Abstract
The correlated discharges of cortical neurons in primary somatosensory cortex are a potential source of information about somatosensory stimuli. One aspect of neuronal correlations that has not been well studied is how the spatiotemporal properties of tactile stimuli affect the presence and magnitude of correlations. We presented single- and dual-point stimuli with varying spatiotemporal relationships to the hands of three anesthetized owl monkeys and recorded neuronal activity from 100-electrode arrays implanted in primary somatosensory cortex. Correlation magnitudes derived from joint peristimulus time histogram (JPSTH) analysis of single neuron pairs were used to determine the level of spike timing correlations under selected spatiotemporal stimulus conditions. Correlated activities between neuron pairs were commonly observed, and the proportions of correlated pairs tended to decrease with distance between the recorded neurons. Distance between stimulus sites also affected correlations. When stimuli were presented simultaneously at two sites, ∼37% of the recorded neuron pairs showed significant correlations when adjacent phalanges were stimulated, and ∼21% of the pairs were significantly correlated when nonadjacent digits were stimulated. Spatial proximity of paired stimuli also increased the average correlation magnitude. Stimulus onset asynchronies in the paired stimuli had small effects on the correlation magnitude. These results show that correlated discharges between neurons at the first level of cortical processing provide information about the relative locations of two stimuli on the hand.
Keywords: area 3b, hand, joint peristimulus time histogram, multi-electrode array, primate
tactile sensations we experience may be suppressed or utilized in our behavior. In a highly simplified experimental analogy, we stimulated two points simultaneously or asynchronously on different hand locations to examine the spatiotemporal stimulus effects on cortical populations in primary somatosensory cortex, area 3b, in three anesthetized New World owl monkeys. Our goal was to determine if spatial and temporal stimulus manipulations affect the presence and magnitude of spike timing correlations in predictable ways related to anatomical connectivity. We focused on correlated firing between pairs of area 3b neurons that may reflect common thalamic input arriving nearly simultaneously to nearby neurons, local intracortical and/or feedback connections within cortex, or both thalamic and cortical connections (Reed et al. 2008). Properties and possible roles of neuronal correlations have been studied in several brain areas, reviewed elsewhere (Jermakowicz and Casagrande 2007; Kohn et al. 2009; Salinas and Sejnowski 2001). Correlated activities between pairs of neurons have been reported on extensively for the visual system (e.g., Bair et al. 2001; Dong et al. 2008; Fries et al. 2001; Jermakowicz et al. 2009; Kohn and Smith 2005), where stimulus properties and attention appear to influence changes in strength of correlations between neurons. Correlations in primate somatosensory cortex have been less extensively studied than those in visual cortex but may serve similar functions (e.g., Blake et al. 2005; Hsiao et al. 2002).
Spike timing correlations were shown to emerge in the 3b hand representations of two owl monkeys following long-term behavioral training in a touch detection task presented to adjacent digits (Blake et al. 2005). Receptive fields expanded, often to include sites on the two adjacent digits that were stimulated; and significant correlations developed between neuron pairs across distances ≤1.4 mm in cortex. We sought to determine how properties of tactile stimuli influenced the presence and magnitude of correlations between neuron pairs in the hand representation of primary somatosensory cortex in monkeys that were not trained in a sensory task.
Area 3b in primates is considered the homologue of S1 in other mammals (Kaas 1983), and correlated spiking activity in S1 has been reported in cats and rats. In anesthetized cats, correlation magnitudes were related to features of air jet stimuli, including location preference and directional selectivity (Roy and Alloway 1999). In anesthetized rats, neuron pairs in S1 showed differential correlation strengths for high or low frequencies of whisker stimulation (Zhang and Alloway 2004). In an extension of that study, Zhang and Alloway (2006) used air jets moving along rows and arcs of the rat whiskers to examine directional selectivity, and they concluded that correlation strength varied with density of intracortical connections. Thus spatiotemporal stimulus properties affect pairwise correlations between neurons in S1 of anesthetized cats and rats.
Therefore, we hypothesized that spike timing correlations would be enhanced by tactile stimulation in predictable ways in area 3b of monkeys. Because the densities of shared thalamic and intrinsic connections have been implicated in affecting the strength of neuronal correlations (e.g., Blake et al. 2005; Roy and Alloway 1999; Zhang and Alloway 2006), we predicted that neurons in close cortical space would tend to have higher correlation magnitudes than distant neurons in area 3b. Furthermore, we expected that simultaneous stimulation of paired locations would result in higher correlations between neuron pairs with common input (compared with asynchronous stimulation) because synchronization of the stimuli would be reflected in the neuron firing. Spatial and temporal parameters might interact such that the strongest correlations occur for neuron pairs closest in cortical space, representing neighboring locations on the hand, when the stimuli were presented closest in time. We expected interactions to vary as a function of spatial and temporal proximity, yielding probable explanations for properties of correlations in area 3b in the primate hand representation. We tested the hypothesis that spatial (different parts of the hand) and temporal (stimulus onset asynchronies) stimulus features would alter correlated discharges in cortical neurons. An initial report from two of the three monkeys described here compared correlation magnitudes for single-site stimulation on a digit with simultaneous stimulation on nonadjacent digit sites for pairs of neurons across the 3b hand representation (Reed et al. 2008). We extend those studies by describing effects of spatiotemporal stimulus variables on the occurrence and magnitudes of spike timing correlations between cortical neurons.
MATERIALS AND METHODS
Preparation
All procedures followed the guidelines established by the National Institutes of Health in accordance with protocols submitted to and approved by the Animal Care and Use Committee of Vanderbilt University. Three adult owl monkeys (Aotus nancymaae) were prepared for electrophysiological recordings following our previously published procedures (Reed et al. 2008, 2010a, 2010b, 2011). Monkeys 1 and 2 were males, each weighing 1 kg, and monkey 3 was a female, weighing 1.2 kg. The same monkeys were part of a study on neuron response properties (Reed et al. 2010a), and monkeys 1 and 2 were part of a previous study related to spike correlations (Reed et al. 2008). A ketamine injection (10–30 mg/kg im) was administered for sedation during surgical preparations. Anesthesia was induced with 2–4% halothane gas and maintained with intravenous propofol (10 mg·kg−1·h−1 iv). A servo-controlled heating pad was used to maintain rectal temperature between 37 and 39°C. The monkey was secured in a stereotaxic device throughout the surgery and experiment. Paralysis was maintained by vecuronium bromide (0.1–0.3 mg·kg−1·h−1 iv) mixed with 5% dextrose and lactated Ringer solution after the initial induction dose of 1–3 ml of vecuronium bromide. Animals were artificially ventilated with a mixture of N2O-O2-CO2 (75:23.5:1.5) at a rate sufficient to maintain peak end-tidal CO2 at ∼4%. Electrocardiograms and electroencephalograms were used to assess anesthetic depth. A craniotomy and duratomy were performed overlying primary somatosensory cortex. A pneumatic array inserter (Blackrock Instruments, Salt Lake City, UT) was set to 600-μm depth to target placement of the electrode tips within layer 3 of cortex. The craniotomy was covered with 1% agar mixed with Ringer solution to prevent desiccation and provide electrode stability. After the surgical procedures, supplemental anesthesia was provided during recordings by 0.3 mg·kg−1·h−1 propofol. In monkey 3, 1.2 mg/kg sufentanil (iv) was added to the Ringer solution during surgery to stabilize anesthetic depth. After delivery of this dose to achieve stable anesthesia, monkey 3 was maintained under propofol anesthesia without sufentanil during the recording period.
Stimulation Procedures
We collected neuron responses to tactile stimulation at single locations on the hand and at paired locations on adjacent and nonadjacent digits or on adjacent and nonadjacent phalanges within a single digit. Two independent force- and position-feedback-controlled motor systems (300B; Aurora Scientific, Aurora, ON, CA) provided the stimuli, controlled by a custom-designed Visual Basic program. Round Teflon probes 1 mm in diameter were secured to the lever arms of the motors to provide the contact surface of stimulation. Stimulus probes indented the skin 0.5 mm for 0.5 s, followed by 2.0 s off of the skin, for 100–120 trials (255–300 s) for each stimulus parameter presented. Data were analyzed from 100 trials. The 500-ms indentation allowed us to record transient and sustained responses (Sur et al. 1984) and used the minimal ramp time allowed by the stimulation equipment, which had a length step response time of 1.3 ms. Dual-point indentations were delivered to selected skin sites simultaneously (0 ms) or with stimulus onset asynchronies of 10, 30, 50, 100, and 500 ms. Both sets of onset asynchronies were tested for each pair of skin sites (i.e., the series stimulating site A first and site B first were included). Each site in a stimulus pair was stimulated separately (single-point stimuli) for comparison. Using methods described previously (Merzenich et al. 1978; Nelson et al. 1980), we mapped receptive fields to relate electrode locations to the hand representation in area 3b and to aid placement of stimulus probes (Reed et al. 2008).
Data Acquisition
Recordings were made using the 100-electrode “Utah” array and Bionics Data Acquisition System (now Blackrock Microsystems) with the following settings. Signals were amplified 5,000 times and bandpass filtered between 250 Hz and 7.5 kHz. Each electrode voltage threshold trigger was set for 3.25 times the mean activity. Waveforms were sampled at 30 kHz for 1.5-ms windows (Samonds et al. 2003).
Histology
After data collection, animals were given an overdose of pentobarbital sodium and perfused transcardially with saline followed by fixative. The brains were removed and prepared for histological analysis as described previously (Jain et al. 2001). The cortex was separated from the thalamus, flattened, and cut frozen at 40 μm parallel to the pial surface. We processed sections for myelin to aid in reconstructing the electrode sites (see Reed et al. 2008, Fig. SI 1). The presence of electrode tracks across serial sections was used to estimate electrode depths (see Reed et al. 2011 for details).
Data Analysis
Spike sorting and data selection.
Details regarding spike sorting procedures are reported fully in supplemental information of Reed et al. (2008 SI). Recording files were opened in Matlab (The MathWorks, Natick, MA), and signals for a given stimulus block were sorted together off-line with an automatic spike classification program based on the t-distribution Expectation Maximization algorithm (Shoham et al. 2003). A second spike-sorting program, Plexon Offline Sorter (Plexon, Dallas, TX), was used to verify quality of unit isolation by requiring that single units had refractory periods ≥1.2 ms and P values ≤0.05 for multivariate analysis of variance (ANOVA) related to spike cluster separation based on waveforms for spikes recorded on the same electrode (Nicolelis et al. 2003). Only well-isolated single units were included, and only one unit per electrode (Ventura and Gerkin 2012) was included in the analysis for each pair to avoid spurious correlations between units on the same electrode based on imperfect unit isolation. When more than one single unit was isolated from an electrode, we selected the single unit that responded to multiple stimulation conditions based on response criteria described later (see Firing rate responses). In addition, we included only those single-unit recordings that met the following conditions: 1) during the sustained period of the stimulus between 100 and 500 ms after stimulus onset, the unit had a mean firing rate >2 spikes/s above the average activity present during the 500 ms before stimulus onset; or 2) the unit responded with a firing rate >5 spikes/s above background activity to at least one of the conditions in a stimulus series; or 3) if the unit did not respond to any of the conditions in a stimulus series based on criterion 2, at least half of the conditions produced firing rates that met criterion 1. These criteria reduced the number of units that would have too few spikes for a valid calculation of the spike timing correlations (Aertsen et al. 1989; Gerstein et al. 1989, 2000). Neurons eliminated by our selection criteria could also have correlated discharges, but the calculation cannot be validated for neurons with very low firing rates. From the selected single units, we generated all of the possible pairs for cross-correlation analysis.
Spike timing correlations.
Methods to obtain peak correlations between neuron pairs using Matlab were the same as those we reported previously (Reed et al. 2008), in which the cross-correlation histogram is derived from joint peristimulus time histogram (JPSTH) analysis (e.g., Aertsen et al. 1989; Gerstein et al. 1989; Ventura et al. 2005), with all spike trains aligned with stimulus onset, similar to other studies of somatosensory cortex (Blake et al. 2005; Ghoshal et al. 2009; Roy and Alloway 1999; Roy et al. 2001, 2007; Zhang and Alloway 2004, 2006). To quantify spike timing synchrony with the JPSTH, small bin sizes between 1 and 5 ms are often used so that each bin contains 1 spike or less (e.g., Aertsen et al. 1989; Alloway et al. 2002; Gerstein et al. 1989; Oram et al. 2001; Roy et al. 2007). We chose 10-ms bins due to the low spontaneous firing rates of neurons under our recording conditions. Bins >5 ms have been used by others (e.g., Ghoshal et al. 2009; Oram et al. 2001; Reed et al. 2008; Vaadia et al. 1995), but bin sizes larger than 20 ms provide coarser temporal correlation (Oram et al. 2001). Almost all of the 10-ms bins in our data contained 1 spike or less, with only an occasional bin with 2 spikes during a transient response of 100 spikes/s or more. The 10-ms bins allowed us to measure correlations when firing rates were relatively low and without this binning adjustment often did not reach an appropriate number of spikes for constructing the JPSTH (Aertsen et al. 1989; Gerstein et al. 1989, 2000). The JPSTH was assembled from 0 to 700 ms poststimulus onset and was normalized by the standard shift-predictor method to correct for effects of firing rate on spike coincidence following the procedure described previously (Reed et al. 2008) and briefly presented here.
Peristimulus time histograms (PSTHs) of spike counts aligned on stimulation onset with 10-ms bins were obtained for each of a pair of neurons:
where ni(k)(u) is the number of spikes per bin in the kth of K total trials. Contribution to the joint peri-event time histogram (JPETH) of spikes in the kth trial is the product of the counts in each bin in the matrix for each neuron:
The average raw JPETH was obtained by
The raw JPETH measures the average incidence of common spike occurrences, but this coincidence is affected by modulation of the firing rates. The shift-predictor is the fraction of coincident spikes due to such modulation, estimated by the cross-product matrix of the individual PETHs:
The normalized JPSTH was determined by subtracting this shift-predictor from the raw JPETH and scaling each bin by the square root of the product of the standard deviations from the individual neurons:
Thus the normalized JPSTH measures probability of coincident spikes in the neuron pair corrected for modulation of firing rates. Values are treated as correlation coefficients, ranging from −1 to +1 (Gerstein et al. 1989). The time-averaged cross-correlogram, our measure of interest, sums JPSTH bins parallel to the main diagonal and measures the average positive or negative correlation across the entire interval of analysis.
To assess possible bias introduced by covariation in neuron excitability over trials and to test reliability of the normalized JPSTH, we repeated the JPSTH calculation on a set of spike trains in which no correlation should be present but where the spike train variability and firing rate are preserved (see Reed et al. 2008 SI). Shuffled JPSTHs were generated by shuffling the order of trials for one of each pair of neurons. Trial-by-trial variability present in the original normalized JPSTH is preserved in the shuffled JPSTH, but shuffling disrupts the relative timing of spikes between the two neurons. Bootstrapping (5,000 iterations) was used to test each normalized JPSTH correlation value against the distribution of shuffled correlation values. This procedure of comparing the shift-predictor normalized JPSTHs with shuffled JPSTHs allowed us to discard inappropriate or unreliable normalizations that did not exceed two standard deviations over the mean of the bootstrapped distribution. Our use of standard corrections in correlation calculations reduces the impact of spurious correlations based on a common stimulus and statistical coincidence due to high firing rates; and examination of shuffled correlations was a test of reliability beyond the standard shift-predictor normalization. Thus we are confident that correlations derived from the normalized JPSTH were reliable indicators of relationships between neurons beyond firing rate modulations.
Firing rate responses.
We used firing rate to determine the relationship of the responsiveness of neurons in the correlated pairs to the stimulus location. We mapped receptive fields; however, the relationship of the receptive fields of both neurons in each correlated pair with the locations of each stimulus probe was complex. Using a response field calculation described previously (Reed et al. 2010a) to assess the firing rate responses of each neuron in a correlated pair, we calculated presence or absence of a response by both neurons to single- or dual-point stimulation. We termed our variable “pair response” to relate the responses of neurons in the correlated pair. If both neurons in the pair responded to a given stimulation, pair response = 2; if only one neuron responded, pair response = 1; and if neither neuron responded, pair response = 0.
For the pair response variable, neurons were considered responsive to stimulation when the peak firing rate met criteria described in Reed et al. 2010a. In brief, spike trains were convolved with a function resembling a postsynaptic potential for smoothing using Matlab. Peak firing rate was calculated from the maximum of the spike density function within 50 ms after stimulation onset (corrected by subtracting average baseline firing rate). A threshold value for excitatory responses was determined as the average baseline firing rate plus two standard deviations of this baseline, at minimum, 5 spikes/s. If peak firing exceeded threshold, the firing was considered a significant response (1 vs. 0). The response designation for each neuron in a correlated pair was combined to give the value for the pair response (2, 1, or 0). This variable is not equivalent to receptive field overlap but relates to the two neurons' receptive fields relative to the stimulus locations. Pair response = 0 does not mean the pair has 0% receptive field overlap; it means the stimuli were outside of the receptive fields of both neurons in the correlated pair.
Summary Statistics
Data from Matlab calculations were summarized in Excel to import into SPSS 17.0 (SPSS, Chicago, IL). Information associated with each correlation included 1) temporal stimulation type of single- or dual-point stimulation at 0-, 10-, 30-, 50-, 100-, or 500-ms stimulus onset delays; 2) spatial proximity of the two probes in the stimulus series of adjacent digits, nonadjacent digits, adjacent phalanges within a digit, or nonadjacent phalanges within a digit; and 3) the pair response variable related to the response to stimulation of each neuron in the pair.
The data distributions differed from the normal and Poisson distributions (Kolmogorov-Smirnov 1-sample test) and had properties that led us to use generalized estimating equations, an extension of generalized linear models (see for review, Tuerlinckx et al. 2006), to analyze the factors contributing to the variance in correlation magnitudes. Data were imbalanced by group and were likely to be correlated rather than independent observations (e.g., Hardin and Hilbe 2003), since some of the same neuron pairs were recorded across several stimulation conditions. This analysis has fewer assumptions regarding data distribution than a parametric repeated-measures ANOVA and accounts for the possibility of non-independent data across the stimulus measures (Liang and Zeger 1986; Zeger and Liang 1986). We estimated sources of variance in the correlation values related to temporal stimulus conditions, spatial proximity of the stimuli, and pair response relationship of the neuron pairs. Bonferroni correction for multiple comparisons was applied within the analysis. The Wald chi-square statistic, based on linearly independent pairwise comparisons among estimated marginal means, was used to test significance of effects of the statistical model. Generalized estimating equations analysis provides estimates for population averages of correlation magnitude (dependent variable) based on the values of predictor variables and estimates for the significance of the effects. For discussion of our use of generalized estimating equations, refer to Reed and Kaas (2010).
RESULTS
Results were obtained from 58,571 pairs of single units from monkey 2, 25,420 pairs from monkey 3, and 2,921 pairs from monkey 1, which recorded the fewest pairs of neurons when we were refining the methods in the shortest duration recording session. These neuron pairs came from well-isolated neuron recordings: 163 neurons collected from 72 electrodes in monkey 2, 83 neurons collected from 65 electrodes in monkey 3, and 32 neurons collected from 29 electrodes in monkey 1. Most of the electrodes were located in the area 3b hand representation (see Fig. 1 for monkey 3 and Reed et al. 2010a, Fig. 2, for all cases). Neurons analyzed were drawn from area 3b, although electrodes near the 3b borders may have recorded neurons from areas 3a or 1.
Fig. 1.
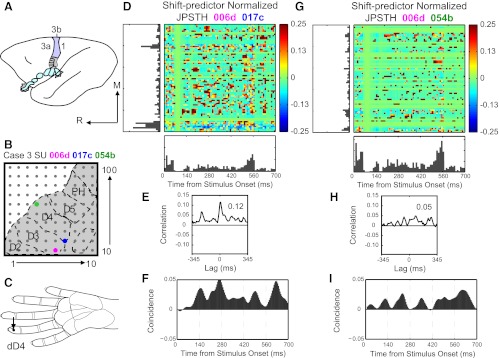
Examples of normalized joint peristimulus time histograms (JPSTH) reveal reliable correlations in spike timing between neuron pairs. A: schematic of owl monkey brain shown with area 3b highlighted in color and hand representation shaded gray. M, medial; R, rostral. B: estimated location of 100-electrode array for case 3 shown with representations of digits (D2–D5) and palm including the hypothenar (PH) pads outlined. Colored dots mark electrodes where single units 006d (pink), 017c (blue), and 054b (green) were recorded. C: arrow points to stimulation location (distal D4, dD4) on hand schematic for results in D–I. D: strong spike timing correlations between 006d and 017c revealed in normalized JPSTH. Color code represents magnitude of normalized correlation. E: correlation histogram derived from normalized JPSTH shows spike times correlated with peak magnitude of 0.12 (shift-predictor correction already applied). F: coincidence histogram shows dynamics of the normalized correlation over the stimulus presentation time. G: weaker spike timing synchrony revealed in normalized JPSTH and correlation histogram (H) between 006d and 054b, which are farther apart in cortex than 006d and 017c. I: coincidence histogram rises variably throughout stimulus duration.
Fig. 2.
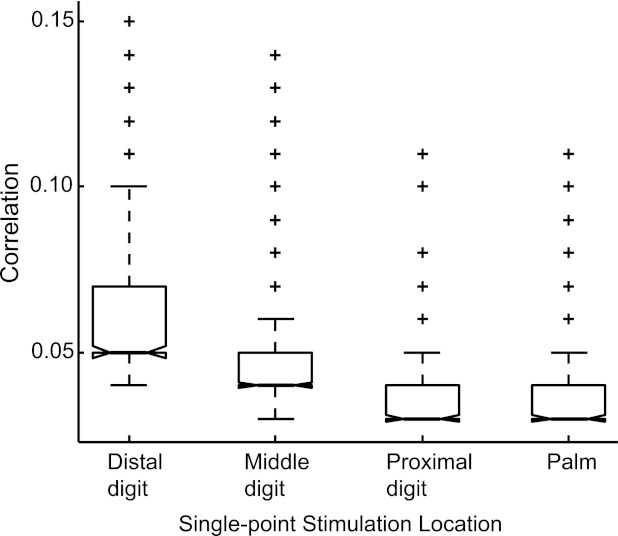
Correlation magnitude variations across stimulus locations. Single-point stimulation locations were grouped (x-axis) and normalized peak correlation values (y-axis) are shown in box plots to determine if stimulation location affected correlation magnitudes. Each group consists of the top 300 rank-ordered normalized correlation values for distal digit, middle digit, proximal digit, or palm locations. Solid horizontal line indicates median in each group. Above the median line is the upper quadrant of the sample, dashed whiskers indicate the range, and plus signs (+) indicate outliers. Box plots are ordered by rank such that distal digits had highest rank and palm had lowest rank.
Not all of the neuron pairs showed significant spike timing correlations, and only neurons that were significantly correlated were used to evaluate spatiotemporal factors contributing to differences in correlation magnitudes. Thus we analyzed 15,662 normalized peak correlations from 4,814 pairs of neurons across multiple stimulus conditions. Note that neuron pairs did not always retain significant correlations across all stimulus conditions. The mean of the observed correlation magnitudes for the data set was 0.0335 (SD 0.0149), the median and mode were both 0.0300, and the range was 0.02–0.22. These correlation magnitudes span the ranges reported in studies within the visual system reviewed by Schulz and Carandini (2010). Whereas multiple studies report correlation magnitudes between nearby cortical neurons in the range 0.1–0.4 (e.g., Bair et al. 2001; de la Rocha et al. 2007; Gutnisky and Dragoi 2008 Kohn and Smith 2005; Nauhaus et al. 2009; Smith and Kohn 2008), other studies have reported average correlations as low as 0.01 (Ecker et al. 2010; Renart et al. 2010). In the somatosensory system, spike timing correlation magnitudes closely match our results, with reports of average correlations from 0.024 to 0.04 (e.g., Alloway et al. 2002; Roy and Alloway 1999).
Figure 1 shows shift-predictor normalized JPSTH matrices from two pairs of neurons recorded in area 3b of monkey 3 (Fig. 1, D and G). Figure 1, D–F, shows results from a pair of neurons recorded from electrodes close in proximity to each other in cortex (Fig. 1B) during single-point stimulation (Fig. 1C). Our measure of interest was the magnitude of the peak correlation, which was large at 0.12 for this neuron pair (Fig. 1E). Inspection revealed that the peaks in the correlation histograms were centered near 0 ms, and the lags were not further analyzed in this report. The coincidence histogram (Fig. 1F) represents the near-coincident firing of the cells time-locked to the event on which the PSTHs are aligned, indicating the dynamics of the correlation between two neurons over time. We did not analyze the coincidence histogram for this study; however, inspection of a subset of the histograms revealed that coincidence is variable over time but tends to be greater during the indentation hold time than at the onset of the stimulus. Thus the calculation effectively normalized correlations that would be expected due to firing rate increases as the stimulus probe first contacts and is removed from the skin.
Results for a pair of neurons separated by greater distance are shown in Fig. 1, G–I. When these neurons were stimulated at a single site, the peak correlation value is lower (0.05) than when two neurons close to each other were examined with the same stimulus at the same time (0.12). In this report, we focus on determining what effects (if any) spatiotemporal stimulus parameter changes have on the presence and magnitude of the peak correlation (from the correlation histogram derived from the normalized JPSTH) and how the relationship of the distance between neuron pairs impacts correlations.
Effects of Single-Point Stimulation on Correlations
From 21,239 neuron pair occurrences recorded in all 3 monkey cases, 3,853 neuron pair occurrences showed significantly correlated spike timings (18.1%). From these 3,853, we tested the overall effects of single-point stimulus location on normalized peak correlation magnitudes. We grouped conditions for single-site stimulation into distal digit, middle digit, proximal digit, and palm locations. We examined correlation magnitudes with Kruskal-Wallis ANOVA (Matlab) using 300 values per group. When 300 values were sampled at random, no differences were found in correlation magnitudes based on groups (Kruskal-Wallis ANOVA: χ2 = 3.1602, P = 0.3676). When the top 300 values were rank-ordered for each group (Fig. 2), we found that distal digit stimulation resulted in higher correlations than middle or proximal digit stimulation and palm stimulation (Kruskal-Wallis ANOVA: χ2 = 479.0878, P < 0.00001).
This result resembles the expected innervation density of the hand (e.g., Darian-Smith and Kenins 1980; Johansson and Vallbo 1979) such that the distal digit has higher innervation density than the middle or proximal digits and palm. The analysis also suggests that many neuron pairs are weakly correlated (no difference when randomly sampling values) but that distinctions can be made when examining correlations with above-average magnitudes (the top 300 rank-ordered values reveal spatial differences expected anatomically). We then refer to results from single-point stimulus conditions when investigating how spatiotemporal parameters of stimuli applied to two points on the hand affect the presence and magnitude of normalized peak correlations.
Effects of Dual-Point Stimulation on Correlation Magnitudes
We used generalized estimating equations to determine the significant sources of variance between correlated pairs, using a factorial model of temporal stimulus asynchrony, spatial stimulus proximity, and the (firing rate) responses of both neurons in the pair to the stimulation. The estimated mean correlation magnitude was 0.034, which was close to the observed mean, 0.0335 (SD 0.0149). All main effects, two-way interactions, and three-way interactions tested were highly significant as determined through generalized estimating equations (Table 1). Frequency-distribution histograms of correlation magnitudes across the pair response categories and the spatial and temporal stimulus conditions for single- and dual-point stimuli (Fig. 3) show that the majority of correlation magnitudes were low (0.03) across all categories, and that higher correlation values were not linked to a single category type. The highest correlation magnitudes were found when dual-point stimuli on adjacent phalanges within one digit activated both neurons in the pair.
Table 1.
Tests of model effects for variance in correlation magnitude classified by spatiotemporal stimulus characteristics and interactions
| Variance Source | Wald Chi-Square | df | P Value |
|---|---|---|---|
| Intercept | 90,087.789 | 1 | <0.0001 |
| Temporal asynchrony | 201.848 | 5 | <0.0001 |
| Spatial proximity | 53.306 | 3 | <0.0001 |
| Pair response | 83.356 | 2 | <0.0001 |
| Temporal × spatial | 181.633 | 15 | <0.0001 |
| Temporal × response | 34.884 | 10 | <0.0001 |
| Spatial × response | 41.224 | 6 | <0.0001 |
| Temporal × spatial × response | 145.001 | 30 | <0.0001 |
Tests of model effects from generalized linear modeling with generalized estimating equations on the dependent variable correlation. df, Degrees of freedom; N = 15,662 observations from single-neuron pairs.
Fig. 3.
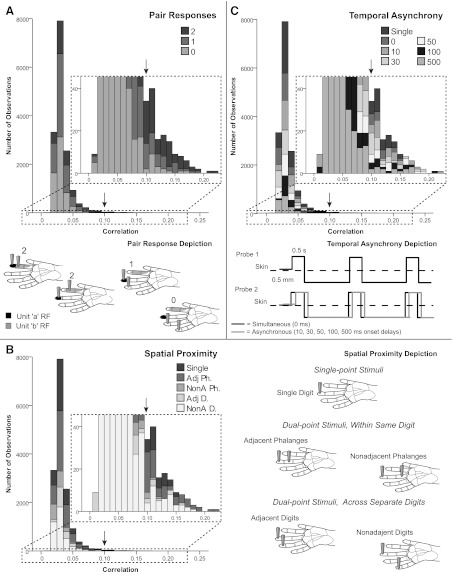
Frequency-distribution histograms for spatiotemporal stimulus relationships. Stacked frequency-distribution histograms show that the majority of observations for all categories had correlation magnitudes between 0.03 and 0.04. Insets marked by dotted lines show close-up of the low frequencies of occurrence. Arrows point to correlation value 0.10 on full-size and inset charts to aid orientation. A, top: for the pair response category, all response categories (0–2) occurred throughout the distribution, even at the high-magnitude correlation tail, but the highest magnitude correlations occurred when pair response = 2. Bottom, pair response schematic depicts possible configurations of stimulation probes in relation to neurons' receptive fields (RFs) for hypothetical units a and b, to categorize pair response relations. When pair response = 2, two types of relationships are possible: RFs of the units can be close in proximity and the stimulus probes can be close in proximity, and both units respond to stimulation; or one stimulus probe is within the RF of one unit while the second probe is in the RF of the second unit. B, left: for the frequency distribution of spatial proximity of stimulus probes, all spatial categories were distributed throughout, even at the high magnitude correlation tail. The highest magnitude correlations were observed when a dual-point stimulus was located on adjacent phalanges (Adj Ph). Adj Ph = 2 probes in the same finger on adjacent phalanges; NonA Ph = 2 probes within same finger on nonadjacent phalanges; Adj D = 2 probes on different, adjacent fingers; and NonA D = 2 probes on different fingers separated by 1 or more digits. Right, spatial proximity schematic illustrates categories for the spatial configuration of the stimulus probes. C, top: for the frequency distribution of temporal asynchrony of stimuli, the high-magnitude correlation tail included all temporal categories, with no clear trends relating to the highest magnitudes. Bottom, temporal asynchrony depiction of stimulation pattern. Solid lines indicate indentation on the skin. Paired stimulation, indicated by probes 1 and 2, may be simultaneous or asynchronous. Two probes are presented to different skin sites; however, the gray solid line (probe 2) shifted relative to the black line depicts overlap in contact time of probes 1 and 2.
Mean correlation magnitudes are shown for each category in Table 2. Note that categories refer to locations of stimulus probes for single- and dual-point stimulation. Categories do not refer to receptive fields of the two neurons in each pair relative to the stimulus probes, except for the simplified pair response measure. See Fig. 3 for schematic depictions of the three categories. Spatial proximity refers to proximity of stimulus probes on the hand surface. All dual-point stimuli on separate digits were matched on distal phalanges (e.g., distal digit 2 with distal digit 3) except for one nonadjacent digit set pairing middle digit 3 with distal digit 1. Distance between neurons (electrodes) in cortex is treated separately from spatial (stimulus) proximity on the hand.
Table 2.
Effects of stimulus relationships on normalized peak correlation magnitude
| Stimulus Category | Correlation | SE | N | P Value |
|---|---|---|---|---|
| Full data set | 0.0335 | 0.00012 | 15,662 | |
| Single point | 0.0340 | 0.00022 | 3,853 | |
| Dual-point stimuli by pair response relationship | ||||
| Pair response 2 | 0.0388 | 0.00039 | 3,063 | |
| Pair response 1 | 0.0336 | 0.00017 | 6,567 | <0.0001 |
| Pair response 0 | 0.0308 | 0.00014 | 6,032 | <0.0001 |
| Dual-point stimuli by spatial proximity parameters | ||||
| Adj Ph | 0.0349 | 0.00023 | 4,849 | 0.001 |
| NonA Ph | 0.0293 | 0.00029 | 1,578 | <0.0001 |
| Adj D | 0.0356 | 0.00034 | 1,444 | <0.0001 |
| NonA D | 0.0324 | 0.00026 | 3,938 | >0.90 |
| Dual-point stimuli by temporal asynchrony parameters | ||||
| 0 ms | 0.0312 | 0.00027 | 2,353 | <0.0001 |
| 10 ms | 0.0359 | 0.00034 | 2,267 | <0.0001 |
| 30 ms | 0.0328 | 0.00033 | 2,284 | >0.90 |
| 50 ms | 0.0327 | 0.00035 | 1,853 | >0.90 |
| 100 ms | 0.0366 | 0.00042 | 1,383 | <0.0001 |
| 500 ms | 0.0320 | 0.00039 | 1,669 | 0.272 |
Correlation values are means and standard error (SE). P values are from multiple comparison tests from Table 1 for stimulus parameters compared with single-point stimulation, except for pair response relationship, which are compared with pair response 2.
Pair response relationships.
The neural responses to tactile stimulation had clear effects on correlation magnitude. When both neurons in the pair responded to the stimulation, the correlation magnitude was higher than when only one neuron in the pair responded or when neither neuron responded (Table 2). The correlation magnitudes we report are those beyond what is expected by chance based on firing rate alone (Reed et al. 2008 SI).
Spatial stimulus proximity.
Neurons coactivated by a single stimulus probe had low correlation magnitudes; however, stimulation of adjacent phalanges increased correlation magnitudes, specifically when the phalanges were on the same or adjacent digits (Table 2). Generally, a spatial gradient existed such that correlation magnitudes showed dependence on the proximity between the paired stimulus sites. Linear regression for the Pearson correlation between the stimulus proximity and correlation magnitude was −0.077 (P < 0.0001; N = 2529) for the subset of dual-point stimulations presented simultaneously (0-ms temporal asynchrony). Thus dual-point stimulation of nearby digit locations increased correlation magnitudes over single-point stimulations, whereas correlation strengths decreased when two distant locations on the hand were stimulated in a weakly negative relationship between correlation magnitude and spatial stimulus proximity.
Temporal stimulus asynchrony.
When stimuli presented at a single point or at two points with stimulus onset asynchronies were compared, correlation magnitudes varied within only a small range. In contrast to our expectations, correlation magnitudes did not decrease predictably as the paired stimuli were separated by longer time delays, and on average, stimulating two points simultaneously resulted in significant decreases in correlation magnitude compared with single-point stimulation (Table 2).
Spatiotemporal interactions.
All spatiotemporal interactions included in our model were significant effects (Table 1). Figure 4 shows factors involved in the three-way interaction (P < 0.0001), which reflects the main effects on correlation magnitudes for temporal asynchrony, spatial proximity, and response categories. Correlation magnitudes were consistently highest when both neurons in a pair responded to stimulation (pair response = 2). Correlation magnitudes tended to increase more for pairs of responding neurons (pair response = 2) when stimulations were presented at longer stimulus onset delays, except when pairs of stimuli were presented to nonadjacent phalanges. Results indicate that magnitudes of spike timing correlations between neuron pairs were affected by the neurons' receptive fields (pair responses) and the relative location of tactile stimuli to each other (spatial proximity), whereas time differences between stimulation onsets (temporal asynchrony) had weaker impact but contributed to the significant three-way interaction (Table 1).
Fig. 4.
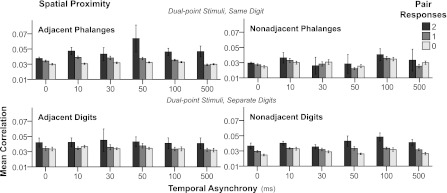
Mean correlation magnitudes across dual-point spatiotemporal stimulation conditions. Bar graphs show mean correlation magnitudes (y-axis) with simultaneous (0 ms) and asynchronous stimulation at 10-, 30-, 50-, 100-, and 500- ms delays (x-axis). Shaded bars indicate pair responses, and separate panels designate spatial proximity of stimulation sites (see Fig. 3). Error bars = 95% confidence intervals. Stimulus factors interacted to contribute to differences in correlation magnitudes, because the patterns are not replicated in the same directions across each condition.
Influence of Stimulus Distance (Spatial Proximity) on Correlation Occurrences
Temporal stimulus parameters affected correlation magnitudes very little; thus subsequent analyses were performed for simultaneous (0-ms asynchrony) dual-point stimulation to focus on spatial parameters. Spatial proximity of stimulation points affected not only magnitudes of correlations (Table 2) but also their presence or frequency of occurrence. Neighboring regions tended to show correlated activity more often than distant regions, as depicted in one aspect of Table 3 (proportion totals). Stimulation of adjacent phalanges resulted in the greatest proportion of correlated pairs (37.2%), followed by nonadjacent phalanges (30.7%). Stimulation of adjacent and nonadjacent digits resulted in similar proportions of correlated neuron pairs (20.6%, 20.8%).
Table 3.
Proportions of correlated neuron pairs across electrode distances when two hand sites were stimulated simultaneously
| Electrode distance, mm |
|||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | Total | |
| Adjacent phalanges | |||||||
| Recorded pairs, n | 435 | 798 | 923 | 389 | 48 | 1 | 2,594 |
| Correlated pairs, n | 196 | 263 | 344 | 141 | 20 | 0 | 964 |
| Proportion, % | 45.1 | 33.0 | 37.3 | 36.3 | 41.7 | 0 | 37.2 |
| Nonadjacent phalanges | |||||||
| Recorded pairs, n | 447 | 835 | 944 | 389 | 42 | 1 | 2,658 |
| Correlated pairs, n | 181 | 224 | 286 | 107 | 17 | 0 | 815 |
| Proportion, % | 40.5 | 26.8 | 30.3 | 27.5 | 40.5 | 0 | 30.7 |
| Adjacent Digits | |||||||
| Recorded pairs, n | 221 | 327 | 299 | 133 | 24 | 0 | 1,004 |
| Correlated pairs, n | 66 | 64 | 58 | 17 | 2 | 0 | 207 |
| Proportion, % | 29.9 | 19.6 | 19.4 | 12.8 | 8.3 | 20.6 | |
| Nonadjacent digits | |||||||
| Recorded pairs, n | 489 | 793 | 942 | 351 | 30 | 0 | 2,605 |
| Correlated pairs, n | 110 | 173 | 181 | 74 | 5 | 0 | 543 |
| Proportion, % | 22.5 | 21.8 | 19.2 | 21.1 | 16.7 | 20.8 | |
| Totals | |||||||
| Recorded pairs, n | 1,592 | 2,753 | 3,108 | 1,262 | 144 | 2 | 8,861 |
| Correlated pairs, n | 553 | 724 | 869 | 336 | 44 | 0 | 2,529 |
| Proportion, % | 34.7 | 26.3 | 28.0 | 26.9 | 30.6 | 0 | 28.5 |
No entry indicates category is undefined.
Relationship of Correlation Magnitude and Average Firing Rate
We examined the relationship between correlation magnitudes and average firing rates of neurons in the correlated pair as another way to assess how responsiveness to stimulus conditions affected correlation strength (Table 4). Geometric mean spike rate is commonly used to average firing rates of the two neurons in a correlated pair (e.g., Bair et al. 2001; de la Rocha et al. 2007; Greenberg et al. 2008; Jermakowicz et al. 2009). We took the average firing rate of each neuron during the epoch when the correlation was measured (0 to 700 ms). The square root of the product of the two neurons' firing rates is referred to as the geometric mean firing rate (GMFR).
Table 4.
Linear regression of correlation magnitude and GMFR by spatiotemporal stimulus parameters
| Stimulus Category | Correlation | SD | GMFR, spikes/s | SD | N | Pearson r | P Value | Spearman ρ | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Full data set | 0.0335 | 0.0149 | 3.006 | 1.262 | 15,662 | 0.440 | <0.0001 | 0.483 | <0.0001 |
| Single point | 0.0340 | 0.0138 | 3.126 | 3.459 | 3,853 | 0.420 | <0.0001 | 0.471 | <0.0001 |
| Dual-Point Stimuli By Spatial Proximity Parameters | |||||||||
| Adj Ph | 0.0349 | 0.0159 | 2.076 | 1.792 | 4,849 | 0.298 | <0.0001 | 0.416 | <0.0001 |
| NonA Ph | 0.0293 | 0.0115 | 1.996 | 1.480 | 1,578 | 0.457 | <0.0001 | 0.502 | <0.0001 |
| Adj D | 0.0356 | 0.0130 | 3.150 | 2.705 | 1,444 | 0.443 | <0.0001 | 0.428 | <0.0001 |
| NonA D | 0.0324 | 0.0162 | 4.385 | 6.216 | 3,938 | 0.646 | <0.0001 | 0.661 | <0.0001 |
| Dual-Point Stimuli By Temporal Asynchrony Parameters | |||||||||
| 0 ms | 0.0312 | 0.0129 | 2.680 | 3.363 | 2,353 | 0.466 | <0.0001 | 0.540 | <0.0001 |
| 10 ms | 0.0359 | 0.0161 | 3.327 | 4.208 | 2,267 | 0.402 | <0.0001 | 0.423 | <0.0001 |
| 30 ms | 0.0328 | 0.0157 | 2.847 | 4.123 | 2,284 | 0.428 | <0.0001 | 0.486 | <0.0001 |
| 50 ms | 0.0327 | 0.0150 | 2.856 | 4.249 | 1,853 | 0.467 | <0.0001 | 0.545 | <0.0001 |
| 100 ms | 0.0366 | 0.0155 | 3.429 | 4.552 | 1,383 | 0.414 | <0.0001 | 0.350 | <0.0001 |
| 500 ms | 0.0320 | 0.0158 | 2.785 | 3.989 | 1,669 | 0.493 | <0.0001 | 0.450 | <0.0001 |
Values indicate linear regression (Pearson's r) and nonparametric correlation (Spearman's ρ) between the spike timing correlation magnitude and geometric mean firing rate (GMFR; spikes/s) for neurons in each correlated pair by spatiotemporal stimulus parameter category.
Linear regression analysis (SPSS) across all spatiotemporal stimulus factors showed that correlation magnitudes weakly increased with larger mean firing rates (Pearson r = 0.440, P < 0.0001, N = 15,662; see Table 4). However, the relationship between firing rates and correlation magnitude was strongest when nonadjacent digit locations were stimulated and weakest when adjacent phalanges within a single digit were stimulated.
Because spatial proximity of stimuli affected relationships between correlation magnitude and average firing rate similarly across temporal conditions, trends are shown in a heat map (Fig. 5) for single-point control and simultaneous dual-point stimulation conditions across digits (adjacent and nonadjacent digits) and within digits (adjacent and nonadjacent phalanges). The relationship between correlation magnitudes and GMFR most resembled single-point stimulation when nonadjacent digits were stimulated. Correlation magnitudes for dual-point stimulation did not vary strongly with firing rate when stimuli were near each other on the hand, whereas correlations were somewhat higher with higher firing rates when nonadjacent digits were stimulated simultaneously. A scatter plot of the data set is shown in Fig. 5, indicating the weak relationship between correlation magnitude and GMFR overall.
Fig. 5.
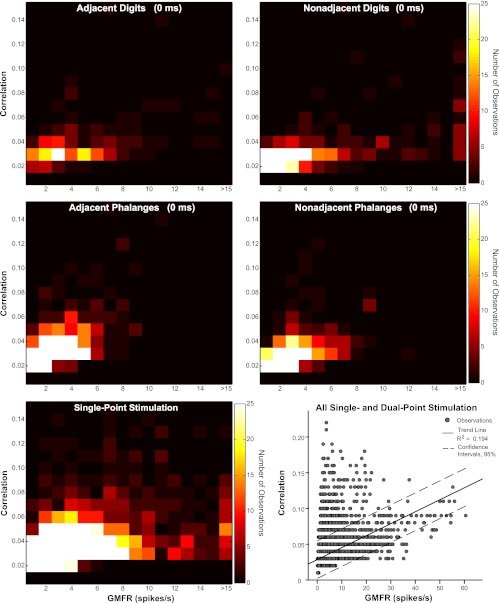
Relationship of correlation magnitudes with average firing rates. Peak correlation magnitudes vs. geometric mean firing rate (GMFR; spikes/s) of neurons in correlated pairs are shown in heat maps generated from scatter plots for single-point stimulation and simultaneous (0 ms) dual-point stimulation. Color scale applies to all maps and indicates frequency of neuron pair observations. Stimulus conditions shown are representative of the full set of results. Scatter plot shows data set (15,662 observations) with trend line (solid) indicating the weak relationship between correlation magnitude and GMFR overall (R2 = 0.194). Dotted lines represent 95% confidence intervals.
Cortical Distance Between Neurons and Stimulus Parameter Effects on Correlations
The configuration of the 100-electrode array allowed patterns of correlations across cortical space to be examined. To represent the relationship of the electrode distance between pairs of recorded neurons with the magnitudes of the spike timing correlations, we developed a visualization of the spatial representation of the region covered by the array (Fig. 6) as published previously (Reed et al. 2008). Figure 6 shows an example of correlation patterns during selected spatiotemporal stimulus conditions. These patterns reveal similarities when two single sites were stimulated, despite the difference in locations (digits 2 and 5).
Fig. 6.
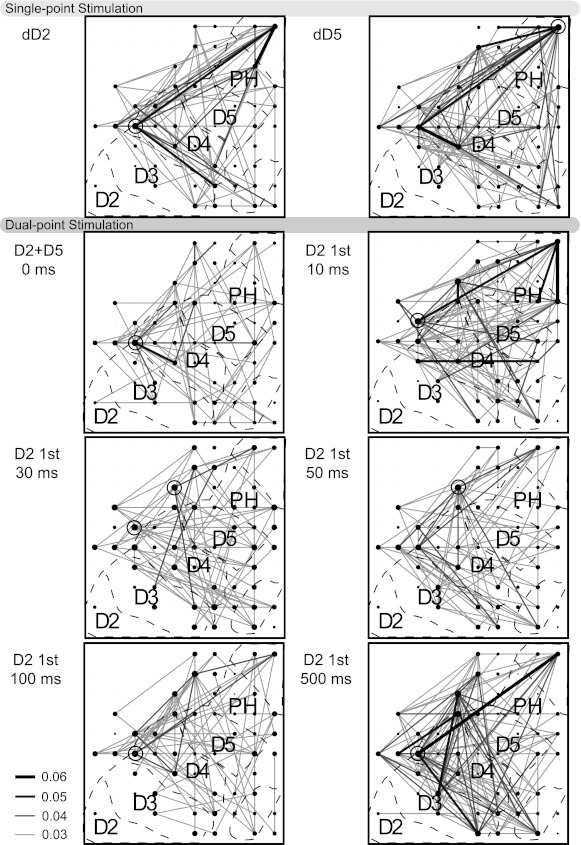
Depiction of correlations between neuron pairs during spatiotemporal stimulations. Stimuli were presented to distal digit 2 (dD2) and 5 (dD5) individually in blocks of 100 trials for monkey 3. These locations were stimulated simultaneously and asynchronously with dD2 stimulation preceding dD5. Black dots indicate electrode sites from which single neurons were recorded. Significant correlations represented by lines connecting the dots. Dot size indicates magnitude of the sum of all correlations with that neuron. Open circles surround dots that represent neurons with the highest magnitude of summed correlations. Shade and thickness of lines represent magnitude of correlations (see key). When correlations are not reliable, no lines are shown, but dot sizes increase to indicate which electrodes recorded single neurons during the stimulation (multi-unit activity = pinpoint dots). Overlay of approximate locations in area 3b is indicated by dashed lines. Networks of correlations were largely stable regardless of stimulus parameters, with a notable exception of reduced correlations when distant digits were stimulated simultaneously.
This suggests that the network of connections that produced correlated activities was minimally dependent on the stimulation site. The numbers of significant correlations decreased when these two sites were stimulated simultaneously, and some correlations between specific pairs appeared that were not present in either of the single-point stimulation conditions. Correlations during the remaining dual-point asynchronous stimulation conditions in Fig. 6 increased in number and magnitude compared with simultaneous stimulation, which is similar to the trends in the population (Table 2). These illustrations of correlated activity emphasize the consistently weak and widespread nature of the correlations between neurons across the hand representation and show the limited changes in correlations across the cortical region as spatial and temporal stimulus parameters on the hand vary.
Cortical distance effects on correlation occurrences.
We used the spacing between electrodes in the array to estimate the cortical distance between neurons and relate this distance to effects on the frequency of occurrence of correlated pairs in our recordings. Because stimulus onset asynchronies had weak and variable effects (Fig. 3, Table 2), we analyzed simultaneous (0 ms) dual-point stimulation to focus on the spatial aspects of stimulus parameters along with cortical distance. The proportions of correlated pairs compared with the total recorded pairs for each category of stimulus spatial proximity are listed in Table 3, grouped by electrode distance between neurons in the pairs. Overall, proportions of correlated pairs decreased with distance between the electrodes in cortex across all categories of spatial stimulus proximity on the hand. Neurons located more closely in cortex were more likely to be correlated, regardless of activation by stimulation on two distant points on the hand.
Cortical distance effects on correlation magnitudes.
We also examined the relationship of the magnitude of correlations and the distance between the electrode pairs from which the correlated neurons were recorded, as in other studies within somatosensory cortex (Alloway et al. 2002; Reed et al. 2008; Roy and Alloway 1999). These paired values of cortical electrode distance and correlation magnitude were categorized by the stimulus proximity on the hand for simultaneous (0 ms) dual-point stimulation. The Pearson correlation from linear regression between electrode distance and the magnitude of correlated activity across all stimulus proximity conditions was −0.083 (P < 0.0005; N = 2,529). Data are shown in heat maps divided by the proximity category (Fig. 7). To determine whether relationships differed for stimulation within a digit and across digits, we calculated the Pearson correlation between electrode distance and magnitude of spike correlations for each spatial proximity category, listed in Table 5. The strongest relationship was found when nonadjacent digits were stimulated, at −0.1567. The electrode distance between correlated neurons most affected correlation magnitudes when the paired stimuli were distant, but there were no strong trends for differences that appear to represent the rostral-caudal dimension in cortex (within a digit) or the medial-lateral dimension (across digits). Weak correlations were found between neuron pairs across all electrode distances from 400 to 4,800 μm. Although stronger correlations tended to occur between nearby neurons (400 μm apart), strong correlations were found between neuron pairs separated by more than 4 mm.
Fig. 7.
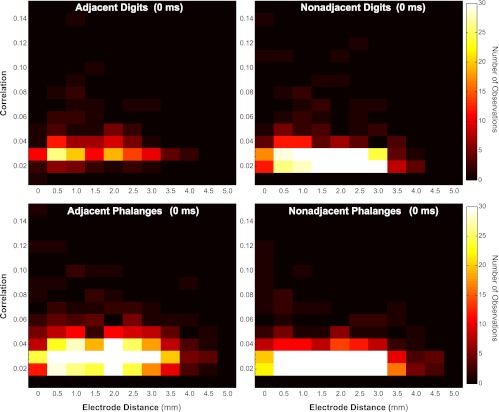
Relationship between correlation magnitude and distance between electrode pairs. Magnitudes of significant correlations plotted (y-axis) on the basis of geometric distance between electrode pairs from which the activity was recorded (x-axis) for each of the dual-point (0 ms) spatial proximity categories are shown in heat maps. Color scale applies to all plots and indicates number of neuron pairs observed. Generally, stronger correlations were found between neurons located on nearby electrodes, but correlations were found between neuron pairs separated by more than 4 mm, especially when 2 sites within 1 digit were stimulated.
Table 5.
Regression analysis of correlation magnitude and electrode distance categorized by stimulus proximity for simultaneous (0 ms) dual-point stimulation
| Spatial Proximity | Pearson r | P Value | N |
|---|---|---|---|
| Adj Ph | −0.0496 | 0.1238 | 964 |
| NonA Ph | −0.0815 | 0.0200 | 815 |
| Adj D | −0.0171 | 0.8071 | 207 |
| NonA D | −0.1567 | 0.0002 | 543 |
| Total (combined) | −0.0830 | <0.0001 | 2,529 |
Values indicate linear regression (Pearson's r) between the spike timing correlation magnitude and electrode distance for neuron pairs.
DISCUSSION
Spike timing correlations indicate that neuron pairs are linked in some way by common inputs or direct synaptic connections. Functional connections measured through spike timing correlations have been described as very dynamic (e.g., Eggermont 2006; Fries et al. 2001; Tiesinga and Sejnowski 2004), and synchronies between pairs of neurons in area 3b have been shown to increase after extensive training on a tactile discrimination task in owl monkeys (Blake et al. 2005). We found that neurons distributed throughout the hand representation of area 3b of owl monkeys exhibit correlated activity during single-point or dual-point tactile stimulation of the hand. Stimulus variations in spatiotemporal properties resulted in small changes in the magnitudes of correlations that varied across correlated pairs (Table 2). Here we compare our results to those of others in an effort to evaluate how spatiotemporal stimulus parameters affect spike timing correlations in primary somatosensory cortex.
Effects of Spatial and Temporal Stimulus Properties on Correlations
In accordance with the hypothesis that correlations in spike timings across neurons in 3b are useful for signaling that stimuli grouped in time and space are part of the same object, we expected that simultaneous stimulation of two locations would increase the magnitudes or numbers of correlations between neuron pairs due to common synaptic input, and that the farther apart in time two paired stimuli were presented, the lower the correlation magnitudes would be. Our results did not match this expectation, because simultaneous (0 ms) stimulation at two sites reduced correlation magnitudes compared with single-point stimulation and dual-point stimulation at onset asynchronies from 10 to 500 ms (Table 2). One explanation for this finding is that simultaneous stimulation on different and discontinuous parts of the hand is disruptive to correlations in a given network. We did not calculate correlations during spontaneous activity to represent the underlying network state due to low spontaneous firing rate in our anesthetized monkeys; however, correlations obtained during driven activity tend to reflect correlations in spontaneous activity when spike rates are high enough to perform the calculation (Reed et al. 2008 SI, Fig. 3). Changes in temporal stimulus presentation had variable effects on correlation magnitudes within a small range (Table 2); therefore, even though temporal parameters were significant factors in the variance of correlation strength according to our statistical model (Tables 1 and 2), we do not suggest that small changes in weak correlations play significant roles in stimulus processing. Further study is required to determine the impact of varying temporal stimulus parameters on properties of spike timing correlations and what role, if any, this plays in processing.
Summarizing over temporal stimulus conditions, proximity of dual-point stimuli on the hand affected the magnitude of correlations (Table 2). The prediction that stimulus locations closer in space would be associated with higher correlation magnitudes was largely supported. Anatomical connections within the area 3b hand representation appear denser in the distal-to-proximal axis within a digit than across digit representations (Fang et al. 2002); thus there is anatomical basis to expect differences in functional connections of these regions. We addressed stimulating points within a digit vs. across two digits. Figure 6 is consistent with the average results, showing reduced correlation strengths in many neuron pairs when distant digits were stimulated. Stimulating nearby digits tended to increase correlations (Table 2). Possibly, if digit sites were stimulated without intervening gaps (D3 and D4 between D2 and D5, Fig. 6) the results may differ.
Although magnitudes of correlations did not match all predictions, when sites closer in spatial proximity were stimulated simultaneously, greater proportions of neurons were correlated, as expected. Simultaneous dual-point stimulation on nearby hand locations resulted in greater percentages of correlated neurons out of total recorded neurons (adjacent phalanges = 37.2%, Table 3) compared with dual-point stimulation on distant locations (nonadjacent digits = 20.8%) and with single-point stimulation (18.1%).
We also evaluated a pair response factor, which is the number of units in a correlated pair responding to a stimulus with significantly increased firing. GMFR does not directly reveal whether a neuron responded to tactile stimulation; therefore, the pair response measure was developed. Pair responses give a simplified measure of overlap between neuron receptive fields with the stimulus locations. We found that when both neurons in the pair responded to stimulation, correlation magnitude was highest, and when neither neuron responded to stimulation, significant correlations were still present but mean correlation magnitude was lowest (Table 2). Based on this pair response factor, our results are similar to others (e.g., de la Rocha et al. 2007, in vitro and model neurons; Greenberg et al. 2008, rat visual cortex; Jermakowicz et al. 2009, primate visual cortex) reporting trends for correlation magnitude to increase with increasing firing rates. Neuron pairs that both responded to stimulation were encountered less often than pairs in which only one neuron or neither neuron responded to stimulation for recordings in area 3b. Observations may differ in higher order areas because area 3b receptive fields tend to be confined to one digit, whereas receptive complexity tends to increase in cortical areas further in the processing stream (e.g., S2), where neurons are more likely to have multi-digit or bilateral receptive fields (see Mountcastle 2005).
Our results from area 3b support the expectation that the relationship between the responsiveness of both neurons in the pair and the stimulation location (pair responses) would play a role in the magnitude of the correlations across all stimulus parameters (Fig. 4). However, our results did not satisfy the expectation that stimulation at longer stimulus onset delays would decrease correlation magnitudes compared with near-simultaneous stimulation. If such effects exist, they were not detectable. Figure 6 reflects the complexity of interactions across the hand representation under selected spatiotemporal stimulation conditions. General localization of correlated pairs remained qualitatively the same, spanning most of the hand representation, throughout the stimulation series. Single-point stimulation on digit 5 did not result in increased correlation numbers or magnitudes localized within the representation of that digit.
Although on average the site of stimulation did not affect correlation magnitudes, the highest correlation magnitudes were found when distal digit sites were stimulated, and the lowest magnitudes were found when the proximal digit sites or palm sites were stimulated (Fig. 2). Possibly, higher correlation magnitudes between pairs of neurons occur where innervation density of the skin is higher and those regions in cortex are highly represented. Also, because weak correlations between neurons across the hand representation are abundant for all spatiotemporal stimulus conditions tested, stronger magnitude correlations between neurons likely promote firing in common target neurons, such as in area 1, and will be important to study.
It should be noted that our studies were performed in animals anesthetized with propofol, which acts primarily as a GABAA receptor agonist (e.g., Trapani et al. 2000), and dose-dependent reductions in amplitudes of somatosensory evoked potentials and prolonged latencies have been reported in propofol-anesthetized rats (e.g., Logginidou et al. 2003). Correlations in anesthetized animals are likely to reflect only the functional connections not silenced by anesthetic-induced inhibition. We might expect higher correlations in awake animals under the same spatiotemporal stimulations.
Relationship of Correlation Magnitude and Average Firing Rate
Several reports describe significant relationships between correlation magnitudes and average firing rates such that higher correlation magnitudes tend to occur when the two neurons in the pair have higher average firing rates (e.g., de la Rocha et al. 2007; Greenberg et al. 2008; Jermakowicz et al. 2009), although others report very weak relationships (Samonds and Bonds 2005). Our data showed weak, positive relationships overall; however, this trend occurred primarily when nonadjacent digit locations were stimulated (Fig. 5). This effect occurred because firing rates were not greatly suppressed by paired stimulation on nonadjacent digits, whereas firing rates tended to decrease (compared to single-point stimulation) when paired stimuli were presented on nearby hand locations. This difference may result from weaker lateral inhibition when distant digits were stimulated compared with sites closer in proximity (Reed et al. 2010a).
Figure 8 summarizes correlations between neuron pairs and average firing rates of the correlated neurons when dual-point stimuli were presented across the hand, averaged over all temporal stimulation conditions. Stimulation on adjacent phalanges within a digit resulted in the highest proportion of correlated pairs and nearly the highest correlation magnitudes; thus the thickest line depicts the relationship between these locations as the strongest based on correlations, followed by a slightly thinner line between adjacent digits. This reflects the lower proportion of correlated pairs and higher correlation magnitudes found when adjacent digits were stimulated. Stimulation on nonadjacent phalanges resulted in the second largest proportion of correlated pairs, but magnitudes were the lowest, reflected by the thin line. The line between nonadjacent digits is similar in thickness, because this group had the second lowest proportion of correlated pairs, and magnitudes of those correlations were the second lowest among the conditions.
Fig. 8.
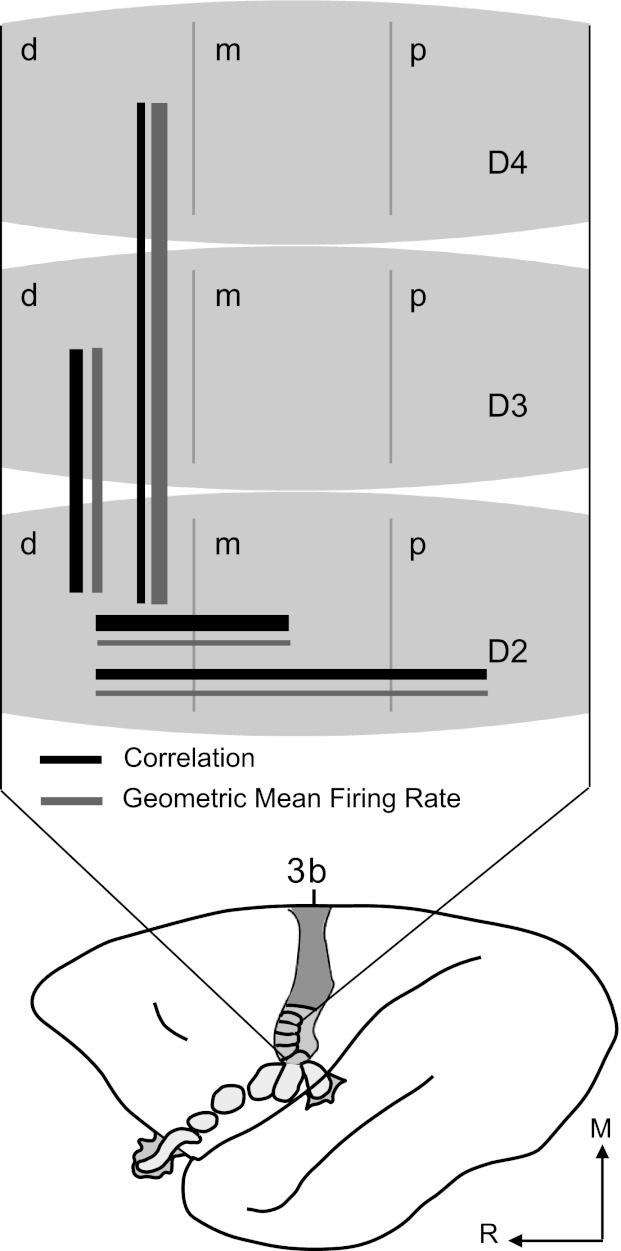
Summary of interactions within hand representation based on correlations and average firing rates. Schematic of owl monkey brain shows area 3b organization. Part of the hand representation is schematized and enlarged to summarize correlation and firing rate results. Digits are divided into distal (d), middle (m), and proximal (p) sections to represent phalanges within each digit. Solid black lines represent relationships between parts of the area 3b hand representation based on the magnitudes of correlations and proportions of correlated neuron pairs. Solid gray lines indicate relationships based on magnitudes of GMFR of correlated pairs. Line thickness approximates relative magnitudes within each category for spatial proximity of paired, dual-point stimuli. Relative strength of correlations between neurons did not strictly follow average firing rates of those neurons when different parts of the hand were stimulated.
Average GMFRs of neurons within the hand representation of area 3b summarized in Fig. 8 did not follow the same patterns as the spike timing correlations. For example, when nonadjacent digits were stimulated, average firing rates showed the largest magnitudes while magnitudes and proportions of correlations recorded were low. Correlation magnitudes do not increase with firing rate under all conditions.
Similarly, Roy and Alloway (1999) found that 17% of neuron pairs they recorded in cat S1 showed changes in correlations related to directional preferences to moving air jet stimulation without corresponding changes in the firing rate. Greenberg et al. (2008) found that despite firing rate decreases, spike timing correlations increased during anesthesia compared with awake periods. Vaadia et al. (1995; sensorimotor cortex, behaving macaques) and deCharms and Merzenich (1996; auditory cortex, anesthetized marmosets), found that spike timing correlations can change without corresponding changes in mean firing rate. Roelfsema et al. (2004; primary visual cortex, behaving macaques) found that firing rates covaried with perceptual grouping of stimuli, but correlation magnitudes varied instead with general task difficulty. Our findings, in conjunction with those of others, indicate that correlation magnitudes do not strictly follow firing rate modulations and thus reveal that different sources of modulation exist.
Our previous report (Reed et al. 2010a) from these same neuron populations examined peak firing rates (rather than average firing rates) of single neurons (rather than neuron pairs). Although it is difficult to compare the measures used in this study with those of the previous study, general comparison may relate to whether firing rate measures or temporal correlation measures, or both, best capture tactile stimulus information. Peak firing rates of single neurons were suppressed by dual-point stimulation, with the greatest suppression for the 30-ms stimulus onset asynchrony (Reed et al. 2010a). These peak firing results resembled the GMFR for neuron pairs (Table 4). Correlations between neuron pairs did not resemble trends found in peak firing rates, since firing rates were more strongly affected by temporal stimulus parameters than were correlations, which varied more strongly with spatial factors. These differences support the assertion that neuron spike rates and spike timing correlations play different roles rather than provide redundant information.
If spike timings are correlated in neurons that project to the same target neurons, target neurons may be activated even when spike rates are low (e.g., Stuart and Häusser 2001). We found dual-point stimulation conditions in which firing rates tended to decrease while correlation strength was maintained near single-point stimulation levels (Table 4). When firing rates decrease and correlation strengths do not follow, the maintained correlated activity may combine to drive postsynaptic shared target neurons.
Relationship of Correlations and Cortical Distance
We also examined how the proximity of dual-point stimuli affected correlations relative to the distance between the two neurons. Generally, proportions of correlated neurons decreased with increasing distance between neuron pairs and when sites closer in spatial proximity were stimulated simultaneously, greater proportions of neurons were correlated (Table 3). These data extend our previous report on nonadjacent digit stimulation (Reed et al. 2008) and resemble reports from others.
Alloway and colleagues (1999, 2002) examined correlations between neuron pairs (of single- and multi-units) in cat somatosensory cortex (S1 and S2) and found a tendency for the proportion of correlated pairs to decrease with increasing distance between electrode pairs. They examined smaller electrode spacing to a maximal distance of about 0.9 mm, whereas our array allowed us to examine 0.4-mm electrode spacing up to a maximal distance of 4.8 mm. We found significant correlations even at distances beyond 4 mm (Fig. 7). Widespread presence of low-magnitude correlations resulted in a linear correlation coefficient that was low and weak (Pearson r = −0.083; Table 5). This trend resembles that found by Greenberg et al. (2008) in visual cortex of awake and anesthetized rats, with weak relationships between correlation magnitude and distance between electrode sites (awake: r = 0.004, P = 0.94; anesthetized: r = −0.07, P = 0.023).
Summary and Conclusions
Overall, these correlations between neurons spanning across the hand representation in primary somatosensory cortex indicate that widespread interactions occur within area 3b of primates, and the magnitude and presence of correlated activity is affected by the spatiotemporal configurations of tactile stimuli in ways that do not mirror changes in firing rate. Strong correlations between neurons within adjacent phalanges of a digit may correspond to a greater density of intrinsic connections along the distal-proximal axis of the digit representation, and strong correlations between adjacent digit representations could correspond to dense intrinsic connections within the hand representation (Fang et al. 2002). Shared thalamic inputs are unlikely to be main contributors to correlations between adjacent digit representations and are even less likely to contribute to correlations between neurons in nonadjacent digit representations (Qi et al. 2011). Lateral intrinsic connections and feedback connections may contribute to correlation magnitude changes with distance between neurons in different digit representations but would likely show correlation peaks not centered on zero.
Although the influence of temporal stimulus relationships on the magnitude of spike timing correlations is unclear, the average strength of correlations suggests that adjacent phalanges and adjacent digits have stronger functional intrinsic or feedback connections than nonadjacent phalanges and nonadjacent digits in the area 3b hand representation of owl monkeys. Neurophysiological results may guide anatomically based experiments to investigate the anatomical underpinnings of our results. For example, future experiments could test how destroying different possible sources of common input and cortical connections would affect the correlations compared with the results reported here. The present neurophysiological findings may be relevant to tactile object perception, because stimuli that contact adjacent skin locations may be more likely to be part of the same object. Our results support the hypothesis that sensory stimulus information can affect correlations between neurons, and these correlations may provide information about stimuli in addition to that provided by changes in firing rate.
GRANTS
This work was supported by the James S. McDonnell Foundation (to J. H. Kaas) and NIH Grants R01 NS16446 (to J. H. Kaas), F31 NS053231 (to J. L. Reed), R03 NS057399 (to H.-X. Qi), and T32 GM07347 (to M. J. Burish).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.R., P.P., H.-X.Q., and J.H.K. conception and design of research; J.L.R., H.-X.Q., Z.Z., M.R.B., and M.J.B. performed experiments; J.L.R. analyzed data; J.L.R., P.P., H.-X.Q., and J.H.K. interpreted results of experiments; J.L.R., P.P., and H.-X.Q. prepared figures; J.L.R. and P.P. drafted manuscript; J.L.R., P.P., H.-X.Q., Z.Z., M.R.B., M.J.B., and J.H.K. edited and revised manuscript; J.L.R., P.P., H.-X.Q., Z.Z., M.R.B., M.J.B., and J.H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. A. B. Bonds for expert guidance, assistance, and use of facilities and equipment; Drs. Omar Gharbawie and Corrie Camalier for help collecting data from two of the three monkeys; and Dr. Ford Ebner for helpful comments on the manuscript. Matlab scripts were written primarily by P. Pouget and John Haitas in collaboration with J. L. Reed and Jurnell Cockhren.
Present address of P. Pouget: Neurologie et Thérapeutique Expérimentale, Université Pierre and Marie Curie, Hôpital de la Salpêtrière, 47 boulevard de l'Hôpital, Paris, France.
Present address of Z. Zhou: Research and Development, Epic Systems Corporation, 1979 Milky Way, Verona WI 53593.
Present address of M. J. Burish: Department of Neurology, University of California, San Francisco, CA 94143.
REFERENCES
- Aertsen AMHJ, Gerstein GL, Habib M, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol 61: 900–917, 1989 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Zhang M, Dick SH, Roy SA. Pervasive synchronization of local neural networks in the secondary somatosensory cortex of cats during focal cutaneous stimulation. Exp Brain Res 147: 227–242, 2002 [DOI] [PubMed] [Google Scholar]
- Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21: 1676–1697, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol 94: 2239–2250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I, Kenins P. Innervation density of mechanoreceptive fibres supplying glaborous skin of the monkey's index finger. J Physiol 309: 147–155, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature 381: 610–613, 1996 [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Doiron B, Shea-Brown E, Josić K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature 448: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- Dong Y, Mihalas S, Qui F, von der Heydt R, Niebur E. Synchrony and the binding problem in macaque visual cortex. J Vis 8: 1–16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science 327: 584–587, 2010 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Properties of correlated neural activity clusters in cat auditory cortex resemble those of neural assemblies. J Neurophysiol 96: 746–764, 2006 [DOI] [PubMed] [Google Scholar]
- Fang PC, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of new world monkeys. J Comp Neurol 454: 310–319, 2002 [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds J, Rorie A, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001 [DOI] [PubMed] [Google Scholar]
- Gerstein GL. Cross-correlation measures of unresolved multi-neuron recordings. J Neurosci Methods 100: 41–51, 2000 [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Bedenbaugh P, Aertsen AM. Neuronal assemblies. IEEE Trans Biomed Eng 36: 4–14, 1989 [DOI] [PubMed] [Google Scholar]
- Ghoshal A, Pouget P, Popescu M, Ebner F. Early bilateral sensory deprivation blocks the development of coincident discharge in rat barrel cortex. J Neurosci 29: 2384–2392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JND. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci 11: 749–751, 2008 [DOI] [PubMed] [Google Scholar]
- Gutnisky DA, Dragoi V. Adaptive coding of visual information in neural populations. Nature 452: 220–224, 2008 [DOI] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman and Hall/CRC, 2003 [Google Scholar]
- Hsiao SS, Lane J, Fitzgerald P. Representation of orientation in the somatosensory system. Behav Brain Res 135: 93–103, 2002 [DOI] [PubMed] [Google Scholar]
- Jain N, Qi HX, Kaas JH. Long-term chronic multichannel recordings from sensorimotor cortex and thalamus of primates. In: Progress in Brain Research, edited by Nicolelis MA. Amsterdam: Elsevier Science, 2001, chapt 5, vol. 130, p. 1–10 [PubMed] [Google Scholar]
- Jermakowicz WJ, Casagrande VA. Neural networks a century after Cajal. Brain Res Rev 55: 264–284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermakowicz WJ, Chen X, Khaytin I, Bonds AB, Casagrande VA. Relationship between spontaneous and evoked spike-time correlations in primate visual cortex. J Neurophysiol 101: 2279–2289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four type of mechanoreceptive units in glaborous skin. J Physiol 286: 283–300, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–231, 1983 [DOI] [PubMed] [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25: 3661–3673, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Zandvakili A, Smith MA. Correlations and brain states: from electro-physiology to functional imaging. Curr Opin Neurobiol 19: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22, 1986 [Google Scholar]
- Logginidou HG, Li BH, Li DP, Lohmann JS, Schuler G, DiVittore NA, Kreiser S, Cronin AJ. Propofol suppresses the cortical somatosensory evoked potential in rats. Anesth Analg 97:1784–1788, 2003 [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Lin CS. Double representations of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). J Comp Neurol 181: 41–74, 1978 [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The Sensory Hand: Neural Mechanisms of Somatic Sensation. Cambridge, MA: Harvard University Press, 2005, p. 281–300, 379–395 [Google Scholar]
- Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci 12: 70–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol 192: 611- 643, 1980 [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram MW, Hatsopoulos NG, Richmond BJ, Donoghue JP. Excess synchrony in motor cortical neurons provides redundant direction information with that from coarse temporal measures. J Neurophysiol 86: 1700–1716, 2001 [DOI] [PubMed] [Google Scholar]
- Qi HX, Gharbawie OA, Wong P, Kaas JH. Cell-poor septa separate representations of digits in the ventroposterior nucleus of the thalamus in monkeys and prosimian galagos. J Comp Neurol 519: 738–758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Kaas JH. Statistical analysis of large-scale neuronal recording data. Neural Netw 23:673–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Pouget P, Qi HX, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc Natl Acad Sci USA 105: 10233–10237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi HX, Kaas JH. Spatiotemporal properties of neuron response suppression in owl monkey primary somatosensory cortex when stimuli are presented to both hands. J Neurosci 31: 3589–3601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi HX, Pouget P, Burish MJ, Bonds AB, Kaas JH. Modular processing in the hand representation of primate primary somatosensory cortex coexists with widespread activation. J Neurophysiol 104: 3136–3145, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi HX, Zhou Z, Bernard MR, Burish MJ, Bonds AB, Kaas JH. Response properties of neurons in primary somatosensory cortex of owl monkeys reflect widespread spatiotemporal integration. J Neurophysiol 103:2139–2157, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science 327: 587–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Synchrony and covariation of firing rates in the primary visual cortex during contour grouping. Nat Neurosci 7: 982–991, 2004 [DOI] [PubMed] [Google Scholar]
- Roy A, Steinmetz PN, Hsiao SS, Johnson KO, Niebur E. Synchrony: a neural correlate of somatosensory attention. J Neurophysiol 98: 1645–1661, 2007 [DOI] [PubMed] [Google Scholar]
- Roy S, Alloway KD. Synchronization of local neural networks in the somatosensory cortex: a comparison of stationary and moving stimuli. J Neurophysiol 81: 999–1013, 1999 [DOI] [PubMed] [Google Scholar]
- Roy SA, Dear SP, Alloway KD. Long-range cortical synchronization without concomitant oscillations in the somatosensory system of anesthetized cats. J Neurosci 21: 1795–1808, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2: 539–550, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperation between area 17 neuron pairs enhances fine discrimination of orientation. J Neurosci 23: 2416–2425, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Bonds AB. Gamma oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J Neurophysiol 93: 223–236, 2005 [DOI] [PubMed] [Google Scholar]
- Schulz DPA, Carandini M. An uncorrelated state for the cortex? F1000 Biol Rep 2: 43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127: 111–122, 2003 [DOI] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci 28: 12591–12603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Häusser M. Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci 4: 63–71, 2001 [DOI] [PubMed] [Google Scholar]
- Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol 51: 724–744, 1984 [DOI] [PubMed] [Google Scholar]
- Tiesinga PH, Sejnowski TJ. Rapid temporal modulation of synchrony by competition in cortical interneuron networks. Neural Comput 16: 251–275, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani G, Altomare C, Sanna E, Biggio G, Liso G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem 7: 249–271, 2000 [DOI] [PubMed] [Google Scholar]
- Tuerlinckx F, Rijmen F, Verbeke G, De Boeck P. Statistical inference in generalized linear mixed models: a review. Br J Math Stat Psychol 59: 225–255, 2006 [DOI] [PubMed] [Google Scholar]
- Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H, Aertsen A. Dynamics of neuronal interactions in monkey cortex in relation to behavioural events. Nature 373: 515–518, 1995 [DOI] [PubMed] [Google Scholar]
- Ventura V, Cai C, Kass RE. Statistical assessment of time-varying dependency between two neurons. J Neurophysiol 94: 2940–2947, 2005 [DOI] [PubMed] [Google Scholar]
- Ventura V, Gerkin RC. Accurately estimating neuronal correlation requires a new spike-sorting paradigm. Proc Natl Acad Sci USA 109: 7230–7235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]
- Zhang M, Alloway KD. Stimulus-induced intercolumnar synchronization of neuronal activity in rat barrel cortex: a laminar analysis. J Neurophysiol 92: 1464–1478, 2004 [DOI] [PubMed] [Google Scholar]
- Zhang M, Alloway KD. Intercolumnar synchronization of neuronal activity in rat barrel cortex during patterned airjet stimulation: a laminar analysis. Exp Brain Res 169: 311–325, 2006 [DOI] [PubMed] [Google Scholar]


