Abstract
Neuronal spike activity was recorded in the medial prefrontal cortex (mPFC) as rats performed an operant spatial delayed alternation task. The sensitivities of neurons to choice, outcome, and temporal information-related aspects of the task were examined. About one-third of neurons were sensitive to the location of delayed responding while animals were at one of two spatially distinct response ports. However, many fewer neurons (<10%) maintained choice information over the delay, each exhibiting persistent differences in firing rates for only a portion of the delay. Another third of cells encoded information about behavioral outcomes, and some of these neurons (>20% of all cells) fired at distinct rates in advance of correct and incorrect responses (i.e., prospective encoding of outcome). Other cells were sensitive to reward-related feedback stimuli (>20%), the outcome of the preceding trial (retrospective encoding, 5–10%), and/or the time since a trial was last performed (10–20%). An anatomical analysis of the recording sites found that cells that were sensitive to choice, temporal, and outcome information were commingled within the middle layers of the mPFC. Together, our results suggest that spatial processing is only part of what drives mPFC neurons to become active during spatial working memory tasks. We propose that the primary role of mPFC in these tasks is to monitor behavioral performance by encoding information about recent trial outcomes to guide expectations and responses on the current trial. By encoding these variables, the mPFC is able to exert control over action and ensure that tasks are performed effectively and efficiently.
Keywords: executive function, working memory, error processing, reward, persistent activity
the goal of this study was to understand the role of prefrontal regions of the rodent cerebral cortex in the performance of spatial delayed alternation (DA) tasks. DA tasks are simple tests of spatial working memory function in which the subject is rewarded for responding at alternating locations after a delay period (e.g., Mishkin and Pribaum 1955). Damage in dorsal parts of the prefrontal cortex of primates produces lasting impairments in spatial DA (e.g., Goldman et al. 1971). Neurons in these cortical regions are thought to maintain information about the spatial choice over delay periods based on persistent and spatially selective spike activity (Fuster and Alexander 1971; Kubota and Niki 1971; Preuss 1995). Rodents do not have dorsal (granular) prefrontal regions, but they do have medial prefrontal regions (mPFC) that may be homologous to cingulate and ventromedial prefrontal regions in primates (Laubach 2011). Lesions in the mPFC of primates have produced mixed results in spatial DA tasks. Some studies have reported impairments (Pribram et al. 1962), while others have reported only transient or variable effects (Murray et al. 1989; Pribram and Fulton 1954; Rushworth et al. 2003). In rodents, damage in the mPFC, especially in the prelimbic area, impairs spatial DA performance in the T maze (Brito et al. 1982) and operant spatial DA tasks (Dunnett et al. 1999; van Haaren et al. 1988). These effects tend to be transient in nature (e.g., Brito et al. 1982; Delatour and Gisquet-Verrier 2001) and may be due to reductions in spontaneous alternation (Delatour and Gisquet-Verrier 1996). Interestingly, lesions of mPFC do not consistently impair performance in another test of spatial working memory, the radial arm maze (Delatour and Gisquet-Verrier 1996; Gisquet-Verrier and Delatour 2006; Ragozzino et al. 1998). Data from primate mPFC, including from studies of human subjects, suggest that this region is involved in conflict monitoring and action selection (reviewed in Seamans et al. 2008). Therefore, a plausible theory of rodent mPFC function is not that it is involved in spatial working memory per se, but that it has a role in performance monitoring or “working-with-memory” to optimize behavioral responding (Moscovitch and Winocur 1992).
The idea of “working-with-memory” fits well with modern views on the functional significance of the mPFC in cognitive and motivational control (e.g., see Rushworth et al. 2011 for review). A deficit in action and/or outcome monitoring following damage in the mPFC would lead to erratic performance of the spatial DA task. A lapse of control over performance, even with an intact spatial memory system, would lead to an increased frequency of errors. Only a few studies that have used spatial DA-style designs have examined any nonspatial effects of mPFC lesions or inactivations. Dunnett et al. (1999) reported that lesions of mPFC do not lead to perseverative responding (i.e., repeated responding at an incorrect location), and this result was recently confirmed in a reversible inactivation study by Horst and Laubach (2009). In that study, we also noted that errors were associated with longer and more variable intertrial intervals and that inactivation of mPFC increased temporal variability prior to correct responding. Control of response timing is a key nonspatial factor for spatial DA performance, as a prompt response (e.g., rapid travel to the required location at the end of the delay period) would minimize demands on working memory.
Neuronal recording studies have, unfortunately, not helped resolve the role of mPFC in spatial DA performance. We are not aware of any recordings in the mPFC of primates performing spatial DA tasks. Similar to the lesion literature reviewed above, recording studies in rodents have resulted in a mixed set of findings. Batuev et al. (1990) claimed to find neurons that fired persistently in a choice-sensitive fashion over the delay period in a maze-based DA task, but they did not control, or even monitor, the animals' behavior in the start box during the delay period. Jung et al. (1998) used several maze-based DA designs and found that few, if any, mPFC neurons showed choice-sensitive persistent activity during the delay period. Finally, Baeg et al. (2003) used maze-based tasks and reported that simultaneous recordings of groups of neurons could be used to predict the spatial location of the delayed response. As in the Jung study, few neurons were both choice sensitive and persistently active over the delay period, and these neurons did not need to be included in the neuronal population for successful decoding of the spatial response.
In the present study, we report data from mPFC neurons that were recorded during an operant spatial DA task. The task was designed to minimize postural variability during the delay period with the same methods as in a recent reversible inactivation study from our laboratory (Horst and Laubach 2009). This issue is important because postural variability could lead to apparent encoding of choice by mPFC neurons, e.g., due to differences in posture and/or heading direction during the delay period (Cowen and McNaughton 2007; Euston and McNaughton 2006). Indeed, behavioral studies have shown that, if allowed, rats tend to use postural strategies to mediate information about the spatial choice over delay periods (Chudasama and Muir 1997; Ennaceur et al. 1997).
Using the operant spatial DA task, we assessed how the spike counts of mPFC neurons encoded choice-, outcome-, and temporally related information at two key time points in the task: 1) when rats entered choice ports after the end of the delay period and 2) during the initial portion of the delay period when rats consumed fluid at a reward port. We found many neurons that encoded information about the spatial choice when rats were in distinct locations in the environment. However, fewer neurons maintained choice information over the delay period. Many other neurons were sensitive to nonspatial aspects of the task (i.e., the outcome of the delayed response and the time since the last trial). Few neurons were jointly sensitive to multiple task attributes or to single task attributes across multiple phases of the task. Together, our findings suggest that mPFC encodes a diverse set of task attributes (spatial and nonspatial) that could be used to monitor behavioral performance in order to control spatial working memory processing.
METHODS
Rats had regulated access to water for 1 wk prior to training and throughout the period of training and testing. Food was available ad libitum. During training sessions, rats were reinforced with water, which was supplemented in the home cage to maintain them at ∼90% of their free access body weight. All procedures were approved by the Animal Care and Use Committee at the John B. Pierce Laboratory and conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were taken to minimize the number of animals used and to reduce pain and suffering.
Behavioral Task
Adult male Long-Evans (N = 5) or Brown Norway (N = 4) rats were trained to perform a spatial DA procedure (Caetano et al. 2012; Horst and Laubach 2009). They were trained in an operant box housed within a sound-attenuating chamber (ENV-008, Med Associates). A spout located between two barriers (which controlled for the posture of rats during lever pressing and reward consumption) was located at one end of the chamber, and two choice ports were located on the opposite wall (Fig. 1A). All response locations were equipped with infrared beams that detected head entries into the choice ports and licks to the reward spout. Behavioral chambers and devices were custom made by the Instruments Shop at the John B. Pierce Laboratory and were controlled with software (MedPC) and a computer interface from Med Associates.
Fig. 1.
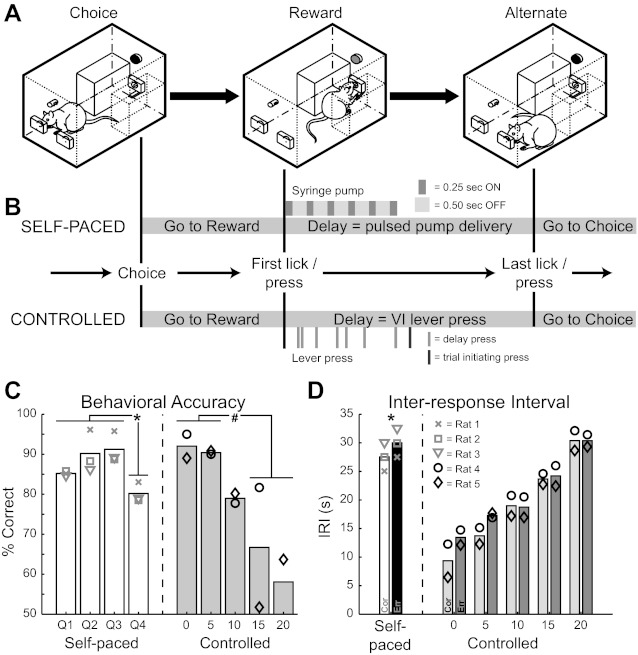
Spatial delayed alternation task and performance. A: schematic of behavioral arena with sequence of primary events. In the “Choice” phase, the rat selects the right nose poke port and then traverses the chamber to collect a reward. Reward collection starts the delay period. At the end of the delay, the rat crosses back to the other side of the chamber to the alternate (left) choice port for another correct response. B: detailed sequence of events in the self-paced and controlled versions of the spatial delayed alternation (DA) task. In the self-paced version (top), after correctly responding in the choice port the rat licks the reward spout, which triggers pulsed delivery of fluid (six 0.25-s pulses of water separated by 0.50-s pauses). The rat is then free to make the next spatial choice. Choosing the opposite choice port results in another reward. In the controlled version (bottom), the rat makes a choice and then travels to the reward spout. In addition to collecting a reward during the ensuing delay, the rat must also press a lever on a variable-interval (VI) schedule (0, 5, 10, 15, or 20 s, randomly interleaved) to initiate the next choice period. Selection of the opposite choice port is rewarded. In either task variation, selection of the same choice port in consecutive trials is scored as an error and no reward is delivered. After an error, rats must initiate the next trial by contacting the reward spout (self-paced) or pressing the lever on a variable interval schedule (controlled). The trial following an error is a correction trial, in which rats must select the choice port that would have been correct. C: performance accuracy of rats in the self-paced (left, N = 3 rats that performed postoperatively) and controlled (right, N = 2 rats with isolated single units only) versions of the spatial DA task, with symbols denoting individual subjects. For the self-paced version, accuracy is presented by interresponse interval (IRI) quartile (Q1, Q2, Q3, Q4). For the controlled version, accuracy is presented by assigned delay (0, 5, 10, 15, 20 s). *P < 0.01 for shortest 3 vs. longest quartile of IRIs in the self-paced version. #P = 0.07 for shortest 2 vs. longest 2 assigned delays in the controlled version. D: median IRI of rats in the self-paced (left, N = 3) and controlled (right, N = 2) task variations, with symbols denoting individual subjects. The overall median of IRIs is presented for self-paced rats, while IRIs are presented by assigned delay for the controlled-delay rats. *P < 0.01 for IRIs preceding correct vs. error responses in the self-paced version. (All P values reported are from t-tests of paired samples).
Five rats were trained and tested in a self-paced version of the spatial DA task that we have previously shown depends upon functional mPFC (Horst and Laubach 2009; Fig. 1B, top). To rule out the possibility that allowing rats to set their own pace would permit them to circumvent the use of spatial working memory, we trained a separate group of four rats in a modified version of the task with greater temporal control over the delays between successive trials (Fig. 1B, bottom).
Self-paced spatial delayed alternation.
Five rats were trained to alternate between spatial choices at their own pace, with methods described by Horst and Laubach (2009). Rats were trained first to collect water from the reward spout at periodic intervals (every 5–20 s). Next, they were required to make head entries into either choice port to trigger a reward. In the final stage, rats were required to alternate their choices between left and right ports in order to receive a reward. An example of a successful alternation trial is shown in Fig. 1A. In this case, the rat has just made a correct response in the right choice port (Fig. 1A, left) and must traverse the chamber to collect the reward (Fig. 1A, center), which is delivered in a pulsatile fashion (six 0.25-s pulses, separated by 0.50-s pauses) to encourage the rat to remain in this position over a short delay. Once contact with the spout is made, the rat is free to make the next spatial choice (Fig. 1A, right). A response in the opposite choice port (in this case, the left port) is rewarded. If the same port is chosen on consecutive trials, it is scored as an error; the lights in the chamber extinguish, and the rat must make contact with the reward spout before making the next choice, although no reward is delivered. After an error, the target location for the correct choice is always in the choice port opposite to the one chosen erroneously.
Modified spatial delayed alternation task with controlled delays.
Four rats were trained in a modified version of the spatial DA procedure that permitted experimenter control over delays by requiring rats to press a lever on a variable-interval schedule between spatial choices, similar to the paradigm used by Caetano et al. (2012). Rats were first trained to press a lever below the reward spout to trigger reward delivery. In the next phase of training, a single lever press initiated the choice phase, in which a head entry into either choice port resulted in availability of fluid at the reward spout. A light above the choice ports illuminated during the choice phase (the “Go” stimulus), and a light above the lever illuminated during the reward/delay phase (the “Collect” stimulus). Rats were then trained to alternate spatial choices. Once rats were alternating well (>65% correct), they were required across separate sessions to press the lever on successively increasing fixed ratio schedules (FR1, 2, 4, 8) to initiate the choice phase. In this phase of training, rats learned to stay at the lever until a press triggered the Go stimulus.
Fully trained rats had to press the lever on a variable-interval schedule (0-, 5-, 10-, 15-, 20-s delays, pseudorandomly interleaved) between spatial choices. The first press occurring after the assigned delay initiated the choice phase (Fig. 1B, darkest tick below delay for the “Controlled” task). Successive choices in the same location were scored as errors. The choice phase was reset via lever pressing on the 0- to 20-s variable-interval schedule, and trial correction proceeded as in the self-paced version of the task.
Surgeries
Rats were trained until they performed the task with an accuracy of >75% correct. They were then given 1 wk of full access to water and implanted with microwire arrays into mPFC. After initial anesthesia with ∼4% halothane, intraperitoneal injections of ketamine (80–100 mg/kg) and diazepam (8–10 mg/kg) or ketamine and xylazine (10 mg/kg) were administered. Supplements (1/3 of the initial dose) of the two drugs administered in the procedure were given approximately every 60 min. By standard methods, arrays of microwire electrodes were placed in mPFC with a craniotomy that was centered at 3.2 mm rostral to bregma and ±1.4 mm lateral to bregma. The arrays were placed to avoid major vessels within the craniotomy and were lowered to a depth of 2.8–3.6 mm at an angle of 12° from the midline. Eight rats were implanted with fixed arrays made with 50-μm stainless steel wire, which had in vitro impedance between 200 and 300 kΩ, arranged in a 2 × 8 configuration with ∼200 μm between electrodes (NB Labs). One rat (trained to perform the self-paced version of the spatial DA task) was implanted with a drivable array of microelectrodes (1 × 8 configuration, CD Neural Technologies), placed at the following coordinates: AP: +3.2, ML: +1.4, DV: −2.8 mm at 12° from the midline. The microdrive was lowered in steps of 0.05 mm every day throughout the period of the recordings (45 days).
Of the five rats trained in the self-paced version of the task, one became ill and a second did not complete any trials after microelectrode implantation. Two of the rats trained in the controlled version of the task did not have clear single-unit activity. Data from these four rats were excluded from all aspects of the study.
Electrophysiological Recordings
One week after surgery, neuronal recordings were made during behavioral sessions with a multielectrode recording system (Plexon). Single units were identified online with an oscilloscope and audio monitor and off-line with the Plexon Offline Sorter. Online sorting was done with the “boxes” feature in the Plexon software, in which waveforms were manually selected on the basis of their amplitude and deviation from background firing. Artifacts due to cable noise and behavioral devices were removed during off-line sorting. Single units were identified as having 1) consistent waveform shape, 2) average amplitude estimated at least three times larger than background activity, and 3) a consistent refractory period of at least 2 ms in interspike interval histograms.
Histological Procedures
Once experiments were complete, rats were anesthetized with 100 mg/kg pentobarbital sodium and transcardially perfused with 10% formalin. Brains were sectioned horizontally on a freezing microtome, mounted on subbed slides, and stained for Nissl with thionin. Histological examination of electrode tracts showed that recording sites were located in the mPFC of all rats (see Fig. 2).
Fig. 2.

Neuronal ensemble recordings in the medial prefrontal cortex (mPFC). A: locations of implanted microwire electrode arrays in the 5 behaving rats with isolated single units are shown on a horizontal section through the mPFC. B: the depth of the section in A is shown in this diagram (as the gray horizontal line), and the region explored with the microdrive in rat 1 is depicted by the blue box. FrA, frontal association area; PrL, prelimbic area.
Data Analyses
Exploratory analyses of neuronal activity and behavior were performed with NeuroExplorer (Nex Technologies). Statistical analyses of neuronal and behavioral data were performed with MATLAB (MathWorks) and R (www.r-project.org). Computer code used for the data analysis in the study is available upon request from the corresponding author.
Behavioral Analyses
Data were averaged across sessions for each subject prior to statistical comparisons. Comparisons were made between variables with t-tests for independent or paired samples, as appropriate. For both versions of the spatial DA task, performance was assessed in terms of accuracy (number of correct trials/total number of trials) and the median length of interresponse intervals (IRIs) preceding correct versus error responses. To determine whether the length of time between successive choices, i.e., the delay, affects performance in the self-paced version of the task, accuracy was assessed for each IRI quartile. For the controlled-delay version of the task, accuracy was assessed for each of the assigned delays (0–20 s). Comparisons were also made between median IRI lengths preceding correct versus error responses in both task versions. Finally, to assess whether there were gross differences in movements depending on the spatial choice made, comparisons between the movement times (“Go to Choice” in Fig. 1B) were made for left versus right choices. To visualize differences in performance across rats, data are presented as bar plots with individual rats represented as symbols (Fig. 1, C and D). Only rats that were subsequently assessed for neuronal responses (i.e., those with clear single-unit activity and sufficient behavioral data during recording sessions) were included in these analyses.
Assessment of Neuronal Sensitivity to Behavioral Events
Neuronal firing patterns around the primary behavioral events in the task (choice port entry and reward collection) were compared between self-paced (N = 91 neurons) and controlled (N = 92 neurons) versions of the spatial DA task to evaluate whether subsequent analyses could be carried out on the combined data set. The average firing rate and 95% confidence intervals (with bootci.m in MATLAB) were calculated and plotted over a 10-s window centered on the event of interest. Deviations from the overall mean firing rate around the event of interest suggest that the population is generally modulated around the event. Divergence versus overlap of the confidence bands can be used to assess differences and similarities between the firing patterns observed in the two task variations.
Nonparametric rank sum and rank correlation tests were used to assess the identity and fraction of neurons that were sensitive to various aspects of DA performance, including the animal's current choice (left vs. right port), the outcome of the current trial (correct vs. error), the outcome of the previous trial, and the (log transformed) time since the last response was made. A neuron was included in the analysis if its mean firing rate was >0.1 Hz (>98% of neurons) and if the rat performed the task with an accuracy of >75% correct and had >10 errors in that session. This minimum error requirement was needed to explore outcome-related differences in firing rates. The analysis was done either around the time of choice port entry or around the time of reward collection with a sliding window of 1 s, in steps of 0.1 s. Neurons were considered sensitive to differences in performance if P < 0.05 (with Bonferroni correction for 4 behavioral variables, P < 0.0125) by a rank sum test (effects of spatial choice and current and past behavioral outcomes) or a rank correlation test (effects of time since the last trial was performed).
The fraction of sensitive neurons was then assessed in each of the 0.1-s bins around the time of the choice or reward collection to determine the task epoch in which particular classes of neurons encoded behavioral information, i.e., when the mean and confidence bands (bootci.m in MATLAB) exceeded a fraction of 0.05.
Conjunction analysis [i.e., in MATLAB: sum(pChoice<0.0125 & pOutcome<0.0125)/length(pChoice)] was used to assess the potential for neurons to encode multiple task variables. The analysis was performed with results from the nonparametric analyses described above in three of the 1-s data windows (−1 to 0 s, −0.5 to 0.5 s, and 0 to 1 s). A criterion of P < 0.0125 (Bonferroni correction, P < 0.05/4) was used. χ2-Tests (proportions tests) were applied to assess relative fractions of cells that were sensitive to each behavioral variable or conjunction of variables.
To examine firing patterns associated with choice, outcome, prior outcome, and pace, we used the MATLAB function imagesc.m to make time-sorted firing rate difference plots. We plotted the normalized differences in spike counts for left vs. right choices (Choice), correct vs. erroneous responses on the current (Outcome) or preceding (Prior) trial, and trials in the upper and lower quartiles for pace defined by the IRIs (Pace). Neurons were sorted based on the time of the maximum difference in spike counts (normalized to have a maximum value of 1).
To examine whether neurons fired more around correct versus incorrect choices, a preference score (PS) was calculated by taking the absolute difference in median spike counts (SC) from trials with correct versus incorrect responses during the 1-s window prior to the choice. The raw preference score was calculated for each neuron as PSraw = median(SCC) − median(SCE), with MATLAB notation. The normalized preference score was calculated for each neuron as PSnorm = [median(SCC) − median(SCE)]/[median(SCC) + median(SCE)]. We examined the distribution of the preference scores using histograms and defined outcome-sensitive neurons as those with preference scores greater than the median[abs(PSraw/norm)]. The preference scores were useful for assessing the fractions of neurons that fired more prior to errors compared with prior to correct responses (i.e., neurons below the median score fired more on error trials). Fractions of these preference scores were compared for correct-preferring and error-preferring neurons with a proportions test.
To examine whether outcome-preceding activity reflected differences in left and right choices in the task, we used the same metric, defined for the difference in median spike counts on trials with correct left and right choices: PSnorm = [median(SCL) − median(SCR)]/[median(SCL) + median(SCR)]. We plotted histograms of the distributions of this metric and compared the distributions of outcome-sensitive and outcome-insensitive neurons, using the Kolmogorov-Smirnov test.
RESULTS
Spatial Alternation Performance Diminished as a Function of Increasing Delay
Rats performed the spatial DA task with high levels of accuracy and tended to perform less accurately as the time between responses increased, a hallmark of tasks that depend on working memory processing. Accuracy in the self-paced version of the task was significantly less at the longest IRIs (fourth quartile = 80.2 ± 1.43%) compared with all other IRIs (quartiles 1–3 = 88.9 ± 1.76%; t-test of paired samples, t = −16.8, P = 0.004). There was a trend toward less accurate performance at the longest assigned delays (15 and 20 s = 62.4 ± 4.67%), compared with the shortest delays (0 and 5 s = 91.2 ± 1.29%) in the task version with controlled delays (t-test of paired samples, t = 8.51, P = 0.07). Accuracy data are presented in Fig. 1C. In the self-paced version, correct trials were preceded by shorter IRIs compared with errors (27.5 ± 1.44 s < 30.0 ± 1.45 s; t-test of paired samples, t = −113.5, P < 0.001; Fig. 1D). There were no differences in IRIs preceding correct versus error responses at any of the temporally controlled delays (Fig. 1D), reflecting the fact that the length of the assigned delay was the dominant factor determining the IRI in this version of the task. These behavioral data indicate a potential role for spatial working memory in the performance of our behavioral procedures and allowed us to examine neuronal activity in mPFC to look for evidence of encoding of the spatial choice, especially during the delay period.
To assess behavioral differences as a potential source of variability in neuronal response patterns, we also compared performance across task variations and analyzed movement time preceding left versus right choices. Accuracy and length of IRIs before correct or error responses did not differ between versions of the spatial DA task. There were no differences in the latency to cross the chamber during the choice phase in either task version, and choice latency did not depend on the location of the response. Thus it appears that, in general, the animals perform the two variations of the spatial DA task in a similar fashion.
Anatomical Distribution of Recording Sites
Most recordings in this study were made in the prelimbic area (PrL, aka Cg3). Some neurons were recorded in the frontal association area (FrA, aka medial agranular cortex). A few neurons were recorded in the more posterior Cg2 region. Recording sites spanned across the superficial and deep layers of the cortex. Recordings from the rat implanted with a microdrivable array of electrodes were all in superficial layers of PrL. Approximate locations of electrode tips are shown in Fig. 2A. Figure 2B depicts the approximate dorso-ventral location of single units recorded in the rat implanted with the microdrive.
Neuronal Population Activity Is Modulated Around Choice and Reward
Neuronal population averages from the self-paced and controlled versions of the spatial DA task both showed evidence for modulation of firing rates around the time of the choice (Fig. 3A) and around reward collection (Fig. 3C). (Perievent histograms of behavioral responses are shown in Fig. 3, B and D, to put the population averages into a behavioral context). In fact, the overall pattern of firing was similar in animals trained in the self-paced (N = 3) and controlled (N = 2) versions of the task, with the exception that neurons from the controlled version showed stronger modulation 1–2 s before the choice (Fig. 3A). This was likely due to the salience of the Go stimulus in those animals, which was presented around that time. The controlled-delay rats were trained to lever press until the Go stimulus was presented, and this stimulus is known to be associated with changes in firing rates in the mPFC in this type of task (e.g., Caetano et al. 2012). Such differences in activity would not be expected to be differentially affected by choice, outcome, or pace. The overall pattern of firing was nearly identical for the two versions of the task around the times of the choice and the reward (Fig. 3C). The only difference between the two task variations was that neurons were more sharply modulated at the end of the delay period in the controlled variation, because of the visual stimulus that served to trigger rapid approaches to the choice ports. Given the overall similarity among neuronal activity in the two task variations, data from all animals in the study were combined for the analyses reported below.
Fig. 3.
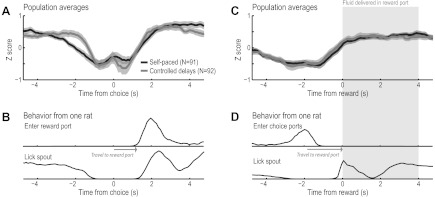
Neuronal population activity around the choice and the reward. A: neuronal population averages around the time of the choice. The overall pattern of firing was similar in animals trained in the self-paced (N = 3) and controlled (N = 2) versions of the task. B: behavioral data from 1 of the rats, showing the distribution of when the rat entered the reward port and licked on the spout in relation to the time of the choice. C: neuronal population averages around the time of the reward. The overall pattern of firing was nearly identical in animals trained in the 2 versions of the task. One difference between the task variations was the visual stimulus that was triggered by lever pressing at the end of the delay period. This stimulus served as a sort of “Go” cue (see Caetano et al. 2012), and neuronal activity was more sharply modulated around the time of the stimulus in the animals tested in the controlled version of the task. D: behavioral data from the same rat in A, showing the distribution of when the rat entered the choice ports and licked on the spout in relation to the time of the reward.
Neurons in Medial Prefrontal Cortex Encode Choice, Outcome, and Temporal Information
Neurons exhibiting choice-related activity were found around the time of choice port entry (Fig. 4A) and near the time of the first lick (Fig. 4D). A large fraction of neurons (>20%) in rat mPFC were sensitive to choice (left vs. right) as rats entered and then departed from the choice ports (Fig. 4B). None of these neurons fired throughout the analysis epoch, but they showed sequential onsets and offsets of differential firing patterns (Fig. 4C) that together spanned the choice period. By contrast, the fraction of choice-sensitive neurons dwindled during the time between the choice and the reward collection, with choice-related firing effectively disappearing at the time of reward collection (Fig. 4E). None of the neurons fired at distinct rates throughout the period of reward consumption based on the preceding choice (Fig. 4F).
Fig. 4.
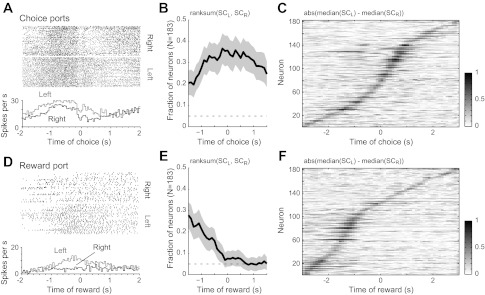
Neurons encoded the choice made at the end of the delay period. A: example of a neuron that fired more when the rat entered the left choice port compared with the right port. Spike rasters are shown at top, and the average firing rates (0.1-s bins) on trials with left and right choice are shown at bottom. The neuron fired at a tonically elevated rate throughout the choice epoch, starting from the end of the delay period. B: fraction of neurons that was sensitive to the spatial location of the choice when rats entered the choice ports. The fraction of cells with P < 0.05 (with Bonferroni correction, P < 0.0125) from a rank sum test of spike counts(SC) in a sliding 1-s window (steps of 0.1 s) is depicted by the black line. The 95% confidence interval is shown as gray shading. C: normalized difference in spike counts around the time of choice port entry for all neurons (1 per row) recorded in the 5 rats. None of the neurons fired at distinct rates throughout the period of the choice. Each pixel represents the difference in spike counts in a 0.1-s bin and was calculated as abs[median(SCL) − median(SCR)]. Differences in spike counts are sorted by the time of maximum difference in spike counts on left and right trials. D: example of a neuron that fired more when the rat entered the reward port after responding in the left choice port. E: fraction of neurons that was sensitive to the spatial location of the preceding choice when rats entered the reward port. F: normalized difference in spike counts based on the location of the preceding choice during the period of travel to the reward port and the initial period of reward consumption. As in C, none of the neurons fired at distinct rates throughout the period of reward consumption based on the preceding choice.
Many neurons were also differentially modulated by the outcome of the current trial around the time of choice and reward collection (Fig. 5). A small but significant fraction (∼10%) of neurons encoded correct versus incorrect choices prior to the time of choice port entry (Fig. 5C, before 0 s), indicating prospective encoding of outcome. Examples of prospective outcome-encoding neurons are shown in Fig. 5A (Effect of outcome, neurons 1 and 2). To examine whether the error trials reflected a “miscoding” of the forthcoming choice, we plotted the spike activity of these neurons for correct trials only and sorted the trials by the location of the choice (left or right; Fig. 5A: Effect of choice, neurons 1 and 2). Both neurons fired at low, equivalent rates during entries into the two ports, indicating that the differences in encoding prior to an error versus a correct response were not the consequence of a miscoding of choice.
Fig. 5.
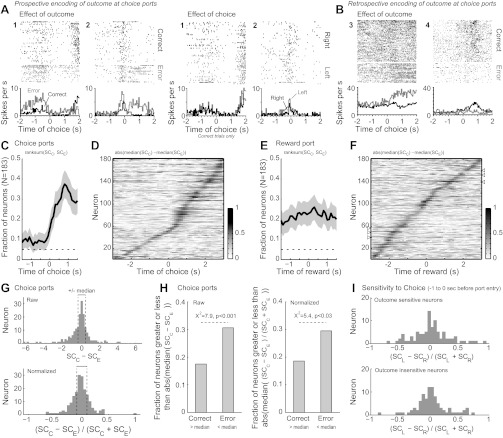
Neurons encoded trial outcomes, both prospectively and retrospectively. A: examples of neurons that fired at distinct rates at the choice ports on trials with correct and incorrect responding. Spike activity is shown for 2 simultaneously recorded neurons. In plots on left, trials were scored as correct or incorrect independent of the location of the choice. Neuron 1 fired persistently at a higher rate during the period before incorrect entries into the choice ports compared with correct port entries. Neuron 2 fired at a higher rate immediately prior to the incorrect port entries and also fired irregularly after the outcome was revealed, during the period when the rat traveled to the reward port. To examine whether the error trials reflected a “miscoding” of the forthcoming choice, in plots on right we plotted the spike activity for correct trials only and sorted the trials by the location of the choice (left or right). Both neurons fired at low, equivalent rates during entries into the 2 ports, providing evidence that neurons in the mPFC can prospectively encode trial outcomes prior to the rat's choice. B: examples of neurons that fired differently after correct and incorrect responding. Neuron 3 fired more spikes after incorrect responses were made. Neuron 4 fired more after correct responses were made. Feedback about the trial outcome was given at a latency of 0.04 s. (Note for A and B: Rasters for effects of outcome were sorted by the travel time to the reward port. Rasters for the effects of choice were plotted in the observed trial orders.) C: fraction of neurons that was sensitive to the trial outcome when rats entered the choice ports. D: normalized difference in spike counts around choice port entry for trials with correct and incorrect responses. As in Fig. 4C, none of the neurons fired at distinct rates throughout the period of the choice based on the outcome of the trial, neither before nor after the choice. Many neurons showed differences in spike counts immediately after feedback was given (0.1–0.5 s after port entry). E: fraction of neurons that was sensitive to the trial outcome when rats entered the reward port. About 20% of neurons were sensitive to outcome throughout this period. F: normalized difference in spike counts around choice port entry for trials with correct and incorrect responses. While most neurons fired selectively after correct and incorrect responses for no more than 1 s, some neurons did fire persistently during this period of the task (arrowheads near 60 and 140 on the y-axis). G: distribution of preference scores for correct and error trials based on raw (top) and normalized (bottom) spike counts for the database of 183 neurons. The median values of the absolute preference scores (shown as dashed lines) were used to characterize whether the cells' firing rates were sensitive to the trial outcome before the choice was made. H: the fractions of cells that showed differences in spike counts that were greater than the median value of the absolute difference in spike counts for the correct and error trials are summarized. Results are summarized as fractions based on raw (left) and normalized (right) measures of activity. For both measures of activity, significantly more cells fired more spikes prior to incorrect responses compared with correct responses based on a proportions test. I: the distributions of preference scores for left and right choices on correct trials were similar for the subpopulations of outcome-sensitive (top) and outcome-insensitive (bottom) neurons.
The fraction of outcome-sensitive neurons increased substantially after the choice was made and feedback (e.g., change in illumination) was given (Fig. 5C, after 0 s), reflecting retrospective encoding of outcome. Examples of neurons retrospectively encoding trial outcome are shown in Fig. 5B (neurons 3 and 4). None of these outcome-sensitive neurons fired at distinct rates throughout the period of the choice based on the outcome of the trial, either before or after the choice (Fig. 5D). Approximately 20% of neurons were differentially modulated by outcome throughout the period surrounding reward collection (Fig. 5E). While most neurons fired selectively after correct and incorrect responses for no more than 1 s, some neurons did fire persistently during this period of the task (Fig. 5F; arrowheads near 60 and 140 on the y-axis denote persistently firing neurons). More neurons in the population fired at a higher rate prior to errors versus correct responses (Fig. 5, G and H; proportions test: χ2 = 7.9, P < 0.001 for raw scores; χ2 = 5.4, P < 0.03 for normalized scores). The histograms in Fig. 5G show the distributions of raw (Fig. 5G, top) and normalized (Fig. 5G, bottom) preference scores that were calculated as the difference between median spike counts in a 1-s window before the time of the choice on trials with correct and error responses. Neurons to the left of the median (Fig. 5G, dashed lines) fired more to errors versus correct responses. Neurons to the right of the median fired more to correct responses. Figure 5H shows the relative proportions of neurons that fired more prior to correct versus error trials.
A similar metric (defined in methods) was used to examine preferences for left and right choices by the outcome-sensitive and outcome-insensitive neurons, as defined by the preference score metric shown in Fig. 5G. The distributions of these preference scores are shown Fig. 5I. Some outcome-sensitive neurons fired at distinct rates before left and right choices. Others, such as the two neurons shown in Fig. 5A, fired at equivalent rates prior to the choice. Overall, there was no difference in the preference scores for choice from the subpopulations of outcome-sensitive and outcome-insensitive neurons (Kolmogorov-Smirnov test statistic: 0.0778, P > 0.9).
Remarkably, there were neurons present in mPFC that fired differentially depending on the outcome of the previous trial. That is, neuronal firing around the time of choice (Fig. 6A) or reward collection (Fig. 6D) reflected whether the previous trial had been correct or incorrect, regardless of the current response. A small but significant fraction of the neuronal population (∼10–20% of all recorded neurons) fired in this manner. Several neurons (Fig. 6A and those denoted by arrowheads in Fig. 6C) fired persistently at distinct rates during the time of the choice following the encoded outcome. Most, however, fired briefly, with outcome-dependent firing occurring sequentially across multiple neurons around both the choice and the reward collection (Fig. 6, C and F).
Fig. 6.
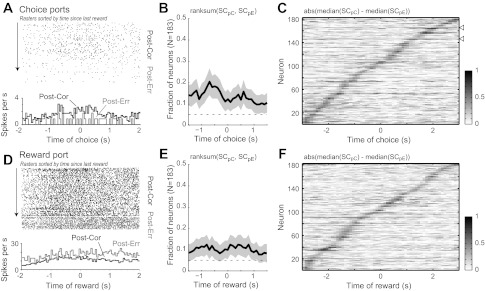
Neurons encoded the outcome of the preceding trial. A: example of a neuron that fired more spikes if the preceding response was correct (Post-Cor) compared with if it was an error (Post-Err). B: fraction of neurons that was sensitive to the outcome of the preceding trial during the period when rats entered the choice ports. C: normalized difference in spike counts around choice port entry for trials that were preceded by correct and incorrect responses. A few cells fired persistently at distinct rates throughout the period of choice (cell in A and those indicated by arrowheads in C). D–F: same plots as A–C for the period of reward port entry and fluid consumption.
As can be seen in Fig. 1D, IRIs varied across trials either because of variation in the rat's self-determined pace or because of the constraints on responding built into the controlled version of the task. Interestingly, there were a number of neurons with firing rates that reflected the time that had passed since the previous choice (see examples around choice port entry and reward collection in Fig. 7, A and D, respectively). Such neurons comprised a small but significant portion of the total neuronal population (Fig. 7, B and E), with differences in firing appearing in sequential neurons during the course of the trial (Fig. 7, C and F).
Fig. 7.
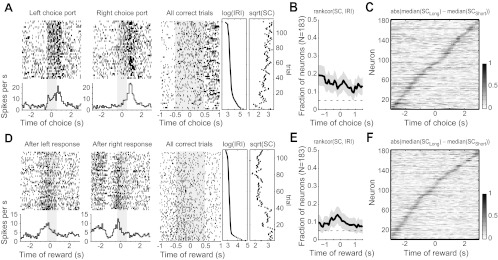
Neurons encoded the time since the last choice was made (i.e., pace). A: a neuron is shown that fired more during left responses compared with right responses (left). Raster on right shows the spike data collapsed over response ports and sorted by the IRI. A clear effect of the time since the last choice was made (“pace”) is revealed in this plot (Spearman's rank-correlation, P < 0.0125). The 2 plots on right show the IRI associated with each row in the raster plot and the spike count (SC) from the neuron during the ±0.5-s epoch around port entry. B: fraction of neurons that was sensitive to the time since the last choice was made when rats entered the choice ports. C: normalized difference in spike counts around choice port entry for trials in the upper and lower halves of the IRI distribution [i.e., Long = IRI > median(IRI)]. D: spike activity from a neuron that showed pace-related activity at the reward port. E and F: same plots as B and C for the period of reward port entry and fluid consumption.
Choice, Outcome, and Temporal Information Are Segregated over Neurons and Phases of the Task
A conjunction analysis was used to summarize the fractions of cells that were sensitive to choice, outcome, and temporal information within and between each phase in the task (response and reward ports) and to examine whether neurons encoded information about single or multiple behavioral variables. We chose to compare neuronal selectivity in two clearly defined task epochs: during three 1-s windows preceding, encompassing, and following either 1) entry into the choice ports, or 2) the start of reward consumption (Fig. 8C). These task epochs showed nearly identical population activity in animals tested in the self-paced and controlled-delay variations of the task (Fig. 3). Over all conjunctions of events, more neurons were sensitive to the individual task variables compared with those that were sensitive to conjunctions of the variables (χ2-test, P < 0.01). The only conjunction that was above the fraction expected by chance (i.e., product of fractions of Choice and Outcome neurons) was the 14.75% of cells that were jointly sensitive to Choice and Outcome during the choice phase of the task (gray asterisk in Fig. 8D, bottom). Notably, the fraction of neurons that were sensitive to Choice was significantly less when rats arrived at the reward port and consumed fluid compared with when rats responded at the choice ports and traveled to the reward port (χ2-test, P < 0.01; blue asterisks in Fig. 8E, middle and bottom).
Fig. 8.
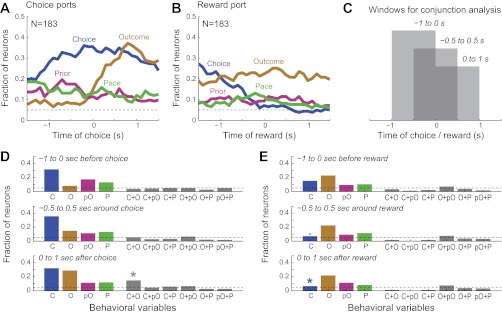
Segregated encoding of choice, outcome, and pace by single mPFC neurons. A and B: fractions of neurons sensitive to choice (blue), outcome (gold), preceding outcome (purple), and pace (green) around the time of choice (A) and reward (B). These data are the same as in the individual summaries in Figs. 4–7 and are shown together here to allow for comparison of the time courses of encoding across variables. C: to assess the degree of overlap in the encodings of the behavioral variables, a conjunction analysis was carried out using 3 of the 1-s data windows. D and E: conjunction analysis at the choice (D) and reward (E) ports. Fractions of neurons that were sensitive to each variable are shown on left (C, Choice; O, Outcome; pO, prior Outcome; P, Pace). Degree of overlap for pairs of behavioral variables is shown on right (e.g., C+O = Choice and Outcome).
Spatial Convergence of Choice-, Outcome-, and Pace-Related Information Within Medial Prefrontal Cortex
Despite the functional segregation of choice and outcome encoding at the level of single-neuron activity, an anatomical mapping of neurons revealed a spatial convergence of choice-, outcome-, and pace-related information within mPFC. This mapping was possible because of our use of microwire arrays and our approach to histological processing. We cut brains in horizontal sections, and this allowed us to localize electrode tips precisely by following electrode tracts to their terminations. A summary of all recording sites is shown in Fig. 2A. A summary of sites that contained neurons that were sensitive to one or more task variables during each epoch is shown in Fig. 9. Some sites (electrodes) allowed for recording more than one neuron.
Fig. 9.
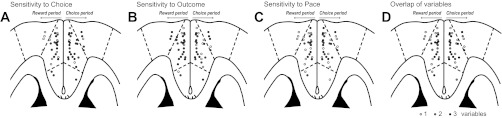
Anatomical convergence of neurons encoding choice, outcome, and pace. The locations of cells with sensitivities to choice, outcome (collapsed over the current and prior trial), and pace are shown with horizontal sections through the mPFC in A–C. In each plot, electrodes where at least 1 cell with a specific type of sensitivity was recorded are depicted as black dots. Electrodes with neurons that encoded other task variables are depicted as white dots. Neurons that encoded the task variables during the reward and choice periods are shown in the left and right hemispheres, respectively. (Note that the neurons were not actually located explicitly in these hemispheres.) D: summary of sensitivities to 1 or more task variables. Electrodes with neurons that were sensitive to 1 variable are depicted as light gray. Those with sensitivity to 2 variables are shown as dark gray. Those with sensitivity to 3 variables are shown as black. Overall, there was no clear segregation of cells with distinct behavioral sensitivities (e.g., choice-encoding cells were found across regions and layers). Instead, there was a commingling of cells with sensitivities to choice, outcome, and pace within the mPFC.
DISCUSSION
The goal of this study was to examine both spatial and nonspatial aspects of mPFC neuronal activity during performance of a spatial DA task that depends on mPFC function (Horst and Laubach 2009). Recordings in mPFC during task performance found many neurons that fired at distinct rates as a function of the spatial location of responding, the outcome of responding (correct or incorrect) on the current and previous trials, and the time since the last response (based on the IRI). Sensitivity of firing rates to the spatial choice was maximal at the time of the delayed response (Fig. 4), and outcome sensitivity was maximal after sensory feedback about the choice was delivered and at the time of reward consumption (Fig. 5). Outcome-related activity was found both before and after feedback about success was given, a finding that is suggestive of a prospective encoding of outcome by mPFC (Fig. 5), and more neurons showed an increase in firing when an error was committed compared with a correct choice (Fig. 5). Neurons also displayed the ability to maintain outcome-related information between trials (Fig. 6) and could encode behavioral pace (Fig. 7). Choice, outcome, or temporal sensitivities were segregated over neurons, as few cells encoded multiple aspects of the task (Fig. 8). Anatomical reconstructions of the electrode recording sites showed that neurons with distinct behavioral sensitivities could be recorded from the same electrodes and were distributed broadly and across layers within mPFC (Fig. 9). These findings suggest that spatial and nonspatial information is encoded by spatially and temporally distributed patterns of neuronal ensemble activity.
Spatial Encoding by Medial Prefrontal Cortex
Our recordings support other studies (Baeg et al. 2003; Chang et al. 2002; Jung et al. 1998; Pratt and Mizumori 2001) that did not find neurons in mPFC that fired persistently in a spatially selective manner during the delay period. By constraining the posture and spatial position of the rats, requiring them to remain within a narrow gap during the delay period, we were able to reduce the potential for behavioral variability associated with differences in posture or position on left and right choice trials. Many neurons (>30% of the population) encoded information about the spatial choice when animals responded at distinct spatial locations at the end of the delay period (Fig. 4B). However, many fewer neurons (∼5% of the population) encoded spatial information during the delay period (Fig. 4E, after 0 s). These findings support the view that mPFC is “not involved in the temporary on-line storage but rather in the control of information required to prospectively organize the ongoing action” (Gisquet-Verrier and Delatour 2006).
A key finding was based on measuring differences in mean firing rates on left and right choice trials, sorting neurons by their peak differences in choice-sensitive firing rates, and plotting the differences in activity over the neuronal population (Fig. 4, C and F). These plots resembled those in recent studies on the hippocampus (MacDonald et al. 2011; Pastalkova et al. 2008) and parietal cortex (Harvey et al. 2012). We interpret these results as evidence for choice information being encoded by brief periods of choice-sensitive firing that are sequentially propagated throughout the neuronal population. We found no evidence for cells in mPFC firing persistently throughout the entire delay period in a spatially mnemonic manner.
Nonspatial Encoding by Medial Prefrontal Cortex
Nonspatial processing was the dominant factor influencing the activity of mPFC neurons during DA. Similar to a recent study by Hyman and colleagues (2012), trial outcomes had a major effect on mPFC activity. Reward feedback was encoded by up to 40% of the population (Fig. 5). Prospective outcome-related activity was also commonly observed and comprised 10–20% of the population. Such neurons fired at distinct rates before rats made errors in the DA task (Fig. 5A). These cells are reminiscent of the persistently active, outcome-encoding cells that we have previously found in mPFC during a simple reaction time task (Narayanan and Laubach 2008). In the context of the spatial DA task, prospective outcome encoding does not necessarily reflect the animal's prediction of the trial outcome. Instead, it may represent uncertainty about whether the forthcoming response will yield reward. This is supported by the observation that behavior (and most likely the neuronal activity underlying behavior) is more variable prior to an incorrect versus a correct response (Horst and Laubach 2009). The greater prevalence of increased firing prior to errors versus correct responses may reflect the increased demands on prefrontal cortex for selecting an appropriate response in the face of an uncertain outcome.
A second novel finding with regard to nonspatial processing in mPFC is based on a subpopulation of neurons (<20%) that showed variability in firing rates associated with the time since the last trial, a measure that we describe as “pace” (Fig. 7). These cells were distinct from the subpopulations that encoded choice- and outcome-related information, as few pace-related neurons (<5%) were sensitive to these other variables (Fig. 8, D and E). As pace-sensitive neurons accounted for temporal variability in task performance, they might have a role in the maintenance of working memory or could reflect changes in motivation over the duration of the trials. In a related reversible inactivation study (Horst and Laubach 2009), we found that behavioral variability was altered after infusions of muscimol into mPFC but not into orbital and insular areas in the lateral frontal cortex. Previous lesion studies in monkeys (Chen et al. 1995; Thaler et al. 1995) and stroke studies in human beings (Stuss et al. 2003) have also reported changes in temporal variability following impairments of mPFC function. To our knowledge, the present study is the first to report pace-related encoding in mPFC during delayed response performance.
Functional Significance of Spatial and Nonspatial Processing by mPFC
Our recordings revealed that both spatial (Fig. 4) and nonspatial (Figs. 5–7) factors were associated with selective firing by neurons in the mPFC. Nonspatial factors included current and prior behavioral outcomes (Figs. 5 and 6) and the time elapsed since the last trial was performed (Fig. 7). Most neurons that encoded these variables did so in an independent manner (Fig. 8). The only conjunction of responses that was more common than expected by chance was the combination of choice- and outcome-related firing immediately following the time of the choice (Fig. 8D), presumably reflecting encoding of the current spatial choice with the sensory feedback about the outcome. Despite the functional segregation of neuronal response types in the mPFC, neurons with different response selectivity were located near one another and sometimes on the same electrode (Fig. 9). That is, there was a segregation of behavioral encoding by single mPFC neurons and an anatomical commingling of neurons with unique behavioral sensitivities in the mPFC. Together, these results suggest that there are multiple overlapping signals encoded by the mPFC, with the majority of neurons tracking the animal's success in performing the task. We therefore propose that the role of mPFC in delayed response tasks is to monitor behavioral performance based on information about the current state of action (including information about the animal's location in space) and the current and prior behavioral outcome (success vs. failure). By encoding these variables, the mPFC is able to ensure that the task is performed effectively (minimizing mistakes and maximizing rewards) and efficiently (minimizing demands on memory encoding and retrieval by monitoring and controlling the pace of task performance).
GRANTS
This research was supported by National Institutes of Health (NIH) Grant 2-T32-NS-007224 (N. K. Horst), the Kavli Foundation (M. Laubach), and NIH Grant P01-AG-030004-01A1 (M. Laubach).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K.H. and M.L. conception and design of research; N.K.H. performed experiments; N.K.H. and M.L. analyzed data; N.K.H. and M.L. interpreted results of experiments; N.K.H. and M.L. prepared figures; N.K.H. and M.L. drafted manuscript; N.K.H. and M.L. edited and revised manuscript; N.K.H. and M.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Instruments Shop at the John B. Pierce Laboratory for technical support throughout this project, Nandakumar Narayanan for assistance with surgeries, and Trevor Bekolay, Marcelo Caetano, and Hannah Clarke for helpful comments on this manuscript.
Present address of N. K. Horst: University of Cambridge, Cambridge, United Kingdom.
REFERENCES
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177–188, 2003 [DOI] [PubMed] [Google Scholar]
- Batuev AS, Kursina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res 41: 95–102, 1990 [DOI] [PubMed] [Google Scholar]
- Brito GN, Thomas GJ, Davis BJ, Gingold SI. Prelimbic cortex, mediodorsal thalamus, septum, and delayed alternation in rats. Exp Brain Res 46: 52–58, 1982 [DOI] [PubMed] [Google Scholar]
- Caetano MS, Horst NK, Harenberg L, Liu B, Arnsten AF, Laubach M. Lost in transition: aging-related changes in executive control by the medial prefrontal cortex. J Neurosci 32: 3765–3777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp Brain Res 142: 67–80, 2002 [DOI] [PubMed] [Google Scholar]
- Chen YC, Thaler D, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. II. The timing and selection of learned movements. Exp Brain Res 102: 461–473, 1995 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 134: 73–82, 1997 [DOI] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol 98: 303–316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behav Neurosci 110: 1282–1298, 1996 [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Involvement of the dorsal anterior cingulate cortex in temporal behavioral sequencing: subregional analysis of the medial prefrontal cortex in rat. Behav Brain Res 126: 105–114, 2001 [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Nathwani F, Brasted PJ. Medial prefrontal and neostriatal lesions disrupt performance in an operant delayed alternation task in rats. Behav Brain Res 106: 13–28, 1999 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113: 509–519, 1997 [DOI] [PubMed] [Google Scholar]
- Euston DR, McNaughton BL. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci 26: 13143–13155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971 [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Delatour B. The role of the rat prelimbic/infralimbic cortex in working memory: not involved in the short-term maintenance but in monitoring and processing functions. Neuroscience 141: 585–596, 2006 [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Physiol Psychol 77: 212–220, 1971 [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484: 62–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience 164: 444–456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Whitman J, Emberly E, Woodward TS, Seamans JK. Action and outcome activity state patterns in the anterior cingulate cortex. Cereb Cortex (May 22, 2012). doi:10.1093/cercor/bhs104 [DOI] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex 8: 437–450, 1998 [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol 34: 337–347, 1971 [DOI] [PubMed] [Google Scholar]
- Laubach M. A comparative perspective on executive and motivational control by the medial prefrontal cortex. In: Neural Basis of Cognitive and Motivation Control, edited by Mars R, Sallet J, Rushworth M, Yeung N. Cambridge, MA: MIT Press, 2011 [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71: 737–749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Pribram KH. Analysis of the effects of frontal lesions in monkey. I. Variations of delayed alternation. J Comp Physiol Psychol 48: 492–495, 1955 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: The Handbook of Aging and Cognition, edited by Salthouse TA, Craik FI. Hillsdale, NJ: Erlbaum, 1992, p. 315–372 [Google Scholar]
- Murray EA, Davidson M, Gaffan D, Olton DS, Suomi S. Effects of fornix transection and cingulate cortical ablation on spatial memory in rhesus monkeys. Exp Brain Res 74: 173–186, 1989 [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol 100: 520–525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science 321: 1322–1327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res 123: 165–183, 2001 [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7: 1–24, 1995 [DOI] [PubMed] [Google Scholar]
- Pribram KH, Fulton JF. An experimental critique of the effects of anterior cingulate ablations in monkey. Brain 77: 34–44, 1954 [DOI] [PubMed] [Google Scholar]
- Pribram KH, Wilson WA, Jr, Connors J. Effects of lesions of the medial forebrain on alternation behavior of rhesus monkeys. Exp Neurol 6: 36–47, 1962 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci 112: 293–303, 1998 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. J Cogn Neurosci 15: 338–353, 2003 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron 70: 1054–1069, 2011 [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res 14: 249–262, 2008 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain 126: 2363–2380, 2003 [DOI] [PubMed] [Google Scholar]
- Thaler D, Chen YC, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. I. Simple learned movements. Exp Brain Res 102: 445–460, 1995 [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Zijderveld G, van Hest A, de Bruin JP, van Eden CG, van de Poll NE. Acquisition of conditional associations and operant delayed spatial response alternation: effects of lesions in the medial prefrontal cortex. Behav Neurosci 102: 481–488, 1988 [DOI] [PubMed] [Google Scholar]


