJ. Number of motor units in human abductor hallucis. Muscle Nerve 43: 895–896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Mazzocchio R, Pénicaud A, Rossi A. Distribution of recurrent inhibition in the human upper limb. Acta Physiol Scand 149: 183–198, 1993 [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 57–62, 2004 [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand 65: 87–100, 1965 [Google Scholar]
- Kim MS, Masakado Y, Tomita Y, Chino N, Pae YS, Lee KE. Synchronization of single motor units during voluntary contractions in the upper and lower extremities. Clin Neurophysiol 112: 1243–1249, 2001 [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Tuck DL, Westgaard RH. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol 327: 105–135, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol 322: 287–314, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischka U, Fajfr R, Fellenberg T, Hess CW. Facilitation of motor evoked potentials from magnetic brain stimulation in man: a comparative study of different target muscles. J Clin Neurophysiol 10: 505–512, 1993 [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, sect. 1, vol. II, pt. 1 chapt. 13, p. 597–666 [Google Scholar]
- Landgren S, Phillips CG, Porter R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol 161: 91–111, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Porter R, Redman SJ. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol 232: 499–510, 1985 [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol 381: 497–527, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Tatton WG, Tasker RR. Electromyographic response to displacement of different forelimb joints in the squirrel monkey. J Neurosci 3: 783–794, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher HR, Vardar U. A comparison of homonymous and heteronymous connectivity in the spinal monosynaptic reflex arc of the cat. Exp Brain Res 74: 480–492, 1989 [DOI] [PubMed] [Google Scholar]
- Maltenfort MG, Heckman CJ, Rymer WZ. Decorrelating actions of Renshaw interneurons on the firing of spinal motoneurons within a motor nucleus: a simulation study. J Neurophysiol 80: 309–323, 1998 [DOI] [PubMed] [Google Scholar]
- Mantel GW, Lemon RN. Cross-correlation reveals facilitation of single motor units in thenar muscles by single corticospinal neurones in the conscious monkey. Neurosci Lett 77: 113–118, 1987 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Stretch reflex and servo action in a variety of human muscles. J Physiol 259: 531–560, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JF, Farmer SF, Halliday DM, Rosenberg JR, Brown P. The unilateral and bilateral control of motor unit pairs in the first dorsal interosseous and paraspinal muscles in man. J Physiol 521: 553–564, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei B, Schmied A, Mazzocchio R, Decchi B, Rossi A, Vedel JP. Pharmacologically induced enhancement of recurrent inhibition in humans: effects on motoneurone discharge patterns. J Physiol 548: 615–629, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain 120: 1199–1216, 1997 [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA, Farmer SF. Physiological tremor in human subjects with X-linked Kallmann's syndrome and mirror movements. J Physiol 530: 551–563, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry 34: 121–131, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol 97: 550–556, 2007 [DOI] [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998 [DOI] [PubMed] [Google Scholar]
- Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol 34: 171–187, 1971 [DOI] [PubMed] [Google Scholar]
- Moore GP, Segundo JP, Perkel DH, Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J 10: 876–900, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RB, Porter R. The effect of a preceding stimulus on temporal facilitation at corticomotoneuronal synapses. J Physiol 228: 749–763, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain 105: 223–269, 1982 [DOI] [PubMed] [Google Scholar]
- Nelson SG, Mendell LM. Projection of single knee flexor Ia fibers to homonymous and heteronymous motoneurons. J Neurophysiol 41: 778–787, 1978 [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 448: 397–412, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol 80: 2475–2494, 1998 [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20, 1979 [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. The pyramidal projection to motoneurones of some muscle groups of the baboon's forelimb. Prog Brain Res 12: 222–245, 1964 [DOI] [PubMed] [Google Scholar]
- Porter R, Hore J. Time course of minimal corticomotoneuronal excitatory postsynaptic potentials in lumbar motoneurons of the monkey. J Neurophysiol 32: 443–451, 1969 [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Oxford Univ. Press, 1993 [Google Scholar]
- Porter R. Early facilitation at corticomotoneuronal synapses. J Physiol 207: 733–745, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol 103: 2821–2832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R. Presence of homonymous recurrent inhibition in motoneurones supplying different lower limb muscles in humans. Exp Brain Res 84: 367–373, 1991 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Marciani MG, Caramia M, Roma V, Zarola F. Nervous propagation along ‘central’ motor pathways in intact man: characteristics of motor responses to ‘bifocal’ and “unifocal” spine and scalp non-invasive stimulation. Electroencephalogr Clin Neurophysiol 61: 272–286, 1985 [DOI] [PubMed] [Google Scholar]
- Schieppati M, Crenna P. From activity to rest: gating of excitatory autogenetic afferences from the relaxing muscle in man. Exp Brain Res 56: 448–457, 1984 [DOI] [PubMed] [Google Scholar]
- Schmied A, Descarreaux M. Influence of contraction strength on single motor unit synchronous activity. Clin Neurophysiol 121: 1624–1632, 2010 [DOI] [PubMed] [Google Scholar]
- Schmied A, Ivarsson C, Fetz E. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res 97: 159–172, 1993 [DOI] [PubMed] [Google Scholar]
- Scott JG, Mendell LM. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol 39: 679–692, 1976 [DOI] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol 263: 357–381, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003 [DOI] [PubMed] [Google Scholar]
- Semmler JG. Motor unit synchronization and neuromuscular performance. Exerc Sport Sci Rev 30: 8–14, 2002 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol 84: 358–366, 2000 [DOI] [PubMed] [Google Scholar]
- Shapovalov AI, Karamjan OA, Kurchavyi GG, Repina ZA. Synaptic actions evoked from the red nucleus on the spinal alpha-motorneurons in the rhesus monkey. Brain Res 32: 325–348, 1971 [DOI] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995 [DOI] [PubMed] [Google Scholar]
- Takei T, Seki K. Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J Neurosci 30: 17041–17050, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Effects of common excitatory and inhibitory inputs on motoneuron synchronization. J Neurophysiol 86: 2807–2822, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Peterson BW. Peripheral and central substrates of vestibulospinal reflexes. Physiol Rev 58: 80–105, 1978 [DOI] [PubMed] [Google Scholar]
- Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH. The rubrospinal tract in the human brain: diffusion tensor imaging study. Neurosci Lett 504: 45–48, 2011 [DOI] [PubMed] [Google Scholar]


