Abstract
This study assessed the effects of stability constraints of a voluntary task on postural responses to an external perturbation in subjects with Parkinson's disease (PD) and healthy elderly participants. Eleven PD subjects and twelve control subjects were perturbed with backward surface translations while standing and performing two versions of a voluntary task: holding a tray with a cylinder placed with the flat side down [low constraint (LC)] or with the rolling, round side down [high constraint (HC)]. Participants performed alternating blocks of LC and HC trials. PD participants accomplished the voluntary task as well as control subjects, showing slower tray velocity in the HC condition compared with the LC condition. However, the latency of postural responses was longer in the HC condition only for control subjects. Control subjects presented different patterns of hip-shoulder coordination as a function of task constraint, whereas PD subjects had a relatively invariant pattern. Initiating the experiment with the HC task led to 1) decreased postural stability in PD subjects only and 2) reduced peak hip flexion in control subjects only. These results suggest that PD impairs the capacity to adapt postural responses to constraints imposed by a voluntary task.
Keywords: postural control, postural set, initial condition
postural responses to unexpected stance perturbations are considered to be automatically controlled. Automatic movements are triggered by external cues and carried by polysynaptic pathways within the spinal cord and brain stem; they are of shorter latency than voluntary movements and are hard to suppress at will (Prochazka et al. 2000). Nevertheless, recent studies have found that automatic postural responses can be influenced by cortical processing associated with learning, prior experience, and initial postural conditions (for a review, see Jacobs and Horak 2007). In addition, we have recently shown that postural responses can be modulated by constraints set by a concomitant voluntary task (de Lima et al. 2010).
Our recent findings support the notion that a higher order level of motor control, “postural set” (as proposed by Prochazka 1989), modulates automatic postural responses based on context set by the manual task. We tested the effects of a manual, voluntary task constraint on postural responses to unexpected motion of the support surface by requiring stabilization of an unstable (rolling) cylinder on a tray (de Lima et al. 2010). The results showed that the constraint imposed by the manual task led to early postural muscle activation and reduced joint motion in the legs. This adaptation of postural responses was paralleled by decreased tray displacement, indicating a two-way interaction between posture control and voluntary control of the arms (for further evidence of a postural and voluntary task interaction, see also Chen et al. 2011; Morioka et al. 2005; Stoffregen et al. 1999; Stoffregen et al. 2000; Wulf et al. 2004; Wulf et al. 2003; Yu et al. 2010).
Previous studies in our laboratory have suggested that the modulation of postural responses based on context is impaired by Parkinson's disease (PD) (Chong et al. 1999b, 1999c, 2000; Horak et al. 1999; Rocchi et al. 2002). Individuals with PD do not promptly adjust the magnitude of their postural responses based on their initial posture (Horak et al. 1999; Rocchi et al. 2006), direction of perturbation (Chong et al. 1999a), instructional set (Chong et al. 1999a, 1999b, 2000), or prior experience (Chong et al. 1999a, 2000; De Nunzio et al. 2007). Of particular interest for the present study, individuals with PD have been found to be impaired at integrating control of postural stability with the goal of voluntary tasks such as step initiation (Burleigh-Jacobs et al. 1997; Gantchev et al. 1996; Rocchi et al. 2006), trunk bending (Alexandrov et al. 1998), and pointing to a target (Tagliabue et al. 2009). Tagliabue et al. (2009) showed that as the stability constraint imposed by a pointing task increases, functional integration between the voluntary and postural tasks decreases in individuals with PD. The high stability constraint condition led to poorer control of the center of body mass (CoM) in subjects with PD, whereas performance on the voluntary task was unaffected (Tagliabue et al. 2009).
Although the integration between postural and voluntary task control has already been investigated in people with PD (Alexandrov et al. 1998; Burleigh-Jacobs et al. 1997; Gantchev et al. 1996; Rocchi et al. 2006; Tagliabue et al. 2009), questions remain about how postural responses to an external perturbation adapt to changes in the voluntary task constraint. Previous studies focused on the integration of self-initiated postural movements rather than externally triggered postural responses. As a neurophysiological question, our experiment is important for understanding interactions between different levels of movement control (automatic and voluntary). In addition, our study addresses an important question related to the flexibility of postural reactive responses in PD participants associated with voluntary task constraints. Recent studies have highlighted the higher fall incidence in patients with PD under conditions in which a voluntary task is accomplished during unexpected external perturbation (Bloem et al. 2001; Grimbergen et al. 2004; Smithson et al. 1998; Willemsen et al. 2000), but the neurophysiological basis for this difficulty is not understood.
We assessed postural responses to backward surface translations while a manual, voluntary task was performed in the context of either a high (HC) or low stability constraint (LC). Postural responses were assessed through the latency of muscular activation and CoM stability. The onset of muscle activation was used as an indicator of the modulation of postural responses by central set. Since the onset of muscular activation occurs at an early phase of postural control, it indicates whether adjustments have been made in a feedforward manner based on the initial constraints of the voluntary task. We predicted increased latencies of postural muscle responses under the HC voluntary task in healthy elderly participants, due to cortical influences on postural control, and no change of postural response latencies in PD participants based on task constraint. As a consequence, we expect PD participants to show decrements in postural stability under the HC voluntary task. Kinematics of the lower limbs were used to assess adjustments in postural strategy in later phases of postural control. Adaptability from previous experience was assessed through repetitive perturbations under the same task constraint, whereas adaptability to context change was assessed through transitions between task constraints.
METHODS
Subjects.
Eleven subjects with idiopathic PD (5 men and 6 women, mean age: 67.2 ± 5.5 yr) and twelve age-matched healthy control subjects (6 men and 6 women, mean age: 68.0 ± 5.0 yr) participated in this study. The diagnosis of idiopathic PD was made by a movement disorders neurologist. The mean illness duration of PD participants was 8.3 yr (±3.3 yr, range: 5–14 yr). The mean score on the unified PD rating scale (Fahn et al. 1987) was 27.5 (±9.4, range: 17.5–46), and the mean Hoehn and Yahr stage (Hoehn and Yahr 1967) was 2.7 (±0.5, range: 2–3). General cognitive function was assessed for both groups with the Montreal Cognitive Assessment (Nasreddine et al. 2005): means were 27.4 ± 1.8 for PD subjects and 27.9 ± 1.5 for control subjects. One participant in the PD group was excluded due to a low score (20) on the cognitive assessment. All participants were tested without pain or other conditions limiting independent stance, and all had vision corrected to 20/40 or better.
Participants with PD were tested while taking their anti-PD medication (“on” levodopa state) for two reasons. First, patients with PD are likely to show tremor and bradykinesia of the upper limbs in the “off” medication state, which could impair their voluntary performance of balancing the cylinder on the tray. Second, unlike the effects on voluntary control, the ability to change postural responses based on the context has been shown not to improve with medication in parkinsonian patients (Bloem et al. 1996; Horak et al. 1996). All participants provided informed consent, and experimental procedures were approved by the Institutional Review Board of Oregon Health and Science University.
Apparatus and task.
Participants performed a dual task of upright standing on a movable force plate while holding a wooden tray (30 × 40 cm, mass: 432 g) with a cylinder (diameter: 9.5 cm, height: 5 cm, mass: 124 g) on it. Their upper arms were to be maintained parallel to the trunk, and elbows were bent at 90° (Fig. 1). The aim of the postural component of the task was to maintain a stable upright stance while the force plate was unpredictably moved 11 cm backward, with mean velocity of 16 cm/s and a peak acceleration of 12 cm/s2. The aim of the manual component of the task was to maintain a cylinder stably at the middle of the tray during force plate motion.
Fig. 1.
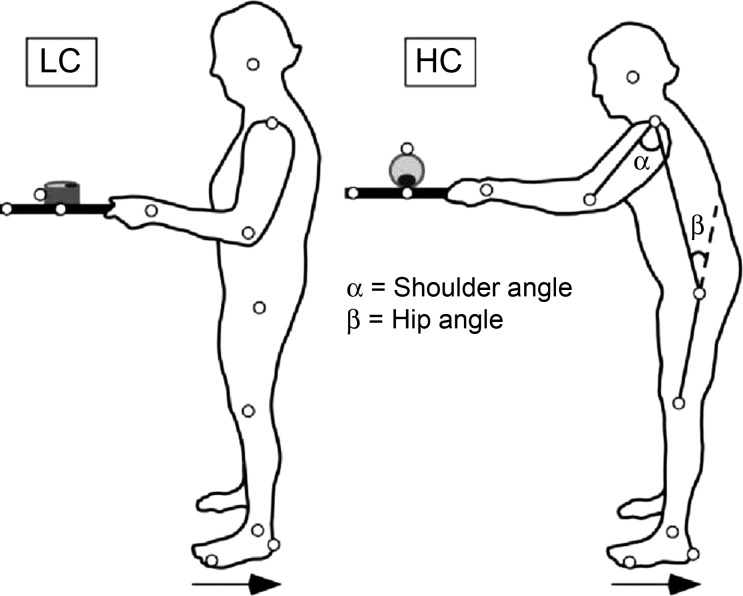
Experimental setup. Schematic representation of the experimental setup for the low constraint (LC) and high constraint (HC) conditions. In the picture on the right, the subject is bending forward to indicate the hip and shoulder angles. Small circles represent kinematic markers. Horizontal arrows indicate the direction of surface translation.
Experimental design.
Both PD and control groups were evaluated in two conditions: LC and HC. In the LC condition, the cylinder lay on its flat side, so that slow horizontal movements of the tray would not lead to a slide of the cylinder on the tray (Fig. 1, left). In the HC condition, the cylinder was placed on its round surface, so that it was free to roll in the anterior-posterior direction, limited in motion to around 90° by a weight attached to its bottom (Fig. 1, right). The heavier the weight, the easier it is to prevent cylinder motion. To find the most challenging situation within individual limitations, subjects were asked to try to prevent the cylinder from moving while holding the tray, starting with a weight of 10 g (most challenging). The weight was increased by steps of 10 g up to the weight at which the participant could equilibrate the tray with the cylinder on it in three consecutive periods of 10 s. This procedure was done to achieve approximately the same challenge in the HC condition for all participants.
The experimental protocol consisted of 3 blocks of 7 trials in each task constraint level, for a total of 42 trials. LC and HC trial blocks were alternated, with the first constraint condition counterbalanced across subjects. Repetition of trials in the same task constraint allowed the assessment of adaptability with practice, whereas the alternation of different conditions between blocks allowed the assessment of adaptability to a change.
Procedures.
Participants wore a harness attached to the laboratory ceiling, and an assistant stood near the participant to assist in cases of loss of balance. The following instructions were given to participants:
Stand straight with your upper arms parallel to your body and your elbows at 90° and look at the cylinder. The platform will move backward. We will not tell you to get ready, so you will not know exactly when the perturbation will start. Please, keep looking at the cylinder and try to avoid any movement of the cylinder. Also, try to do not step during the perturbation.
Additionally, participants were asked to maintain the same balance of pressure on the ground beneath their feet, which was monitored on an oscilloscope by the experimenter immediately before triggering each force plate displacement. Participants were asked to place their feet in a comfortable position (mean of 13.4 ± 2.6 cm apart). Foot positions were marked with tape to make sure the same position was maintained throughout the experiment. On finishing each trial, participants gave the tray back to experimenter and waited on the platform during its automatic (slow) repositioning. When the initial position was achieved, the tray was given back to the participant, and the cylinder was repositioned for the next trial. Rest breaks lasting 1 min were given between blocks. After three blocks, a longer rest was allowed, during which participants sat for 15 min. No refamiliarization was provided between blocks.
Data collection and analysis.
Electromyographic (EMG) activity was measured from the medial heads of the right gastrocnemius (GAS) and tibialis anterior (TA) muscles using 2.5-cm surface electrodes attached 2–4 cm apart. The skin was shaved, cleaned, and scrubbed before application of the electrodes. Online, EMG signals were amplified at a gain of 2,000–10,000, band-pass filtered from 15 to 2,000 Hz, and sampled at 480 Hz. Offline, they were rectified and low-pass filtered at 10 Hz. The center of pressure (CoP) position over time was sampled from the force plate with a sample frequency of 480 Hz and filtered at 10 Hz.
A motion analysis system with eight Falcon video cameras (Motion Analysis, Santa Rosa, CA) provided three-dimensional coordinates of the reflective markers on the body, tray, cylinder, and movable surface, sampled at 60 Hz. Reflective markers were placed bilaterally at the fifth metatarsophalangeal joints, heels, lateral malleoli, lateral knee joint centers, greater trochanters, acromions, lateral epicondyles, wrist joint centers, and jaw joint centers. Additional markers were attached at the moving platform and the steady floor as reference to define the platform movement. Twenty-six anthropometric measures of head, limb, and trunk segments (length, width, and perimeter) were collected from each participant (Chandler et al. 1975). These anthropometric measures, together with the motion analysis data, were used to estimate the sagittal plane CoM.
Measurements.
Peak tray velocity was used to compare performance of the voluntary task between LC and HC conditions. Low peak tray velocity was interpreted as better performance on the voluntary task, since this variable was positively correlated (r = 0.68) with the total path of the cylinder marker in the HC trials.
Postural reactive responses were quantified by the latency of GAS and TA muscle activation. The onsets of muscle bursts were identified as the first sustained EMG activity (>25 ms) greater than 2 SDs above the baseline.
Postural equilibrium was quantified by two variables: automatic postural response stability (APR) and magnitude of the first CoM peak. The APR was calculated by integrating the difference (normalized to participant height) between the CoP and CoM time series over 150 ms, starting 50 ms after the onset of the first GAS burst (agonistic muscle). The APR provides a measure of the margin of stability, showing the force generated by the postural response to arrest the falling CoM (Winter et al. 1998), with high values being interpreted as increased postural stability.
Interaction between voluntary and postural tasks was characterized by coordination between the shoulder and hip joints. Interjoint coordination was assessed by fitting an ellipse embracing 95% of the values of the angle-angle plot for the hip and shoulder joint displacement from the perturbation onset until the end of the trial. First, we calculated the ratios of the short axis versus the long axis of each ellipse. This variable was used to detect different modes of coordination between the joints in two ways. Second, we conducted regression analysis to find the slopes of the fitted ellipses. Higher slopes indicate relatively larger participation of the hip joint, and lower values indicate relatively larger participation of the shoulder joint.
The relative phase between shoulder and hip joints was calculated with the cross-spectral density method (Duarte 2002; Bennett et al. 2005). Values of the relative phase ranged from 0 to 180°. Values around 0° correspond to in-phase coordination, values around 180° correspond to antiphase coordination, and values that are not close to 0 or 180° indicate out-of-phase coordination.
Statistical analysis.
Adaptation across blocks was measured for all variables, comparing the averages obtained in the first block with the last block of trials. Preliminarily, data were analyzed through four-way 2 (group) × 2 (sequence: low-high × high-low) × 2 (constraint: HC × LC) × 2 (block: first × last) ANOVAs with repeated measures on the last two factors. The results showed an absence of a block effect except for shoulder and peak hip flexion. For this reason, the other variables were analyzed through three-way 2 (group) × 2 (sequence) × 2 (constraint) ANOVAs with repeated measures on the last factor. The level of significance was set at 0.05. Post hoc comparisons were made through the Tukey test. Only significant effects are reported.
RESULTS
Tray velocity.
Figure 2 shows average tray velocity curves for control (A and B) and PD (E and F) participants. Tray velocity was not different between groups. The main effect of constraint was significant [F(1,20) = 63.08, P = 0.01] due to reduced tray velocity in the HC condition (mean: 16.36 ± 0.86 cm/s) compared with the LC condition (mean: 12.10 ± 0.72 cm/s). In Fig. 3A, group average values were compared as a function of task constraint and sequence.
Fig. 2.
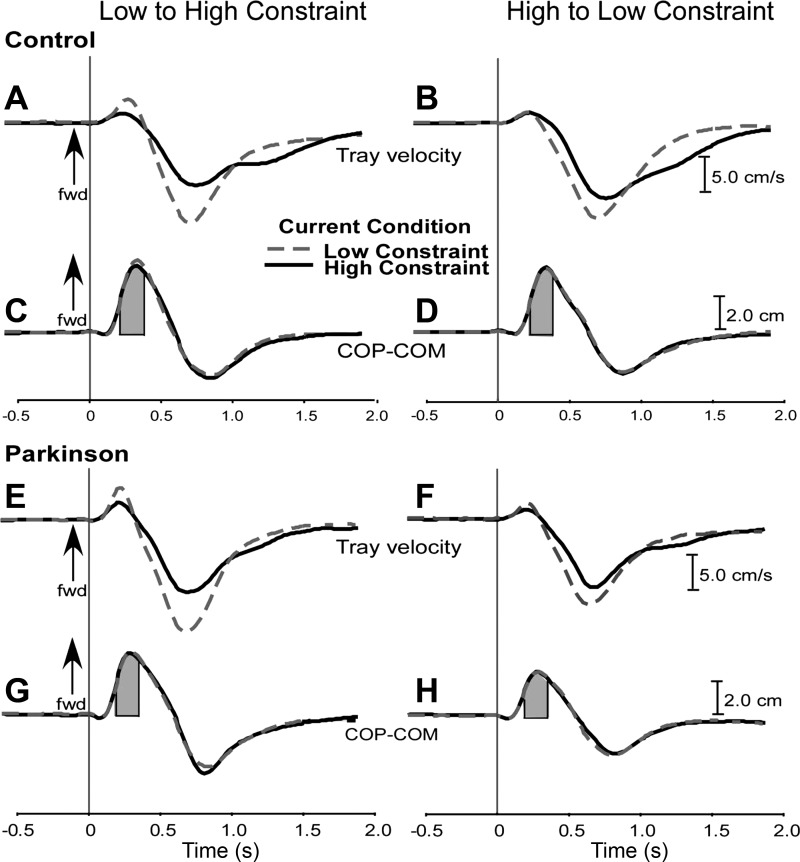
Grand means of tray velocity and difference between center of pressure (COP) and center of body mass (COM), representing postural stability for control participants (A–D) and subjects with Parkinson's disease (PD; E–H). A, C, E, and G: means for participants performing the low-high (L-H) sequence. B, D, F, and H: means for participants performing the high-low (H-L) sequence. Dashed lines represent the LC current condition and solid lines denote the HC current condition. Arrows indicate forward (fwd) direction. Shaded areas represent the region below the COP-COM curve taken to calculate the margin of stability.
Fig. 3.
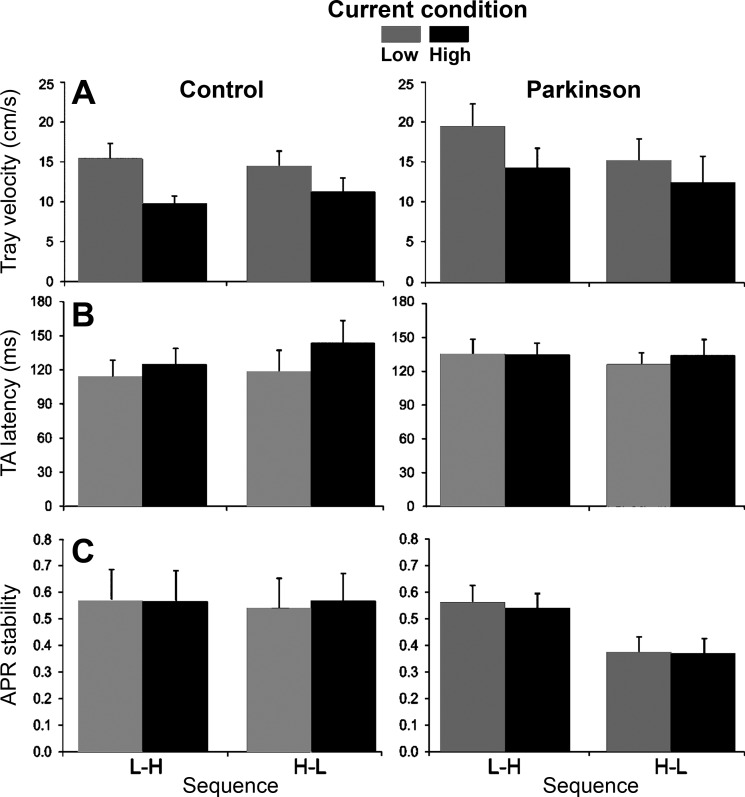
Postural and voluntary performance. A–C: averages and SEs across subjects for tray velocity (A), tibialis anterior (TA) latency (B), and margin of automatic postural response (APR) stability (C) for current constraint and constraint sequence for PD and control groups (high values are interpreted as increased postural stability).
Postural muscle latency.
TA muscle latency showed a significant main effect of constraint [F(1,20) = 6.21, P = 0.02], with later activation in the HC condition (mean: 134.22 ± 7.02 ms) than in the LC condition (mean: 123.47 ± 6.82 ms). Although analysis did not indicate a significant group by constraint interaction, the results shown in Fig. 3B suggests that task constraint affected the control group more than the PD group. For this reason, we performed two-way ANOVA with two groups and two constraints. Analysis showed a significant interaction [F(1,22) = 6.68, P = 0.02] due to higher TA latency in the HC condition than in the LC condition for the control group but not for the PD group. GAS muscle latency was not affected by constraint or order.
Postural stability.
APR CoP-CoM stability curves are shown in Fig. 2 for control (C and D) and PD (G and H) participants. Stability was significantly affected by group [F(1,20) = 8.60, P = 0.008], sequence [F(1,20) = 4.39, P = 0.04] and group by sequence interaction [F(1,20) = 5.40, P = 0.03]. APR stability was lower in the high-low sequence than in the low-high sequence for the PD group, whereas no significant difference was detected for the control group (Fig. 3C).
Hip-shoulder coordination.
Representative examples of shoulder-hip joint angle plots are shown in Fig. 4A. Analysis of the ellipse slope indicated a significant main effect of constraint [F(1,20) = 13.60, P = 0.001] and a group by sequence interaction [F(1,20) = 5.46, P = 0.03]. The high-low sequence was associated with a smaller slope than the low-high sequence in control participants, whereas no significant difference was found between sequences for PD participants (Fig. 4B).
Fig. 4.
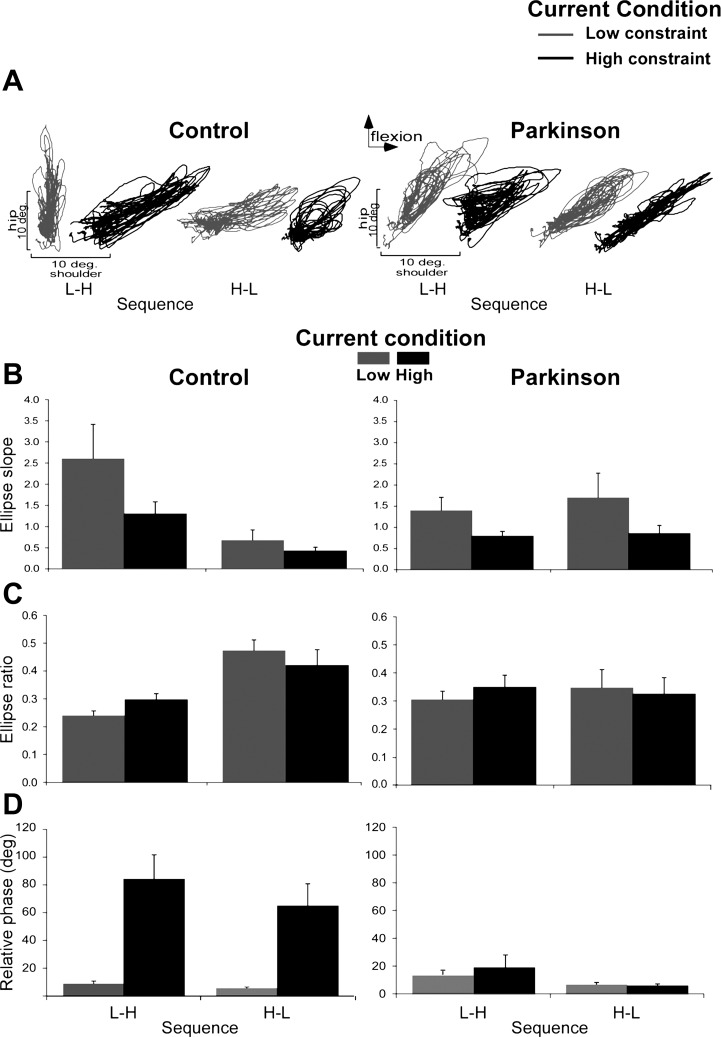
Interjoint coordination for PD and control participants as a function of current constraint and sequence of constraints. A: representative examples of shoulder and hip coordination of 21 trials for control subject 4 (L-H sequence) and subject 5 (H-L sequence) as well as for PD subject 6 (L-H sequence) and subject 4 (H-L sequence). B: means and SEs of the ellipse slope. C: means and SEs of the ratio of ellipse. D: relative phases between the shoulder and hip (high values are interpreted as more out-of-phase coordination).
Interjoint coordination of hip and shoulder displacement during the task was objectified with ellipse ratios. A significant main effect of sequence [F(1,20) = 5.60, P = 0.03] and a group by sequence interaction [F(1,20) = 4.55, P = 0.04] were found for ellipse ratios. Higher values for the high-low sequence than the low-high sequence for the control group were found, whereas no significant difference was found between sequences for PD participants (Fig. 4C).
The relative phase between the shoulder and the hip is shown in Fig. 4D. Analysis indicated a significant main effect of constraint [F(1,20) = 17.14, P < 0.01] and a group by constraint interaction [F(1,20) = 12.33, P < 0.01]. The control group showed out-of-phase coordination in the HC condition (mean: 74.20 ± 15.90°) compared with in-phase coordination in the LC condition (mean: 7.70 ± 1.34°), whereas the relative phase in the PD group did not significantly differ according to voluntary task constraint level (LC mean: 10.80 ± 2.23° and HC mean: 13.53 ± 6.61°).
Adaptation across blocks.
Figure 5A shows adaptation curves of peak shoulder flexion as a function of sequence of task constraints. Shoulder flexion adapted over time, as shown by a significant main effect of block [F(1,40) = 32.26, P < 0.01]. Post hoc comparisons indicated smaller values of peak shoulder flexion in the last block than in the first block. The difference between the groups of the rate of adaptation of shoulder peak flexion was more subtle than for peak hip flexion, with control subjects reducing values across time by 39% and PD subjects reducing values by 31%. Although analysis did not indicate a significant group by constraint interaction, the results shown in Fig. 5A suggest that task constraint had a stronger effect on the control group, which showed larger shoulder flexion in HC conditions than in LC conditions.
Fig. 5.
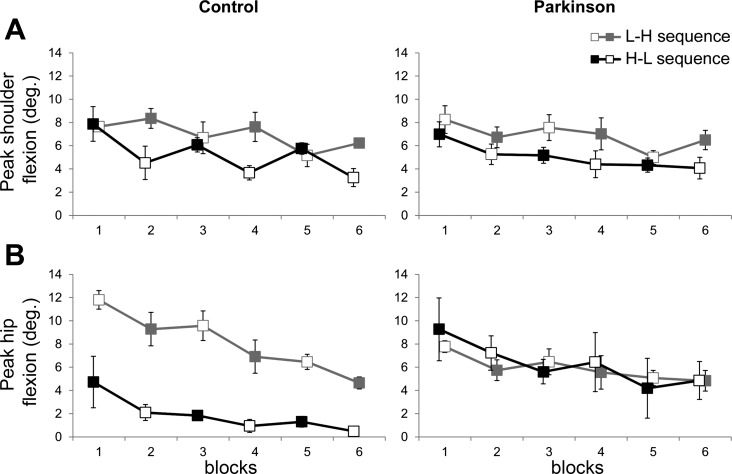
Peak joint flexion for PD and control participants as a function of current constraint and sequence of constraints. A: shoulder flexion. B: hip flexion. Shaded squares represent HC conditions, and open squares represent LC conditions.
Figure 5B shows adaptation curves of peak hip flexion as a function of sequence of task constraints. Analysis indicated significant main effect of group [F(1,40) = 5.82, P = 0.02], sequence [F(1,40) = 10.84, P < 0.01], and block [F(1,40) = 32.07, P < 0.01], in addition to significant group by sequence [F(1,40) = 18.15, P = 0.002] and group by block interactions [F(1,40) = 10.29, P < 0.01]. The high-low sequence resulted in smaller hip flexion than the low-high sequence for the control group but not for the PD group. Post hoc comparisons for the effect of block indicated smaller hip flexion in the last block compared with the first block. Control participants decreased hip flexion across time by 54%, whereas PD participants decreased hip flexion by only 18% from the first trial to the last trial.
DISCUSSION
This study examined the effects of stability constraint imposed by a voluntary task (balancing an object on a tray) on postural responses in individuals with PD compared with age-matched healthy participants. The results showed that the stability constraint from the voluntary task affected performance of both voluntary and postural tasks in the two groups, but less effectively in the PD group. In the voluntary task, both groups showed decreased tray velocity and increased participation of shoulder movements in response to perturbations during the HC task, as seen by the change in the shoulder-hip relationship. In contrast, the latency of postural muscle activation increased in HC conditions in control subjects but not in PD subjects. These results suggest that PD leads to a decreased capacity to adjust postural control for constraints imposed by a voluntary task.
Subjects with PD also showed a significant inability to flexibly change kinematic strategy based on constraints imposed by a voluntary task and by prior experience. The sequence of task presentation and varying stability constraint context led to different coordination modes between the hip and shoulder involved in the postural and voluntary goals in control subjects but not in PD subjects. In addition, the first presentation of the HC condition led to decreased hip motion in control subjects only, which persisted in trials throughout the series. That effect was not observed in participants with PD, who displayed similar hip motion across the series regardless of the first presentation of the LC or HC conditions of the voluntary tasks.
Voluntary task performance and its integration with postural responses.
PD and control participants achieved the voluntary task goal of minimizing cylinder motion by reducing tray velocity under the HC task. Kinematic analysis showed that both the PD and control groups reduced tray velocity by increasing participation of the shoulder in stabilizing the tray under the HC context. In contrast, previous studies in individuals with PD have shown poor voluntary task performance in challenging tasks, such as tasks imposing time pressure (Doan et al. 2006; Tunik et al. 2004) or high accuracy demand (Rand et al. 2000; Weiss et al. 1997). Our PD participants, however, kept an adequate performance in the voluntary task associated with decreased postural control. Considering that controls and PD participants did not differ in voluntary task performance, one could assume that it was possible to achieve cylinder stability by adopting either the kinematic strategy showed by control subjects or the one showed by PD subjects. However, PD subjects performing the high-low sequence suffered decreased postural stability, suggesting that they used a less than optimal postural strategy.
The finding of appropriate voluntary movement adjustments in PD subjects might be surprising. A possible explanation is that motion of the arms to stabilize the cylinder may have been triggered by sensory stimuli from vestibular and proprioceptive receptors signaling loss of stable balance from the abrupt platform motion. Previous studies have shown that PD participants use external cues to trigger difficult voluntary movements (Darmon et al. 1999; Gueye et al. 1998; Kelly et al. 2002). In this regard, additional sensory information derived from postural perturbation might have been used to facilitate a voluntary task under different constraints. An alternative explanation is that voluntary performance in PD subjects was ameliorated by taking levodopa (Haslinger et al. 2001; Kelly et al. 2002; Rascol et al. 1994). Our participants were under the effect of levodopa, which has been shown to be of greater benefit for voluntary behavior than for postural control (Bloem et al. 1996; Horak et al. 1996; Horak et al. 1992). From these results, it is apparent that voluntary control during postural perturbations is not disturbed in individuals with PD in the on levodopa state.
In contrast to voluntary control subjects, PD subjects were found here to have impaired ability to adapt postural responses from voluntary task constraints. Control but not PD subjects delayed the latency of their TA activation in response to postural perturbation based on the voluntary task constraint. That behavior supports our hypothesis of modulation of postural responses from higher-order, feedforward, central set in control subjects but not PD subjects (Jacobs and Horak 2007). These results are consistent with previous findings indicating that individuals with PD have a reduced ability to immediately adjust postural responses to context based on feedforward control (Chong et al. 1999a, 1999b, 2000; Haslinger et al. 2001; Horak et al. 1992). Our results thus support a disruption of the interaction between voluntary actions and automatic postural responses in individuals with PD, consistent with a loss of the “posture first” strategy seen in healthy subjects (Bloem et al. 2006).
Influence of the constraint sequence.
We found that the sequence of task constraints affected kinematics (peak hip flexion) and coordination (shoulder-hip ratio) of postural and voluntary task control in the control group. Kinematic strategies, on the other hand, were not affected by current task constraint. Our results showed that once a shoulder-hip coordination mode and hip strategy is selected by the central nervous system to accomplish both the postural and voluntary tasks, it is maintained across constraint conditions. Generalization of postural strategies has also been seen in situations in which people are exposed to discrete, unpredictable postural perturbations, like in a push or slip. In those situations, individuals generate motor responses that tend toward a default value corresponding to a medium-sized perturbation (Horak et al. 1989) or to a size appropriate to withstand the largest perturbation (Van Ooteghem et al. 2008, 2009, 2010). Previous studies have also shown that for sequences of similar voluntary actions, individuals often reuse a motor plan rather than generating a new one (Cohen and Rosenbaum 2004; Rosenbaum et al. 2001). It has been suggested that this strategy is more computationally efficient (even if less biomechanically efficient) and might be used in postural control.
Unlike control subjects, PD subjects were insensitive to sequence of task constraints. We presented evidence that PD participants were unable to adjust the shoulder-hip mode of coordination and hip strategy in accordance with the sequence of voluntary task constraint. This behavior highlights the reduced flexibility of PD individuals to generate appropriate motor responses under different contexts. This assertion is strengthened by the finding that in the PD group postural stability was lower in the high-low sequence than in the low-high sequence. Previous studies have shown that performance in challenging voluntary tasks leads to decreased postural stability in individuals with PD, whereas no influence on voluntary task performance was observed (Bloem et al. 2006; Marchese et al. 2003; Tagliabue et al. 2009). A possible explanation for that behavior is that, under challenging conditions, PD individuals prioritize the voluntary task over postural control (Bloem et al. 2006). It is plausible that individuals with PD allocated more neural resources to accomplish the voluntary task, leading to deteriorated postural control, whereas healthy individuals implement a more balanced distribution of resources between voluntary and manual tasks. Alternatively, the less effective postural control demonstrated by the PD group could have been caused by a loss of ability to flexibly adopt different modes of coordination and by reduced hip mobility.
Adaptation of postural kinematics.
In agreement with prior studies showing a gradual reduction of postural responses after repetitions of perturbation (Keshner et al. 1987; Timmann and Horak 1997; Van Ooteghem et al. 2009), our results showed that control subjects gradually decreased hip and shoulder flexion across blocks of trials. Decreasing the magnitude of postural responses across trials is thought to be a result of habituation, leading to increased energy efficiency (Horak and Nashner 1986; Keshner et al. 1987). Depending on the sequence, the control group showed different participation of the hip, starting at the very first block. Control participants who started with the HC condition used less hip flexion and adapted faster than those who started with the LC condition. In contrast to the sequence effect on hip flexion, control subjects modulated use of shoulder movements depending on the current constraint from the voluntary task. That shoulder behavior as a function of constraint condition is consistent with results from our relative phase analysis. Unlike PD subjects, control subjects adapted the coordination mode between the hip and shoulder to the voluntary task constraint: control subjects showed interjoint coordination closer to an in-phase mode of coordination in the LC condition and closer to an out-of-phase mode in the HC condition. Since each joint motion was affected by different factors (hip by prior experience and shoulder by current context), shoulder (voluntary movement) and hip joints (postural control) may be controlled by separated neural mechanisms (Torres et al. 2011).
Subjects with PD showed neither a sequence effect for the hip nor a constraint effect for the shoulder kinematics or for relative phase of the hip-shoulder motions. The same behavior of shoulder and peak hip flexion regardless of current constraint condition or task sequence in the PD group supports the notion of reduced flexibility in adapting postural responses based on a central set. Taken together, our findings indicate that healthy elderly subjects are able to modulate postural responses consistent with stability constraints imposed by a voluntary task, whereas PD leads to decreased adaptability of postural control to deal with voluntary task constraints. This decreased adaptability may be harmful to maintenance of the upright stance when unpredictable postural perturbations occur during the performance of voluntary tasks.
GRANTS
This work was supported by National Institute on Aging Grant R37-AG-006457 (to F.B. Horak) and Brazilian National Council for Scientific and Technological Development Grant 200321/2010-2 (to A. C. de Lima-Pardini).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C.d.L.-P., S.P., R.G.C., L.A.T., B.A.S., and F.B.H. conception and design of research; A.C.d.L.-P. and S.P. performed experiments; A.C.d.L.-P., S.P., R.G.C., and F.B.H. analyzed data; A.C.d.L.-P., S.P., R.G.C., L.A.T., B.A.S., and F.B.H. interpreted results of experiments; A.C.d.L.-P. and S.P. prepared figures; A.C.d.L.-P. drafted manuscript; A.C.d.L.-P., S.P., R.G.C., L.A.T., B.A.S., and F.B.H. edited and revised manuscript; A.C.d.L.-P., S.P., R.G.C., L.A.T., B.A.S., and F.B.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all the participants, Elizabeth Murdock for recruiting subjects, Marina Brito Silva for data collection assistance, Edward King for technical assistance, and Patty Carlson-Kuhta for general assistance.
REFERENCES
- Alexandrov A, Aurenty R, Massion J, Mesure S, Viallet F. Axial synergies in parkinsonian patients during voluntary trunk bending. Gait Posture 8: 124–135, 1998 [DOI] [PubMed] [Google Scholar]
- Bennett BC, Abel MF, Wolovick A, Franklin T, Allaire PE, Kerrigan DC. Center of mass movement and energy transfer during walking in children with cerebral palsy. Arch Phys Med Rehabil 86: 2189–2194, 2005 [DOI] [PubMed] [Google Scholar]
- Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord 11: 509–521, 1996 [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol 248: 950–958, 2001 [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson's disease. J Neurol Sci 248: 196–204, 2006 [DOI] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord 12: 206–215, 1997 [DOI] [PubMed] [Google Scholar]
- Chandler RF, Clauser CE, McConville JT, Reynolds HM, Young JW. Investigation of Inertial Properties of the Human Body. AMRL Technical Report. Ohio: Wright-Patterson Air Force Base, 1975 [Google Scholar]
- Chen FC, Tsai CL, Stoffregen TA, Wade MG. Postural responses to a suprapostural visual task among children with and without developmental coordination disorder. Res Dev Disabil 32: 1948–1956, 2011 [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Frank J, Kaye J. Sensory organization for balance: specific deficits in Alzheimer's but not in Parkinson's disease. J Gerontol A Biol Sci Med Sci 54: M122–128, 1999a [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Time-dependent influence of sensorimotor set on automatic responses in perturbed stance. Exp Brain Res 124: 513–519, 1999b [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson's disease impairs the ability to change set quickly. J Neurol Sci 175: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- Chong RK, Jones CL, Horak FB. Postural set for balance control is normal in Alzheimer's but not in Parkinson's disease. J Gerontol A Biol Sci Med Sci 54: M129–135, 1999c [DOI] [PubMed] [Google Scholar]
- Cohen RG, Rosenbaum DA. Where grasps are made reveals how grasps are planned: generation and recall of motor plans. Exp Brain Res 157: 486–495, 2004 [DOI] [PubMed] [Google Scholar]
- Darmon A, Azulay JP, Pouget J, Blin O. [Posture and gait modulation using sensory or attentional cues in Parkinson's disease. A possible approach to the mechanism of episodic freezing]. Rev Neurol (Paris) 155: 1047–1056, 1999 [PubMed] [Google Scholar]
- de Lima AC, de Azevedo Neto RM, Teixeira LA. On the functional integration between postural and supra-postural tasks on the basis of contextual cues and task constraint. Gait Posture 32: 615–618, 2010 [DOI] [PubMed] [Google Scholar]
- De Nunzio AM, Nardone A, Schieppati M. The control of equilibrium in Parkinson's disease patients: delayed adaptation of balancing strategy to shifts in sensory set during a dynamic task. Brain Res Bull 74: 258–270, 2007 [DOI] [PubMed] [Google Scholar]
- Doan J, Whishaw IQ, Pellis SM, Suchowersky O, Brown LA. Motor deficits in Parkinsonian reaching: dopa-sensitivity influenced by real-world task constraint. J Mot Behav 38: 45–59, 2006 [DOI] [PubMed] [Google Scholar]
- Duarte M. Relphase (online). http://demotu.org/software2/relphase.m [18 June 2012].
- Fahn S, Marsden M, Goldstein M, Calne DB. Recent Developments in Parkinson's Disease. New York: MacMilan, 1987 [Google Scholar]
- Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in Parkinsonian patients. Electroencephalogr Clin Neurophysiol 101: 110–120, 1996 [DOI] [PubMed] [Google Scholar]
- Grimbergen YA, Munneke M, Bloem BR. Falls in Parkinson's disease. Curr Opin Neurol 17: 405–415, 2004 [DOI] [PubMed] [Google Scholar]
- Gueye L, Viallet F, Legallet E, Trouche E. The use of advance information for motor preparation in Parkinson's disease: effects of cueing and compatibility between warning and imperative stimuli. Brain Cogn 38: 66–86, 1998 [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 124: 558–570, 2001 [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkisonism: onset, progression, and mortality. Neurology 17: 427–442, 1967 [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol 62: 841–853, 1989 [DOI] [PubMed] [Google Scholar]
- Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Exp Neurol 193: 504–521, 1999 [DOI] [PubMed] [Google Scholar]
- Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol 75: 2380–2396, 1996 [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55: 1369–1381, 1986 [DOI] [PubMed] [Google Scholar]
- Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci 111: 46–58, 1992 [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 114: 1339–1348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly VE, Hyngstrom AS, Rundle MM, Bastian AJ. Interaction of levodopa and cues on voluntary reaching in Parkinson's disease. Mov Disord 17: 38–44, 2002 [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Pfaltz CR. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res 69: 77–92, 1987 [DOI] [PubMed] [Google Scholar]
- Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov Disord 18: 652–658, 2003 [DOI] [PubMed] [Google Scholar]
- Morioka S, Hiyamizu M, Yagi F. The effects of an attentional demand tasks on standing posture control. J Physiol Anthropol Appl Human Sci 24: 215–219, 2005 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res 130: 417–432, 2000 [DOI] [PubMed] [Google Scholar]
- Rand MK, Stelmach GE, Bloedel JR. Movement accuracy constraints in Parkinson's disease patients. Neuropsychologia 38: 203–212, 2000 [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Fabre N, Senard JM, Montastruc JL, Celsis P, Marc-Vergnes JP, Rascol A. Normal activation of the supplementary motor area in patients with Parkinson's disease undergoing long-term treatment with levodopa. J Neurol Neurosurg Psychiatry 57: 567–571, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry 73: 267–274, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson's disease: influence of initial stance conditions. Neurosci Lett 406: 128–132, 2006 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Meulenbroek RG, Vaughan J. Planning reaching and grasping movements: theoretical premises and practical implications. Motor Control 5: 99–115, 2001 [DOI] [PubMed] [Google Scholar]
- Smithson F, Morris ME, Iansek R. Performance on clinical tests of balance in Parkinson's disease. Phys Ther 78: 577–592, 1998 [DOI] [PubMed] [Google Scholar]
- Stoffregen TA, Gorday KM, Sheng YY, Flynn SB. Perceiving affordances for another person's actions. J Exp Psychol Hum Percept Perform 25: 120–136, 1999 [DOI] [PubMed] [Google Scholar]
- Stoffregen TA, Hettinger LJ, Haas MW, Roe MM, Smart LJ. Postural instability and motion sickness in a fixed-based flight simulator. Hum Factors 42: 458–469, 2000 [DOI] [PubMed] [Google Scholar]
- Tagliabue M, Ferrigno G, Horak F. Effects of Parkinson's disease on proprioceptive control of posture and reaching while standing. Neuroscience 158: 1206–1214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Horak FB. Prediction and set-dependent scaling of early postural responses in cerebellar patients. Brain 120: 327–337, 1997 [DOI] [PubMed] [Google Scholar]
- Torres EB. Two classes of movements in motor control. Exp Brain Res 215: 269–283, 2011 [DOI] [PubMed] [Google Scholar]
- Tunik E, Adamovich SV, Poizner H, Feldman AG. Deficits in rapid adjustments of movements according to task constraints in Parkinson's disease. Mov Disord 19: 897–906, 2004 [DOI] [PubMed] [Google Scholar]
- Van Ooteghem K, Frank JS, Allard F, Buchanan JJ, Oates AR, Horak FB. Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res 187: 603–611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooteghem K, Frank JS, Allard F, Horak FB. Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res 204: 505–514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooteghem K, Frank JS, Horak FB. Practice-related improvements in posture control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp Brain Res 199: 185–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P, Stelmach GE, Hefter H. Programming of a movement sequence in Parkinson's disease. Brain 120: 91–102, 1997 [DOI] [PubMed] [Google Scholar]
- Willemsen MD, Grimbergen YA, Slabbekoorn M, Bloem BR. [Falling in Parkinson disease: more often due to postural instability than to environmental factors]. Ned Tijdschr Geneeskd 144: 2309–2314, 2000 [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol 80: 1211–1221, 1998 [DOI] [PubMed] [Google Scholar]
- Wulf G, Mercer J, McNevin N, Guadagnoli MA. Reciprocal influences of attentional focus on postural and suprapostural task performance. J Mot Behav 36: 189–199, 2004 [DOI] [PubMed] [Google Scholar]
- Wulf G, Weigelt M, Poulter D, McNevin N. Attentional focus on suprapostural tasks affects balance learning. Q J Exp Psychol A 56: 1191–1211, 2003 [DOI] [PubMed] [Google Scholar]
- Yu Y, Yank JR, Katsumata Y, Villard S, Kennedy RS, Stoffregen TA. Visual vigilance performance and standing posture at sea. Aviat Space Environ Med 81: 375–382, 2010 [DOI] [PubMed] [Google Scholar]


