Abstract
There has been extensive debate as to whether muscle synergies in motor tasks reflect underlying neural organization or simply correlations in muscle activity that are imposed by the task. One possible means of distinguishing these two alternatives is through the analysis of variability in the electromyogram (EMG). Here, we simulated EMG in eight lower-limb muscles and introduced hypothetical neural coupling between specific muscle groups. Neural coupling was simulated by introducing correlations in the neural activation commands to different muscles (positive, negative, or zero coupling). When the entire EMG signal was used for analysis, the extracted synergies reflected only simultaneous muscle activity, regardless of the neural coupling between the muscles. On the other hand, examining the variability in the EMG after subtracting the ensemble average was successful in identifying the simulated neural coupling. The extracted synergies from these two methods were also different when we analyzed data from participants during treadmill walking. The results emphasize the importance of examining EMG variability to understand the neural basis of muscle synergies.
Keywords: synergy, simulation, principal component analysis, task constraints
the problem of how the nervous system coordinates the numerous degrees of freedom of the body has been a long-standing issue in motor control (Bernstein 1967). One hypothesis is that the nervous system simplifies control by functionally linking multiple muscles to act as a single unit (Turvey et al. 1978). These units have been termed muscle synergies (although see Latash et al. 2007 for a different interpretation), and different decomposition techniques have been used to uncover the structure of these synergies in a variety of tasks including posture and locomotion (Clark et al. 2010; d'Avella et al. 2003; Ivanenko et al. 2004; McKay and Ting 2008; Torres-Oviedo and Ting 2010).
However, there has been a debate over whether such muscle synergies reflect the intrinsic organization of the motor system or whether they simply reflect “task-related coupling,” i.e., correlations in muscle activity that are induced by the task (Buchanan et al. 1986; Tresch and Jarc 2009). In other words, task constraints limit the set of possible muscle activations that can be used to perform the task successfully. These task constraints combined with biomechanical constraints may mean that certain muscles have to be activated simultaneously to perform the task, which in turn may create the impression that these muscles are coupled (Kutch and Valero-Cuevas 2011). For example, in locomotion, both the knee extensors and the hip abductors have to be activated in the loading phase to achieve the task constraint of providing body support during stance (Perry 1992). However, this simultaneous activation does not necessarily imply that these muscles are neurally coupled. Therefore, an important issue in identifying muscle synergies is to distinguish simultaneous muscle activity that is dictated by the task from a neural control strategy of coupling muscle activity.
In this regard, a stronger test of the neural origins of muscle synergies is to examine the structure of motor variability (Kutch et al. 2008; Valero-Cuevas et al. 2009). The rationale here is that if a group of muscles are coupled together as part of a neural control strategy, then not only should the muscle activity be correlated, but also the variations in muscle activity in these muscles should be correlated. Using the same example, if the knee extensors and the hip abductors are positively coupled, then, apart from having simultaneous activity, a higher than average activation in the knee extensors at any given point in the stride must also be associated with a higher than average activation in the hip abductors instantaneously. However, to examine the structure in this variability, a detrending procedure is needed to remove the average muscle activity observed in each muscle because in the absence of detrending, the correlation in the average electromyogram (EMG) due to their simultaneous activation would dominate the correlations in the EMG variations.
To illustrate the difference between examining correlations in average EMG vs. correlations in the EMG variability, consider a system consisting of two muscles. Figure 1 shows four hypothetical situations that reflect different neurophysiological relationships between the two muscles. Consider the scenario where the two muscles have to be simultaneously activated to achieve the task. In this situation, the correlations between the actual muscle activities of these two muscles would be highly positive in all four cases regardless of the actual coupling between the two muscles. On the other hand, if the variations are analyzed (after detrending the average muscle activity computed across strides), the correlations would now accurately reflect the coupling (or the lack thereof) between the muscles. For example, in cases A and B, the variations in EMG between the two muscles would be highly positively correlated (i.e., whenever M1 has higher than average EMG amplitude, M2 would also have a higher than average EMG amplitude). In case C, the variations would be negatively correlated (higher than average EMG in M1 would be associated with lower than average EMG in M2), whereas in case D, the variations in EMG would show little or no correlation.
Fig. 1.
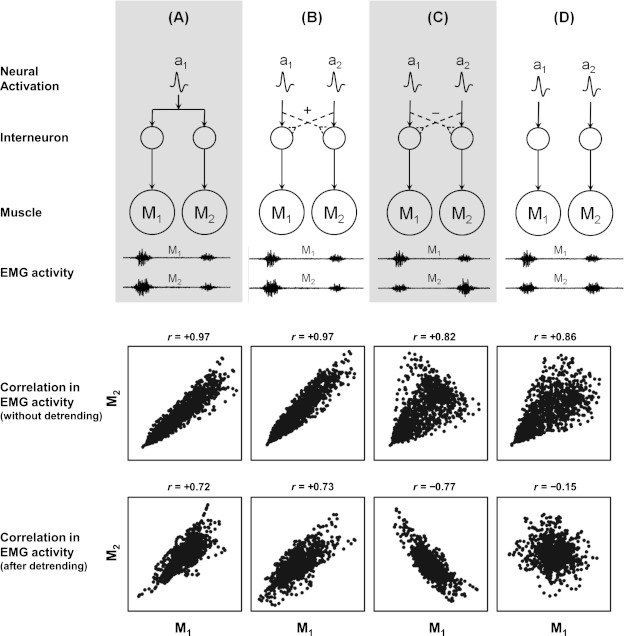
Top: schematic representation of 4 hypothetical cases representing different neurophysiological relationships in a simple system of 2 muscles (M1 and M2). In all cases, the neural activation commands (ai) are propagated to the individual muscles through a neural network that results in electromyogram (EMG) activity in both muscles. A: there is only a single neural activation for both muscles. B: there are 2 separate neural activations for each muscle, and these are positively coupled through an interneuronal network. C: there are 2 separate neural activations for each muscle, and these are negatively coupled through an interneuronal network. D: there are 2 separate neural activations for each muscle that are not coupled. Consider the scenario where the neural activations for both muscles are simultaneous. The hypothetical EMG activity for each case is shown. Even though all cases exhibit simultaneous firing of both muscles, the relationship between the EMG amplitudes varies. For example, in cases A and B, a bigger burst in M1 is associated with a bigger EMG burst in M2, whereas this is reversed in case C. In case D, there is no relationship between the EMG burst in M1 and that in M2. Bottom: scatterplots depicting the correlations between the 2 muscles when using the EMG without detrending compared with the EMG after detrending. Examining the EMG activity without detrending would result in positive correlations in all 4 cases because both muscles fire simultaneously. However, examining the EMG variability using the detrending procedure would accurately distinguish the positive coupling in A and B from the negative coupling in C and the lack of any coupling in D.
Here, we simulated EMG in eight lower-limb muscles during walking. We then introduced three types of neural coupling between different muscle groups: 1) positive coupling; 2) negative coupling; and 3) zero coupling. In all cases, we maintained the timing of the simulated muscle activity similar to normal human gait to examine whether different methods of extracting synergies can distinguish between simultaneous activity (i.e., the zero coupling condition) and coupled activity. We then compared the differences between two methods of extracting synergies: 1) using the entire muscle activity (full EMG); and 2) using the detrended muscle activity (detrended EMG) obtained after subtracting the ensemble average of all strides from each stride. We show that the conventional way of extracting synergies from the full EMG is not sensitive to the hypothetical neural coupling between muscles, whereas extracting synergies from the detrended EMG that examines the structure in the variability is able to distinguish the coupling conditions. Furthermore, we also analyzed data acquired from human subjects during treadmill walking and show that the muscle synergies extracted from these two methods are significantly different, indicating that some of the muscle synergies observed in gait are due to simultaneous muscle activity, which might be due to task constraints.
MATERIALS AND METHODS
Simulation of EMG During Gait
We first simulated muscle activity in 8 lower-limb muscles that have been used commonly in the study of gait: vastus medialis (VM), rectus femoris (RF), medial hamstrings (MH), lateral hamstrings (LH), tibialis anterior (TA), medial gastrocnemius (MG), soleus (SO), and gluteus medius (GM). The simulated EMG data consisted of 60 strides sampled at a frequency of 1,000 Hz with each stride lasting 1 s.
The EMG simulation was performed using the following algorithm (Fig. 2A):
Fig. 2.
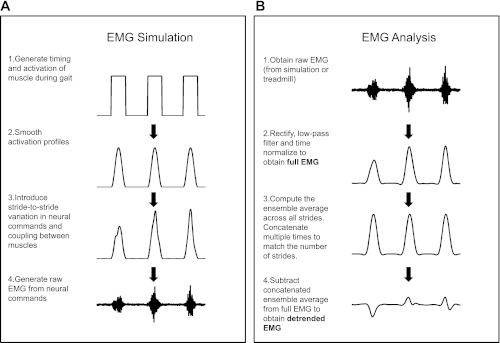
Schematic of EMG simulation (A) and EMG processing (B). The various steps involved in each stage are described in the figure.
We simulated neural activation commands, ai(t), for each of the eight muscles by using square waves to simulate the onset and offset of muscle activity during the gait cycle (on = 1, off = 0). The timing and amplitude of these commands were chosen to resemble closely previously published data (Hof et al. 2002). Since RF and TA typically exhibit a smaller second burst within each gait cycle, a second activation command was used for these two muscles. We also added timing errors in the onset and duration of each burst that resulted in variation in the timing of peak muscle activity from stride to stride (SD = 3%, range = 14%). Since all strides were of the same duration, this was equivalent to a timing error in data that is time-normalized. Finally, we smoothed out the abrupt change in activation due to the square wave by multiplying it with a Hanning window.
For each neural activation command, we introduced variability in the amplitude of the EMG (in addition to the timing error introduced above) by using a Gaussian white noise process ni(t) with a SD of 3. This noise was added to the muscle activation under the assumption of signal-dependent noise (Harris and Wolpert 1998; Schmidt et al. 1979):
| (1) |
This resulting activation was then passed through a zero-phase low-pass filter (2nd order Butterworth, 6 Hz) to simulate physiologically realistic activation patterns during gait. Any negative values of activation were set to zero. It is important to note that even though the SD is three times the magnitude of the signal, the effective amplitude of the noise was substantially reduced by the subsequent low-pass filtering and was selected to match previously observed stride-to-stride variability in EMG for these muscles (coefficient of variation ≈ 35%; Winter and Yack 1987). It is to be noted that even though the noise is added to all time points in the data, it effectively results in stride-to-stride variation because the noise is signal-dependent (i.e., the noise has a noticeable influence only when each activation command is on, which is only once per stride).
Once the neural activations (with stride-to-stride variations) for each muscle were determined, we simulated the raw EMG as follows (Kleissen and Zilvold 1994):
| (2) |
The carrier signal wi was white Gaussian noise with unit amplitude that was band-pass filtered in the range from 10 to 500 Hz. We also added baseline noise ηi that was white Gaussian noise with SD 0.1 times the amplitude of the square wave. In case of RF and TA, which each had two activation commands, the EMG component for each activation command was generated separately and then summed together. The choice of SD value for the baseline noise component was selected so that the EMG had a signal-to-noise ratio of 17 dB.
Neural Coupling to Create Synergies
We identified four muscle synergies that have been previously reported in locomotion (Clark et al. 2010): 1) VM, RF, and GM; 2) MG and SO; 3) MH and LH; and 4) RF and TA. To simulate neural coupling between these muscles in each synergy, we introduced correlations between the associated noise components of each muscle (ni) in Eq. 1. Three levels of neural coupling were used: 1) positive coupling (Pearson correlation coefficient r = 0.9); 2) negative coupling (r = −0.9); and 3) zero coupling (r = 0). The strong positive and negative correlations (in the positive and negative coupling conditions) effectively constrain the mutual relationship of the EMG amplitudes for all muscles within a synergy. For example, consider the muscle activity of MG-SO in the positive coupling condition (Fig. 3, left). In this case, a higher (or lower) activity in MG on a given stride is also associated with higher (or lower) activity in SO on the same stride. On the other hand, in the negative coupling condition, this situation is exactly reversed. Here, a higher activity in MG is associated with a lower activity in SO (Fig. 3, middle). For the synergy with three muscles, the negative correlation was introduced between VM-RF and VM-GM. It is important to note that the neural coupling introduced between these muscles is separate from the fact that these muscles already have simultaneous muscle activity (which is observed in all 3 neural coupling conditions as shown in Fig. 3).
Fig. 3.
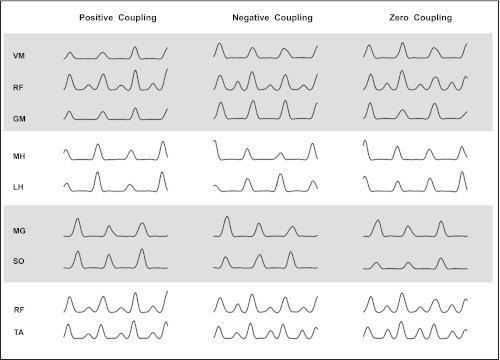
Full EMG in the 8 muscles (grouped into 4 muscle synergies) in the positive, negative, and 0 coupling conditions (shown for 3 strides). The coupling affects the relation between EMG variations for muscles within a synergy. For example, consider the medial gastrocnemius (MG)-soleus (SO) synergy. In the positive coupling condition, a greater than average activation in the MG muscle is also associated with a greater than average activation in the SO muscle. This situation is exactly reversed in the negative coupling condition, and in the 0 coupling condition there is no such relation. It is important to note that regardless of the coupling condition, the timing of muscle activity in a synergy was always simultaneous. Note that rectus femoris (RF) appears in 2 synergies and is therefore repeated twice in the figure [with the larger bump being coupled to vastus medialis (VM) in synergy 1 and the smaller bump being coupled to tibialis anterior (TA) in synergy 4]. MH, medial hamstrings; LH, lateral hamstrings; GM, gluteus medius.
Treadmill Walking
In addition to our EMG simulation, we also collected surface EMG data from the aforementioned muscles of the dominant leg (preferred leg to kick a ball) on six young healthy participants (30 ± 4 yr) when walking on a treadmill at 1.1 m/s. All subjects provided informed written consent, and procedures were approved by the Institutional Review Board at Northwestern University.
EMG signals were sampled at 1,000 Hz, and data were collected for 2 min when the participants walked over a split-belt ADAL treadmill (Techmachine, Andrézieux-Bouthéon, France) with embedded force plates. EMG electrodes were applied over the test muscles using a standard protocol. After cleaning the skin over the electrode placement sites with alcohol swabs, surface EMG electrodes (model MA-311; Motion Lab Systems, Baton Rouge, LA; 20× gain, >100-dB minimum common mode rejection, input impedance > 100,000 MΩ, noise < 1.2-μV root mean square) were placed over the eight muscles and tightly secured to the skin using self-adhesive tapes and cohesive flexible bandages (CoFlex; Andover Healthcare, Salisbury, MA). Electrode placement sites were standardized according to the guidelines established by the international SENIAM initiative (http://www.seniam.org) except for SO, for which the electrode was placed at 2/3rd of the line between lateral femoral condyle and lateral malleolus.
Data Analysis
EMG processing.
The steps involved in EMG processing are shown in Fig. 2B. The raw EMG data (both simulated and treadmill) were digitally band-pass filtered (40–500 Hz), rectified, and smoothed with a zero-phase low-pass Butterworth filter (2nd order, 4 Hz). For the sake of clarity, we will refer to the filtered EMG pattern (i.e., the linear envelope) obtained from the raw EMG as the full EMG to contrast it against the detrended EMG (see details below). In the data collected on participants, the EMG data from each stride was time-normalized to 101 points and concatenated together for each muscle. Gait events were identified using data recorded from the force plates. We also eliminated the first and last stride of the data; therefore, the number of data points for each muscle, N, was 58,000 for the simulation data, and it was (n − 2) × 101 for the treadmill data, where n represents the total number of strides on the treadmill.
Synergy extraction using full EMG.
In the first analysis, we used the full EMG to extract synergies. The matrix of muscle activity (N × 8) was subject to a principal component analysis (PCA) followed by a varimax rotation (Wakeling and Horn 2009). We extracted four invariant muscle synergies with time-varying activation patterns for each synergy (similar to Clark et al. 2010). The PCA was done using the correlation matrix since we were interested in coupling without regard to the absolute amplitude of the EMG. Previous results show that this analysis yields similar results compared with other extraction methods such as nonnegative matrix factorization (NMF; Cappellini et al. 2006). The reason we chose PCA over NMF was because our subsequent method of using detrended EMG involved data that had both negative and positive values.
Synergy extraction using detrended EMG.
To detrend the EMG, we first computed the ensemble average EMG for each muscle. Then, for each muscle, we subtracted the ensemble average of that muscle from the muscle activity in each stride (by concatenating the ensemble average over multiple strides and then subtracting this from the full EMG) to capture effectively only the variation in muscle activity while eliminating the task-related muscle activity. Note that our method of detrending involves subtracting the ensemble average from each muscle (instead of the usual procedure of subtracting a single mean value). Therefore, this procedure is not equivalent to a simple translation of the data. We then ran the PCA with varimax rotation on the detrended EMG and extracted four muscle synergies. Therefore, this method effectively computes the correlation structure between muscles at every instant in time over all strides.
Statistical Analyses
Simulated data.
We ran each simulation condition 10 times to generate 10 data sets. The set of 4 muscle synergies that was extracted from a data set is referred to as a synergy set. To compare synergy sets across different conditions, we used the following procedure: first, we examined whether the mean variance accounted for (VAF) by the 4 synergies in that condition was >80%. Any condition where the 80% threshold was not achieved was excluded from the synergy comparison. This threshold was set to avoid comparing synergy sets that did not account for sufficient variance in the data (i.e., if they were simply fitting noise).
If the VAF was >80%, we used a bootstrapping procedure to compare the synergies obtained. The largest principal angle between the vector subspaces spanned by the synergy sets was used to measure the similarity between synergies (Cheung et al. 2009). For example, to compare the synergy sets in the positive and negative coupling condition, we chose at random two synergy sets (without replacement) from each condition: P1 and P2 and N1 and N2, respectively. Then, we computed the principal angle between the synergy sets in the two different conditions and subtracted the maximum of the principal angles computed between synergy sets within the same condition [i.e., θP1−N1 − max (θP1−P2, θN1−N2)]. A greater difference in the principal angle would imply that the synergy sets in the 2 conditions were less similar. This was repeated for 50 iterations to obtain a mean and SD for the difference in principal angle. This measure was then statistically tested for whether it was significantly >0 (at the 0.05 significance level).
Treadmill Data
For the treadmill data, we computed the VAF by four synergies using the two methods. Similar to the simulation data, we computed the similarity between the synergy sets extracted using the largest principal angle.
RESULTS
Our simulated EMG resulted in qualitatively similar profiles of individual muscle activity, both compared with our experimental data as well as those that have been reported in the literature (Fig. 4). Although more detailed EMG simulations are available (Farina and Merletti 2001), our results are fairly robust to changes in these parameters as the analyses were performed only using the linear envelope.
Fig. 4.
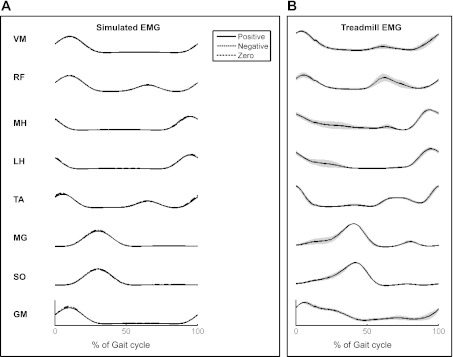
A: ensemble average of simulated EMG in the 8 muscles from heel strike (0%) to subsequent heel strike (100%). Note that RF and TA have a 2nd burst ∼60% into the gait cycle. Note that the ensemble average was similar for all 3 coupling conditions (positive, negative, and 0) and almost overlap completely. B: ensemble average of EMG for the 8 muscles from 6 healthy adults during treadmill walking at 1.1 m/s. The gray bands indicate 1 SE (across-participant).
Comparing Synergies from the Two Methods
We found that when the analysis was done using the full EMG, the extracted synergies were similar even though the three levels of coupling were widely different (Fig. 5). The VAF was also >80% in all cases (Fig. 6A). The principal angles between synergy sets in different coupling conditions were quite small, although two out of the three comparisons were statistically significant (P < 0.05; Fig. 6B).
Fig. 5.
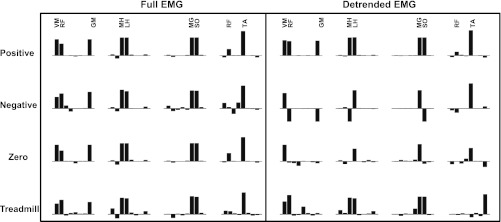
Comparison of the 4 synergies (averaged across simulations or subjects) extracted using the full EMG and the detrended EMG in the positive coupling, negative coupling, 0 coupling, and the treadmill conditions. For the simulated data, synergies extracted using the full EMG were relatively insensitive to changes in the simulated coupling, whereas the synergies extracted from the detrended EMG showed clear differences depending on the coupling. The treadmill data also showed differences in the structure of the synergies that were extracted using the 2 methods.
Fig. 6.
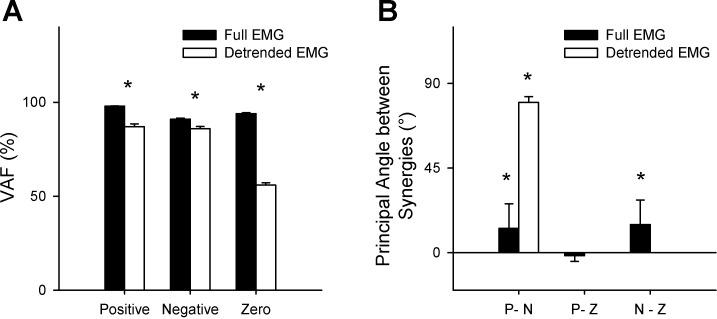
A: variance accounted for (VAF) for by 4 synergies in the 3 coupling conditions using the 2 methods. With the exception of the synergies in the 0 coupling condition using the detrended EMG, the VAF of all other conditions were >80%. In all cases, the VAF using the full EMG method was significantly higher than the VAF from the detrended EMG method (*P < 0.05). B: principal angles between synergies for the 3 coupling conditions. P, N, and Z indicate the positive, negative, and 0 coupling conditions. For the full EMG, differences in angles are small, indicating that the analysis was not very sensitive to the different values of coupling. However, the results show a clear difference between coupling conditions when using the detrended EMG. For the detrended EMG, the comparisons involving the 0 coupling condition (P-Z and N-Z) were not computed because the VAF in this condition was close to 50% (i.e., essentially the synergies in these conditions are fitting noise). Error bars indicate 1 SD. *Statistically >0 (P < 0.05).
On the other hand, when we used the detrended EMG for analysis, there were significant differences in synergies between the 3 different coupling conditions (Fig. 5). First, the zero coupling condition was identified using the VAF, which was only ∼55% (Fig. 6A). Between the positive and negative coupling conditions that did show VAF of over 80%, the principal angle between the synergy sets was high, indicating a significant change (P < 0.05; Fig. 6B).
In the analysis of the treadmill data, there was a significant drop in the VAF by 4 synergies when comparing the full EMG (92 ± 2%) with the detrended EMG (68 ± 4%; P < 0.05). The largest principal angle between synergy sets extracted using the 2 methods was 48 ± 25°, indicating that the synergies were qualitatively different (since the maximum possible angle between any 2 synergy sets is 90°; Fig. 5). Interestingly, the muscle synergy (VM-RF-GM) that involved muscles spanning different joints and not innervated by the same nerve was observed in the full EMG but not in the detrended data. This suggests that at least some of these muscle synergies previously identified may be related to simultaneous muscle activation rather than being reflective of coupling at a neural level.
DISCUSSION
In our simulated data, we found that when we used the usual method of extracting synergies using the full EMG, the results picked up only on the simultaneous activation of different muscles, regardless of the value of the neural coupling. However, when the detrended EMG was used to analyze the structure of the variability, the analysis was able to distinguish correctly the different neural coupling conditions. Although this result was expected, the purpose of our simulation was to demonstrate how the method of extracting muscle synergies using the full EMG is incapable of capturing features in the EMG variability.
On the other hand, when we analyzed the data from participants walking on a treadmill using the two methods, the results were not predictable a priori. We found that there were qualitative differences between the synergies extracted using the two methods, reinforcing the idea that simultaneous activation of muscles does not necessarily reflect a neural control strategy. Although two out of the four muscle synergies (MH-LH and MG-SO; Fig. 5) were similar in both methods, it is important to recognize that they arise from different features of the data: in the case of the full EMG, the muscle synergies predominantly reflect the correlations in the average muscle activity (i.e., simultaneous muscle activity), whereas in the case of the detrended EMG, these muscle synergies reflect correlations in the EMG variations (i.e., whether the variations in activity of 1 muscle were correlated with variations in the other). The finding that the 2 methods yield different conclusions are consistent with other studies that have shown that when the analyses are performed using variability, there has not been much support for the hypothesis of invariant muscle synergies (Kutch et al. 2008; Valero-Cuevas et al. 2009). Although we analyzed data from only 1 speed condition, the focus of our study was to emphasize the difference in the 2 methods of extracting synergies.
Why does the analysis of the full EMG not provide the complete picture to infer neural control strategies? As we mentioned in the Introduction, task and biomechanical constraints may effectively reduce the redundancy in the system (Buchanan et al. 1986; Newell 1986). In the case of locomotion, the task includes constraints (although these are harder to identify analytically compared with static tasks) such as having to propel the center of mass forward and maintaining body support during stance. These constraints impose certain restrictions on the set of possible muscle activation patterns that can be used for locomotion. Although it has been argued that the nervous system satisfies each of these constraints by using a specific muscle synergy (McGowan et al. 2010; Neptune et al. 2009), this can only be considered a control strategy if it can be shown that the system is redundant enough to do these subtasks without having to activate these muscles simultaneously (Kutch and Valero-Cuevas 2011). It is important to note that although not all simultaneous muscle activity is dictated by task or biomechanical constraints, any task-imposed muscle activation patterns will alter the correlations observed in the muscle activity, possibly masking any underlying control strategy. In this regard, the EMG variability provides a distinct advantage because the variability is relatively uninfluenced by the task demands (since the task can be achieved regardless of the structure in the variability as long as there is not excessive variability). Therefore, the presence or absence of significant structure in the variability provides a more direct window into the control strategy used (Valero-Cuevas et al. 2009).
In tasks such as locomotion, where it is cumbersome to derive analytically the muscle activations imposed by task constraints, the detrended EMG could be used as a tool to identify task constraints. For example, across different variations of the task, if there is a correlation structure in the full EMG but none when using the detrended EMG, this suggests that the muscles are simply firing simultaneously and could be reflective of task constraints. However, if the detrended EMG shows a correlation structure, then it could be considered a first step to infer a neural control strategy. Here, it has to be noted that the term neural control strategy does not necessarily imply that there are physical neuroanatomic connections between these muscles; rather, it only suggests that the nervous system functionally coordinates these muscles as a single unit. Therefore, although the exact causes behind the correlation structure in the detrended EMG require further investigation, this method provides a stronger test of the neural basis of muscle synergies compared with using the full EMG.
This analysis also has implications for studying muscle synergies in pathological conditions. For example, when using full EMG in stroke survivors, there has been evidence that there may be merging of muscle synergies and that the extent of merging correlates with degree of impairment (Clark et al. 2010). These authors proposed that neural mechanisms such as increased recruitment of brain-stem pathways and/or altered reflexes and afferent processing may explain these changes in muscle synergies. As mentioned before, if these neural mechanisms were indeed responsible for alterations in muscle synergies, then extracting synergies using the detrended EMG should also yield similar conclusions as the full EMG. On the other hand, if the differences observed are more a manifestation of constraints induced by the pathology (e.g., prolonged activation of muscles to compensate for muscle weakness), then the detrended EMG would not show the same correlation structure as the full EMG.
The method of detrending EMG described here parallels similar methods used in analyzing redundancy in isometric force production (Latash et al. 2001; Ranganathan and Newell 2009). Furthermore, we assume that this coupling between muscles is stable throughout the duration of the task, and therefore the analysis is performed on the data across the entire time series (Ranganathan and Newell 2008; Valero-Cuevas et al. 2009) rather than being computed separately at particular phases in the gait cycle. However, there are certain limitations to our approach: we make the assumption that the ensemble average muscle pattern is a desired pattern (with variability being deviations from this mean) and do not make a distinction between task-relevant (i.e., EMG variability that has an influence on the task outcome) and task-irrelevant variability (i.e., EMG variability that has no influence on the task outcome; Cusumano and Cesari 2006; Scholz and Schöner 1999; Todorov and Jordan 2002). Therefore, although we can identify coupling relationships between the different muscles [cf. M-modes (Krishnamoorthy et al. 2003)], we do not explicitly know whether this coupling is used for any particular biomechanical function (e.g., keeping speed constant at every stride; Dingwell et al. 2010). A future study using a complete EMG-driven musculoskeletal model for walking could be used to make this distinction between task and null-space variability. Such a model could also be used to derive the task constraints in locomotion, which could provide a more conclusive way to show that certain muscle activity arises from task constraints (Kutch and Valero-Cuevas 2011). Other methodological considerations when using the detrended EMG are to ensure a reasonably high signal-to-noise ratio in the EMG (which would affect the VAF) and to minimize cross talk between muscles to avoid spurious correlations.
In summary, we would like to emphasize that the two types of analysis are complementary and give rise to different interpretations about movement control. Extracting synergies using the full EMG is useful in quantifying timing (and in some methods, amplitude) relationships between different muscles, which can be considered an index of intermuscular coordination. However, caution should be exercised in interpreting these relationships as part of a neural control strategy since the constraints involved in performing the task may significantly influence the muscle activation patterns observed. On the other hand, extracting synergies from the EMG variability provides a stronger test of neural control strategies that is not confounded by task requirements. Both these levels of analysis may be critical for gaining further insight into the control of normal and pathological locomotion.
GRANTS
This work was supported in part by National Institute on Disability and Rehabilitation Research Grant H133E070013.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
All experiments were performed at the Rehabilitation Institute of Chicago. R.R. and C.K. contributed to the conception and design of the experiments; R.R. and C.K. collected, analyzed, and interpreted the data; R.R. and C.K. wrote the manuscript; both authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. William Z. Rymer, Howard Zelaznik, and Maura Casadio and two anonymous reviewers for their insightful comments on an earlier version of the manuscript.
REFERENCES
- Bernstein NA. The Co-Ordination and Regulation of Movements. Oxford: Pergamon Press, 1967 [Google Scholar]
- Buchanan TS, Almdale DP, Lewis JL, Rymer WZ. Characteristics of synergic relations during isometric contractions of human elbow muscles. J Neurophysiol 56: 1225–1241, 1986 [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006 [DOI] [PubMed] [Google Scholar]
- Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci USA 106: 19563–19568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusumano JP, Cesari P. Body-goal variability mapping in an aiming task. Biol Cybern 94: 367–379, 2006 [DOI] [PubMed] [Google Scholar]
- d'Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6: 300–308, 2003 [DOI] [PubMed] [Google Scholar]
- Dingwell JB, John J, Cusumano JP. Do humans optimally exploit redundancy to control step variability in walking? PLoS Comput Biol 6: e1000856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R. A novel approach for precise simulation of the EMG signal detected by surface electrodes. IEEE Trans Biomed Eng 48: 637–646, 2001 [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature 394: 780–784, 1998 [DOI] [PubMed] [Google Scholar]
- Hof AL, Elzinga H, Grimmius W, Halbertsma JP. Speed dependence of averaged EMG profiles in walking. Gait Posture 16: 78–86, 2002 [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556: 267–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleissen RF, Zilvold G. Estimation uncertainty in ensemble averaged surface EMG profiles during gait. J Electromyogr Kinesiol 4: 83–94, 1994 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Goodman S, Zatsiorsky V, Latash ML. Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biol Cybern 89: 152–161, 2003 [DOI] [PubMed] [Google Scholar]
- Kutch JJ, Kuo AD, Bloch AM, Rymer WZ. Endpoint force fluctuations reveal flexible rather than synergistic patterns of muscle cooperation. J Neurophysiol 100: 2455–2471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Muscle redundancy does not imply robustness to muscle dysfunction. J Biomech 44: 1264–1270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Danion F, Schöner G. Structure of motor variability in marginally redundant multifinger force production tasks. Exp Brain Res 141: 153–165, 2001 [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schöner G. Toward a new theory of motor synergies. Motor Control 11: 276–308, 2007 [DOI] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: adaptations to altered mechanical demands. J Biomech 43: 412–419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JL, Ting LH. Functional muscle synergies constrain force production during postural tasks. J Biomech 41: 299–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: a simulation study. J Biomech 42: 1282–1287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KM. Constraints on the development of coordination. In: Motor Development in Children: Aspects of Coordination and Control, edited by Wade M, Whiting HT. Dordrecht, The Netherlands: Martinus Nijhoff, 1986, p. 341–360 [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack, 1992 [Google Scholar]
- Ranganathan R, Newell KM. Influence of augmented feedback on coordination strategies. J Mot Behav 41: 317–330, 2009 [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Newell KM. Motor synergies: feedback and error compensation within and between trials. Exp Brain Res 186: 561–570, 2008 [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Zelaznik HN, Hawkins B, Frank JS, Quinn JT. Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86: 415–451, 1979 [PubMed] [Google Scholar]
- Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999 [DOI] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol 103: 3084–3098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 19: 601–607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey MT, Shaw RE, Mace W. Issues in the theory of action: degrees of freedom, coordinative structures and coalitions. In: Attention and Performance VII, edited by Requin J. Hillsdale, NJ: Erlbaum, 1978, p. 557–595 [Google Scholar]
- Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol 102: 59–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling JM, Horn T. Neuromechanics of muscle synergies during cycling. J Neurophysiol 101: 843–854, 2009 [DOI] [PubMed] [Google Scholar]
- Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol 67: 402–411, 1987 [DOI] [PubMed] [Google Scholar]


