Abstract
Recent findings implicate the central lateral amygdala (CeL) in conditioned fear. Indeed, CeL contains neurons exhibiting positive (CeL-On) or negative (CeL-Off) responses to fear-inducing conditioned stimuli (CSs). In mice, these cells differ in their expression of protein kinase Cδ (PKCδ) and physiological properties. CeL-Off cells are PKCδ+ and late firing (LF), whereas CeL-On cells are PKCδ− and express a regular-spiking (RS) or low-threshold bursting (LTB) phenotype. However, the scarcity of LF cells in rats raises questions about the correspondence between the organization of CeL in mice and rats. Therefore, we studied the PKCδ expression, morphological properties, synaptic responsiveness, and fear conditioning-induced plasticity of rat CeL neurons. No PKCδ+ LF cells were encountered, but ≈20–25% of RS and LTB neurons were PKCδ+. Compared with RS neurons, a higher proportion of LTB cells projected to central medial amygdala (CeM) and they had fewer primary dendritic branches, yet the amplitude of excitatory postsynaptic potentials (EPSPs) evoked by lateral amygdala (LA) stimulation was similar in RS and LTB cells. In contrast, LA-evoked inhibitory postsynaptic potentials (IPSPs) had a higher amplitude in LTB than RS neurons. Finally, fear conditioning did not induce plasticity at LA inputs to RS or LTB neurons. These findings point to major species differences in the organization of CeL. Since rat LTB cells are subjected to stronger feedforward inhibition, they are more likely to exhibit inhibitory CS responses than RS cells. This is expected to cause a disinhibition of CeM fear output neurons and therefore an increase in fear expression.
Keywords: amygdala, fear conditioning, GABA
classical fear conditioning is an experimental paradigm used to investigate how animals learn to fear new stimuli by experience. The underlying mechanisms are studied intensely because findings from animal and human studies converge to suggest that anxiety disorders result from abnormalities in the network that supports this form of learning. Early studies established that the lateral (LA) and central (Ce) nuclei of the amygdala are critical nodes in this network (Davis 1993; LeDoux 1993). Indeed, LA is the main entry point of sensory inputs to the amygdala (LeDoux et al. 1990), whereas Ce, particularly its medial sector (CeM), is the main source of amygdala outputs to fear effector neurons in the brain stem (Hopkins and Holstege 1978; Veening et al. 1984). However, transmission of information from LA to CeM is indirect, involving a multilayered intra-amygdaloid network of glutamatergic and GABAergic connections (reviewed in Pare and Duvarci 2012).
Indeed, LA sends divergent glutamatergic projections to several amygdala nuclei that regulate CeM activity (Krettek and Price 1978; Pitkänen et al. 1997; Smith and Paré 1994). One of these is the lateral sector of Ce (CeL). Principal CeL neurons use GABA as a transmitter and form inhibitory synapses with each other (Lopez De Armentia and Sah 2004; Sun et al. 1994) and CeM cells (Haubensak et al. 2010; Petrovich and Swanson 1997). A proportion of CeL neurons target CeM cells that project to different fear effector structures of the brain stem such as the periaqueductal gray or the dorsal vagal complex (Viviani et al. 2011).
On the surface, since the LA neurons projecting to CeL are glutamatergic and the CeL cells projecting to CeM are GABAergic, LA inputs are expected to trigger an excitation of CeL cells and a consequent inhibition of CeM neurons. However, recordings of mouse (Ciocchi et al. 2010) and rat (Duvarci et al. 2011) CeL neurons 24 h after fear conditioning disclosed two populations of cells with inhibitory (CeL-Off) or excitatory (CeL-On) responses to auditory conditioned stimuli (CSs). In rats, the incidence of CeL-Off cells was two times higher than that of CeL-On cells (Duvarci et al. 2011). In contrast, CeM cells only showed positive responses to the CS (Ciocchi et al. 2010; Duvarci et al. 2011). It was further shown in mice that CeL-Off and CeL-On cells correspond to PKCδ+ and PKCδ− neurons and that these two subsets of CeL cells express different electroresponsive properties (Haubensak et al. 2010).
These findings raise a number of interesting questions. First, since there are major species differences between the electroresponsive properties of CeL cells (Dumont et al. 2002), is the correspondence between the physiological properties of mice CeL neurons and PKCδ expression preserved in rats? Second, are LA inputs differentially contacting affecting different subtypes of CeL neurons? Are particular subtypes of CeL neurons subjected to differing amounts of feedforward inhibition when LA inputs are activated? Third, does fear conditioning alter the efficacy of LA synapses onto CeL neurons? The present study was undertaken to address these questions.
MATERIALS AND METHODS
Experiments were performed in male Sprague-Dawley rats (26–40 days old) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Rutgers University (Newark, NJ).
Slice preparation.
Rats were anesthetized with ketamine, pentobarbital, and xylazine (respectively 80 mg/kg, 60 mg/kg, and 12 mg/kg ip). After abolition of all reflexes, they were perfused through the heart with a cold (4°C) modified artificial cerebrospinal fluid (aCSF) that contained (in mM) 126 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 glucose. Their brains were then extracted and cut into 400-μm-thick slices with a vibrating microtome while submerged in the same solution as above. After cutting, slices were transferred to an incubating chamber, where they were allowed to recover for at least 1 h at room temperature in a control aCSF with the same composition as above with the exception that NaCl was substituted for choline chloride (pH 7.3, 300 mosM). The slices were then transferred one at a time to a recording chamber perfused with the latter solution (7 ml/min). Before the recordings began, the temperature of the chamber was gradually increased to 32°C.
Electrophysiology.
Under visual guidance with differential interference contrast and infrared videomicroscopy, we obtained whole cell patch recordings of CeL neurons with pipettes (7–10 MΩ) pulled from borosilicate glass capillaries and filled with a solution containing (in mM) 130 K-gluconate, 10 HEPES, 10 KCl, 2 MgCl2, 2 ATP-Mg, and 0.2 GTP-Tris (pH 7.2, 280 mosM). Biocytin (0.5%) was added to the intracellular solution when studying the morphology of recorded cells or correlating electroresponsive properties with PKCδ expression. The liquid junction potential was 10 mV with this solution, and the membrane potential was corrected accordingly. Current-clamp recordings were obtained with an Axoclamp 2B amplifier and digitized at 10 kHz with a Digidata 1200 interface (Axon Instruments, Foster City, CA).
To characterize the electroresponsive properties of recorded cells, a graded series of depolarizing and hyperpolarizing current pulses (20 pA steps, 500 ms in duration) was applied from rest and other prepulse potentials. The input resistance (Rin) of the cells was estimated in the linear portion of current-voltage plots.
To activate LA inputs to CeL, two closely spaced (100 μm) stimulating electrodes were positioned in LA. To minimize variability between experiments, we always selected the same coronal level (corresponding to ≈2.7 mm posterior to bregma) and positioned the stimulating electrodes at the same site (0.3 mm medial to the external capsule and 0.7 mm ventral to the dorsal tip of LA). In some experiments, we tested the effects of various drugs on LA-evoked responses. These included the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (AP5; 100 μM), the non-NMDA glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM), and the GABAA blocker picrotoxin (100 μM). All drugs were obtained from Sigma (St. Louis, MO).
Biocytin revelation for morphological identification of recorded cells.
Depending on the experiment, biocytin was revealed in one of two ways: diaminobenzidine (DAB) to study the morphology of CeL cells and fluorescent streptavidin to determine whether recorded cells expressed PKCδ. The rationale for using different biocytin revelation methods when studying neuronal morphology versus PKCδ expression is the following. In other cell types, we have observed that the prolonged recording times that are optimal to fully reveal the dendritic arbors and axons of recorded cells are incompatible with immunohistochemistry. It appears that with prolonged recording times, equilibration between the pipette solution and the cells' interior leads to a reduction in antigen levels, typically resulting in lack of immunoreactivity. Also, our experience in other cell types has revealed that even when recording times are kept short, the DAB reaction product interferes with the immunoreaction and/or visualization of the second chromogen. This is why we opted to study 1) PKCδ expression with streptavidin, keeping the recording times short, and 2) dendritic/axonal morphology with longer recording times. In the latter case, we could have used fluorescent streptavidin or DAB to reveal the biocytin, but we find that the long-term stability of the DAB reaction product (relative to fluorescent streptavidin) is more convenient and thus used this approach to study neuronal morphology.
We first describe the approach used to reveal the morphology of recorded cells. At the conclusion of the recordings, the slices were removed from the chamber and fixed for 1–3 days in 0.1 M phosphate-buffered saline (pH 7.4) containing 4% paraformaldehyde. Slices were then embedded in agar (3%) and sectioned on a vibrating microtome at a thickness of 100 μm. Unless noted otherwise, all histological processing was carried out at room temperature. Sections were washed several times in phosphate buffer (PB, 0.1 M, pH 7.4) and then transferred to a H2O2 solution (0.5%) in PB for 15 min. After numerous washes in PB, sections were incubated for 12 h in a solution containing 0.5% Triton, plus 1% of solutions A and B of an ABC kit (Vector, Burlingame, CA) in PB. On the next day they were washed in PB (5 × 10 min). Biocytin was visualized by incubating the sections in a 0.1 M PB solution that contained DAB tetrahydrochloride (0.05%, Sigma), 2.5 mM nickel ammonium sulfate (Fisher), and H2O2 (0.003%) for 5–10 min. The sections were then washed in PB (5 × 10 min), mounted on gelatin-coated slides, and air-dried. The sections were then counterstained with cresyl violet and coverslipped with Permount for later reconstruction.
The morphology of recorded cells was reconstructed by using all available sections (typically three). Camera lucida drawings of all labeled segments were performed. The labeling found in different sections was aligned by using blood vessels present on consecutive sections and by using the matching position of the ends of multiple dendritic and/or axonal segments present in different sections. The cresyl violet staining was used to identify the outline and border of CeL and CeM. A micrometric graticule inserted in one of the eyepieces of the microscope was used to measure the soma, dendrites, and axons of the cells.
Biocytin revelation combined with PKCδ immunohistochemistry.
The polyclonal antibody we used was produced in rabbits and directed against a synthetic peptide (SFVNPKYEQFLE) corresponding to amino acids 662–673 of rat PKCδ conjugated to bovine serum albumin (BSA). It was obtained from EMD Millipore (Billerica, MA; catalog no. 539532). The EMD Millipore website mentions that cross-reactivity with a variety of other molecules (including many other PKC isoforms) was tested by ELISA and found to be negligible. In rat, this antibody produced a pattern of amygdala immunostaining identical to that seen in previous mouse studies (Haubensak et al. 2010). Moreover, we verified that preincubation of the antibody with the control peptide (1:1,000 diluted antibody solution and 0.1 mg/ml control peptide) abolished all immunolabeling in the amygdala.
The fixation and sectioning procedures were as above. The sections were washed repeatedly in PB, preincubated for 1 h in a blocking solution (1% normal goat serum, 3% BSA, Triton X-100, 0.3%), and incubated overnight at 4°C with the PKCδ antibody from EMD Millipore (1:500, in the blocking solution described above). The sections were then washed repeatedly in PB and incubated in the secondary antibody solution (1:1,000, Invitrogen, Carlsbad, CA) conjugated with Alexa 488 for 1 h. After repeated washes in PB, the sections were incubated with Alexa 594-conjugated streptavidin (1:100,000, Invitrogen) for 2 h. Finally, the sections were washed in PB (5 × 10 min), mounted on gelatin-coated slides, and coverslipped with Fluoromount (Sigma-Aldrich, St. Louis, MO).
Behavior.
In some experiments, rats were subjected to a classical fear conditioning paradigm, 24 h prior to the in vitro experiments. Three groups of rats were used in these experiments. We first describe the conditioning apparatus and then explain how these three groups differed. We used conditioning chambers (Coulbourn Instruments, Allentown, PA) with metal grid floors and Plexiglas walls. All rats were first habituated to the conditioning chamber and the tone CS (30 s, 4 kHz, 80 dB, presented 5 times). Then the experimental group (“FC group”) was subjected to an auditory fear conditioning protocol that consisted of five presentations of the CS, each coterminating with a foot shock unconditioned stimulus (US; 0.5 mA, 1 s). After habituation, the second group of rats (“CS-only group”) received five additional presentations of the CS alone whereas the third group of rats (“US-only group”) received five presentations of the US alone. The investigator was blind to the group identity of the rats during the in vitro experiment. To achieve this, a number of rats were subjected to the same three behavioral training procedures described above but not used for physiological experiments. An individual (otherwise not involved with the present experiments) randomly picked one of two rats to be used for physiological experiments. The remaining rat was presented with one-tone CS later the same day. The behavioral data described in results were obtained from these rats. The rats used for the physiological experiments were not presented with the CS the day after conditioning.
RESULTS
Relationship between electroresponsive properties and PKCδ expression in CeL neurons.
On the basis of variations in the temporal dynamics of current-evoked spiking, previous patch recording studies identified three main cell types in CeL: regular spiking (RS; Fig. 1A1), low-threshold bursting (LTB, Fig. 1A2), and late firing (LF, Fig. 1A3). However, marked species differences were observed in the relative incidence of these cell types. In guinea pigs (Martina et al. 1999), cats (Dumont et al. 2002), and mice (Haubensak et al. 2010), LF cells account for 55–60% of CeL neurons. In contrast, the incidence of LF cells is much lower in rats (9–29%; Dumont et al. 2002; Lopez de Armentia and Sah 2004), while the incidence of RS and LTB neurons is higher. The differing incidence of LF cells is likely not a consequence of methodological variations since all these reports but one (Lopez de Armentia and Sah 2004) relied on the same recording methods, including a K-gluconate-based intracellular solution, as used here.
Fig. 1.
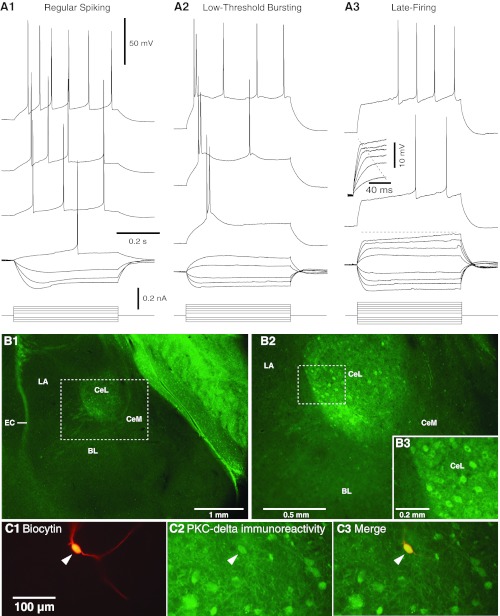
Electroresponsive properties of central lateral (CeL) amygdala neurons. A: voltage responses to depolarizing and hyperpolarizing current pulses in 3 types of CeL cells: regular spiking (RS, A1), low-threshold bursting (LTB, A2), and late firing (LF, A3). When sufficiently depolarized, RS cells generate spike trains that display marked spike frequency accommodation. Although LTB cells also generate accommodating spike trains when depolarized from membrane potentials (Vm) positive to −70 mV, they generate high-frequency (≥100 Hz) spike bursts or doublets followed by single spikes from more negative Vm. In response to suprathreshold depolarizations, LF neurons display a conspicuous delay to firing that is especially pronounced from Vm negative to −75 mV. Also characteristic of LF cells is a marked change in the rising phase of voltage responses to depolarizing current pulses as the stimulus intensity is increased (inset in A3). Prepulse potentials in A1–A3 were −68, −75, and −80 mV, respectively. B: PKCδ immunoreactivity on coronal sections at low (B1) and high (B2) power. Dashed rectangle in B1 is expanded in B2. Dashed rectangle in B2 is expanded in B3. CeM, medial sector of central amygdala; LA, lateral nucleus of amygdala; EC, external capsule; BL, basolateral nucleus of amygdala. C1: CeL neuron labeled with biocytin. C2: post hoc immunohistochemistry revealed that this cell expressed PKCδ (arrowhead). C3: overlay of C1 and C2.
The results of the present study in rats are consistent with the above: only 12.1% of CeL neurons (21 of 173) were of the LF type, whereas RS and LTB cells accounted for 54.3% (94 of 173) and 33.5% (58 of 173), respectively, of our sample. We ran analyses of variance (ANOVAs) to determine whether the physiological properties (see Table 1) of the three cell types varied. This revealed significant group differences in time constant, spike threshold, and membrane potential (see F and P values in Table 1). Post hoc t-tests with significance thresholds corrected for multiple comparisons revealed that the time constant of LF cells was significantly lower than that of RS and LTB cells (P = 0.001), with no differences between the latter two. Presumably, the expression of a d-like K+ current by LF cells underlies this effect. In addition, spike threshold was significantly more negative in LTB than RS or LF cells (P = 0.0001), likely resulting from the contribution of a t conductance in LTB cells. Finally, the differences in resting membrane potentials, although significant (LF ≈ RS < LTB), were very small and will not be considered further. Note that because of the low incidence of LF cells in rats (12.1%), the remainder of this study focuses on LTB and RS neurons.
Table 1.
Physiological properties of CeL neurons
| Action Potential |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Type | n | Incidence, % | Rest, mV | Rin, MΩ | Threshold, mV | Amplitude, mV | Duration, ms | Time Constant, ms |
| RS | 94 | 54.3 | −81.3 ± 0.6 | 465.2 ± 41.9 | −51.7 ± 0.3 | 85.7 ± 1.1 | 1.31 ± 0.03 | 32.2 ± 1.32 |
| LTB | 58 | 33.5 | −78.9 ± 0.7 | 459.1 ± 19.2 | −57.4 ± 0.4 | 87.1 ± 1.0 | 1.31 ± 0.04 | 37.9 ± 1.63 |
| LF | 21 | 12.1 | −82.8 ± 1.2 | 319.3 ± 18.2 | −50.9 ± 0.4 | 85.2 ± 1.9 | 1.27 ± 0.06 | 27.1 ± 2.30 |
| F = 5.58 | F = 1.77 | F = 63.62 | F = 0.24 | F = 0.15 | F = 6.81 | |||
| P = 0.005 | P = 0.17 | P = 0.0001 | P = 0.79 | P = 0.86 | P = 0.001 | |||
Values are means ± SE. CeL, central lateral amygdala; RS, regular spiking; LTB, low-threshold bursting; LF, late firing; Rest, resting potential; Rin, input resistance.
Since in mice PKCδ is prevalently expressed by LF cells, the rarity of LF cells in rats raises the question of whether the incidence of PKCδ-expressing CeL cells is lower in rats or whether PKCδ is expressed by a different physiological type of neuron. The former possibility appears unlikely because a cursory examination of sections processed to reveal PKCδ immunoreactivity indicates that PKCδ-positive neurons account for a sizeable proportion of rat CeL cells (Fig. 1B).
To address the second possibility, we recorded CeL neurons with a pipette solution containing biocytin and then verified whether PKCδ immunofluorescence (Fig. 1C) correlated with the physiological properties of the recorded cells. A total of 18 LTB and 24 RS neurons were tested in this manner, and the incidence of PKCδ-immunopositive cells was similar in both groups (28% and 21% of cells, respectively; Fisher exact test, P = 0.25). Only four LF neurons were encountered in this series of experiments, and none of them expressed PKCδ.
Morphological correlates of electroresponsive properties.
In different samples of RS (n = 16) and LTB (n = 10) neurons, biocytin was revealed with DAB and their morphological properties were examined (Figs. 2 and 3). RS and LTB neurons were similar in most respects (see Table 2). All recovered cells had a medium-size soma and moderately to highly branched dendritic trees. In addition, most RS (69%) and LTB (80%) cells displayed a high density of dendritic spines (Fig. 2D), but some had a markedly lower spine density (Fig. 2, A2 and A3). Finally, when the axons of RS or LTB cells could be seen, they invariably bore numerous axonal varicosities (Fig. 2, B2 and C).
Fig. 2.
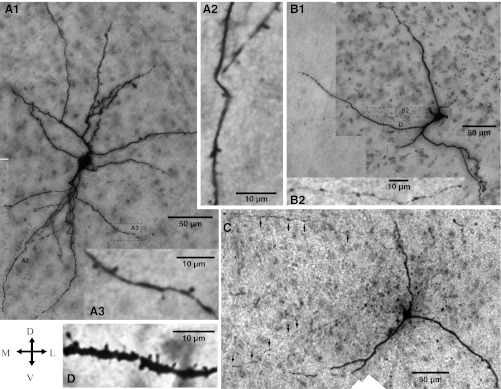
Morphological properties of LTB and RS neurons recorded in CeL. Cross (bottom left) indicates orientation of the micrographs (D, dorsal; V, ventral; M, medial; L, lateral). A1: RS neuron. The dendritic segments enclosed in the dashed rectangles are shown at a higher magnification in A2 and A3. Although this cell had a low spine density, other RS cells displayed a very high spine density, similar to the LTB cell displayed in D. B and C: LTB neurons. The rectangles in B1 enclose axonal (top) and dendritic (bottom) segments shown at a higher magnification in B2 and D, respectively. Arrows in C point to axonal varicosities.
Fig. 3.
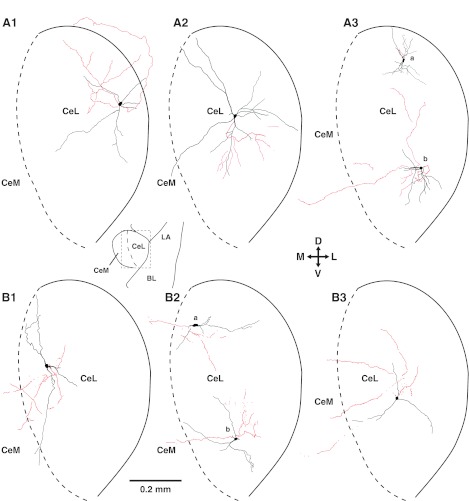
Dendritic (black) and axonal (red) patterns of CeL neurons. A: RS cells. B: LTB cells. Scheme between A1 and A2 (bottom) depicts a low-power view of the amygdala; rectangle indicates region depicted in A1–A3 and B1–B3. Cross in center indicates orientation of the drawings.
Table 2.
Morphological properties of CeL neurons
| Soma Diameter, μm |
Primary Dendrites |
||||||
|---|---|---|---|---|---|---|---|
| Cell Type | n | Maximum | Minimum | Number | Diameter, μm (40 μm from soma) | Distance to First Dendritic Branching from Soma, μm | Length of Intervaricose Axonal Segments, μm |
| RS | 16 | 15.1 ± 1.3 | 10.3 ± 0.9 | 3.8 ± 0.3 | 1.1 ± 0.1 | 24.3 ± 6.0 | 8.9 ± 0.8 |
| LTB | 10 | 15.1 ± 0.6 | 10.2 ± 0.7 | 2.7 ± 0.3 | 1.2 ± 0.1 | 32.7 ± 7.7 | 10.2 ± 0.7 |
Values are means ± SE. For the analysis of distance to first dendritic branching, we only considered primary dendrites that could be seen to branch at least once.
However, RS and LTB neurons differed in two respects. First, despite conspicuous variations within each cell type, the number of primary dendrites was significantly higher in RS than LTB neurons (Mann-Whitney U-test, P = 0.016; Table 2). Second, the incidence of cells with axons reaching CeM was significantly lower in RS (25% or 4 of 16; Fig. 3A) than LTB (80% or 8 of 10; Fig. 3B; Fisher exact test, P = 0.01) neurons. The above contrast (lower incidence of RS cells with axons to CeM yet higher number of primary dendrites in RS cells) strongly suggests that the quality of the biocytin labeling was not inferior in RS cells compared with LTB neurons. Moreover, there were no significant differences between the average length of RS versus LTB axons (RS: 750 ± 149 μm; LTB: 752 ± 117 μm) or in the distance between the somata of the two cell types from the CeL/CeM border (RS: 282 ± 24 μm; LTB: 275 ± 25 μm).
The axon of many RS cells was similar to the case depicted in Fig. 3A3a: a short axonal segment that exited the plane of the slice very close to the parent soma. When the axon of RS cells arborized within the plane of the slice (Fig. 3, A1, A2, and A3b), all contributed varicose axons that branched in CeL, with a few continuing dorsally to the sublenticular region (Fig. 3A1). RS cells with axons reaching CeM, as in the case depicted in Fig. 3A3b, were rare. We were concerned that differences in the orientation of RS and LTB axons might explain the apparent scarcity of CeM projections in RS cells. For instance, it is conceivable that prior to turning toward CeM the axon of RS cells course rostrally or caudally, in which case we would erroneously conclude that they lack projections to CeM. This led us to record an additional group of four RS cells in horizontal slices. However, the proportion of CeM-projecting RS cells was not higher in this sample; only one of them contributed an axon collateral to CeM.
While these results suggest that LTB neurons form stronger connections with CeM than RS cells, these results should be interpreted cautiously because another possibility, namely that the axon of RS cells reaches CeM after coursing through the stria terminalis, cannot be excluded at this time. Indeed, prior tracing (see Price and Amaral 1981) and electrophysiological (Nagy and Paré 2008) studies have revealed that the axon of some Ce neurons reach their brain stem target after a circuitous path in which they first course caudally, then rostrodorsally along the stria terminalis, then ventrocaudally toward the brain stem. It is conceivable that some RS axons emit a branch in CeM along the latter part of this roundabout trajectory. Such axons could not be visualized with the approach used here.
Impact of LA inputs on LTB vs. RS cells.
Transmission of CS information to CeL is thought to depend, at least in part, on the activation of glutamatergic LA neurons projecting to CeL, yet it was observed that CS presentation inhibits a proportion of CeL cells (Ciocchi et al. 2010; Duvarci et al. 2011), corresponding to PKCδ+ neurons (Haubensak et al. 2010), and excites others (Ciocchi et al. 2010; Duvarci et al. 2011), corresponding to PKCδ− neurons (Haubensak et al. 2010). These findings raise the possibility that the impact of LA inputs is not uniform among CeL neurons. To examine this question, we compared the amplitude of excitatory postsynaptic potentials (EPSPs) elicited by electrical stimulation of LA in LTB (n = 14) versus RS (n = 27) neurons from a membrane potential of −75 mV, as determined by DC current injection (see Supplemental Data Set for the behavior of the rare LF cells encountered during these experiments).1 It should be noted that all tested cells were responsive to LA stimuli and that all were included in the analyses provided below.
As shown in Fig. 4A, LA-evoked EPSPs had similar amplitudes in RS and LTB cells (F = 0.56, P = 0.46). Since the latter part of LA-evoked EPSPs is presumably contaminated by inhibitory postsynaptic potentials (IPSPs), we also compared EPSP rising slopes (first 2 ms following response onset). However, this approach also failed to reveal significant differences between RS and LTB neurons (t-test, P = 0.77). To determine whether the initial part of LA-evoked EPSPs is contaminated by IPSPs, we also compared EPSP rising slopes before versus after application of picrotoxin (n = 5) but observed no significant effect (paired t-test, P = 0.25). Therefore, these results suggest that LA axons form similar connections with CeL neurons expressing different intrinsic electroresponsive properties.
Fig. 4.
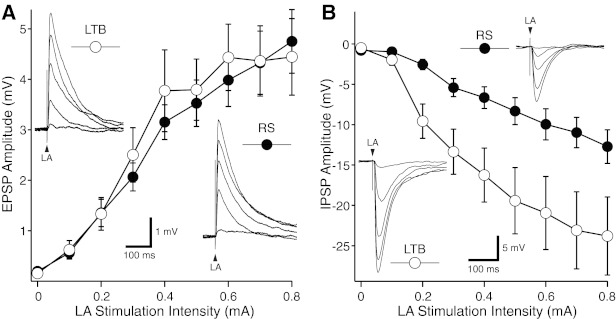
LA-evoked responses in LTB and RS CeL neurons. A: average ± SE excitatory postsynaptic potential (EPSP) amplitude (y-axis) as a function of LA stimulation intensity (x-axis) in samples of 27 RS and 16 LTB neurons. In all cases, prestimulus Vm was set to −80 mV. Insets: representative examples of responses seen in LTB (top left) and RS (bottom right) neurons with LA stimulus intensities ranging between 0.2 and 0.8 mA (each response is the average of ≥3 trials). B: average ± SE inhibitory postsynaptic potential (IPSP) amplitude (y-axis) as a function of LA stimulation intensity (x-axis) in samples of 12 RS and 10 LTB neurons. In all cases, prestimulus Vm was set to 0 mV. Insets: representative examples of responses seen in LTB (bottom left) and RS (top right) neurons with LA stimulus intensities ranging between 0.2 and 0.8 mA (each response is the average of ≥3 trials). In all tested cells, LA shocks were 0.1 ms in duration.
Alternatively, it is possible that the heterogeneous CS responsiveness of CeL neurons is due to variations in the recruitment of feedforward inhibition by LA inputs. Indeed, CeL neurons form inhibitory synapses with each other (Lopez de Armentia and Sah 2004) and they receive additional GABAergic afferents from intercalated cells (Amir et al. 2011; Paré and Smith 1993), themselves targeted by LA axons (Royer et al. 1999). To test this idea, we next compared the amplitude of LA-evoked inhibition in LTB (n = 8) versus RS (n = 15) neurons. These tests were carried out from a prestimulus potential of 0 mV to facilitate observation of the IPSPs. As shown in Fig. 4B, LA-evoked IPSPs had significantly higher amplitudes in LTB than RS cells (F = 5.21, P = 0.034).
One potential caveat in the above experiments is the possibility that the IPSPs elicited by LA stimuli resulted from current diffusion from the stimulation site to GABAergic neurons located in the intercalated clusters or CeL itself. However, if this were the case, one would expect LA-evoked IPSPs to persist after blockade of ionotropic glutamate receptors. We therefore examined the effect of adding CNQX (20 μM) and AP5 (100 μM) to the aCSF in six CeL neurons (4 RS and 2 LTB cells), using the same range of stimulation intensities as in the above experiments (0.1–0.8 mA). In all cases, blockade of ionotropic glutamate receptors nearly or completely abolished LA-evoked responses (>90% reduction), indicating that the IPSPs we observed in control conditions were not caused by current diffusion from the stimulation site but by the glutamatergic activation of GABAergic cells projecting to the recorded cells.
Overall, these results suggest that while the direct impact of LA inputs is similar in RS and LTB neurons, the differential recruitment of inhibition by LA inputs in these two cell types results in stronger inhibition of LTB than RS cells. Such differences could potentially contribute to generate the heterogeneous CS responsiveness of CeL cells, as documented in previous studies (Ciocchi et al. 2010; Duvarci et al. 2011).
Impact of fear conditioning on responsiveness of CeL cells to LA inputs.
It has been reported that inactivation of Ce globally (Wilensky et al. 2006), of CeL only (Ciocchi et al. 2010), or of PKCδ+ neurons selectively (Haubensak et al. 2010) interferes with subsequent expression of conditioned fear. These findings were interpreted as evidence that CeL is a critical site of synaptic plasticity for CS-US associations. However, the nature of this plasticity remains unclear. Since LA inputs to CeL constitute a likely candidate, we sought to determine whether fear conditioning alters their impact. To this end, rats were randomly assigned to one of three groups. All rats were first habituated to the training context and tone CS. They then received either five presentations of the CS alone (tone-only group, n = 12), of the US alone (shock-only group, n = 11), or of the CS and US (FC group, n = 17), the two coterminating. The next day, the rats were deeply anesthetized, their brain was extracted, and coronal sections of the amygdala were prepared as in the previous experiments.
Figure 5A shows the behavior of the three rat groups. These rats were not used for physiological experiments but ran through the behavioral protocol to ensure that the investigator was blind to the group identity of the experimental subjects. However, they were subjected to the exact same behavioral protocols with the exception that they received an additional CS presentation the day after fear conditioning (see details in materials and methods). As expected, significant group differences in percentage of time spent freezing to the CS were seen on days 1 (F = 44.46, P < 0.0001) and 2 (F = 13.77, P = 0.0003). On day 1, rats belonging to the FC and shock-only groups displayed higher freezing levels than the tone-only group (t-test, P < 0.0001 for FC vs. tone and shock vs. tone). On day 2, the FC rats had significantly higher freezing levels than both of the other groups (t-test, P < 0.0001 for FC vs. tone, P = 0.014 for FC vs. shock).
Fig. 5.
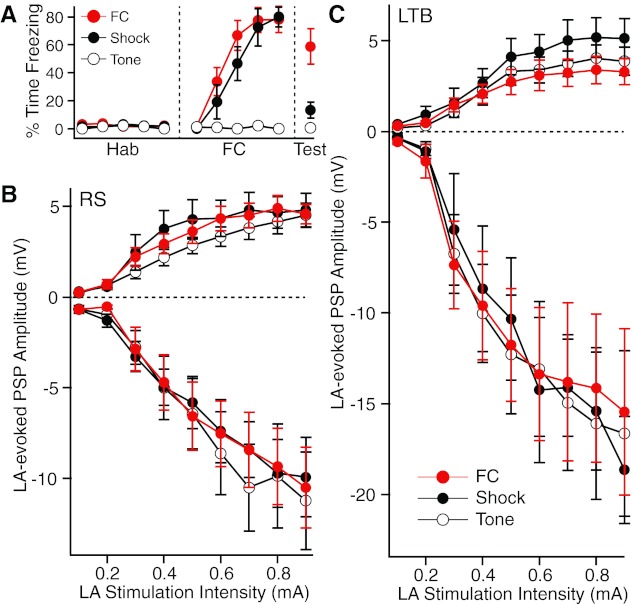
Impact of fear conditioning on the responses of CeL neurons to electrical stimuli delivered in LA. A: after habituation (Hab) to the tone conditioned stimulus (CS), 3 groups of rats were either subjected to fear conditioning (FC) or presented with the same number of shocks (Shock) or tones (Tone). Graph plots % time freezing in the 3 groups. A recall test (Test, right) was performed the next day. These animals were not used for the physiological experiments but were treated exactly as the experimental subjects (see materials and methods). B and C: amplitude (y-axis) of LA-evoked EPSPs (positive values) or IPSPs (negative values) as a function of stimulation intensity (x-axis) in RS (B) or LTB (C) neurons.
Using the same methods and testing conditions as above for naive animals (Fig. 4), we compared the amplitude of LA-evoked IPSPs and EPSPs in samples of RS (n = 12–22; Fig. 5B) and LTB (n = 8–14; Fig. 5C) neurons obtained from the three rat groups. Group differences in inhibition did not even approach significance in either cell types (RS: F = 0.024, P = 0.976; LTB: F = 0.068, P = 0.934). ANOVAs on LA-evoked EPSP amplitudes also failed to detect significant group differences in RS (F = 0.347, P = 0.708) and LTB (F = 0.823, P = 0.449) neurons. Negative results were also obtained when we compared the rising slope of LA-evoked EPSPs.
Because we were concerned that potential group differences in LA-evoked EPSPs might have been obscured by training-induced changes in the passive properties of CeL neurons, we next compared the membrane potential, time constant, and Rin of RS and LTB neurons in the three groups. ANOVAs revealed no significant group differences in the resting potential and time constant of both cell types (P ≥ 0.18). However, there were significant group differences in the Rin of LTB cells (F = 10.55, P = 0.0003). Post hoc t-tests corrected for multiple comparisons revealed that the Rin of LTB cells in the tone-only group (371.9 ± 21.6 MΩ) was significantly lower (t-test, P = 0.001) than that in the shock-only group (565 ± 35.5 MΩ) but not the FC group (423 ± 33.9 MΩ). The ANOVA on the Rin of RS cells narrowly missed significance (F = 2.21, P = 0.12). In these cells, the trend was for the Rin of the FC group (366.8 ± 22.9 MΩ) to be lower than in the shock-only (438.1 ± 30.5 MΩ) and tone-only (410.2 ± 20.8 MΩ) groups.
Therefore, to minimize the impact of these group differences in Rin, we normalized the amplitude of LA-evoked EPSPs and IPSPs to the average Rin of the tone-only group. This normalization was carried out for RS and LTB neurons separately. Nevertheless, training-induced differences in EPSP and IPSP amplitudes remained insignificant for both cell types (EPSPs: RS, F = 0.4, P = 0.67; LTB, F = 0.19, P = 0.83; IPSPs: RS, F = 0.27, P = 0.77; LTB, F = 0.21, P = 0.81).
Finally, we considered the possibility that fear conditioning alters the short-term dynamics of transmitter release by LA inputs to CeL neurons. To test this, we applied two LA stimuli separated by 50 ms and recorded evoked responses in voltage-clamp mode from a holding potential of −80 mV. These tests were carried out in LTB and RS cells from the same three behavioral groups analyzed above. As shown in Fig. 6A, when two LA stimuli were applied in rapid succession we routinely observed that the second excitatory postsynaptic current (EPSC) had a higher amplitude than the first. This facilitation was statistically significant in both cell types (RS, n = 30, paired t-test, P < 0.0001; LTB, n = 19, paired t-test, P < 0.0001). However, its magnitude was similar in the two cell types (t-test, P = 0.16), and it did not vary significantly across the three behavioral groups (RS, F =1.385, P = 0.27; LTB, F =0.336, P = 0.72; Fig. 6B).
Fig. 6.
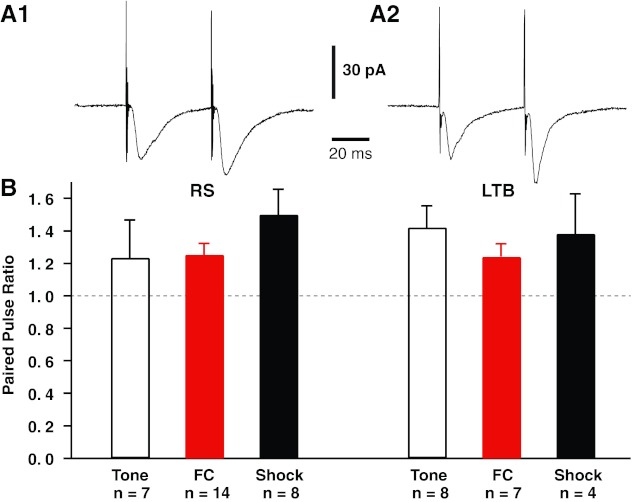
Impact of fear conditioning on the responses of CeL neurons to 2 LA stimuli delivered in rapid succession. Rats were subjected to fear conditioning or control procedures (tone only or shock only). The next day, they were deeply anesthetized, their brain was extracted, and coronal sections of the amygdala were prepared as in the previous experiments. In each tested cell, 2 LA stimuli were applied in rapid succession (50-ms intershock interval) and responses were recorded in voltage-clamp mode from a holding potential of −80 mV. A: responses to paired LA stimuli in representative RS (A1) and LTB (A2) neurons. In both cell types, the second LA stimulus generally elicited EPSCs of greater amplitudes than the first. For all cells, we computed a paired-pulse ratio (EPSC2/EPSC1). B: paired-pulse ratio (y-axis) for RS (left) and LTB (right) cells of the 3 behavioral groups (tone only, open bars; FC, red bars; shock only, black bars).
DISCUSSION
The present study was undertaken to compare properties of synaptic transmission and fear conditioning-induced plasticity in different types of rat CeL neurons. The significance of these questions derives from the fact that CeL is well positioned to regulate conditioned fear expression. Indeed, CeL receives glutamatergic inputs from LA (Krettek and Price 1978; Smith and Paré 1994; Pitkänen et al. 1997), a critical site of plasticity for conditioned fear (Sah et al. 2003; Pape and Pare 2010), and from the pontine parabrachial nucleus (Bernard et al. 1993; reviewed in Neugebauer et al. 2009). The latter structure is known to relay plastic nociceptive inputs from the spinal cord and trigeminal nucleus to CeL (Bernard et al. 1993; Neugebauer and Li 2002), and convergence of these parabrachial inputs with CS information from LA could potentially contribute to induction of fear conditioning-related synaptic plasticity in CeL. Finally, CeL sends GABAergic projections to CeM (Haubensak et al. 2010; Petrovich and Swanson 1997), the main source of amygdala outputs to downstream fear effectors (Hopkins and Holstege 1978; Veening et al. 1984).
In keeping with this, several studies have implicated CeL as an important site of plasticity for CS-US associations (Ciocchi et al. 2010; Haubensak et al. 2010; Wilensky et al. 2006). Moreover, recordings of mouse and rat CeL neurons (Ciocchi et al. 2010; Duvarci et al. 2011) revealed two populations of cells with inhibitory (CeL-Off) or excitatory (CeL-On) responses to auditory CSs, corresponding in mice to PKCδ+ and PKCδ− neurons, respectively (Haubensak et al. 2010). In contrast, CeM cells only showed positive CS responses (Ciocchi et al. 2010; Duvarci et al. 2011). The presence of reciprocal inhibitory connections between CeL-On and CeL-Off neurons led to the proposal that the excitation of CeL-On cells by the CS causes the inhibition of CeL-Off neurons resulting in the disinhibition of CeM fear output neurons (Ciocchi et al. 2010; Haubensak et al. 2010).
Species differences in PKCδ expression and electroresponsive properties of CeL cells.
Previous studies have identified three main physiological cell types in CeL (RS, LTB, LF) with major species differences in their relative incidence. In guinea pigs (Martina et al. 1999), cats (Dumont et al. 2002), and mice (Haubensak et al. 2010), LF cells constitute the majority (≈60%) of CeL neurons compared with 9–29% in rats (Dumont et al. 2002; Lopez de Armentia and Sah 2004; present results: 12%). Since in mice and rats, PKCδ+ neurons account for a considerable proportion of CeL cells, and PKCδ is prevalently expressed by LF cells in mice, the rarity of LF cells in rats raised the possibility that PKCδ is expressed by a different physiological type of neurons in rats. Consistent with this, we found that a similar proportion of rat RS (21%) and LTB (28%) neurons expressed PKCδ; no PKCδ+ LF cells were encountered. These results indicate that there are important species differences in the organization of CeL, not only in the relative incidence of different physiological cell types, as previously reported, but in their profiles of peptide expression. Moreover, these results suggest that in rat CeL neurons there is no consistent relationship between PKCδ expression, electroresponsive properties, and (likely) CS responsiveness.
Contrasting morphological properties and synaptic responsiveness of RS and LTB neurons.
In previous studies in mice, it was found that both CeL-On and CeL-Off neurons project to CeM (Ciocchi et al. 2010; Haubensak et al. 2010). While we also found some CeM-projecting cells among rat RS and LTB neurons, this was seen much more frequently in LTB cells. LTB neurons also differed from RS cells in that they displayed fewer primary dendrites. However, LA-evoked EPSPs had similar amplitudes in the two cell types. The main difference between RS and LTB cells resided in the larger amplitude of LA-evoked IPSPs in LTB neurons, suggesting that they are subjected to more feedforward inhibition than RS cells. On this basis, one would expect that LTB cells are more likely to exhibit inhibitory CS responses than RS cells. Since many LTB cells project to CeM, this would translate into a disinhibition of CeM cells, and increased fear expression.
Impact of fear conditioning on synaptic responsiveness of CeL neurons.
It was suggested that CeL is a critical site of synaptic plasticity for CS-US associations because manipulations that reduce CeL excitability during fear conditioning interfere with the subsequent expression of conditioned fear responses (Ciocchi et al. 2010; Haubensak et al. 2010; Wilensky et al. 2006). In light of prior work, a likely candidate for this plasticity is a conditioning-induced change in the efficacy of LA synapses onto CeL neurons. To test this idea, we performed an ex vivo study of LA-evoked responses in CeL cells from rats that underwent fear conditioning or were only presented with the CS or US. However, neither LA-evoked EPSPs or IPSPs varied significantly between the three rat groups in RS or LTB cells.
Although we previously obtained positive results with this ex vivo approach in different cell types (Amano et al. 2010), the present results should be interpreted with caution. They do not necessarily imply that LA synapses onto CeL neurons do not undergo plasticity as a result of fear conditioning. Indeed, it is conceivable that the plastic properties of LA inputs vary depending on the type of CeL cells they contact. Although we separately considered RS and LTB neurons here, it is possible that physiological properties are not the relevant dimension. It could be that plasticity of LA synapses varies with PKCδ expression profile, presence versus absence of CeM projections, or yet another unidentified property. Alternatively, it could be that LA synapses onto CeL neurons truly are not a site of plasticity in fear conditioning or that other properties than those examined here were modified. These possibilities should be tested in future experiments.
GRANTS
This work was supported by National Institute of Mental Health R01 Grant MH-083710.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A. and S.G. performed experiments; T.A., A.A., S.G., and D.P. analyzed data; T.A., A.A., and S.G. edited and revised manuscript; T.A., A.A., S.G., and D.P. approved final version of manuscript; A.A., S.G., and D.P. prepared figures; D.P. conception and design of research; D.P. drafted manuscript.
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13: 489–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105: 3054–3066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329: 201–229, 1993 [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala circuits. Nature 468: 277–282, 2010 [DOI] [PubMed] [Google Scholar]
- Davis M. Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res 26: 235–260, 1993 [PubMed] [Google Scholar]
- Dumont É, Martina M, Samson R, Drolet G, Pare D. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci 15: 545–552, 2002 [DOI] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci 31: 289–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 32: 529–547, 1978 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–280, 1978 [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann NY Acad Sci 702: 149–57, 1993 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10: 1062–1069, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez De Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol 92: 1285–1294, 2004 [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol 82: 1843–1854, 1999 [DOI] [PubMed] [Google Scholar]
- Nagy FZ, Paré D. Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. J Neurophysiol 100: 3429–3436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 87: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 89: 716–727, 2003 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 60: 226–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol 22: 222–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57: 1077–1090, 1993 [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 763: 247–254, 1997 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523, 1997 [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1: 1242–1259, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19: 10575–10583, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with post-embedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248, 1994 [DOI] [PubMed] [Google Scholar]
- Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol 340: 43–64, 1994 [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res 303: 337–357, 1984 [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333: 104–107, 2011 [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 2387–12396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


