Abstract
The mouse is an important model system for investigating the neural circuits mediating behavior. Because of advances in imaging and optogenetic methods, head-fixed mouse preparations provide an unparalleled opportunity to observe and control neural circuits. To investigate how neural circuits produce behavior, these methods need to be paired with equally well-controlled and monitored behavioral paradigms. Here, we introduce the choice ball, a response device that enables two-alternative forced-choice (2AFC) tasks in head-fixed mice based on the readout of lateral paw movements. We demonstrate the advantages of the choice ball by training mice in the random-click task, a two-choice auditory discrimination behavior. For each trial, mice listened to binaural streams of Poisson-distributed clicks and were required to roll the choice ball laterally toward the side with the greater click rate. In this assay, mice performed hundreds of trials per session with accuracy ranging from 95% for easy stimuli (large interaural click-rate contrast) to near chance level for low-contrast stimuli. We also show, using the record of individual paw strokes, that mice often reverse decisions they have already initiated and that decision reversals correlate with improved performance. The choice ball enables head-fixed 2AFC paradigms, facilitating the circuit-level analysis of sensory processing, decision making, and motor control in mice.
Keywords: head-fixed mouse, two-alternative forced-choice, psychophysics, reaction-time, head-fixed behavior, auditory discrimination
a central goal in systems neuroscience is to establish the neural circuit mechanisms underlying behavior. By relating the firing of cell populations to perceptual and cognitive behaviors, studies in head-fixed monkeys (e.g., Newsome et al. 1989; Treue and Maunsell 1996) and freely moving rodents (e.g., Erlich et al. 2011; Jaramillo and Zador 2011; Kepecs et al. 2008) have provided cellular level insights into the mechanics of cognition. The psychophysical tasks used in these studies provide precise, quantitative measures of behavioral events that are necessary to interpret behavior and infer the relationships of mental variables to brain function (Parker and Newsome 1998).
To probe the neural circuits underlying behavior, the mouse has emerged as an ideal model system due to its genetic flexibility. Mice can be engineered to express fluorescent reporters of neural activity in genetically targeted circuit elements (Li et al. 2005; Zariwala et al. 2012). In addition, Cre-driver mouse lines targeting specific cell types (Lindeberg et al. 2004; Tanahira et al. 2009; Taniguchi et al. 2011; Zariwala et al. 2012) have made a growing array of circuit components genetically targetable for studies seeking to examine their respective roles in neural computation. In recent years, experiments combining use of imaging techniques with behavior in mice have exploited these advantages using various head-fixed preparations (Andermann et al. 2010; Dombeck et al. 2007; Komiyama et al. 2010). Studies conducted in awake head-fixed mice that require a behavioral choice readout have previously been limited to Go/No-Go behavioral responses (Andermann et al. 2010; Histed et al. 2012; Komiyama et al. 2010; Mehta et al. 2007) or virtual navigation (Harvey et al. 2009). Although considerable progress has been made using these classes of behavior in the rodent, epochs of disengagement and impulsivity in Go/No-Go tasks complicate the interpretation behavioral choices (Schwarz et al. 2010; Stüttgen et al. 2006; Stüttgen and Schwarz 2008).
Although previously unavailable for head-fixed mouse, two-alternative forced-choice (2AFC) behaviors have been favored in the neuroscience of decision making. The 2AFC design permits more informative interpretation of incorrect choices. In Go/No-Go tasks, impulsive response behavior appears as a bias toward the Go choice, whereas impulsivity in a 2AFC discrimination task can cause performance to decrease without affecting the readout of choice preference. Likewise, epochs of relative disengagement in Go/No-Go tasks are difficult to differentiate from a preference for No-Go responses. For these reasons, the process of making an active choice response on each trial makes behavior in 2AFC tasks considerably easier to interpret (Schwarz et al. 2010). Moreover, the symmetrical reward contingencies of 2AFC tasks enable reaction time measurements that are not confounded by choice-specific motivational factors typical in Go/No-Go task designs (Zariwala et al. 2006). Indeed, a growing number of studies in freely moving rodent have taken advantage of 2AFC tasks using choice ports for choice readout with high temporal resolution. Rat 2AFC tasks using ports have been used to study the neural correlates of mental variables in decision making (Kepecs et al. 2008), sensory processing (Cury and Uchida 2010; Otazu et al. 2009; Yoshida and Katz 2011), and motor control (Erlich et al. 2011; Felsen and Mainen 2008). Similar 2AFC tasks have also been developed for freely moving mice (Busse et al. 2011; Rinberg et al. 2006). However, due to the small size of the mouse, head-fixed behavior greatly facilitates the use of contemporary techniques such as brain imaging, awake patch electrode recordings, high-channel-count microdrives, and optogenetics.
Here, we introduce a novel way for head-fixed mice to communicate their decisions: the choice ball, a modified computer trackball that rotates about an axis and is operated by short lateral motions of the animal's front paws. We demonstrate the advantages of this response device using an auditory discrimination task and show that head-fixed mice can be trained in a robust two-alternative choice paradigm yielding psychophysical measures.
METHODS
All animal procedures were conducted in accordance with National Institutes of Health (NIH) guidelines and with the approval of the Cold Spring Harbor Laboratory (CSHL) Institutional Animal Care and Use Committee.
Apparatus.
A stable platform to support the hindpaws of a mouse was positioned such that when head-fixed, the animal's front paws rested on a modified computer trackball as shown in Fig. 1A. The platform was made from a polycarbonate tube (50.8-mm outer diameter; McMaster) cut to 100 mm in length and milled to expose the animal's front paws to the ball as depicted in Fig. 1B (a photo diagram of the setup). A custom-designed optical lickometer was placed within reach of the animal's tongue. A lickometer is a device specialized for precise delivery of liquid droplets and measurement of lick events. Water reward was dispensed from a reservoir above the animal into the lickometer through silicone elastic tubing. Water flow was controlled using a solenoid pinch valve (NResearch) with pulse timing calibrated such that a single reward measured 5 μl. Sounds were delivered using a set of speakers (HP 5187-2105; Harman Kardon) positioned on either side of the animal's head. Speakers were calibrated to 70-dB sound pressure level (SPL) within a 5- to 40-kHz range using a pressure-field microphone (Brüel & Kjær, Sound & Vibration Measurement, Nærum, Denmark). Auditory stimuli generated in MATLAB were sampled at 200 kHz and delivered to the speakers using a Lynx L22 sound card (Lynx Studio Technology). A white light-emitting diode (LED) to indicate trial onset was mounted on the top face of the lickometer, pointing toward the mouse. An infrared camera was positioned 20 cm in front of the animal, and its output was shown on a liquid crystal display (LCD) for monitoring purposes. The trackball was placed on a lab jack (Fisher Scientific) to allow fine-scale adjustment of its height relative to the mouse's paws. The entire apparatus was enclosed in a dark acoustic isolation chamber (Industrial Acoustics).
Fig. 1.
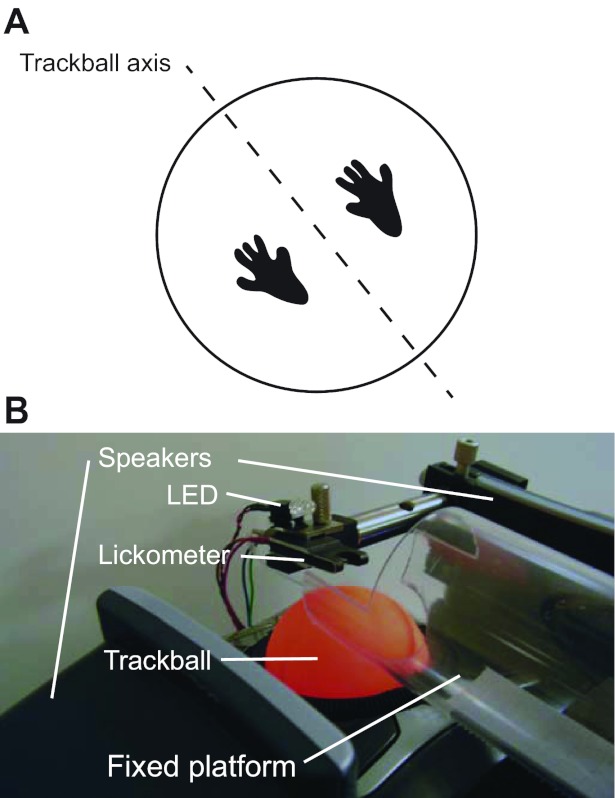
The choice ball apparatus. A: orientation of the choice ball. A ping-pong ball replacing a commercial trackball was fitted with a steel rod and secured in the trackball chassis such that it rotated freely about its axis in range of the trackball optical motion sensor. At the beginning of each trial, the mouse's front paws rested on the ball approximately as shown (also see Supplemental Video S1 available in the data supplement online at the Journal of Neurophysiology web site). B: photo diagram of task setup. A head-fixed mouse is positioned with its front paws on the choice ball and its hindpaws on a fixed platform. A water-dispensing lickometer is positioned within reach of its tongue. A light-emitting diode (LED) on the lickometer indicated the no-movement period to the mouse at the start of each trial. Speakers positioned laterally on either side of the mouse were used to present the stimulus (head fixture beams omitted for clarity).
Choice ball.
The choice ball apparatus employs a series of modifications to a commercially available Universal Serial Bus (USB) trackball (Expert Mouse, K64325; Kensington). The 55-mm diameter trackball is large enough that a laboratory mouse can position both paws comfortably and stably on its surface. We replaced the original trackball with an oversized 55-mm diameter ping-pong ball (Joola) to reduce the force required for the mouse to rotate it. To restrict the trackball motion to rotation about a single axis, we secured a precision-aligned hypodermic tube through the ball, creating mechanical guidance to the two acceptable choice responses. The hypodermic tube was anchored to metal inserts mounted in grooves cut into the trackball chassis such that the ball could freely rotate about this axis and remain within range of the trackball optical sensor. To prevent undesired visual cues, we disabled the trackball time-delayed automatic LED shut-off feature by clipping the trackball LED leads from its printed circuit board, rewiring it to an external power source, and securing it back on to the circuit board with epoxy. The raw trackball position was read out by a dedicated personal computer (PC) running the Windows XP operating system (Microsoft) using a script in MATLAB r2008a (The MathWorks). Pointer enhancement was disabled in the operating system to ensure a linear readout of the trackball position. In our setup, a single pixel registered by the trackball computer corresponded to 0.26° of rotation or 0.13 mm of lateral movement along the circumference. Ball position was monitored and logged to the computer hard drive in real-time. During each trial, the computer measured ball movement toward predetermined choice boundaries. Choice boundary crossing events were communicated in real-time from the trackball computer to our behavior system using parallel port logic lines. Position data were returned over an Ethernet connection following each trial to the computer governing our behavior system. The modified trackball module itself was thus inexpensive and easy to integrate with our existing behavior system based on Bcontrol (C. D. Brody, Z. F. Mainen, and A. M. Zador, CSHL), an open-source, real-time state machine framework (e.g., Erlich et al. 2011; Felsen and Mainen 2008). The MATLAB code used on the dedicated trackball computer to acquire trackball position data and interface choice responses with our behavior system is provided in a GitHub repository (https://github.com/KepecsLab/ChoiceBallSystem). On this site, we also provide code and setup instructions for a microcontroller-based choice ball system that is functionally identical to the one used here but does not require an additional, dedicated computer.
Subjects.
Data are reported from three male B6129SF2/J white-bellied agouti mice aged 12 wk at the onset of training. Mice were housed individually on a 12-h reversed light/dark schedule. Four additional mice were used but never advanced beyond the earliest phases of training due to low performance in the auditory task and persistent side bias. Food was available ad libitum, and the mice were placed on a liquid restriction schedule with daily body weight monitoring to ensure that body mass remained within 85% of mass before restriction. Mice were provided with ≥1 ml water/day. One hour following training, if the water delivered in the task did not exceed the daily water allowance, the remaining portion was provided to each mouse in its home cage.
Surgery.
Mice were anesthetized with intraperitoneal injection of ketamine (150 mg/kg) and xylazine (12 mg/kg). The skull was exposed, and a horizontally oriented titanium bar (20 × 3 × 1 mm) was centered 2 mm above bregma and secured to the skull surface with acrylic cement. Three bone screws spaced among the occipital and parietal bones were used to ensure the stability of the implant, and the remaining exposed skull was sealed with acrylic cement. For postoperative analgesia, ketoprofen (5 mg/kg) was administered intraperitoneally. Mice were given 1 wk to recover from surgery with water and food available ad libitum.
Behavioral training.
After surgery recovery, mice were introduced to the apparatus following 3 days on a liquid restriction schedule. For each mouse, we optimized the orientation of the lickometer before each session such that licks interrupted the lickometer photo-gate and the mouse's tongue touched the drink tube when extended. We also adjusted the elevation of the hindpaw platform for each mouse such that the animal's anterior/posterior axis was parallel to the floor with its front paws resting on the trackball. The objective of training was for mice to use the choice ball device to classify two Poisson click streams by indicating which stream had a faster underlying rate. Training was implemented in six phases. The first phase was designed to train head-fixed mice to lick for water. Mice were trained to lick by intermittently rewarding their licking behavior. Mice were advanced to the subsequent stage once they were rewarded for licking ≥200 times in a single 30-min session. In the 2nd phase, mice learned to lick preferentially in the period following an auditory cue. Each trial started with a random delay between 1 and 3 s in length. Following the delay, a 1-s long train of random clicks was presented at 100-Hz mean click frequency from both left and right speakers. Clicks were 1-ms white noise pulses flanked by 100-μs linear ramps between silence and 70-dB SPL. Mice were rewarded for licking in the second following the click-train offset. Mice were advanced once they licked in the second following sound offset for ≥90% of 200 consecutive trials. The 3rd phase was intended to teach mice to use the ball to dispense reward. Parameters were identical to the 2nd phase except that the click train was extended to 2 s in length and mice were required to move the trackball in either direction while the click train was being presented to terminate the click train and dispense reward. Sessions in this phase of training were repeated twice daily until mice responded with a 6.5-mm lateral trackball movement (∼13° about the ball axis) at the correct time for ≥50% of the 1st 100 trials in a session. In the 4th phase of training, sound was presented from only 1 side on each trial, and mice learned to push the ball selectively toward the side with sound. The rewarded side with sound was alternated trialwise between left and right. Choosing the wrong direction resulted in repeating the same stimulus on the subsequent trial until the animal chose correctly (to prevent choice bias). During this phase, we trained mice to move the ball for increasing distances to register a response. Mice were initially required to move the ball 6.5 mm (∼13°) in the chosen direction to respond, and once accuracy on this phase surpassed 90% per session, the distance to choice threshold was gradually increased to 26 mm (∼50°, 200 pixels). We initially chose 26 mm as the rewarded choice boundary for our task based on early observations that mice were unwilling to paw repeatedly for distances >26 mm over hundreds of trials per session (data not shown). A session would end once the mouse failed to cross a choice threshold for 10 sequential trials following trial 100. Training on the 4th phase was repeated twice daily until choice accuracy surpassed 90% for a single session of >100 trials with the choice movement boundary set at 26 mm. In the 5th training phase, left and right trials were randomly interleaved so that mice could not perform well by simply alternating choices trialwise and ignoring the stimulus. The antibias algorithm was disabled for this phase and all subsequent phases. The 6th training phase (multiple difficulty) was identical to the 5th except that Poisson-distributed click train pairs were generated at 3 levels of stimulus difficulty. The rates used to generate the left and right click trains always summed to 200 Hz as follows for each difficulty level: 200/0 Hz, 0/200 Hz (easy left, easy right), 150/50 Hz, 50/150 Hz (medium left, medium right), and 125/75 Hz, 75/125 Hz (difficult left, difficult right). Mice were returned to the 5th stage of training (single difficulty) for the remainder of the experiment following several multiple difficulty sessions. In these subsequent sessions, the boundary for reward delivery was extended beyond 26 mm in a manner dependent on performance, and LEDs positioned on top of each speaker indicated the chosen direction to the mouse once the ball passed 26 mm. Outcome scoring for these sessions in our analyses used the earlier 26-mm decision threshold.
RESULTS
Design and implementation of two-choice response device for head-fixed mice.
We designed a two-choice response device suitable for head-fixed mice that pairs the high-resolution event timing required for psychophysical analysis with a pair of well-defined, laterally symmetrical motor actions by which mice can report choices among alternatives. We sought a device that head-fixed mice could learn to operate with a short period of training and that they would operate continuously despite limited forelimb strength. Based on these criteria, we chose to train mice to operate a choice ball, a modified computer trackball, with their front paws (Fig. 1). Using the choice ball, mice reported their classification of auditory stimuli by rotating the ball laterally toward a fixed choice boundary ∼50° from the start point. By rapidly sampling the trackball position, our device could report the animal's choice in real-time. Using the position record for each trial, we were subsequently able to reconstruct the time course of the animal's rotation of the trackball for further analysis, exposing an additional dimension of behavioral information to inform our understanding of the decision-making process.
An auditory two-choice discrimination task using the choice ball.
To demonstrate the usefulness of the choice ball and examine its performance characteristics, we trained mice in an auditory discrimination task (Fig. 2). In designing the behavior, we chose a task that provided graded decision difficulty and ease of operation allowing for hundreds of trials, permitting within-session analysis at each difficulty level. As an analog to the visual random-dot task that has been used successfully in primates to probe decision making (Newsome et al. 1989; Roitman and Shadlen 2002), we designed an auditory task using random click trains as stimuli (Erlich et al. 2012). Mice listened to independent random click trains delivered from speakers on their left and right and were rewarded for rolling the trackball toward the side with the faster underlying click rate. Each trial was initialized by illuminating an LED positioned in front of the animal (Fig. 2C) to indicate to the animal that they must cease moving the ball for a period of 1 s to continue the trial. This ensured that any paw movements captured during a trial were initiated from resting position at some time following stimulus onset. Although we did not store a record of trackball movement before trial start, we observed in video records that mice frequently shifted their paws, often in the opposite direction of their paw movement in the previous trial as would be necessary to regain balance on the ball. Once a 1-s period had passed with no ball motion, the LED was extinguished, and two Poisson-distributed click trains of different rates were delivered from the left and right speakers. Mice were not required to sample the stimulus for a fixed period and were permitted to respond as soon as they had made a choice. To qualify as a response, mice had to move the choice ball by 26 mm (∼50°) about its axis. Failure to respond within the 2-s stimulus delivery period resulted in termination of the current trial and initialization of the next trial after a 3-s delay. Mice were rewarded for pushing the ball in the direction of the click train for which the underlying rate was faster as shown in Fig. 2B. A correct response was immediately rewarded with 5 μl of water dispensed from the lickometer. An incorrect response resulted in a 5-s punishment delay before the next trial was initialized.
Fig. 2.
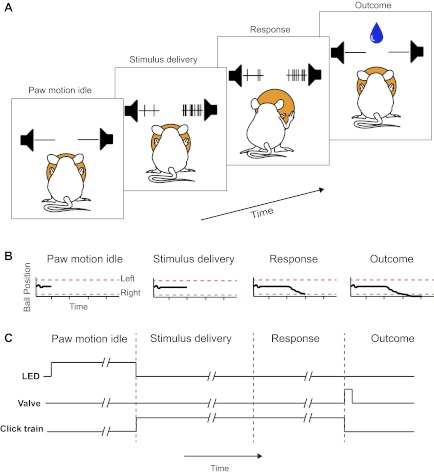
A 2-alternative forced-choice task for head-fixed mice. A: trial stages. 1) Paw motion idle: a mouse must cease paw motion for 1 s to initiate a trial. 2) Stimulus delivery: the mouse listens to 2 random click streams and discriminates the faster clicking side. 3) Response: the mouse rotates the trackball with its paws in the chosen direction until a response threshold is crossed. The click train was not terminated until a choice was registered, allowing ongoing clicks to inform paw movement. 4) Outcome: the mouse was rewarded with a water droplet if it responded correctly or punished with a 5-s time-out delay if it responded incorrectly. B: time course of ball position record. In each trial, a fixed threshold was set at ±26 mm of lateral paw movement (∼50° about the ball axis) such that ball rotation past threshold in the correct direction will register a correct response. C: illustrated time course of trial-start LED, water valve, and the auditory stimulus.
We report on 3 mice trained in the 2AFC task. Following the 1st 3 phases of training, we collected 68 behavior sessions from our animals, averaging 283 completed trials per session. Although the task is not explicitly self-initiated, mice responded reliably, rotating the trackball by a sufficient amount to be counted as a valid response (26 mm, 200 pixels) on >90% of all trials in behavioral sessions. The paw movement component of choice responses was rapid, averaging 189 ms of ball motion to register a choice (Fig. 3, A and B). Mice typically learned to discriminate among the easiest stimuli in <2 wk. Figure 4A shows an example plot illustrating the improvement in discrimination accuracy over the course of training for a single mouse. Three types of sessions are shown, reflecting the order and difficulty of trial types: sessions for which trials with the easiest stimuli to classify were alternated trialwise between left and right correct responses, sessions for which the easiest stimuli were randomly interleaved, and randomly interleaved sessions containing stimuli of multiple difficulty categories. Mice typically approached 90% proficiency at classifying the easiest stimulus over the course of the 1st 5,000 trials as shown in Fig. 4B. For sessions with multiple difficulties, difficult stimuli were created by configuring the relative rates of clicks coming from the 2 speaker channels. Psychometric performance varied across difficulties, indicating that mice had mastered the intended click-train discrimination task, as shown for all 10 multiple difficulty sessions and for a single session of 466 trials in Fig. 5, A and B.
Fig. 3.
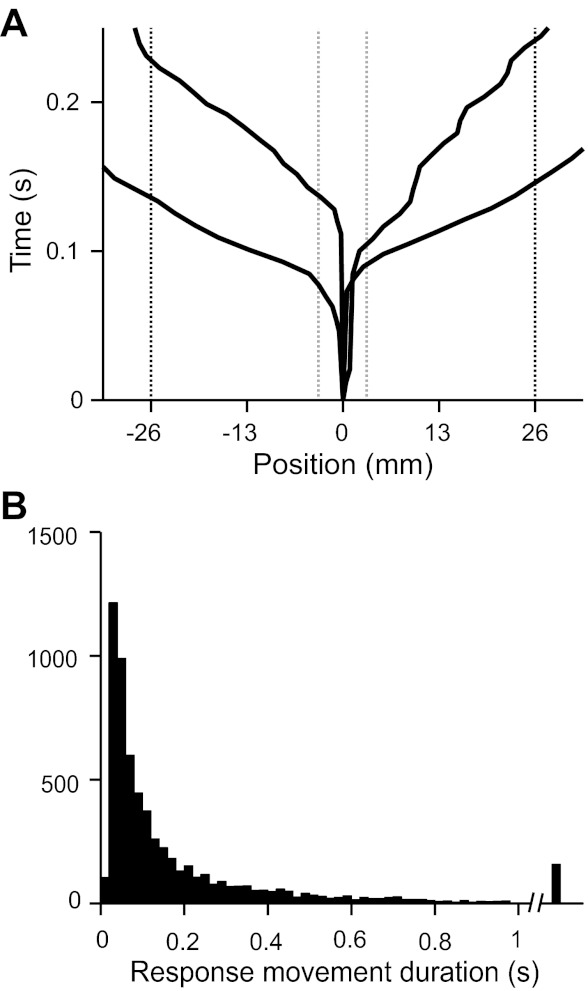
Rapid readout of choice responses. A: trackball position data. The trackball position record allows for high-resolution reconstruction of the animal's motor response. Ball movement trajectories for 4 example trials are shown with respect to auditory stimulus onset. Choices are registered when the trackball has rotated by 50° in either direction (shown as 26 mm along the ball circumference with respect to the position at trial start). B: timing of paw movement responses. Variability in ball position was calculated for the 1st 50 ms following auditory stimulus onset of 10,575 posttraining trials of 3 mice. The SD of position during this prereaction period was 1.65 mm. The duration of paw sweeps was considered during the period of fixation from ±3.3 mm (2 SD, gray dotted lines in A) to the ±26-mm decision boundary (black dotted lines) as a measure of the speed with which the mice operated the ball to report their decisions. Trials in which a mouse moved 6.5 mm in 1 direction and ultimately responded in the opposite direction were considered decision-reversal trials (Fig. 6) and omitted from this analysis. Mean response movement duration was 189 ms.
Fig. 4.
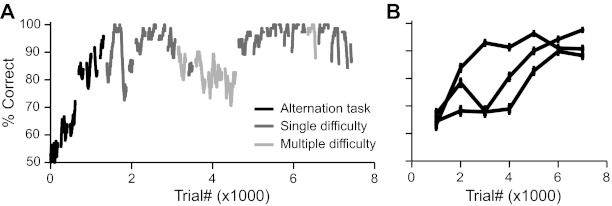
Mice learn the trackball task consistently and with high accuracy. A: sliding window performance for a single mouse (window length = 100 trials). Mice were initially trained, using only the easiest stimuli (100% left/right contrast), to alternate left and right responses (black bins). When accuracy approached 90%, mice were advanced to sessions with interleaved 100% contrast stimuli (dark gray bins) and subsequently sessions with multiple difficulty levels (light gray bins). B: performance records for 3 mice, considering only trials with 100% left/right contrast (bin size = 1,000 trials). Error bars show SE.
Fig. 5.
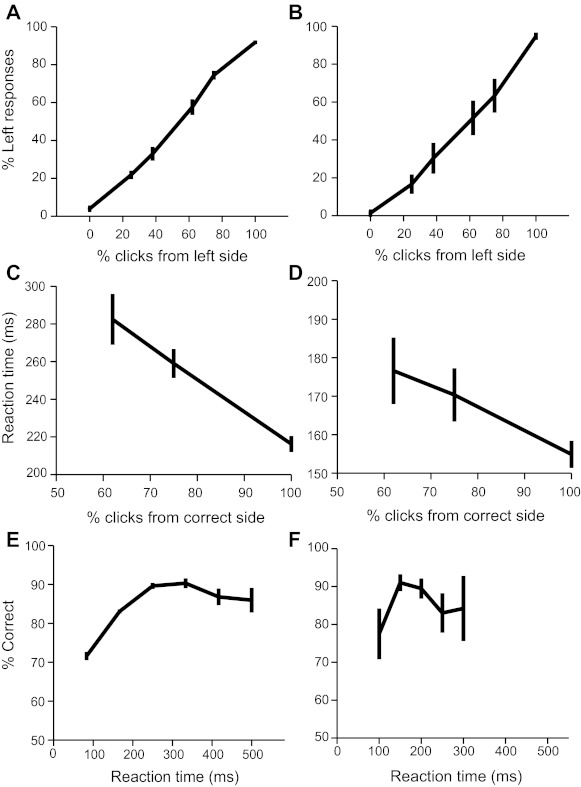
Choice reaction times correlate with stimulus difficulty and performance. A: pooled psychometric performance for all sessions across mice for sessions with multiple difficulties showing a full range of accuracy in classifying stimuli of varying difficulty. Left-side click percentage reflects a total of 200 Hz distributed between the left and right channels. Error bars show 95% binomial confidence interval. B: the psychometric function for an example session of 466 trials. C: averaged reaction time with respect to difficulty considering only correct trials across all multiple difficulty sessions showing that mice spend more time sampling the stimulus before responding on difficult trials. Error bars in C and D show SE. D: reaction time with respect to difficulty for the example session (same as in B). E: pooled reaction time conditional accuracy for 3 animals. Accuracy peaks when animals respond 250 ms following stimulus onset. Error bars show 95% binomial confidence interval. F: reaction time conditional accuracy for the same example session as in B and D.
In our task, due to temporal variability in the stimulus, optimal performance requires an evidence integration strategy (Palmer et al. 2005). Specifically, mice could gain an accuracy advantage by using a decision-making strategy where more ambiguous evidence is intentionally sampled for longer periods. With respect to easy stimuli (200-Hz contrast), mice spent significantly more time sampling medium (100-Hz contrast) and difficult stimuli (60-Hz contrast), as shown in Fig. 5C for all sessions averaged across mice (ANOVA and post hoc Tukey-Kramer test, P < 1e−6). Using the same test, the difference between medium and difficult stimulus sampling time was not significant (P > 0.05). For the example session shown in Fig. 5D, the mouse also spent significantly less time sampling easy stimuli than difficult stimuli (P < 0.05). An additional prediction of integration in our task is higher performance on trials where mice spent more time sampling the binaural click train before responding. Figure 5, E and F, shows that over the 1st 200 ms, accuracy improves as reaction time increases for the easiest stimulus condition across sessions (note nonoverlapping 95% binomial confidence intervals for the increasing trend over the 1st 200 ms in Fig. 5E and a similar trend for the 1st 200 ms of sampling in the example session in Fig. 5F).
Choice reversals during decision making.
The choice ball affords us an additional window into the decision-making process, the opportunity to reconstruct the time course of the animal's report of its decision. In analyzing ball movement data, we noticed that mice occasionally reversed the decisions they had initiated. If reversal trials were used strategically to correct errors made as part of the decision-making process, we anticipated that they would be correlated with improved performance and would become less necessary as the mice became more proficient in the task. To test these predictions, we defined a choice reversal as a response in which the mouse moved the trackball 6.5 mm in one direction but ultimately chose the other direction. The boundary for choice reversals was placed at 6.5 mm, corresponding to 4 SD from the mean of lateral ball movement during the 1st 50 ms following stimulus onset. The time courses of trackball movement for 5 choice-reversal trials are shown in Fig. 6A overlaid on 45 surrounding nonreversal trials from the same session. In total, 6.9% of all trials qualified as reversals by these criteria. Performance on reversal trials within the same difficulty category was slightly but significantly enhanced with respect to nonreversal trials (Fig. 6B; χ2 test, P < 0.001), consistent with the prediction that mice can reverse their choices to improve accuracy. If mice use decision reversals to resolve errors made as part of learning, we reasoned that reversal probability would diminish as a function of experience in the task. For this analysis, we excluded alternation sessions and considered only sessions with randomly interleaved trials, where the rewarded choice boundary was set at 26 mm. The latter restriction was necessary because the decision to reverse movement during the response has a cost for the animal that depends on the total distance to choice boundary. Figure 6C shows that reversal probability decreases during the 1st 2,500 interleaved trials of the easiest decision difficulty. The combined reversal probability for the 3 mice was significantly higher in the 1st 500 trials than the final 500 trials of the period observed (15.7 vs. 9.3%; χ2 test, P < 1e−7).
Fig. 6.
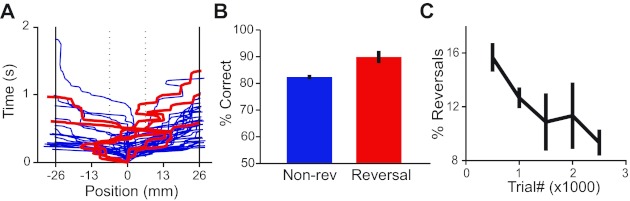
Choice reversals in mice. A: ball movement time course plot for 50 consecutive trials. Dotted line at ±6.5 mm indicates the reversal detection threshold. Solid lines at ±26 mm indicate decision threshold. Detected reversal trials are shown in red. B: for the easiest decision category, accuracy is greater on reversal trials than on nonreversal (Non-rev) trials averaged across all sessions for 3 mice. Error bars show 95% confidence intervals. C: reversal probability for the easiest decision category decreases with task experience (average of 3 mice shown in blocks of 500 trials). Error bars show SE.
DISCUSSION
We designed a simple response device for acquiring two-choice decisions from head-fixed mice. Our device has several advantages over previously used methods. By divorcing the animal's method of acquiring reward (licking) from its method of choice response (paw motion), our paradigm does not require conditioning the animal to suppress anticipatory licking (Andermann et al. 2010; Schwarz et al. 2010) and leaves lick rate available as an additional behavioral metric. By providing high-resolution trackball movement data, our device opens an additional facet of the animal's choice response to analysis that is lost in experiments with a binary readout, the ability to detect and study changes of mind. Furthermore, the ability to reward simple lateral paw strokes affords us greater temporal resolution than head-fixed analogs of traditional rodent decision-making tasks based on directed navigation (Harvey et al. 2012).
To demonstrate the usefulness of this response device, we designed an auditory psychophysics task based on binaural random click discrimination. The task was inspired by the random dot task, a simple and extensively researched paradigm in primate visual neuroscience, used to study decision making (Newsome et al. 1989; Roitman and Shadlen 2002). Although we chose to use audition for superior stimulus control in head-fixed mouse, our task is fundamentally similar in that a subject is trained to integrate stochastic information supporting two hypotheses over a sampling period and makes a discrete choice between them on each trial, providing both a choice and a reaction-time measure. Our posttraining sessions averaged several hundred completed trials. High trial counts are necessary to characterize complex response profiles of neurons to different behavioral contingencies.
In keeping with findings in primates (Mazurek et al. 2003; Palmer et al. 2005), mice spent more time sampling difficult stimuli (Fig. 5C) and discriminated more accurately on trials with longer sampling durations (Fig. 5E). Under idealized conditions in perceptual discrimination, a performance increase is realized through continued sampling of a stimulus (Link and Heath 1975; Mazurek et al. 2003; Ratcliff 1988). Although these effects are consistent with a speed-accuracy tradeoff strategy exploiting integration of temporal evidence, further experiments specifically designed to test this hypothesis will be required (Kiani et al. 2008; Zariwala et al. 2006).
We have also shown that mice sometimes reverse the direction of choice responses they have initiated. Reversal trials correlated with performance gain, and reversal probability decreased with training. Changes of mind in human test subjects have been attributed to conflicting information in early sensory processing that is considered after a decision has been initiated (Resulaj et al. 2009). Since after initiating a response, mice in our task were permitted to sample the stimulus continuously until their final choice was registered, the precise source of evidence used to trigger decision reversals remains unresolved. Nevertheless, our results suggest that in addition to humans, mice can use choice reversals to optimize decision accuracy strategically. Whether the reversals we observed are true changes of mind or corrections of motor errors in support of the animal's original choice remains an open question.
The choice ball provides an opportunity to combine information-rich 2AFC psychophysical behavior with a stable, head-fixed configuration ideal for emerging imaging and optogenetic techniques. Studies in the monkeys have leveraged simple, information-rich motor responses (saccades) to uncover clearly defined set of motor control circuits in the frontal eye fields and superior colliculus (Schall and Thompson 1999), governing choice responses. A similar anatomic link has been exploited in a Go/No-Go task for head-fixed mice, the ALM and PPM regions of primary motor cortex that govern licking (Komiyama et al. 2010). Since the motor response in our task is a simple lateral shift of the forelimbs, we anticipate that these stereotyped movements will provide a tractable paradigm for understanding the neural circuits that control choice responses in mice.
GRANTS
This research was supported by grants from the NIH National Institute of Neurological Disorders and Stroke, Merck and Sloan Foundations, and the Louis Morin Charitable Trust to A. Kepecs and the Gerry family fellowship of the Watson School of Biological Sciences to J. I. Sanders.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.I.S. and A.K. conception and design of research; J.I.S. performed experiments; J.I.S. analyzed data; J.I.S. and A.K. interpreted results of experiments; J.I.S. prepared figures; J.I.S. drafted manuscript; J.I.S. and A.K. edited and revised manuscript; J.I.S. and A.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Eifert for machine shop support and Drs. G. Turner and S. Jaramillo for comments on the manuscript.
REFERENCES
- Andermann ML, Kerlin AM, Reid C. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci 4: 2–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses 24: 637–645, 1999 [DOI] [PubMed] [Google Scholar]
- Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Schölvinck ML, Zaharia AD, Carandini M. The detection of visual contrast in the behaving mouse. J Neurosci 31: 11351–11361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron 68: 570–585, 2010 [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron 72: 330–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Duan CA, Ly R, Brunton BW, Hanks TD, Brody CD. The frontal and parietal cortex play distinct roles in accumulation of evidence: inactivations and modeling. Society for Neuroscience, New Orleans, LA, 42: 289.22, 2012 [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60: 137–148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci 5: 458–462, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484: 62–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461: 941–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Carvalho LA, Maunsell JH. Psychophysical measurement of contrast sensitivity in the behaving mouse. J Neurophysiol 107: 758–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo S, Zador AM. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat Neurosci 14: 246–251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature 455: 227–231, 2008 [DOI] [PubMed] [Google Scholar]
- Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. J Neurosci 28: 3017–3029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464: 1182–1186, 2010 [DOI] [PubMed] [Google Scholar]
- Li Z, Burrone J, Tyler WJ, Hartman KN, Albeanu DF, Murthy VN. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc Natl Acad Sci USA 102: 6131–6136, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40: 67–73, 2004 [DOI] [PubMed] [Google Scholar]
- Link SW, Heath RA. A sequential theory of psychological discrimination. Psychometrika 40: 77–105, 1975 [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex 13: 1257–1269, 2003 [DOI] [PubMed] [Google Scholar]
- Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5: 309–322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature 341: 52–54, 1989 [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci 12: 646–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis 5: 376–404, 2005 [DOI] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21: 227–277, 1998 [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res 40: 2201–2209, 2000 [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Continuous versus discrete information processing: modeling accumulation of partial information. Psychol Rev 95: 238–255, 1988 [DOI] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature 461: 263–266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron 51: 351–358, 2006 [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci 22: 241–259, 1999 [DOI] [PubMed] [Google Scholar]
- Schwarz C, Hentschke H, Butovas S, Haiss F, Stüttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C. The head-fixed behaving rat–procedures and pitfalls. Somatosens Mot Res 27: 131–148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüttgen MC, Rüter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci 26: 7933–7941, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C. Psychophysical and neurometric detection performance under stimulus uncertainty. Nat Neurosci 11: 1091–1099, 2008 [DOI] [PubMed] [Google Scholar]
- Tanahira C, Higo S, Watanabe K, Tomioka R, Ebihara S, Kaneko T, Tamamaki N. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci Res 63: 213–223, 2009 [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71: 995–1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382: 539–541, 1996 [DOI] [PubMed] [Google Scholar]
- Wiesenfeld Z, Branchek T. Refractive state and visual acuity in the hooded rat. Vision Res 16: 823–827, 1976 [DOI] [PubMed] [Google Scholar]
- Wood C, Jennings J. Speed-accuracy tradeoff functions in choice reaction time: experimental designs and computational procedures. Atten Percept Psychophys 19: 92–102, 1976 [Google Scholar]
- Yoshida T, Katz DB. Control of prestimulus activity related to improved sensory coding within a discrimination task. J Neurosci 31: 4101–4112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci 32: 3131–3141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Kepecs A, Uchida N, Mainen ZF. Dissociation of accuracy and reaction time in a two alternative odor mixture discrimination task. Computational and Systems Neuroscience Conference, Salt Lake City, UT, 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


