Abstract
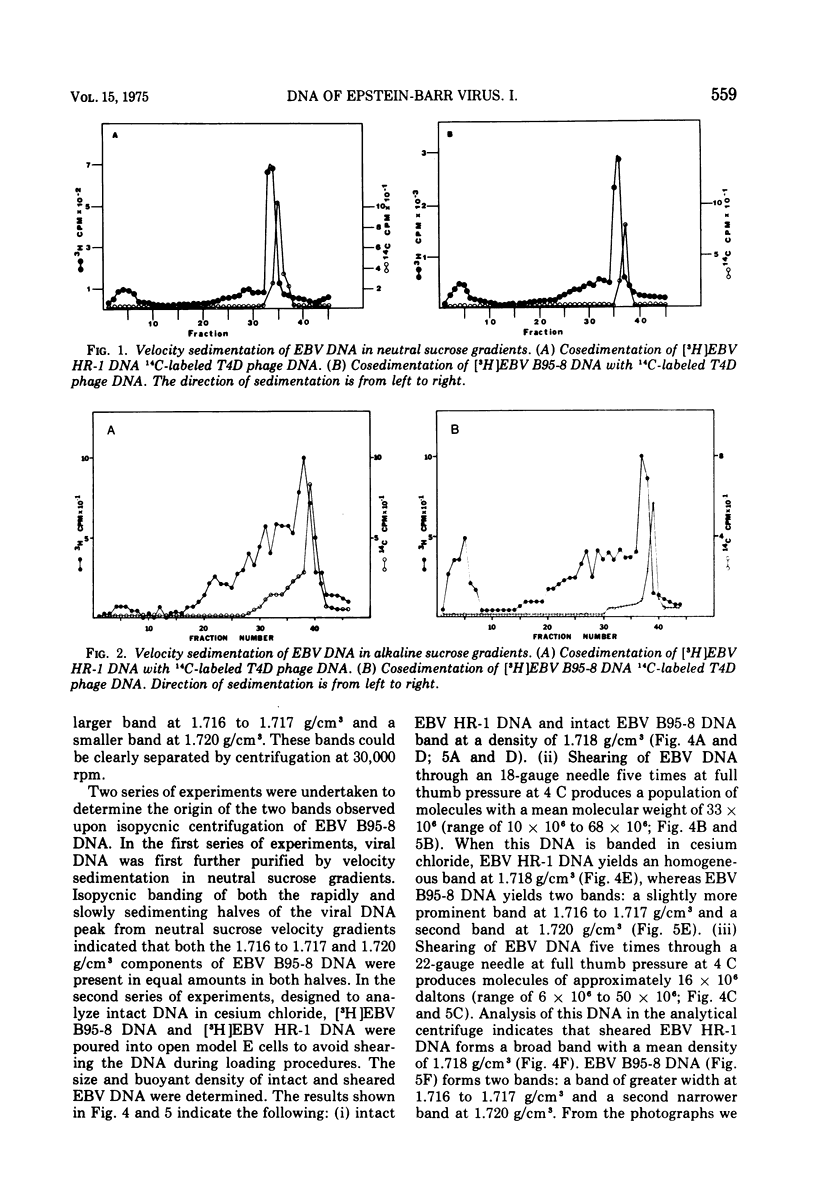
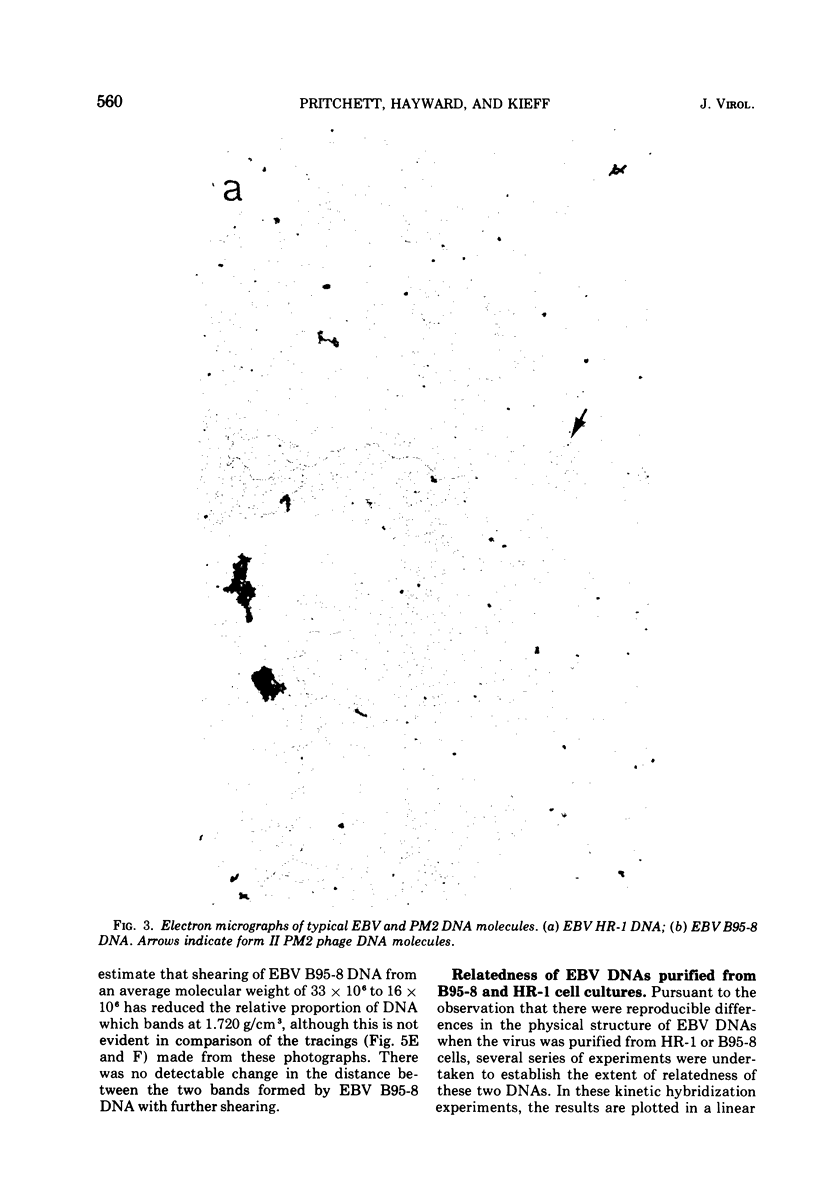
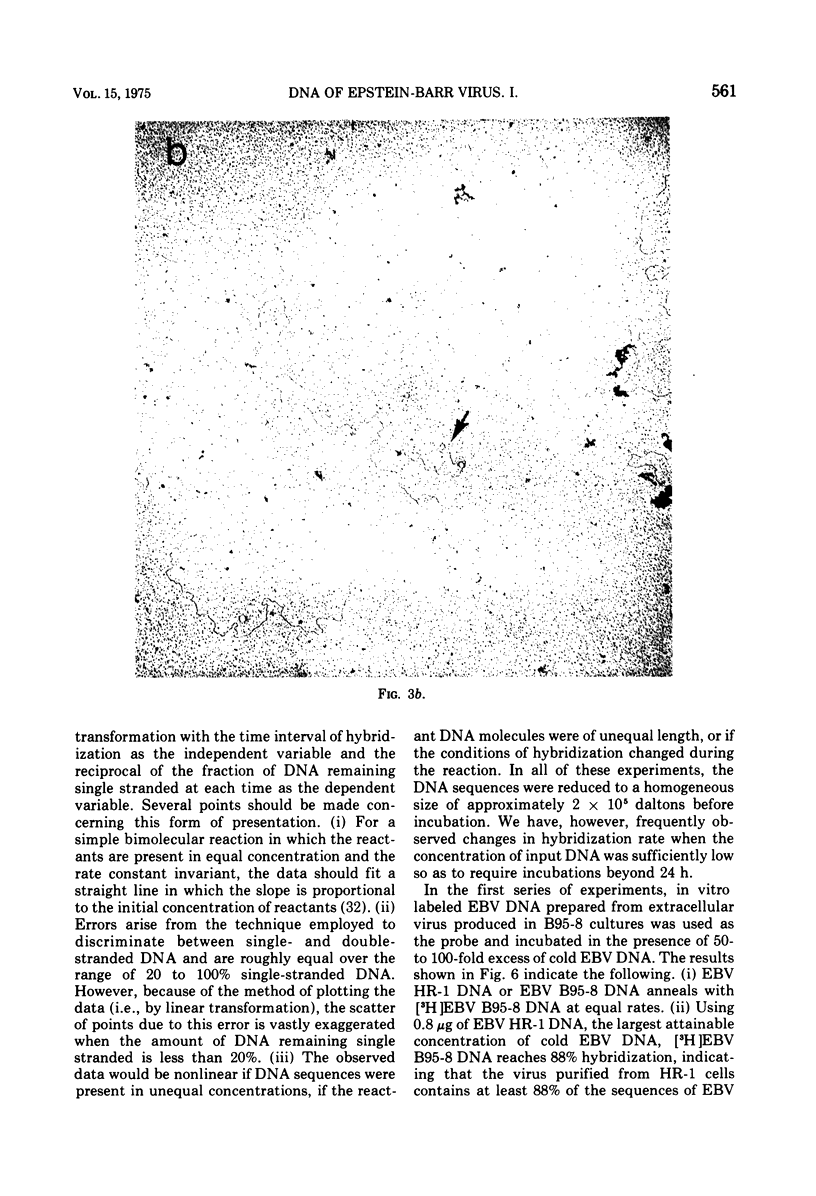
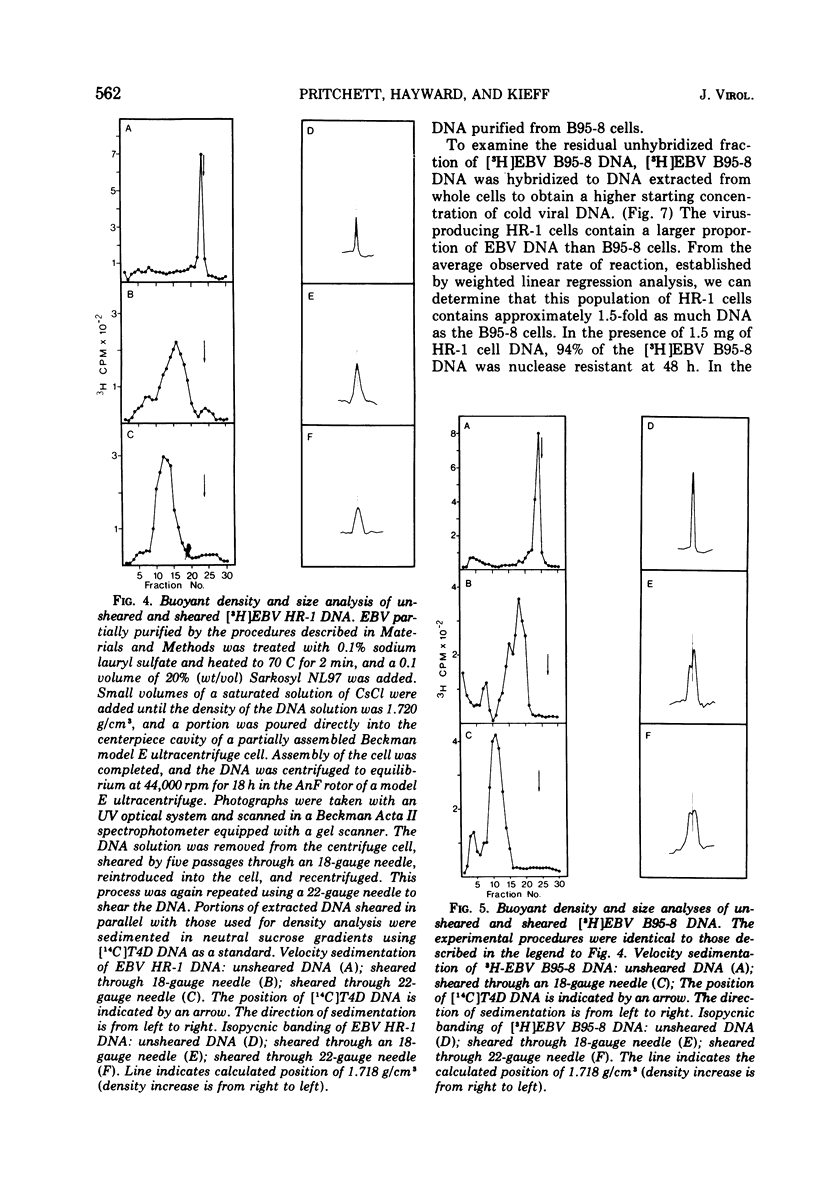
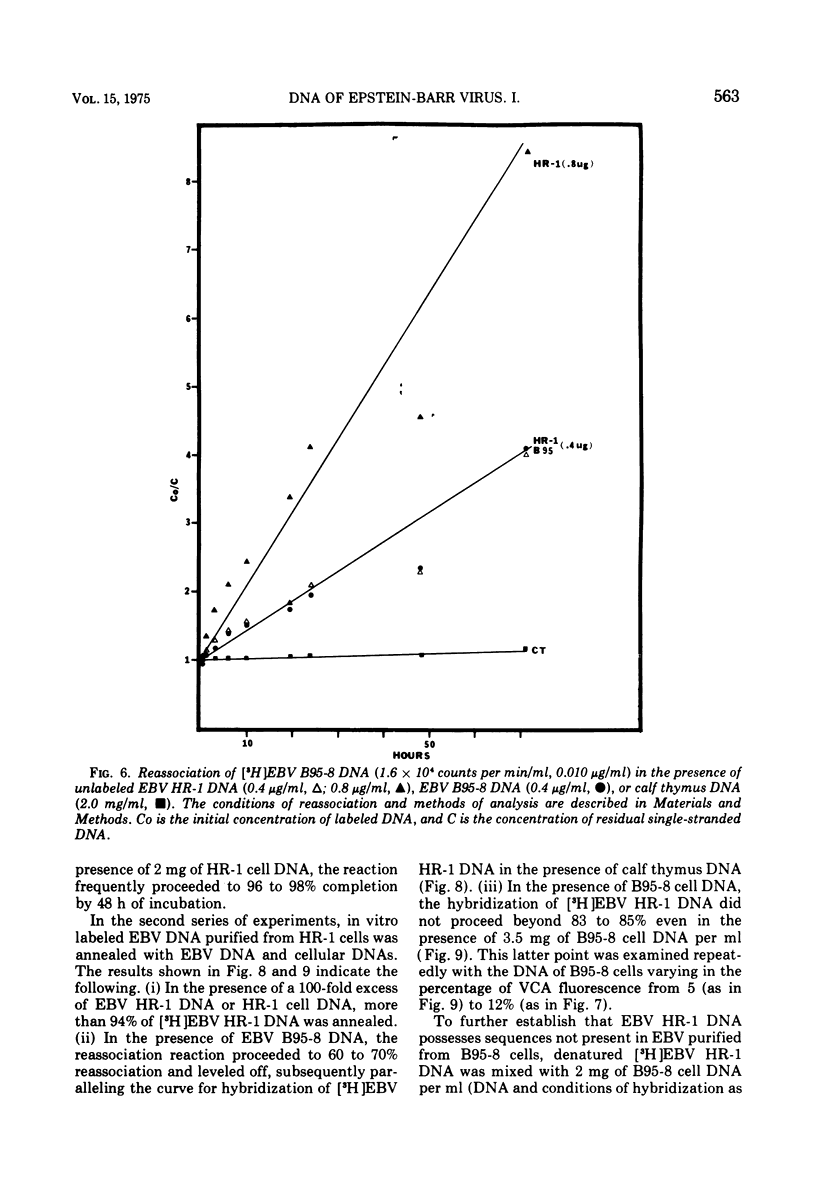
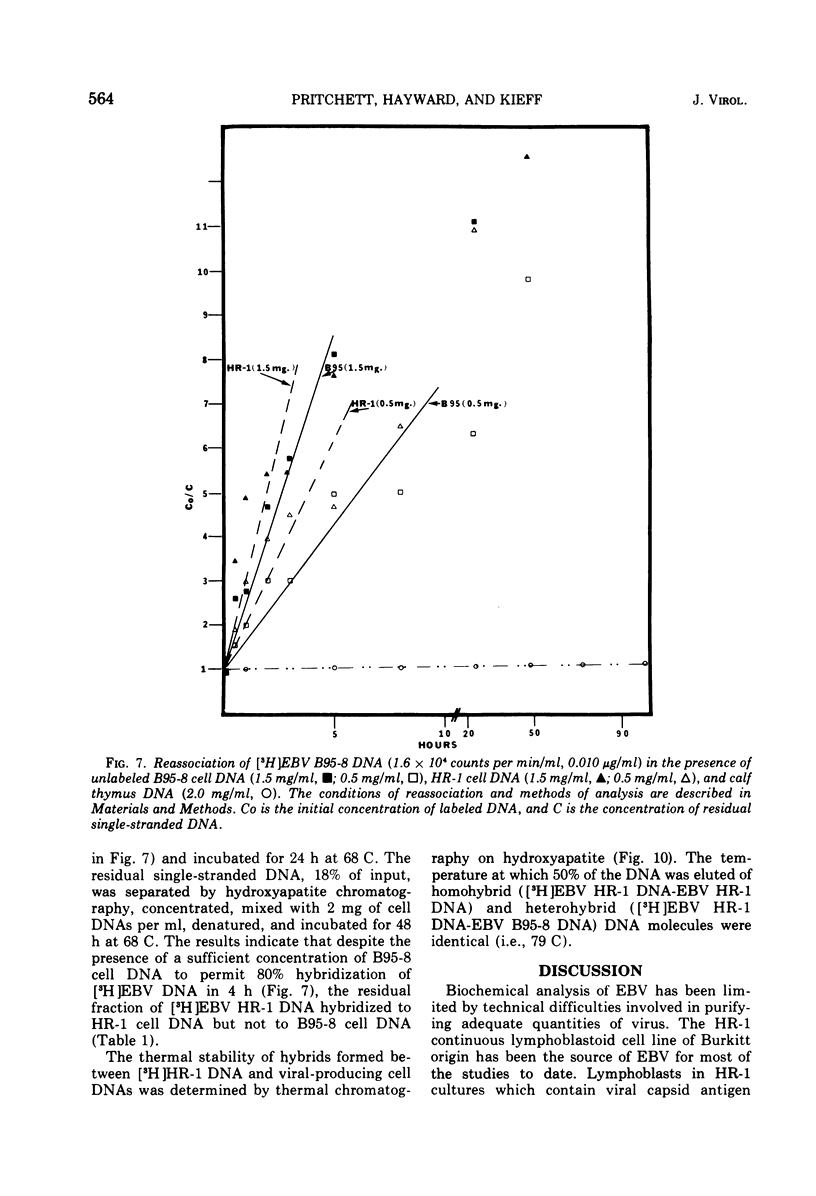
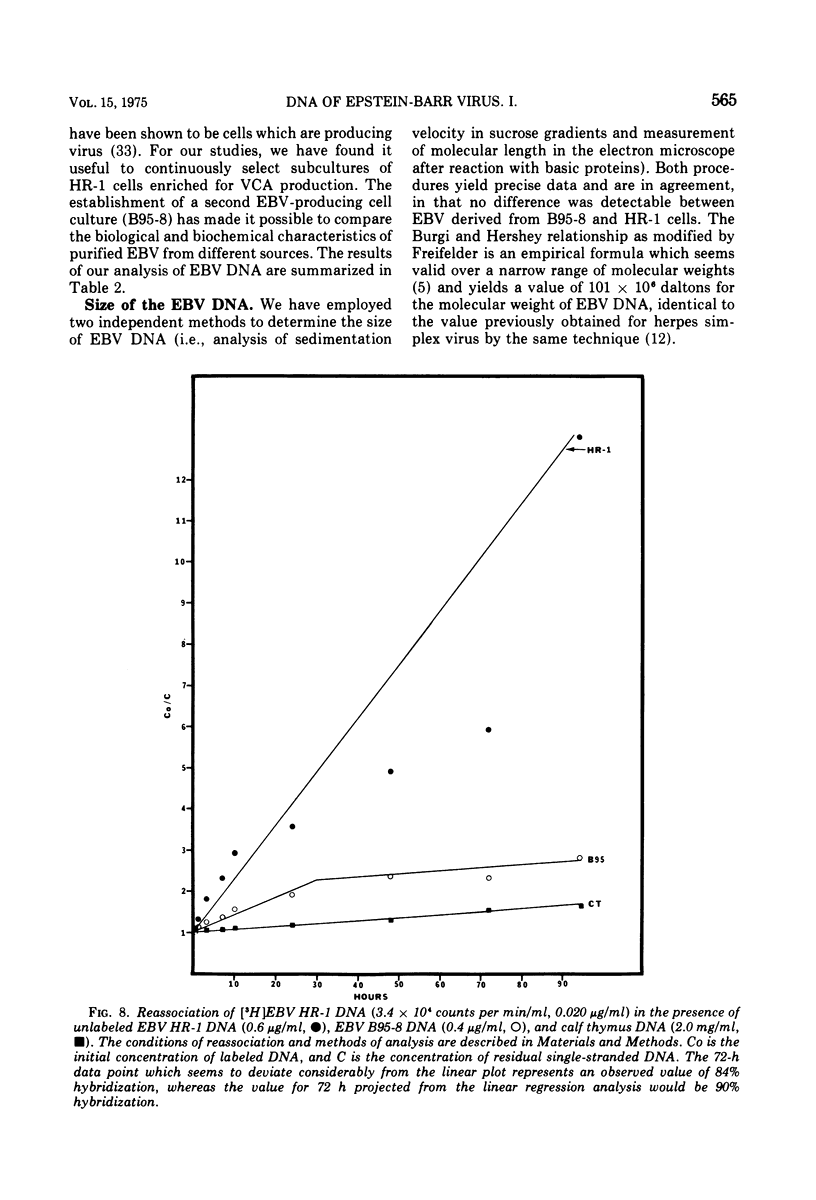
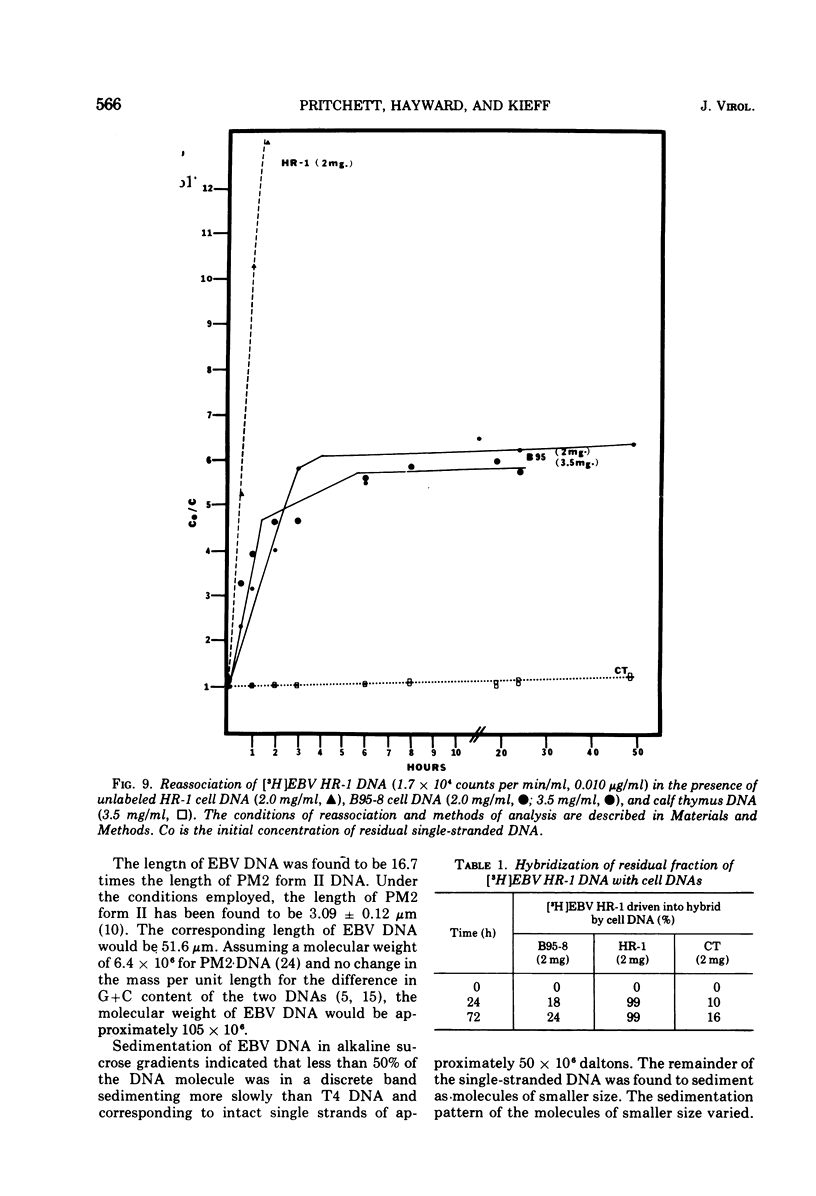
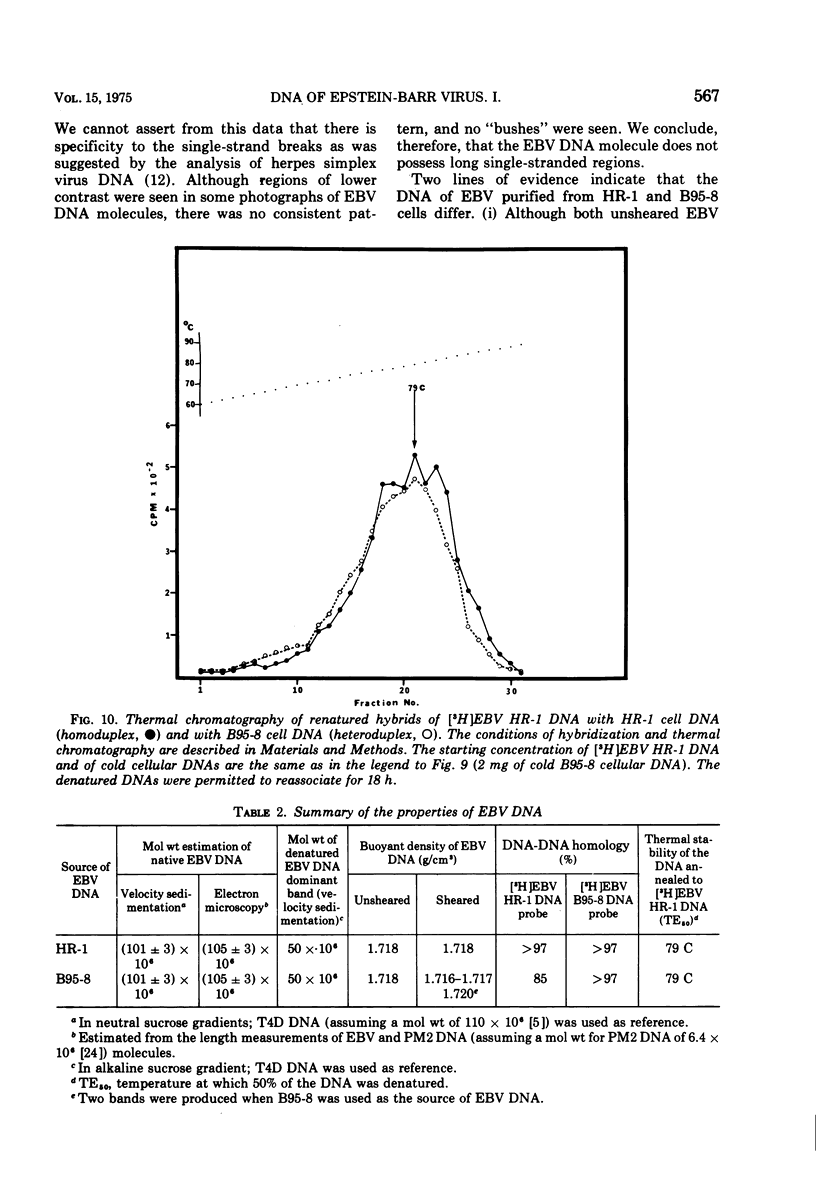
We have compared the properties of the DNA of Epstein-Barr virus (EBV) purified from HR-1 (EBV HR-1 DNA) and B95-8 (EBV B95-8 DNA) continuous lymphoblast cultures. Our data indicate that (i) the S suc of native EBV DNA relative to T4D DNA is 55S. Using the modified Burgi-Hershey relationship (5), we estimate the molecular weight of native EBV DNA is 101 (plus or minus the molecular weight of native FBV DNA by measurement of the length of 3) times 106. Estimation of the molecule relative to form II PM2 DNA yields a value of 105 (plus or minus 3) times 106. (ii) After alkali denaturation, less than 50% of EBV DNA sediments as a single band in alkaline sucrose gradients in the region expected for DNA of 50 times 406 daltons. (iii) Intact EBV HR-1 and EBV B 95-8 DNAs band at 1.718 g/cm3 and a smaller band (approximately 25% of the DNA) AT 1.720 G/CM3. (IV) EBV HR-1 DNA possesses greater than 97% of the sequences of EBV B95-8 DNA. Hybrid DNA molecules formed between (3H)EBV HR-1 DNA and EBV HR-1 DNA or EBV B95-8 DNA had identical thermal stability. EBV B95-8 DNA lacks approximately 15% of the DNA sequences of EBV HR-1 DNA. We interpret these data to mean that EBV B95-8 is derived from a parental EBV through loss of genetic complexity. This defect may be linked to the ability of EBV B95-8 to "transform" lymphocytes invitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Gerber P., Monroe J. H. Studies on leukocytes growing in continuous culture derived from normal human donors. J Natl Cancer Inst. 1968 Apr;40(4):855–866. [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Kleinschmidt A. K. Locating interrupted hydrogen bonding in the secondary structure of PM2 circular DNA by comparative denaturation mapping. J Virol. 1974 Jun;13(6):1176–1185. doi: 10.1128/jvi.13.6.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn U., Lindahl T., Klein C. Fate of virus DNA in the abortive infection of human lymphoid cell lines by Epstein-Barr virus. J Gen Virol. 1972 Sep;16(3):409–412. doi: 10.1099/0022-1317-16-3-409. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Levine J. Homology between Burkitt herpes viral DNA and DNA in continuous lymphoblastoid cells from patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):355–358. doi: 10.1073/pnas.71.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Leighton S. B., Rubenstein I. Calibration of molecular weight scales for DNA. J Mol Biol. 1969 Dec 14;46(2):313–328. doi: 10.1016/0022-2836(69)90424-0. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. E., Grace J. T., Jr, Citron P., Gerner R., Burns A. Leukocyte cultures of patients with leukemia and lymphomas. N Y State J Med. 1966 Nov 1;66(21):2757–2764. [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Huang C. H., Levine P. Absence of Epstein-Barr viral DNA in Amercian Burkitt's lymphoma. N Engl J Med. 1973 Dec 27;289(26):1395–1399. doi: 10.1056/NEJM197312272892604. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. Z., Preiss B., Fraser M. J. A nuclease from Neurospora crassa conidia specific for single-stranded nucleic acids. Prep Biochem. 1971;1(4):283–307. doi: 10.1080/00327487108081946. [DOI] [PubMed] [Google Scholar]
- Rapp F., Li J. L., Jerkofsky M. Transformation of mammalian cells by DNA-containing viruses following photodynamic inactivation. Virology. 1973 Oct;55(2):339–346. doi: 10.1016/0042-6822(73)90173-6. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B., Savage T., Spear P. G., Mizell M., Durr F. E., Sypowicz D. Characterization of the DNA of herpesviruses associated with Lucké adenocarcinoma of the frog and Burkitt lymphoma of man. Virology. 1970 Sep;42(1):257–261. doi: 10.1016/0042-6822(70)90265-5. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Becker Y. Studies on EB virus of Burkitt's lymphoblasts. Virology. 1969 Oct;39(2):312–321. doi: 10.1016/0042-6822(69)90051-8. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Henle W., Hummeler K., Diehl V., Henle G. Comparative study of cultured Burkitt tumor cells by immunofluorescence, autoradiography, and electron microscopy. J Virol. 1967 Aug;1(4):830–837. doi: 10.1128/jvi.1.4.830-837.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]