Abstract
Systemic Lupus Erythematosus (SLE) is a chronic systemic autoimmune disease characterized by the production of anti-nuclear autoantibodies (ANA). ANA development is recognized as one of the initial stages of disease which often results in systemic inflammation, kidney disease and death. The etiology is complex, but it is clear that innate pathways may play an important role in disease progression. Recent data has highlighted an important role for the TLR family, particularly TLR7 in both human disease and murine models. In the studies presented here, we have presented a low copy conditional TLR7 transgenic (Tg7) mouse strain which does not develop spontaneous autoimmunity. When we combine Tg7 with the Sle1 lupus susceptibility locus, the mice develop severe disease. Using the CD19Cre-recombinase system, we normalized expression of TLR7 solely within the B cells. Using this method we demonstrated that overexpression of TLR7 within the B cell compartment reduces the marginal zone B cell compartment and increases B and T cell activation but not T follicular helper cell development. Moreover, this enhanced B-cell TLR7 expression permits the specific development of antibodies to RNA/protein complexes and exacerbates SLE disease.
Introduction
Systemic lupus erythematosus (SLE) is a complex chronic autoimmune disease that is classically associated with the production of pathogenic autoantibodies to a wide spectrum of nuclear antigens (1, 2). These antinuclear antibodies can also be detected in a high frequency of individuals in the general population (3). A subset of individuals with high titered ANA transition to severe disease and this progression is accompanied by increased numbers of antibodies (4). We have focused our recent efforts in understanding how this transition from benign autoimmunity to severe disease occurs.
We have used the B6.Sle1 model as the first step in disease, which develops a mild splenomegaly, enhanced B and T cell activation and high titers of ANAs while rarely developing glomerulonephritis (GN) (5). Sle1, present on chromosome 1, was one of 3 lupus susceptibility regions originally identified from the NZM2410 lupus prone mouse strain through a backcrossing strategy. In these original analyses sle2 and sle3 were also identified and data since then has shown that the presence of these loci leads to enhanced B cell activation and Toll like receptor (TLR) hyperactivity respectively (6). Over the years it has become increasingly apparent that the region encompassing Sle1 is fundamental for driving autoimmunity in a number of other autoimmune prone strains (termed Nba1, Sbw1, Lbw1, Cgnz1, Bxs1 and Bxs2, reviewed earlier (7). However, the progression of Sle1 associated-benign autoimmunity to severe disease depends on an additional immune alteration. Combination of Sle1 with other disease susceptibility loci, particularly with those affecting innate pathways, such as Yaa (y-linked autoimmune accelerating) and Sle3, results in severe disease (6, 8).
The Yaa locus is located on the y-chromosome, driving an aggressive SLE-like disease in male mice. This mutation was proven to be a translocation of approximately 16 genes from the X-chromosome, onto the Y-chromosome (8, 9), which resulted in a 2-fold increase in expression and function of the translocated genes. In particular, there were several interesting immune genes present, including Tlr7, Tlr8 and Rab9 in the translocated DNA. Therefore, we and others went on to show that the upregulation of TLR7 was necessary for the progression to severe disease in different Yaa-containing SLE murine models, using knock-out strategies (10–12). Studies by ourselves and the Izui group also suggested that other genes within this region play a role, since not all severe autoimmune phenotypes were prevented with TLR7 normalization. Data demonstrating the importance of TLR7 in disease progression is not restricted to Yaa-models. Inhibition of TLR7 with immunoregulatory sequences (IRS) inhibits disease in both NZB/W and MRLlpr mice (13, 14). Further, when TLR7 is overexpressed to 8–16 gene copies, it results in acute severe inflammation, kidney disease and mortality (11).
Although genomic analysis of adult patients has not supported a role for a translocation of TLR7 (15), recent studies have shown an association of SLE with an X-chromosome linked TLR7 single nucleotide polymorphism (SNP) in Asian males (16, 17). In addition, an examination in pediatric patients from Mexico revealed a higher copy number of TLR7 in both male and female patients, which could point to an explanation of why pediatric patients demonstrate such an aggressive disease compared to adults (18). Other data suggests TLR7 mRNA levels are increased in adult patients with SLE, correlating with increased RNA expression of IFNα (19). Furthermore, additional evidence suggests that enhanced activation of this pathway is important in human disease, with polymorphisms in its downstream transcription factor, IRF5 being found in multiple studies (20–23). Immunological studies have also demonstrated a role for TLR7 in NETosis, the process by which neutrophils empty their nuclear contents trapping pathogenic material (24). NETosis is enhanced in SLE, and this may be amplified by stimulation with anti-snRNP antibodies (25, 26). This may provide the antigenic material which contributes to the propagation of the inflammatory response.
Since earlier work has focused on eliminating the additional copy of TLR7 to demonstrate its necessity, and given the presence of other immune candidate genes within the Yaa locus (eg Rab9, Tlr8), we sought to determine (a) whether moderate upregulation of TLR7 alone is sufficient to drive disease progression, and (b) how B cells contribute to the later stages of disease progression. We developed a modified bacterial artificial chromosome (BAC) transgenic mouse strain carrying a low copy number of TLR7 (Tg7). The TLR7 gene is flanked by loxP sites so that the transgene can be deleted by Cre-recombinase. When the Tg7 is present on the B6 background and in the absence of Cre, TLR7 mRNA levels are similar to the Yaa strain, and mice do not exhibit any evidence of disease. However, when combined with the Sle1 locus (B6.Sle1Tg7) the mice display disease characteristics similar to the B6.Sle1Yaa strain. To investigate the role of increased TLR7 expression in B cells, we crossed the B6.Sle1Tg7 strain with CD19Cre mice (CD19Cre.B6.Sle1Tg7). When the additional TLR7 copies encoded in the transgene were deleted within B cells, expression of TLR7 was normalized solely within this population. Our data demonstrates many autoimmune traits associated with disease are dependent on an intrinsic upregulation of TLR7 within B cells. Moreover, we show a requirement of increased B cell TLR7 expression for antibodies to RNA/protein complexes, without affecting anti-DNA/chromatin ANAs. This increase in B cell TLR7 expression exacerbates inflammatory infiltrate into the kidney and subsequent disease progression.
Materials and Methods
Reagents and mice
Mice were bred in the University of Texas Southwestern Medical Center or in the Biomedical Resource Centre, Singapore. Breeding pairs for C57BL/6J (B6), B6.129.CD19Cre, and B6.ROSA.EYFP [Gt(ROSA)26Sortm1(Smo/EYFP)Amc] mice were originally obtained from the Jackson Laboratory (27, 28). Cre mice were purchased at n10 B6, but were also checked for any contaminating 129 autoimmune alleles using SNP analyses. None were found near known autoimmune susceptibility regions. The derivation of the B6.Sle1 and B6.Yaa strains has already been described (8, 29). For aging studies to detect the development of disease, mice were 6–9 months old. For all other studies mice used were 6–8 weeks. The modified TLR7 BAC transgene was introduced by pronuclear injection into fertilized eggs and was performed by the Transgenic Core at UT Southwestern Medical Center. The care and use of laboratory animals conformed to the National Institutes of Health guidelines and all experimental procedures conformed to an IACUC approved animal protocol. All antibodies were purchased from BD Biosciences or Ebiosciences with the exception of PE-Texas-Red and QDOT conjugates, which were from Invitrogen and Neu-7/4 which was purchased from Serotec.
Modification of BAC RP23-92P
The B6 derived BAC clone, RP2392P6 was purchased from the BACPAC Resources Center at Children’s Hospital Oakland Research Institute, CA. It contained the full sequence for both TLR7 and PRPS2 (phosphoribosylpyrophosphate synthetase subunit 2). It was modified using a “Counter-Selection BAC Modification Kit” by Red®/ET® Recombination produced by Gene Bridges, Dresden, Germany. The 50bp homology primer sequences for TLR7 were as follows: 5′Fwd: AGC TCA AAG GCT CTG CGA GTC TCG GTT TTC TGT TGC CTT CTC TCT GTC TC; 5′Rev: TAA AAT ACC TTT CTT GAA GGC ATA GAG TGG TTC TAT AGA TGG AGT CCT CT; 3′Fwd; CTC TCT GAA GAA TGT CAC CAC CTA GGA CAT GCC TTG GTA CCT GAA GTT TT; 3′Rev: ATG ACA ACT GTT AGG AAA AAT TCA GAC CTT CAT TTA TGG AAA CCT TTA TG. The loxP sequence inserted into the BAC consists of a [core] flanked by palindrome sequences; 5′-ATAACTTCGTATA [GCATACAT] TATACGAAGTTAT-3′ and 3′-TATTGAAGCATAT [CGTATGTA] ATATGCTTCAATA-5′. Efficient modification of the BAC was examined using genomic sequencing and multiple enzyme digestion with pulse field gel analyses. Integration of the modified BAC by pronuclear injection into C57BL/6J fertilized eggs was completed at the UT Southwestern Transgenic Core.
Assessment of Renal Disease
Blood Urea Nitrogen (BUN) was assessed using the QuantiChrom Urea Assay Kit (BioAssay Systems, CA). Following euthanasia, kidneys were fixed in formalin, and embedded in paraffin for blinded analysis by an independent pathologist, as previously described (30). The severity of GN was graded using the following guidelines set by the World Health Organization, based on light microscopy: 0, normal; 1, mild increase in mesangial cellularity and matrix; 2, moderate increase in mesangial cellularity and matrix, with thickening of the glomerular basement membrane (GBM); 3, focal endocapillary hypercellularity with obliteration of capillary lumina and a substantial increase in the thickness and irregularity of the GBM; and 4, diffuse endocapillary hypercellularity, with segmental necrosis, crescents, and hyalinized end-stage glomeruli. Tubular interstitial nephritis (T&N) was graded on a 0–4 scale, as described (30).
Serology
Serum autoantibodies were analyzed using ELISAs for 1) ANAs, detecting histones and dsDNA) 2) anti-dsDNA and 3) anti-U1 snRNP with B6.Sle1Yaa pooled serum as standard, as described previously (10, 30). Further autoantibody specificity of serum was measured in the Immunomonitoring Core at the Singapore Immunology Network (SIgN), using the AtheNA Multi-Lyte ANA-III Test System (Zeus Scientific), as per manufacturer’s instructions, using a donkey anti-mouse IgG detection antibody (Jackson Immunoresearch). Hep2 staining was performed on selected samples at a 1:200 dilution as per manufacturer’s instructions, with a goat-anti-mouse IgG-DyLight®488 secondary antibody. Cytokine Multiplex Analysis was performed the Bio-Plex cytokine assay kit (Bio-Rad) as per manufacturer’s protocol at BIIR Luminex Core, Dallas.
Cell Purification and RT-PCR
TLR7 copy number from tail DNA lysates was assessed using premixed RTPCR Taqman® primer sets for GAPDH, Mm99999915_g1 and custom designed primers and probe TLR7 from ABI TLR7F: CAGAGGCCCATGTGATCGT; TLR7R: GCCCTCAGGGATTTCTGTCA; TLR7Probe (TAMRA™): ACTGCACAGACAAGC. B cells were labeled using the CD19 positive selection kit from Miltenyi Biotec, and separated using an AutoMACs. Purity was determined to be >97%. Cells were processed for RNA using the trizol/chloroform extraction and a Qiagen RNA-easy kit. RNA message was analyzed using Applied Biosystems Taqman® Gene Expression Assays using RNA-specific primers for B2M (Mm00437762_m1), TLR7 (Mm00446590_m1) and TLR9 (Mm00446193_m1). Absolute MAX QRT-PCR mix from Thermo Scientific was used for amplification, as per the manufacturer’s instructions. An ABI 7300 Real Time System, using Applied Biosystems Sequence Detection Software, Version 1.2.3 was used for amplification and analysis. The message levels for TLR7 and TLR9 were expressed after normalization to B2M expression levels.
Cell Preparation, Flow Cytometry and Microscopy
Peripheral Blood was taken retro-orbitally, or by cardiac puncture. Kidneys were prepared as described previously (10, 31). Briefly, they were minced and resuspended into 0.75ml PBS. Cells were spun down and the supernatant was kept at −20°C for cytokine analysis. This is referred to as kidney plasma in Figure 2f. Cells were resuspended in digestion buffer, consisting of 1mg/ml collagenase IV (Sigma-Aldrich) and DNase I (1ug/ml) in RPMI Complete Media (RPMI CM) and incubated at 37°C for 30mins. Cells were centrifuged, filtered through a 70uM mesh and then mixed 1:1 with 40% Percoll solution. This was centrifuged at 3000 rpm for 20 minutes at RT with the brake off. The loose pellet was washed, counted using a Cellometer® Auto T4 from Nexcelom Bioscience.
Figure 2. An epistatic interaction between Sle1 and Tg7 results in severe autoimmune pathology.
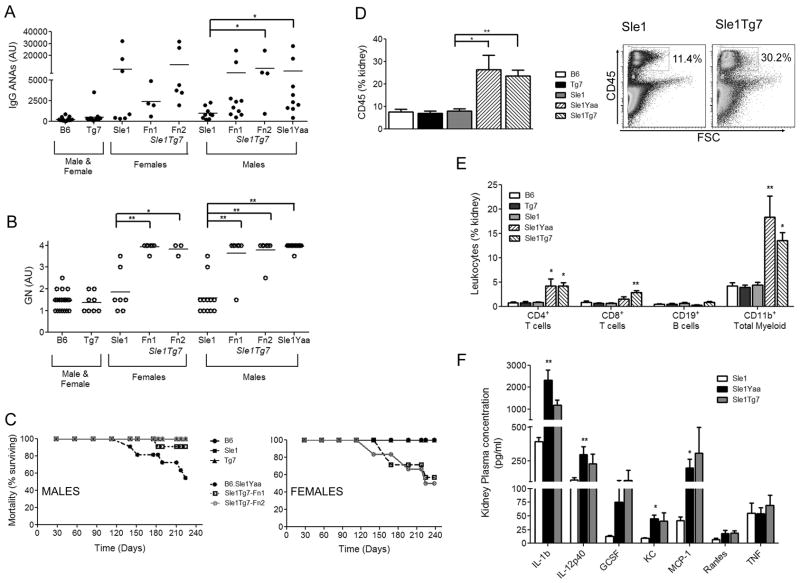
The Tg7 was crossed to the autoimmune-prone B6.Sle1 strain and IgG anti-chromatin/dsDNA antibodies (ANA) were measured by ELISA (Tg7 alone, Fn1/Fn2) (A). Glomerulonephritis (GN) was analyzed by an independent pathologist (B). Mortality curve of male and female mice were significantly different (C). Total leukocyte infiltration into the kidney was measured by CD45 expression. The cumulative data (n≥6 per strain) and gating strategy is shown in (D). Specific infiltrating leukocytes into the kidney were identified with the lineage markers, CD4, CD8, CD19 and CD11b (n≥6 per strain) (E). Inflammatory cytokines released by the kidney were measured by luminex, n=3 per strain (F). Horizontal bars in (A) and (B) represent the mean of the data. Results in (D), (E) and (F) are from male mice, Tg7 = Fn1/2, mean +/− SEM. Significant differences are shown compared to B6.Sle1 control *p<0.05, ** p<0.01.
Splenic or kidney cells were resuspended in staining buffer and stained with a combination of up to 9 directly conjugated antibodies (FITC, PE, PE-Texas-Red, PE-Cy5 or PerCP-Cy5.5, PE-Cy7, allophycocyanin (APC), Alexa700, APC-Cy7, Pacific Blue) and one biotinylated antibody. CFSE and YFP were evaluated in the FITC channel (in the absence of a monoclonal-FITC marker). Secondary detection of the biotinylated antibody was with streptavidin QDOT655. Red blood cell lysis was completed using BD FACS Lysing solution as per manufacturer’s instructions. Acquisition and analysis was completed using a BD LSR II with Flowjo 7.6 for Windows (Treestar). Confocal images were captured with an Olympus FV-1000 Confocal system and processed with Olympus FV10-ASW Fluoview v2.0b.
Statistical Analyses
Results are expressed as the arithmetic mean ± standard error of the mean (SEM). Normality was tested using the Kolmogorov and Smirnov test. For comparing 2 groups, an unpaired 2-way Student’s t-test was used and for multiple comparisons, a 1-way parametric ANOVA & Tukey post hoc comparison was used. If normality test failed, a Mann-Whitney U test compared two groups, and 1-way non-parametric ANOVA and Dunn’s multiple comparison test was used to test multiple comparisons. For time-treatment or time-strain comparisons, a 2-way ANOVA with Bonferroni posthoc analyses was used. Analyses of survival was completed using the Log-rank Mantel-Cox test. Comparisons were made between Tg7 or Yaa containing strains and their wild-types (either B6 or B6.Sle1). Analyses were completed using Prism 5.0 for Windows (GraphPad Software, San Diego, CA, USA) or Microsoft Excel.
Results
TLR7 Transgenic (Tg7) mice are similar in expression and function to Yaa mice
In order to determine whether an upregulation of TLR7 was sufficient to cause disease progression we generated a conditional BAC transgenic mouse (Tg7) using the BAC vector shown in Figure 1A. Analysis of genomic DNA from 4 initial founder lines (Fn) showed 2 additional copies of TLR7 within these mice (Figure 1B), with 3 exhibiting a similar doubling in B cell mRNA expression to the Yaa strain (Figure 1C). Two lines were studied in parallel (Fn1 and Fn2) to ensure that there were no differences between them with regard to disease manifestations, and then we concentrated only on one Tg7 strain (Fn1) for the remaining investigations. The increase in B cell TLR7 transcription was associated with an increase in response to TLR7 ligands R837 and R848 in B cells, as determined by the significant upregulation of CD69 overnight, and associated trend in proliferation at 72hrs (Figure 1D and E). Additional data from a mixed sex population (Fn1 and Fn2) showed similar trends (n=4, p=0.076, supplemental figure 1A). These Tg7 mice did not develop significant levels of ANAs, or glomerulonephritis (Figure 2A and 2B).
Figure 1. TLR7 Transgenic (Tg7) mice are similar in expression and function to Yaa mice.
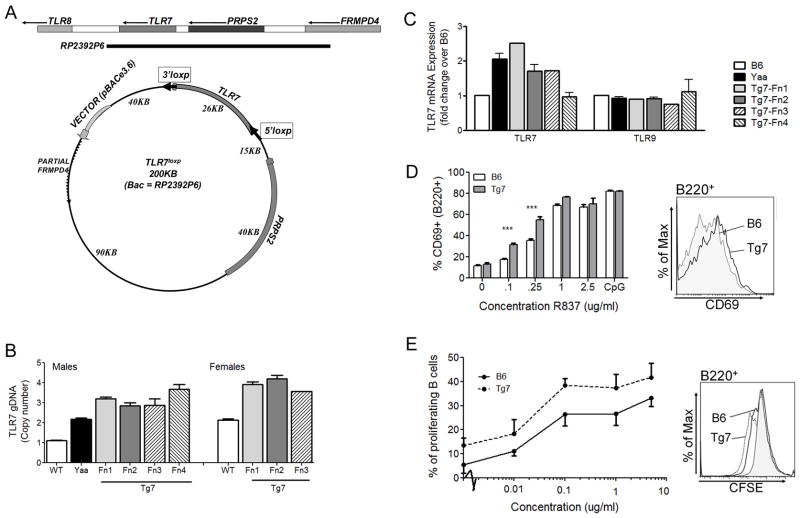
BAC RP23-92P6 was modified as described in the methods to include 2 loxP sites, flanking the TLR7 gene (A). Mice carrying the transgene carried two additional genomic DNA copies (B). Multiple founders (Fn) show 2x TLR7 expression compared to B6 mice (C). Male mice (Fn1 shown) were examined for an increase in the TLR7 functional response. Tg7 (Fn1) mice demonstrate an increase in CD69+ B cells (B220+ cells) after TLR7 stimulation, by R837 (D) and enhanced proliferation of B cells, by R848 as detected by CFSE dilution (E). 2 way ANOVA demonstrated significance for both D and E, post hoc significance is shown by *, p<0.05. Bars represent SEM, n=3 mice per strain in each separate experiment.
Thus we developed a low copy TLR7 transgenic, similar to the Yaa strain, which did not exhibit any overt autoimmune traits.
An epistatic interaction between Sle1 and Tg7 results in severe autoimmune pathology
We then crossed our TLR7 transgenic strain (Tg7) to the B6.Sle1 to examine whether the upregulation of TLR7 recapitulated the effects observed in the B6.Sle1Yaa strain. We have previously shown that the addition of Yaa onto B6.Sle1, increases the penetrance and titer of autoantibodies to nucleosomes (histones/dsDNA) (32). Our B6.Sle1Tg7 mice developed a similar ANA profile in both female and male mice, this was significant in male mice analyzed (Figure 2A). Pathological analysis indicated that female and male B6.Sle1Tg7 mice also developed severe glomerulonephritis (GN), similar to their B6.Sle1Yaa counterparts (Figure 2B). Analysis of mortality demonstrated that male B6.Sle1Tg7 mice survived significantly longer than their B6.Sle1Yaa counterparts (Figure 2C) suggesting additional genes within the Yaa locus may contribute to disease. When examining female B6.Sle1Tg7 mice, we observed an increase in mortality when compared to B6.Sle1 females and B6.Sle1Tg7 males, which is probably due to the previously reported female sex bias of the Sle1 region, as evidenced by the sex bias in IgG ANA levels (Figure 2A).
We have previously shown that the CD45+ leukocyte infiltration associated with kidney disease in experimental and spontaneous SLE models is measurable using flow cytometry (10, 31, 33). Using this technique we were able to confirm that the B6.Sle1Tg7 mice had increased CD45+ cells within the kidney, and that this infiltrate was composed of CD4+ and CD8+ T cells, and CD11b+ myeloid cells, in a similar manner to B6.Sle1Yaa mice (Figure 2D and 2E). Analysis of the supernatant from freshly isolated kidney cells using multi-plex luminex determined several inflammatory cytokines and chemokines that were significantly increased in the B6.Sle1Yaa compared to B6.Sle1 controls, with B6.Sle1Tg7 mice showing similar trends.(Figure 2F). These included IL-12p40, KC (keratinocyte chemoattractant) and MCP-1 (monocyte chemoattractant protein-1), as we detected in an earlier cohort of B6.Sle1Yaa mice (10).
Therefore we demonstrated that the epistatic interaction of the Sle1 lupus susceptibility region and our TLR7 transgene resulted in severe kidney pathology, in a similar manner to the B6.Sle1Yaa strain.
B6.Sle1Tg7 mice develop splenic disease similarly to the B6.Sle1Yaa strain
Splenomegaly is a common feature of lupus prone murine models, including the B6.Sle1Yaa and this was also detected in the B6.Sle1Tg7 strain (Figure 3A). Cellular expansion involved CD4+ T cells, CD19+ B cells and in particular, CD11b+ myeloid cells (Figure 3B). Spleens from B6.Tg7 mice were also slightly larger than B6 wild-type controls but there was no detectable leukocyte population expanding (Figure 3A and 3B). An expansion of splenic CXCR5+ T follicular helper cells is characteristic of Yaa models and this was also found to be a trait of the B6.Tg7 itself, and was also evident on the B6.Sle1 background, with no observable difference when comparing the B6.Sle1Yaa and B6.Sle1Tg7 (Figure 3C). Other mouse models containing the Yaa translocation also show decreases in the percentage of marginal zone B cells (34) and it has been hypothesized that this may play a significant role in disease progression through the associated reduction in IL-10 (35). This reduction in B cell marginal zone cells was also apparent in aged B6.Sle1Tg7 mice (Figure 3D).
Figure 3. B6.Sle1Tg7 mice develop splenic disease characteristics associated with the B6.Sle1Yaa strain.
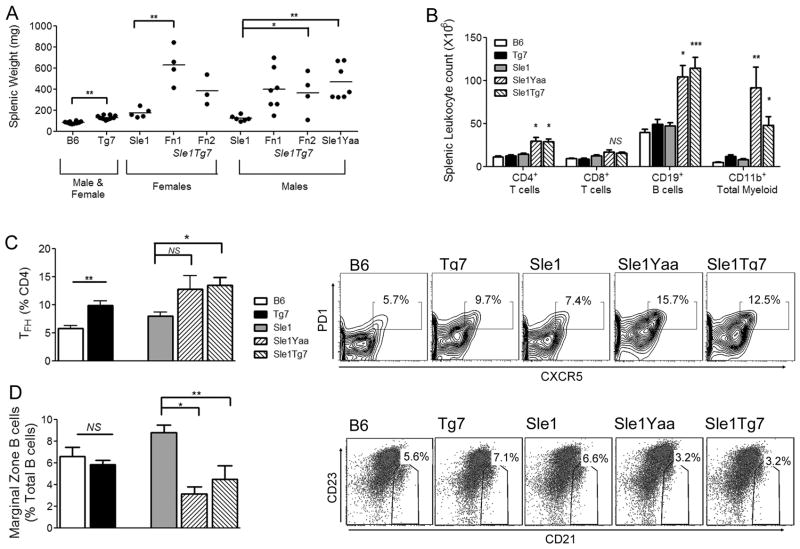
Male mice were aged to 7–9 months and splenic autoimmune traits were analyzed. (Figure 3A and 3B). B6.Sle1Tg7 mice exhibit splenomegaly in a similar manner to B6.Sle1Yaa over their B6.Sle1 counterparts (A). Analysis of expanding leukocytes in the spleen was analyzed using flow cytometry with antibodies to CD3, CD4, CD8, CD19 and CD11b to identify CD4 and CD8 T cells, B cells and the total myeloid population (CD11b+) (B). T follicular helper (TFH) cells were identified by PD-1 and CXCR5 expression on CD4+ T cells (C). Marginal zone B cells (Mz) were identified using B220, CD23 and CD21 monoclonal antibodies (D). Data are represented as mean +/− SEM. Data are cumulative from 2 independent aging cohorts, with a minimum of 3 mice per group for each cohort. (n=6 for B6, B6.Sle1, n=7 for B6.Tg7 (Fn1/2), n=11 for B6.Sle1Tg7, n=7 for B6.Sle1Yaa) Data from (B) and (C) are representative from one cohort. *p<0.05, **p<0.01.
Therefore, our data so far demonstrated that the Tg7 mice which carry additional copies of TLR7 behave similarly to the corresponding Yaa SLE model in terms of all investigated autoimmune traits. Using Tg7 SLE mice we showed that an upregulation of TLR7 is sufficient to promote autoimmunity in B6.Sle1 mice. Comparison of female and male B6.Sle1Tg7 mice did not identify any other differences in autoimmunity other than an increased rate of mortality.
Effective elimination of the additional TLR7 copy in B cells
Since our transgenic system promotes disease progression in a similar manner comparable to the B6.Sle1Yaa model, we then sought to utilize the loxP modifications of this transgene to determine the necessity of the upregulation of TLR7 within the B cells to drive full pathogenicity. To eliminate the additional copies of TLR7 introduced by the BAC, we used the Cre recombinase system (36). When the B6.Sle1Tg7 strain was crossed with the CD19Cre (28) strain to generate CD19.B6.Sle1Tg7, the normal mRNA expression of TLR7 was restored only within the B cells, without a corresponding reduction in CD11b+ myeloid populations (Figure 4A). Analysis of the deletion using a YFP reporter, using the ROSA/EYFP strain (27) showed that <1% of non-B cells expressed YFP, confirming high specificity, and that over 90% of B cells did express YFP, showing high efficiency (0.96±0.11, 90.03±2.46 respectively; representative plots Figure 4b).
Figure 4. Effective elimination of the additional TLR7 copy in B cells.
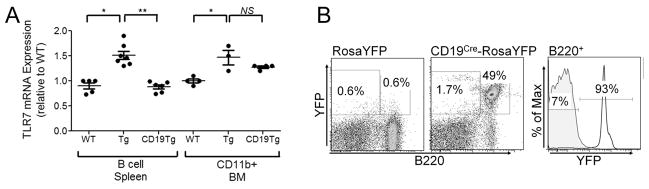
Transgenic mice (Tg7) were crossed Cre recombinase mice (CD19Cre). B cells or CD11b+ cells from bone marrow (BM) were purified in Tg7, wild type (WT) or CD19Tg7, and the mRNA purified and assessed for TLR7 expression. (A). CD19Cre mice were crossed with a ROSA-eYFP reporter strain, and the fluorescence evaluated by flow cytometry using B220 monoclonal antibodies and YFP emission (B).
Increases in B cell TLR7 result in enhanced splenic B and T cell activation, and marginal zone B cell depletion
Using this effective deletion of excess copies of TLR7 by CD19Cre, we analyzed disease progression in female CD19.B6.Sle1Tg7 mice, and compared them to B6.Sle1 and B6.Sle1Tg7 counterparts, to determine the necessity of B cells in the augmentation of autoimmune phenotypes. Analysis of splenomegaly, overall splenic numbers and leukocytes using common lineage markers CD19, CD3, CD4, CD8 and CD11b by flow cytometry showed that B cell normalization of TLR7 did not alter the disease profile of the spleen (Table 1). Analysis of splenic weights from 7–9 month old male mice also replicated these findings and determined that there was no observable effect from the CD19Cre itself (Supplemental Figure 1B).
Table 1.
Normalization of TLR7 in CD19+ cells does not impact splenic leukocyte expansion
| B6 | Tg7 | Sle1 | Sle1Tg7 | CD19Sle1Tg7 | |
|---|---|---|---|---|---|
|
| |||||
| Spleen Weight | 84 ± 4 | 114 ± 7 | 134 ± 6 | 467 ± 28 | 335 ± 31 |
| Count (x107) | 12.9 ± 1.0 | 15.0 ± 1.1 | 17.8 ± 1.3 | 55.4 ±8.2 | 37.1 ±4.5 |
| Sample size (n) | 18 | 19 | 25 | 26 | 14 |
|
| |||||
| Splenic Leukocytes (%CD45) | |||||
|
| |||||
| CD19 | 50.3 ± 7.2 | 47.9 ± 6.4 | 48.1 ±7.3 | 38.3 ± 8.4*** | 33.7 ± 6.9*** |
|
| |||||
| CD4 T | 18.1 ± 3.7 | 20.6 ± 4.1 | 19.2 ± 4.6 | 17.5 ± 2.8 | 19.7 ± 4.8 |
|
| |||||
| CD8 T | 13.3 ±2.2 | 13.4 ± 3.2 | 13.0 ± 2.3 | 7.7 ± 1.7*** | 10.6 ±3.9 |
|
| |||||
| CD11b | 7.4 ± 1.8 | 9.2 ± 1.9 | 9.8 ± 2.3 | 22.1 ± 7.4*** | 26.0 ±6.2*** |
|
| |||||
| Sample size (n) | 13 | 19 | 20 | 23 | 11 |
p<0.001 compared to B6.Sle1 mice. Comparisons between B6.Sle1Tg7 and CD19.B6.Sle1Tg7 did not reveal any significant differences.
Further flow cytometry analysis showed that the restoration of normal TLR7 expression within B cells prevented the loss of the marginal zone compartment (Figure 5A and supplemental Figure 1B). In addition, immunohistochemistry of spleen confirmed a completely disrupted follicular architecture in B6.Sle1Tg7 with no real marginal B cell area. CD19.B6.Sle1Tg7 spleens had marginal B cells however, the zone was discontinuous around the follicle. Both flow and microscopy demonstrated no differences in either germinal center cells (GL7+) or plasma cells within these mice (Figure 5A, 5B).
Figure 5. Increases in B cell TLR7 result in enhanced splenic B and T cell activation, and marginal zone B cell depletion.
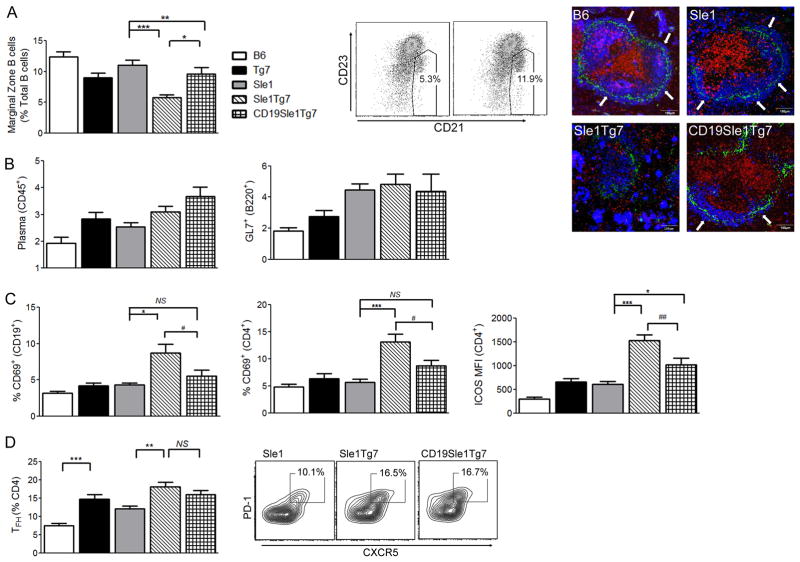
Female mice were aged to 6 months and the percentage of splenic B cell marginal zone cells were determined as described earlier (a, with representative plot). Microscopy using MOMA-1 (green), CD5 (red) IgM (blue) shows continuous IgM+ marginal B cell zone (white arrows) in B6 and B6.Sle1 controls. There is a completely disrupted follicular architecture in B6.Sle1Tg7 with no definitive marginal zone. The IgM bright cells are plasma cells which are numerous in B6.Sle1Tg7. In B6.CD19. Sle1Tg7 there are marginal B cells although the zone is discontinuous around the follicle. FACs analysis of plasma cells (B220−CD138+) and GL7+ germinal center B cells showed no difference between B6.Sle1Tg7 and B6.CD19.Sle1Tg7 (b). The expression of CD69 B cell and CD4+ T cells, and ICOS on CD4+ T cells shows a decrease in activation with CD19Cre B cell normalisation (c). Analyses of splenic CD4+ TFH population, gated using PD-1 and CXCR5 shows no difference between the B6.Sle1Tg7 and B6.CD19Sle1Tg7 (d). Data is representative of mean +/− SEM; 1-Way ANOVA, *p<0.05, **p<0.01, ***p<0.001. (Mann-Whitney, # p<0.05 when B6.CD19Sle1Tg7 is compared to B6.Sle1Tg7). NS, not significant.(n≥8 per strain)
Analysis of activation markers in splenic B and T cells revealed that B6.Sle1Tg7 lymphocytes were activated, with increased CD69 and ICOS, compared to B6.Sle1 controls (Figure 5C). When TLR7 was normalized by CD19Cre the upregulation of CD69 in both lymphocyte populations, and ICOS in CD4+ T cells was prevented (Mann-Whitney comparison, p<0.05; Figure 5C and 5D).
Normalization of TLR7 in B cells specifically decreases antibodies to RNA/protein complexes
The ANA production by the B6.Sle1 model has been measured repeatedly over the years by ELISA detecting IgG autoantibodies to histones, chromatin and double stranded DNA, since these appear to be predominant. We determined that the increase in titers in the B6.Sle1Tg7 strain was dependent on overexpression of TLR7 in B cells (IgG ANA, Figure 6A), since levels in CD19.B6.Sle1Tg7 6 month old female mice were similar to B6.Sle1. However, when we analyzed the penetrance of IgG anti-dsDNA in CD19.B6.Sle1Tg7 mice, we observed no difference when compared with the B6.Sle1Tg7 controls (anti-dsDNA). Since TLR7 has been associated with U1_snRNP autoreactivity, we determined serum levels of these autoantibodies using an ELISA (anti-snRNP) (37–39). The increase in anti-snRNP (U1) shown in the B6.Sle1Tg7 strain was prevented by B cell-TLR7 normalization (Figure 6A). Furthermore, analysis of 7–9 month old male mice showed identical findings (Supplemental Figure 1B). Assessment of antibodies to 10 different autoantigens by Luminex, showed a significant reduction in the titers of anti-SSA52 autoantibodies (Figure 6B). In this assay, the snRNP analyte includes both anti-U1 and the anti-B/B core proteins, unlike the ELISA which detects anti-U1 alone. Further analysis of anti-nuclear antibodies using staining of the HEp2 cell line confirmed that the CD19.B6.Sle1Tg7 had similar titers to the B6.Sle1 mice and there was no change in staining pattern (Figure 6C).
Figure 6. Normalization of TLR7 in B cells specifically decreases antibodies to RNA/protein complexes.
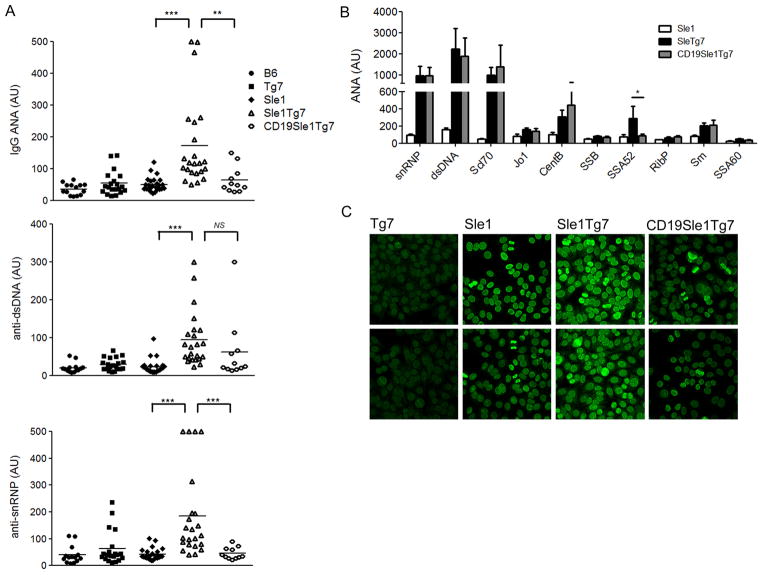
Sera were analyzed for IgG dsDNA/histone/chromatin autoantibodies (IgG ANAs) or IgG anti-dsDNA antibodies (anti-dsDNA), or IgG anti-snRNP antibodies (anti-snRNP) by ELISA (a). The standard curve for each ELISA was done with pooled B6.Sle1Yaa sera to give arbitrary units (AU) for each ELISA. Multiple ANAs were also assayed using luminex (b). Hep-2 staining was completed on 5–6 mice per strain and analyzed by confocal microscopy. Representative images are shown (c). Comparisons to B6.Sle1 control, *p<0.05, ***p<0.001. # p<0.05. Bars represent SEM, NS, not significant.
Overall this data indicates that the B-cell TLR7 expression is responsible for the high titers of antibodies to RNA/protein complexes.
Normalization of B cell TLR7 expression moderately reduces kidney inflammation
Blinded analysis of renal histology by an independent pathologist demonstrated a marginal reduction in GN from CD19.B6.Sle1Tg7 mice when compared to B6.Sle1Tg7 control mice (Figure 7A; p<0.05). However, 85% of the mice still developed severe GN (≥score 3; 6/7 mice) suggesting that other cell types are critical for initiation of kidney disease. Assessment of the blood urea nitrogen (BUN) suggested that the increase in levels shown by the B6.Sle1Tg7 mice, was prevented in the CD19.B6.Sle1Tg7, although a comparison between the 2 strains was not significant (Figure 7B).
Figure 7. Normalization of B cell TLR7 expression moderately reduces kidney inflammation.
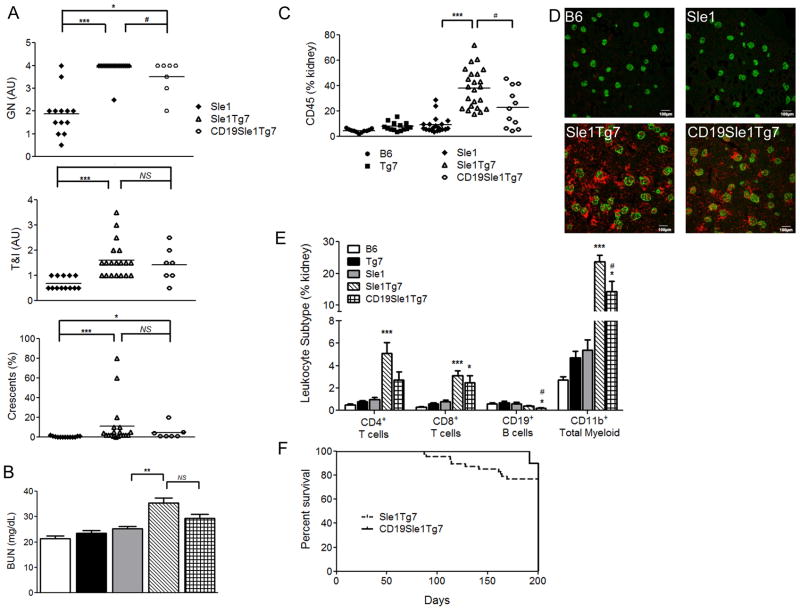
Female mice were aged to 6 months and kidneys were assessed for histological nephritis (a). BUN was also assessed as a measure of kidney function (n≥6 per strain) (b). Total CD45+ leukocyte infiltration into the kidney was assessed using flow cytometry as described earlier (n≥6 per strain) (c), with microscopy demonstrating equivalent infiltration into the glomeruli between B6.Sle1Tg7 and B6.CD19Sle1Tg7 mice, CD45-apc (red), nephrin-Alexa488 (green) (d). Analyses of leukocyte lineages infiltrating the kidney shows a reduction in CD3+CD4+, CD19+ and CD11b+ cells when B6.CD19Sle1Tg7 mice are compared to B6.Sle1Tg7 (#, p<0.05) (e). There was a slight reduction in mortality in the B6.CD19Sle1Tg7 (n=10) mice compared to B6.Sle1Tg7 (n=48) (f). Comparisons to B6.Sle1 control, *p<0.05, **p<0.01, ***p<0.001. Graph bars represent SEM.
Examination of the leukocyte infiltrate in the kidney using flow cytometry and microscopy showed a reduction in CD45+ cells in the CD19.B6.Sle1Tg7 model when compared to the B6.Sle1Tg7, although there was still considerable infiltrate in both strains (Figure 7C and D). Further characterization revealed that CD11b+ and CD19+ cellular recruitment to the kidney was lower in the CD19Cre expressing mice than in the parental B6.Sle1Tg7 strain, with CD4+ recruitment showing a similar trend (Figure 7E, p=0.067 (CD4). When we calculated mortality of mice aged beyond 200 days, we determined that the CD19.Sle1Tg7 strain had a penchant to survive longer than their B6.Sle1Tg7 counterparts, although this was not significant (Figure 7F). Thus we had demonstrated that an increase in TLR7 expression within B cells enhances leukocyte infiltrate resulting in an exacerbation of kidney disease and increased mortality.
Discussion
In this report, we have described a new conditional TLR7 BAC transgenic mouse strain to investigate the role of increased TLR7 expression in SLE. These mice carry two additional copies of the TLR7 gene which results in a two fold increase in TLR7 mRNA levels. Without the addition of other susceptibility loci, these mice do not develop disease. When we combined the TLR7 transgene with the B6.Sle1 susceptibility region, B6.Sle1Tg7 mice develop a severe autoimmune profile almost indistinguishable from the B6.Sle1Yaa strain. Since the TLR7 transgene is floxed it can be used to assess the contribution of upregulated TLR7 in specific leukocyte lineages. In the studies presented here we have assessed the contribution of TLR7 upregulation in CD19+ cells. We have demonstrated that an increase of TLR7 specifically in B cells is responsible for high titers of antibodies to RNA/protein complexes. Furthermore, that increased B-cell TLR7 expression results in increased inflammation of the kidney, leukocyte recruitment and exacerbation of disease.
Since the discovery of the translocation of TLR7 in the Yaa model there has been a burgeoning interest in the role of this receptor in SLE. Recent genetic studies in humans have demonstrated TLR7/TLR8 associations with SLE at genome wide significant levels in multiple populations (16, 17). Further, immunological analyses have shown that anti-snRNP antibodies can increase NETosis, a process which is enhanced in SLE (25, 26). Data from recent investigations using knockout mice, demonstrates an underlying requirement for TLR7 for the development of autoimmunity (40, 41). Our findings from the B6.Sle1 model also support these findings (manuscript in preparation). In our original multi-step hypothesis of how SLE progresses there is an initial loss of tolerance to self which results in the production of autoantibodies (7). Thus, previous data supports a dependency for TLR7 at this stage. The second step to a progression of severe disease depends on an additional immune alteration. Studies using Yaa-containing SLE models have shown that an increase in TLR7 expression is required for the initiation of severe disease (10–12). Furthermore, other non-Yaa SLE models show a role for TLR7 in pathogenesis. Inhibition of both TLR7 and TLR9, or single inhibition of TLR7 reduce autoimmune pathology in both the NZB/W strain and the MRLlpr (13, 14, 42). Furthermore, exogenous administration of a TLR7 ligand, imiquimod, results in an augmentation of kidney disease (43).
In our studies presented here, we demonstrated that a moderate chronic upregulation of TLR7 is sufficient to drive kidney pathogenesis in the lupus susceptible strain, B6.Sle1. Since we know that IFNα induces TLR7 expression, this suggests that a viral initiation or exacerbation of severe disease is a plausible mechanism in SLE (44, 45).
A moderate increase in TLR7 alone is not sufficient for the development of severe disease and the additional susceptibility loci, such Sle1 is required. Polymorphisms of the signaling lymphocyte activation molecule family (SLAMFZ) are responsible for the mild autoimmune traits conferred by this region (46, 47). Recent studies have supported a role for both Ly108 and CD84 in this model (46–48). Ly108 is important for B and T cell autoreactivity, with an autoimmune-resistant haplotype (Ly108-H1) existing in CD4 T cells in B6 mice which prevents ANA production (47). Additionally, CD84 is critical for optimum germinal center formation, efficient T and B cell interaction and the generation of T follicular helper cells (49). Moreover, differential expression or signaling of SLAMF members have been associated with human disease. Different haplotypes of 2 family members, CS1 and 2B4, exist in the human population and differential expression is associated with disease (50–52). Furthermore, associations in Ly9 have been shown to correlate with T cell differences in UK and Canadian SLE populations, and ligation of CD352 (SLAMF6) results in impaired cytokine responses in SLE T cells (53, 54). Therefore, there is an increasing evidence that the SLAM family and its downstream signaling adaptors are significant immunomodulatory receptors, regulating normal B, T and NK cell function, and this may play a significant role in SLE (55). Our murine data has shown us that an epistatic interaction exists between the SLAMF and TLR7, since an immune alteration in either does not result in more severe disease, but the combination of SLAMF polymorphisms and TLR dysregulation results in early onset aggressive nephritis. It is less clear on how these receptors interact with the TLR pathway and impact immune cell function.
A comparison of mortality between B6.Sle1Tg7 and B6.Sle1Yaa mice clearly demonstrate that additional genes within the Yaa locus contribute to disease. These findings are consistent with our earlier investigations and with the results from Izui and colleagues, which show that additional Yaa-genes may contribute to ANA titer and autoimmune pathology (10, 12). These data are in contrast to those reported by another group, who describe a deletion of all autoimmune phenotypes with TLR7 normalization (11). This inconsistency may be due to the additive autoimmune effect from the FcγRIIb deletion which is not present in the other two murine strains.
In these studies we also examined the contribution of B cells to the autoimmunity traits conferred by increased TLR7 expression. We demonstrated that the level of TLR7 expression, in the context of SLAMFZ, dictates the capacity for antigen specificity to RNA. In addition, in this environment, TLR7 confers an increase in the expression of ICOS, CD69, CXCR4 on B and T cells, which are molecules important in leukocyte emigration and activation. However, B cell normalization of TLR7 did not significantly reduce splenomegaly or the cellular composition of the spleen. Furthermore T follicular helper cell (TFH) development was unaffected by TLR7 normalization. A recent study by Tangye and colleagues demonstrated that although the SLAM adaptor protein SAP is required for the B-T cell dependent TFH development, in the case of excess antigen, the T-DC interaction becomes dominant and overrides the necessity of SAP for TFH production. However, SAP is required for the T dependent antibody response (56). Therefore, in SLE, where there is persistent antigen activation from immune complexes containing nuclear components, the DCs may be driving TFH development and may be the central player of disease.
Aside from the role the DC may play, it is also possible that there are TLR7-dependent inflammatory effects intrinsic to the kidney which may contribute to pathogenic disease, since we have previously shown that the kidney itself, can produce IFNα in response to autoantibody-mediated glomerulonephritis (33). However recent studies suggest that TLR7 is expressed by renal macrophages and not kidney mesangial renal cells (14, 43). The combination of our model with the Vav1Cre system will normalize TLR7 expression in all leukocytes and help to elucidate the contribution of the target organ (57).
Overall, we have demonstrated that a chronic moderate upregulation of TLR7 is sufficient to driving severe disease in a murine model of lupus. Further, we demonstrated that upregulation of TLR7 within B cells results in a high titer of antibodies to RNA/protein complexes, a B cell marginal zone defect and an escalation of disease. Our findings have important consequences for clinical therapeutics. SLE is a heterogenous polygenic disease with patients displaying a wide range of disease characteristics. Therefore, it is likely that aberrant activation of different inflammatory pathways may lead to disease. Based on our findings we might hypothesize that patients who exhibit antibodies to RNA/protein complexes, possess an upregulated TLR7 signaling pathway within their B cells, and possibly within other leukocytes. If we could preselect patients for specific autoimmune mechanisms, such as enhanced TLR7 expression or function, using clinical parameters, e.g., anti-snRNP ANAs, we could move to a personalized medicine approach based on the individual needs of the patients, reducing side effects and improving efficacy.
Supplementary Material
Acknowledgments
We thank Lucia Mori (SIgN) for critical review of the manuscript, Michael Poidinger, head of the Bioinformatics core at SIgN for careful review of statistics, Nana (Xiang-Hong) Tian for technical assistance, and Jose Casco DVM, for maintenance and transport of the UT Southwestern murine colony. Further, we would like to thank the flow cytometry cores at UT Southwestern and SIgN.
Grant Support: This work was supported by grants from the NIH and the Alliance for Lupus Research to EKW and core funding from the Singapore Immunology Network (SIgN) at A*STAR, Singapore to AMF.
References
- 1.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 3.Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, Karp D, Wakeland EK, Olsen NJ. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27:153–160. doi: 10.1016/j.jaut.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 5.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci U S A. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 10.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, Wakeland EK. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago-Raber ML, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, Izui S. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 13.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 14.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 15.Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum. 2007;56:3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]
- 16.Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, Wu YL, Yu CY, Tang Y, Chen JY, Yang W, Wong M, Kawasaki A, Tsuchiya N, Sumida T, Kawaguchi Y, Howe HS, Mok MY, Bang SY, Liu FL, Chang DM, Takasaki Y, Hashimoto H, Harley JB, Guthridge JM, Grossman JM, Cantor RM, Song YW, Bae SC, Chen S, Hahn BH, Lau YL, Tsao BP. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki A, Furukawa H, Kondo Y, Ito S, Hayashi T, Kusaoi M, Matsumoto I, Tohma S, Takasaki Y, Hashimoto H, Sumida T, Tsuchiya N. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res Ther. 2011;13:R41. doi: 10.1186/ar3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Ortiz H, Velazquez-Cruz R, Espinosa-Rosales F, Jimenez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69:1861–1865. doi: 10.1136/ard.2009.124313. [DOI] [PubMed] [Google Scholar]
- 19.Komatsuda A, Wakui H, Iwamoto K, Ozawa M, Togashi M, Masai R, Maki N, Hatakeyama T, Sawada K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Immunol. 2008;152:482–487. doi: 10.1111/j.1365-2249.2008.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigurdsson S, Nordmark G, Garnier S, Grundberg E, Kwan T, Nilsson O, Eloranta ML, Gunnarsson I, Svenungsson E, Sturfelt G, Bengtsson AA, Jonsen A, Truedsson L, Rantapaa-Dahlqvist S, Eriksson C, Alm G, Goring HH, Pastinen T, Syvanen AC, Ronnblom L. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Hum Mol Genet. 2008;17:2868–2876. doi: 10.1093/hmg/ddn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly JA, Kelley JM, Kaufman KM, Kilpatrick J, Bruner GR, Merrill JT, James JA, Frank SG, Reams E, Brown EE, Gibson AW, Marion MC, Langefeld CD, Li QZ, Karp DR, Wakeland EK, Petri M, Ramsey-Goldman R, Reveille JD, Vila LM, Alarcon GS, Kimberly RP, Harley JB, Edberg JC. Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes Immun. 2008;9:187–194. doi: 10.1038/gene.2008.4. [DOI] [PubMed] [Google Scholar]
- 23.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48:1080–1092. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]
- 31.Fairhurst AM, Mathian A, Connolly JE, Wang A, Gray HF, George TA, Boudreaux CD, Zhou XJ, Li QZ, Koutouzov S, Banchereau J, Wakeland EK. Systemic IFN-alpha drives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008;38:1948–1960. doi: 10.1002/eji.200837925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian S, Yim YS, Liu K, Tus K, Zhou XJ, Wakeland EK. Epistatic suppression of systemic lupus erythematosus: fine mapping of Sles1 to less than 1 mb. J Immunol. 2005;175:1062–1072. doi: 10.4049/jimmunol.175.2.1062. [DOI] [PubMed] [Google Scholar]
- 33.Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, Cibotti R, Coyle A, Connolly JE, Wakeland EK, Mohan C. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009;183:6831–6838. doi: 10.4049/jimmunol.0900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amano H, Amano E, Moll T, Marinkovic D, Ibnou-Zekri N, Martinez-Soria E, Semac I, Wirth T, Nitschke L, Izui S. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J Immunol. 2003;170:2293–2301. doi: 10.4049/jimmunol.170.5.2293. [DOI] [PubMed] [Google Scholar]
- 35.Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, Hirohashi T, Lei J, Yang M, Markmann JF, Deng S. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10:796–801. doi: 10.1111/j.1600-6143.2010.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birling MC, Gofflot F, Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol. 2009;561:245–263. doi: 10.1007/978-1-60327-019-9_16. [DOI] [PubMed] [Google Scholar]
- 37.Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47–54. doi: 10.1080/08916930400022715. [DOI] [PubMed] [Google Scholar]
- 38.Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 39.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, Reith W, Mohan C, Kotzin BL, Izui S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenert P, Yasuda K, Busconi L, Nelson P, Fleenor C, Ratnabalasuriar RS, Nagy PL, Ashman RF, Rifkin IR, Marshak-Rothstein A. DNA-like class R inhibitory oligonucleotides (INH-ODNs) preferentially block autoantigen-induced B-cell and dendritic cell activation in vitro and autoantibody production in lupus-prone MRL-Fas(lpr/lpr) mice in vivo. Arthritis Res Ther. 2009;11:R79. doi: 10.1186/ar2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patole PS, Pawar RD, Lichtnekert J, Lech M, Kulkarni OP, Ramanjaneyulu A, Segerer S, Anders HJ. Coactivation of Toll-like receptor-3 and -7 in immune complex glomerulonephritis. J Autoimmun. 2007;29:52–59. doi: 10.1016/j.jaut.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 45.Thibault DL, Chu AD, Graham K, Balboni I, Lee LY, Kohlmoos C, Landrigan A, Higgins JP, Tibshirani R, Utz PJ. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008 doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Keszei M, Detre C, Rietdijk ST, Munoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, Wu YY, Shin DM, Sancho J, Zubiaur M, Morse HC, 3rd, Morel L, Engel P, Wang N, Terhorst C. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–822. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 49.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JK, Boles KS, Mathew PA. Molecular and functional characterization of a CS1 (CRACC) splice variant expressed in human NK cells that does not contain immunoreceptor tyrosine-based switch motifs. Eur J Immunol. 2004;34:2791–2799. doi: 10.1002/eji.200424917. [DOI] [PubMed] [Google Scholar]
- 51.Kumaresan PR, Mathew PA. Structure of the human natural killer cell receptor 2B4 gene and identification of a novel alternative transcript. Immunogenetics. 2000;51:987–992. doi: 10.1007/s002510000237. [DOI] [PubMed] [Google Scholar]
- 52.Kim JR, Mathew SO, Patel RK, Pertusi RM, Mathew PA. Altered expression of signalling lymphocyte activation molecule (SLAM) family receptors CS1 (CD319) and 2B4 (CD244) in patients with systemic lupus erythematosus. Clin Exp Immunol. 2010;160:348–358. doi: 10.1111/j.1365-2249.2010.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatterjee M, Kis-Toth K, Thai TH, Terhorst C, Tsokos GC. SLAMF6-driven co-stimulation of human peripheral T cells is defective in SLE T cells. Autoimmunity. 2011;44:211–218. doi: 10.3109/08916934.2010.530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunninghame Graham DS, Vyse TJ, Fortin PR, Montpetit A, Cai YC, Lim S, McKenzie T, Farwell L, Rhodes B, Chad L, Hudson TJ, Sharpe A, Terhorst C, Greenwood CM, Wither J, Rioux JD. Association of LY9 in UK and Canadian SLE families. Genes Immun. 2008;9:93–102. doi: 10.1038/sj.gene.6364453. [DOI] [PubMed] [Google Scholar]
- 55.Cannons JL, Tangye SG, Schwartzberg PL. SLAM Family Receptors and SAP Adaptors in Immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 56.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


