Abstract
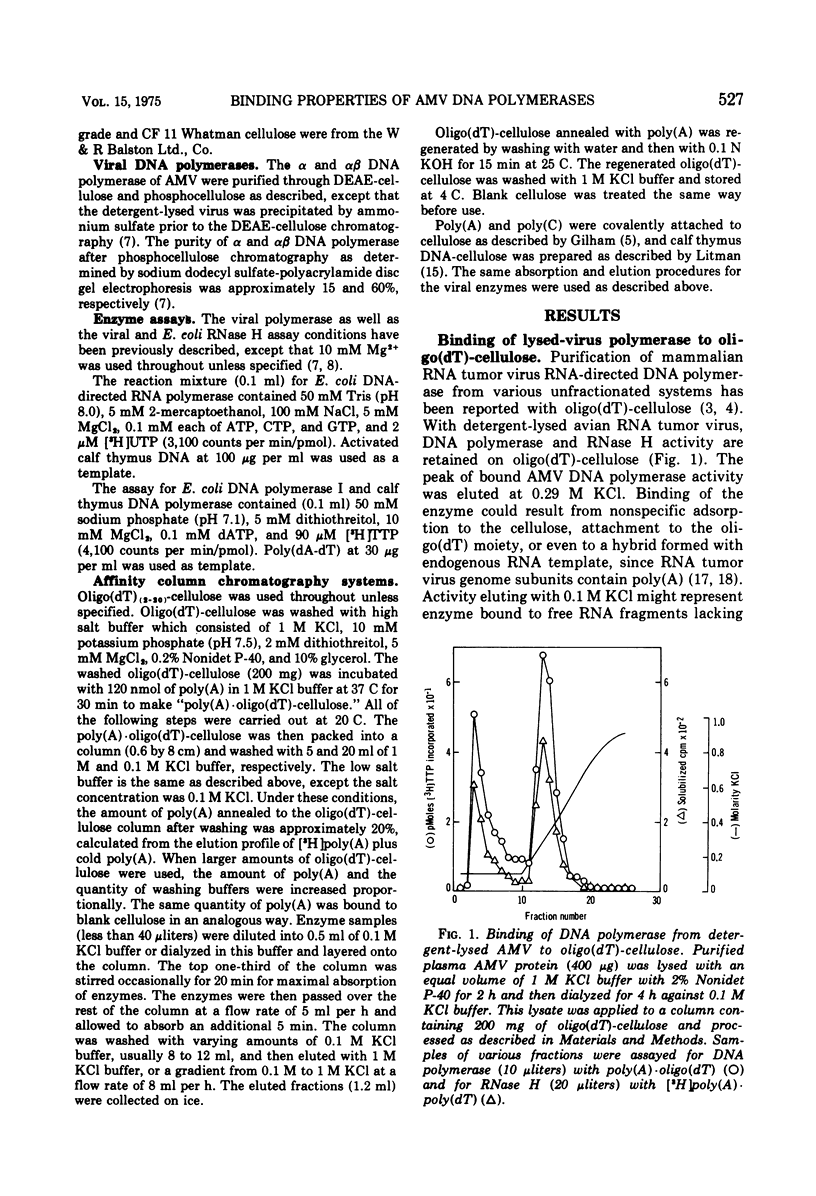
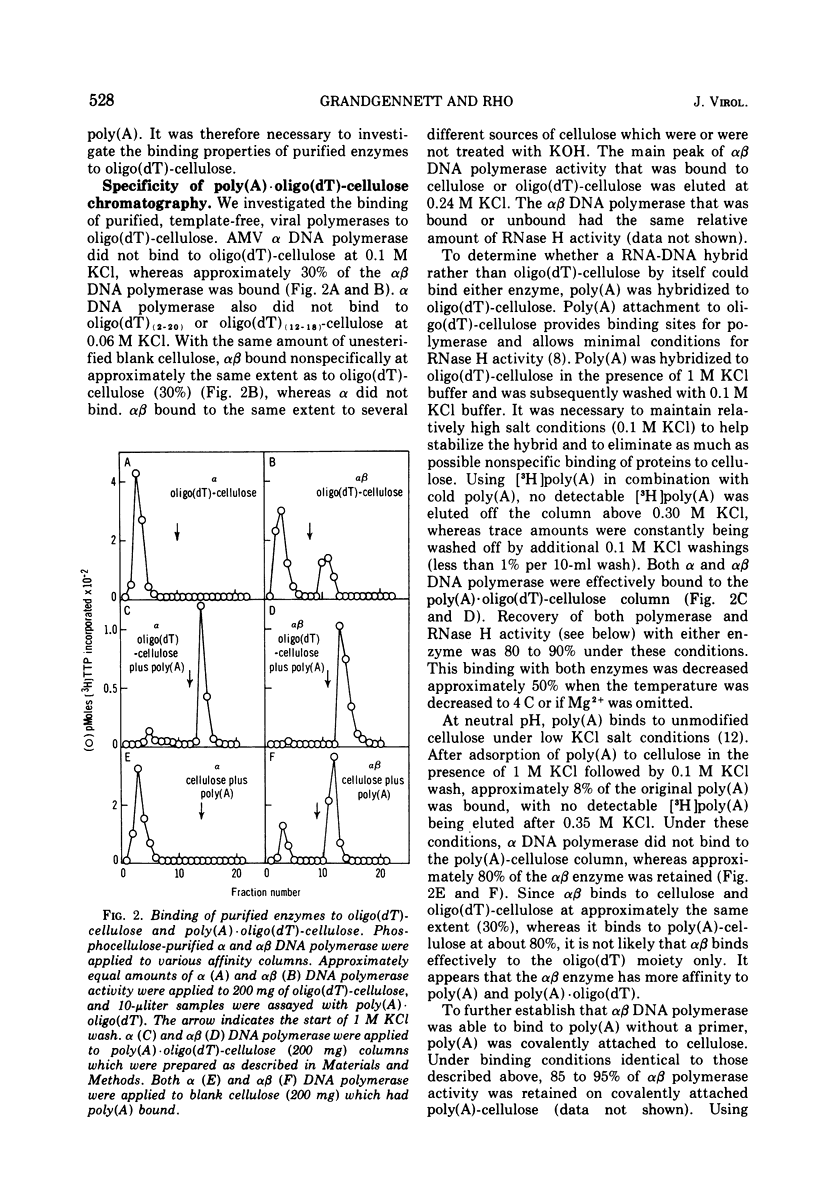
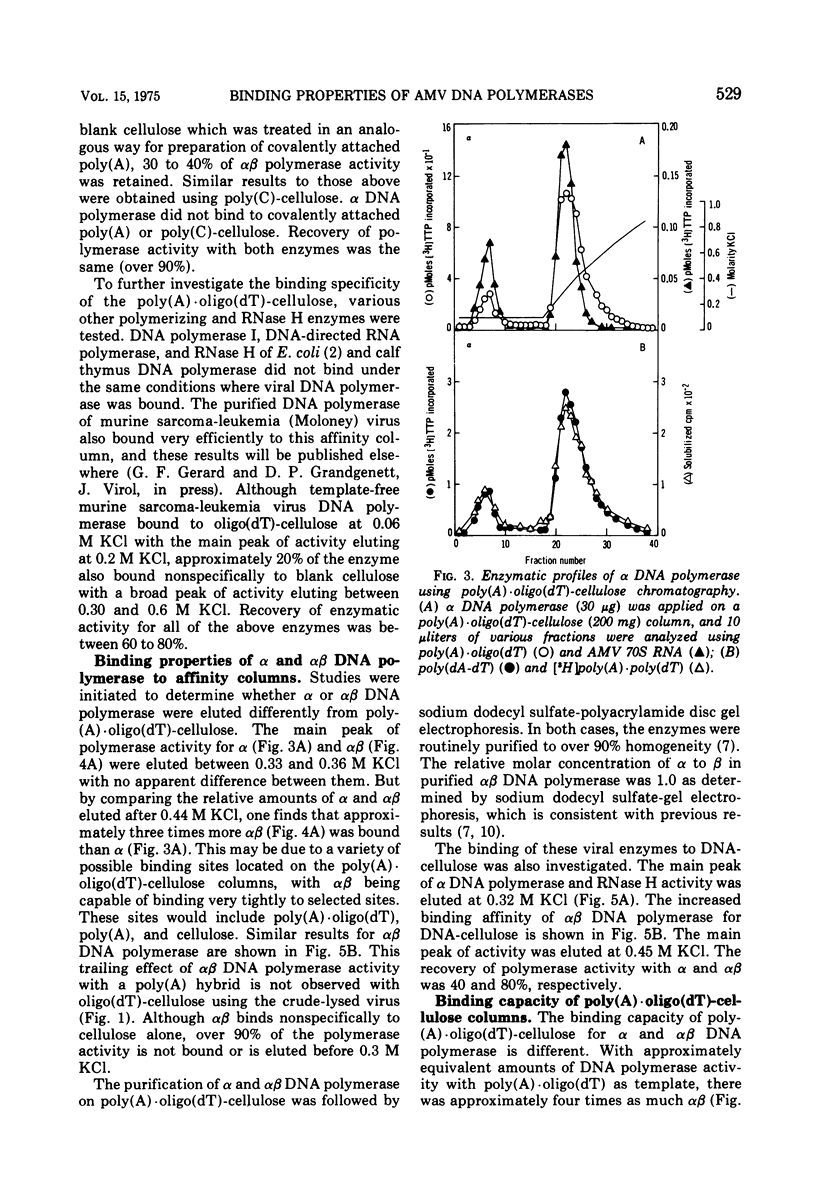
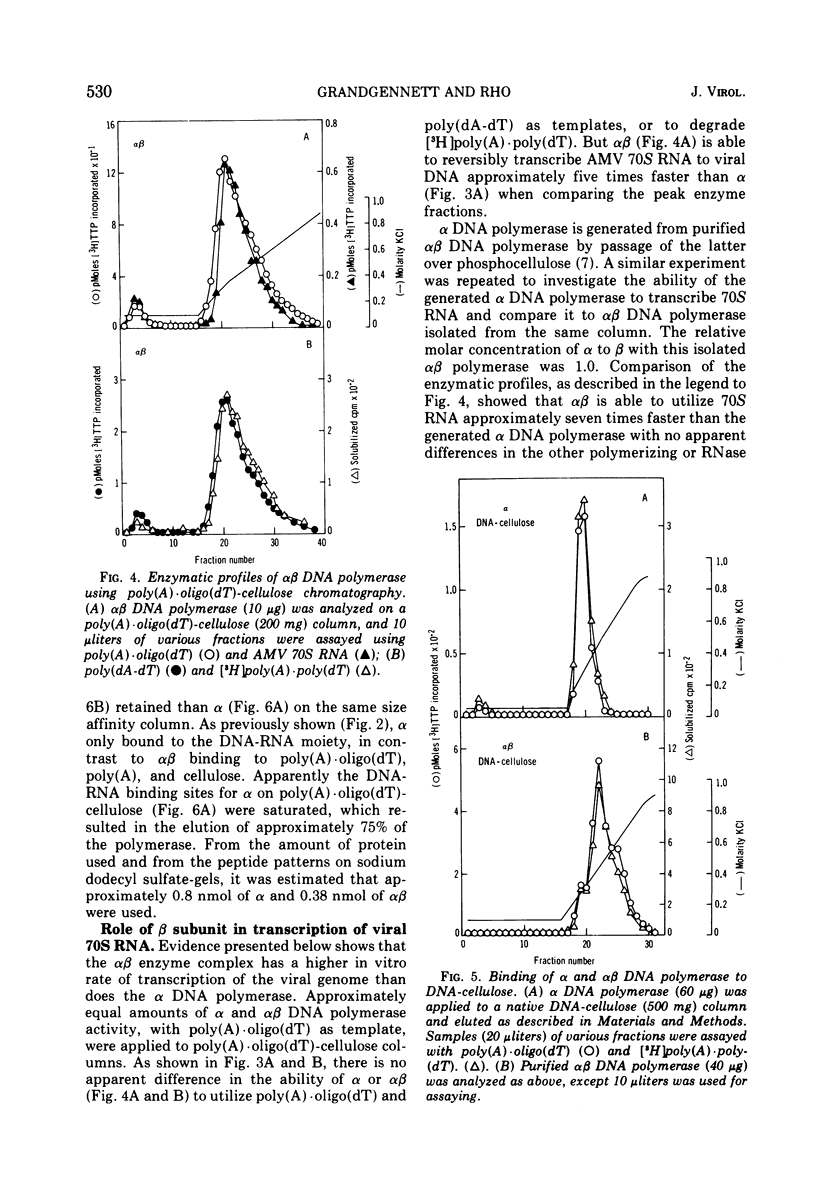
A new method for the analysis and purification of the RNA-directed DNA polymerase of RNA tumor viruses has been developed. This nucleic acid affinity chromatography system utilizes an immobilized oligo (dT) moiety annealed with poly (A). The alpha and alphabeta DNA polymerases of avain myeloblastosis virus bound effectively to poly (A) oligo (dT)-cellulose. Alpha DNA polymerase did not bind effectively to poly (A) oligo (dT)-cellulose, poly (A)-cellulose, or to cellulose. Alphabeta bound to oligo (dT)-cellulose and cellulose at the same extent (approximately 30%), indicating that this enzyme did not bind specifically to the oligo (DT) moiety only. However, alphabeta bound to poly (A)-cellulose two to three times better than to cellulose itself, showing that alphabeta could bind to poly (A) without a primer. Alphabeta DNA polymerase also bound to poly (C)-cellulose, whereas alpha did not. These data show that the alpha DNA polymerase is defective in binding to nucleic acids if the beta subunit is not present. Data is presented which demonstrates that the alphabeta DNA polymerase bound tighter to poly (A). oligo (DT)-cellulose and to calf thymus DNA-cellulose than the alpha DNA polymerase, suggesting that the beta subunit or, at least part of it is responsible for this tighter binding. In addition, alphabeta DNA polymerase is able to reversibly transcribe avian myeloblastosis virus 70S RNA approximately fivefold faster than alpha DNA polymerase in the presence of Mg2+ and equally efficient in the presence of Mn2+. alpha DNA polymerase transcribed 9S globin m RNA slightly better than alphabeta with either metal ion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Gerwin B. I., Bassin R. H. Detection and isolation of a new DNA polymerase from human breast tumor cell line HBT-3 by (dT)12-18-cellulose chromatography. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2453–2456. doi: 10.1073/pnas.70.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Milstien J. B. An oligonucleotide affinity column for RNA-dependent DNA polymerase from RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2599–2603. doi: 10.1073/pnas.69.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham P. T. The synthesis of celluloses containing covalently bound nucleotides, polynucleotides, and nucleic acids. Biochemistry. 1968 Aug;7(8):2809–2813. doi: 10.1021/bi00848a016. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Brackmann K., Green M. Large-scale purification of ribonucleic acid tumor viruses by use of continuous-flow density gradient centrifugation. Appl Microbiol. 1973 Sep;26(3):452–454. doi: 10.1128/am.26.3.452-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Green M. Different mode of action of ribonuclease H in purified alpha and alpha beta ribonucleic acid-directed deoxyribonucleic acid polymerase from avian myeloblastosis virus. J Biol Chem. 1974 Aug 25;249(16):5148–5152. [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitos P. A., Fuller T., King G. S., Hersh R. T. Cations and the binding of polyadenylate to cellulose. Biochim Biophys Acta. 1974 Jul 11;353(3):362–374. doi: 10.1016/0005-2787(74)90030-6. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Green M. The homopolyadenylate and adjacent nucleotides at the 3'-terminus of 30-40s RNA subunits in the genome of murine sarcoma-leukemia virus. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2386–2390. doi: 10.1073/pnas.71.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Scott J. F., Zamecnik P. C. Evidence that the polyadenylic acid segment of "35S" RNA of avian myeloblastosis virus is located at the 3'-OH terminus. Biochem Biophys Res Commun. 1973 Nov 1;55(1):8–16. doi: 10.1016/s0006-291x(73)80052-x. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Watson K. F., Mölling K., Bauer H. Ribonuclease H activity present in purified DNA polymerase from avian myeloblastosis virus. Biochem Biophys Res Commun. 1973 Mar 5;51(1):232–240. doi: 10.1016/0006-291x(73)90533-0. [DOI] [PubMed] [Google Scholar]



