Abstract
It is well established that the prefrontal cortex is involved during memory-guided tasks whereas visually guided tasks are controlled in part by a frontal-parietal network. However, the nature of the transition from visually guided to memory-guided force control is not as well established. As such, this study examines the spatiotemporal pattern of brain activity that occurs during the transition from visually guided to memory-guided force control. We measured 128-channel scalp electroencephalography (EEG) in healthy individuals while they performed a grip force task. After visual feedback was removed, the first significant change in event-related activity occurred in the left central region by 300 ms, followed by changes in prefrontal cortex by 400 ms. Low-resolution electromagnetic tomography (LORETA) was used to localize the strongest activity to the left ventral premotor cortex and ventral prefrontal cortex. A second experiment altered visual feedback gain but did not require memory. In contrast to memory-guided force control, altering visual feedback gain did not lead to early changes in the left central and midline prefrontal regions. Decreasing the spatial amplitude of visual feedback did lead to changes in the midline central region by 300 ms, followed by changes in occipital activity by 400 ms. The findings show that subjects rely on sensorimotor memory processes involving left ventral premotor cortex and ventral prefrontal cortex after the immediate transition from visually guided to memory-guided force control.
Keywords: electroencephalography, event-related potentials, visuomotor system, working memory, low-resolution brain electromagnetic tomography
many daily activities require humans to produce force or perform movements with visual feedback and then transition to performing a similar motor output without visual feedback. One example is when, while driving a car, an individual applies a force to the accelerator to maintain the car speed and applies forces to the steering wheel to keep the car position within the lanes. The driver receives visual feedback of the other cars and the road ahead. At times, however, the driver's eyes may transition away from the road to either pick up a drink or change the radio station, but the voluntary forces applied to the steering wheel and accelerator must be maintained.
Prior studies have found that the production of accurate force output depends on whether or not visual information is available (Slifkin et al. 2000) and on the amount of visual information available (Vaillancourt et al. 2006). Visually guided force control tasks have been associated with activity in frontal and parietal cortices (Grol et al. 2007; Jeannerod et al. 1995; Kuhtz-Buschbeck et al. 2001). Ehrsson and colleagues (2001) identified several areas of the frontal and parietal cortices active during precision grip force control, including primary motor cortex, premotor cortex, supplementary motor area, cingulate motor area, and intraparietal cortex. Neuroimaging techniques that include positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have identified the anatomical regions that are activated during memory-guided motor tasks (Cohen et al. 1997; Debaere et al. 2003; Jahanshahi et al. 1995; Mueller et al. 2007; Vaillancourt et al. 2003). The neural processes that underlie the generation of memory-guided force control have been specifically linked to the dorsolateral prefrontal cortex, ventral prefrontal cortex, and anterior cingulate cortex during isometric grip force control by fMRI (Vaillancourt et al. 2003). Studies using electroencephalography (EEG) have investigated memory processes within long delay periods of memory retention (Bender et al. 2010; Sauseng et al. 2002). However, the neural circuits that are utilized during the immediate transition from visually guided to memory-guided force control have not been well elucidated.
It is well established that the prefrontal cortex is a key brain region that is involved with memory-guided tasks. Neurophysiological studies in monkeys (Fuster and Alexander 1971; Kubota and Niki 1971; Miller et al. 1996) and fMRI studies in humans (Cohen et al. 1997; McCarthy et al. 1996) report persistent neuronal activity in the prefrontal cortex during the delay period of a working memory task. Lesion studies in monkeys and humans indicate that lesions to the prefrontal cortex impair working memory (Curtis and D'Esposito 2004; Müller and Knight 2006). Vaillancourt and colleagues (2003) were able to isolate memory-related processes to the prefrontal cortex during a precision grip force task using fMRI by separating motor memory processes from visual-only and motor-only activations. Studies using transcranial magnetic stimulation (TMS) over the dorsolateral prefrontal cortex (DLPFC) (Hamidi et al. 2009; Mottaghy et al. 2002; Oliveri et al. 2001; Postle et al. 2006) further confirm the role of the prefrontal cortex in working memory. A recent study using EEG examined the delay phase during a memory-guided saccade task and localized prefrontal activity only during the initial part of the delay period (Brignani et al. 2010). The extent and potential timing of prefrontal activity during the immediate transition from a visually guided motor task to a memory-guided motor task are still not well established. Determining the relative timing of prefrontal activation is an important step toward revealing the specific contribution of prefrontal areas during motor memory tasks.
The present study examines force performance and the spatiotemporal pattern of brain activity that occur during the transition from a visually guided to a memory-guided force control task using event-related potentials (ERPs) and low-resolution electromagnetic tomography (LORETA). As a control for the role of visual feedback, a second experiment was conducted that did not require memory but instead altered the spatial amplitude of visual feedback. In accordance with the above-mentioned studies on the role of prefrontal cortex during accurate memory maintenance, we hypothesize that prefrontal event-related activity changes during the transition from visually guided to memory-guided force control. Previous behavioral work suggests that the temporal capacity of short-term visuomotor memory can range between 0.5 and 1.5 s during force production (Vaillancourt and Russell 2002) and can extend up to 2 s during pointing tasks (Binsted et al. 2006; Elliott and Madalena 1987). As such, we expect to observe behavioral changes between 0.5 and 1.5 s during the subjects' memory-guided force production, and further predict that prefrontal activity changes before changes in behavior. Because experiment 2 does not require memory, we predict that prefrontal event-related activity is unique to memory-guided force control (experiment 1) and is not observed during decreases in the gain of visual feedback (experiment 2).
METHODS
Subjects.
A total of 12 participants were in experiment 1 (6 men, 6 women; aged 19–34 yr, mean = 23.5, SD = 4.47) and 11 participants in experiment 2 (6 men, 6 women; aged 19–30 yr. mean = 22.73, SD = 3.82). All participants were healthy right-handed subjects with normal or corrected vision. Self-reported measures of handedness and medical history were used. Each participant participated in only one of the two experiments. Subjects were asked not to consume any caffeine and to refrain from using any hair products on the day of testing. All subjects provided informed consent prior to the experiment. This study was approved by the local Institutional Review Board and is in accordance with the Declaration of Helsinki.
Experimental design.
Subjects sat upright in a chair with their right forearm supported by a rigid armrest and their thumb and index finger in a pinch grip position against two force transducers (Measurement Specialties, Hampton, VA) (Fig. 1A). Both experiments were carried out in a normally illuminated room with a computer monitor that was placed ∼130 cm (52 in.) in front of the subjects. Before the experimental task, all subjects performed three 3-s trials of maximal isometric pinch grip force. The largest force output of the three trials was used as the individual's maximal voluntary contraction (MVC). Next, subjects in experiment 1 performed five practice trials that consisted of 20 s of rest followed by 30 s of force production at 15% of their MVC, and subjects in experiment 2 performed 11 s of rest followed by 6 s of force production at 15% of their MVC. Subjects were asked to practice a pinch grip force task so that 1) the subjects could become familiarized with the equipment and general requirements of the study and 2) the visual display during force production could be recorded and reproduced on the screen for the subjects during a vision-only condition of the task.
Fig. 1.
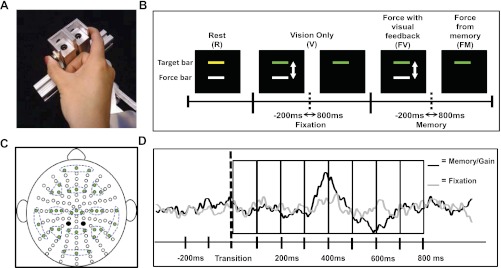
A: precision grip apparatus pressed with the subject's thumb and index fingers. B: sequence of conditions for each trial in experiment 1 along with the visual display viewed by the subject. The transition periods that were examined are also shown. C: configuration of electrodes. Clusters of 3 electrodes (green-filled circles) are highlighted with dashed lines showing the 13 regions of interest (ROIs). Black-filled circles are the two reference electrodes used during data collection. D: eight 100-ms time bins used to compare between fixation and memory transitions in experiment 1 and fixation and gain transitions in experiment 2.
Procedure for experiment 1.
Figure 1B shows one experimental trial with the following sequence of conditions: 1) rest (R, 5 s), 2) vision only with no force production (V, 6 s), 3) force with visual feedback (FV, 5 s), and 4) force from memory (FM, 4 s). Each condition is described in further detail below.
1) R: Subjects were asked to rest and look straight ahead at the computer screen. A yellow stationary target bar was displayed and set at 15% of the subjects' MVC. A white stationary force bar was also displayed during the rest condition.
2) V: Subjects were asked not to produce any force and to focus their attention on the screen as the yellow target bar turned green and the white force bar fluctuated in real time according to the reproduction of the subjects' force output during practice trials. Subjects were asked to continue looking straight ahead at the screen as the white force bar disappeared and only the green target bar remained on the screen. This is a reproduction of a similar visual stimulus that subjects observed during force with visual feedback (FV) and force from memory (FM) practice sessions.
3) FV: Subjects were asked to produce force at 15% of their MVC when the white force bar reappeared, matching the white force bar to the green target bar using online visual feedback of the force output.
4) FM: Subjects were asked to continue producing the same amount of force from memory after the white force bar disappeared.
Each trial lasted 20 s, with the trials repeated 25 times in one block. Each trial followed the same order of conditions described above. To minimize a possible increase in electrocortical activity due to muscle fatigue (Johnston et al. 2001), subjects received a break of at least 3 min after every block in addition to R and V conditions that required no force production within each trial. A total of eight blocks, equaling 200 trials, were performed by each subject. Subjects were instructed to minimize blinking during V, FV, and FM conditions. Because the focus of this study was on the transition from visually guided to memory-guided force control, the analyses were focused on the transition within V and from FV to FM. The transition during V conditions will be referred to as the fixation transition. The transition from FV to FM will be referred to as the memory transition (Fig. 1B). The fixation transition served as a control task for this study, in which subjects viewed a visual stimulus identical to that observed during the memory transition. This allowed us to parse out the neural activity associated with stimulus-related perception and isolate the neural activity relating to memory-guided force control.
Procedure for experiment 2.
In contrast to experiment 1, in which visual feedback was removed completely at the transition, experiment 2 consisted of a decrease in the gain of visual feedback. The visual gain, which is the sensitivity of a system to error, can be varied by manipulating the distance of the eye to the computer monitor and/or changing the spatial amplitude of force output through a white force bar on the computer monitor. For this experiment, the distance between the subject and the computer monitor was kept constant; therefore visual gain was manipulated by changing the size of force output as viewed by the subject. The height of the force fluctuations viewed by the subject was manipulated with the following formula:
| (1) |
in which Fp is the force produced by the subject, Ft is the target force, and G is the gain level. Visual gain was calculated by assuming a set force output standard deviation (SD) of 0.3 N (Vaillancourt et al. 2006). The full range (±3 SD) of the estimated variance for the height of force fluctuation was approximated by multiplying the SD value by 6 (0.3 N × 6 = 1.8 N). The visual gain for each gain level was then calculated with the following formula:
| (2) |
in which α is the visual gain, D is the distance to the monitor, and H1 is the height of the total range of motion in the top half of the visual field. The low and high visual gain levels correspond to visual angles of 0.026° and 2.908°. The selected visual gains were well below and above 1°, spanning the range across which a dramatic change in force performance will occur (Coombes et al. 2010; Vaillancourt et al. 2006). The two levels of visual gain will be referred to as low gain (0.026°) and high gain (2.908°) throughout the remainder of this article. The high gain used in experiment 2 was also used in experiment 1 during force production with visual feedback.
Trials in experiment 2 consisted of the following conditions: 1) rest (R, 5 s), 2) vision only from high to low gain (V, 6 s), 3) force with visual feedback at high gain (HFV, 5 s), 4) force with visual feedback at low gain (LFV, 4 s). Each condition is described in further detail below:
R: Subjects were asked to rest and look straight ahead at the computer screen. A yellow stationary target bar was displayed and set at 15% of the subjects' MVC. A white stationary force bar was also displayed during the rest condition.
V: Subjects were asked not to produce any force and to focus their attention on the screen as the yellow target bar turned green and the white force bar fluctuated in real time according to a reproduction of the subjects' force output during a practice trial from high to low gain. Thus the subjects viewed a similar visual stimulus that they observed during high to low gain transition.
HFV: Subjects were asked to produce force at 15% of their MVC when the white force bar reappeared on the bottom of the screen, matching the white force bar to the green target bar using online visual feedback of the force output set at the high gain level.
LFV: Subjects were asked to continue matching the white force bar to the green target bar using online visual feedback of the force output set at the low gain level.
Each trial lasted 20 s, with the trials repeated 25 times in one block. Subjects received a break of at least 3 min after every block of 25 trials. A total of eight blocks, equaling 200 trials, were performed by each subject. Subjects were instructed to minimize blinking during V, HFV, and LFV conditions. The transition during V conditions will be referred to as the fixation transition. The transition from HFV to LFV will be referred to as the gain transition. The fixation transition served as a control task for this experiment where subjects viewed an visual stimulus identical to that observed during the gain transition.
Behavioral data acquisition.
The force transducers used were ELFF-B4 model load cells constructed from piezoresistive strain gauges measuring force up to 100 N (Measurement Specialties). Force data were collected by Coulbourn Instruments type B V72-25 amplifiers at an excitation voltage of 5 V. The force signal was transmitted via a 16-bit A/D converter and digitized at 200 Hz. The output from the force transducers was presented to the subject with a visual display on the computer screen (white force bar in Fig. 1B). The force output was displayed on the screen at a resolution of 1,024 × 768 pixels and a refresh rate of 60 Hz. Digital triggers identifying the start of each condition (i.e., R, V, FV, and FM in experiment 1 and R, V, HFV, and LFV in experiment 2) were sent from a program written in LabVIEW to the ActiveTwo (BioSemi, Amsterdam, The Netherlands) acquisition software.
Electrophysiological data acquisition.
The EEG was collected with the ActiveTwo system with 128 Ag-AgCl Active Two electrodes. The active electrodes were connected to a cap that was in a preconfigured montage covering the entire scalp surface (Fig. 1C). One of three cap sizes was selected for the subjects depending on their head circumference (i.e., 50–54 cm, 54–58 cm, or 58–62 cm). The signals were amplified through the electrodes at the source and had an output impedance of <1 Ω. EEG signals were digitally amplified at DC and sampled at 2,048 Hz. Electrical potentials were recorded between each electrode and the common mode sense (CMS) electrode, which is analogous to a ground. The CMS and a driven right leg (DRL) electrode were located toward the center of the other electrodes as seen in Fig. 1C (black-filled circles). The CMS and DRL electrodes were used to drive the average potential of the subject as close as possible to the AD-box reference potential electrode. The electrode offsets, a running average of the voltage measured between the CMS and each active electrode, were evaluated before the start of each block and during data collection to be within the acceptable range of ±40 mV (BioSemi). The electrode offset served as an indirect measure of impedance tolerance to ensure that a stable and high-quality signal was recorded from each active electrode.
Behavioral data analysis.
Individual force trials were first visually inspected with a custom-written program in LabVIEW to ensure that subjects were completing the requirements of the task (i.e., producing force during memory and gain transitions and not producing force during fixation transitions). The force data were low-pass filtered with a fourth-order dual-pass Butterworth filter at 10 Hz. Force output was examined in 100-ms time bins from 200 ms before to 800 ms after the memory and gain transitions. Four dependent measures were calculated: 1) mean force output, 2) SD of force, 3) coefficient of variation (CV) of force, and 4) force error. Force error was calculated with the root mean squared error (RMSE) of the force in newtons. This reflected how accurate force production was relative to the target force output. The effect of time on force output was analyzed with repeated-measures ANOVA using Greenhouse-Geisser corrections. Significance was determined with a P value of <0.05. Significant effects were followed by Tukey's honestly significant difference (HSD) test to determine the first 100-ms time bin that was significantly different after changes in visual feedback.
Electrophysiological analysis.
All EEG data were imported into the EMSE software suite (Source Signal Imaging, San Diego, CA) for analysis. The data were first re-referenced to a common average reference. The average reference was chosen to provide the best approximation of an absolute reference with a net source of zero (Srinivasan et al. 1998). This allows us to avoid the violation of quasistationarity for source estimation (Michel et al. 2004). Slow drifts within EEG signals were removed by polynomial detrend and baseline corrected to DC offset. Next, channels were band-pass filtered at 0.5–70 Hz with a notch filter at 60 Hz. Then signals were downsampled from 2,048 Hz to 512 Hz. Trials were manually inspected for movement and eye artifacts and discarded from further analyses if they contained visible artifacts. In addition, clear instructions were provided to subjects to fixate and focus on the force and target cursor on the screen; therefore horizontal eye movements were minimized. Vertical eye blinks and movements were carefully examined during individual inspection of each trial, and trial acceptance was conservative. An average of three individual noisy channels were corrected with the EMSE spatial interpolation filter in 8 of the 12 subjects in experiment 1, and an average of two channels were corrected in 7 of the 11 subjects in experiment 2. The specified channels were recreated by interpolation using all other channels in the file and weighted as a function of its distance from the channel to be reconstructed. For experiment 1, the average number of valid trials per subject was 149 trials (SD = 36.27) for the fixation transitions and 154 trials (SD = 35.9) for the memory transitions. For experiment 2, the average number of valid trials per subject was 121 trials (SD = 36.43) for the fixation transitions and 132 trials (SD = 36.3) for the gain transitions.
ERPs were extracted by averaging across all valid trials for each subject from 0 to 800 ms after the fixation and memory transitions in experiment 1 and the fixation and gain transitions in experiment 2 (Fig. 1D). The transitions were carefully designed to control for the visual properties of the stimuli and to exclude neural activity relating to motor output production by removing visual feedback or manipulating visual gain during the maintenance of force production. A total of eight 100-ms time bins were analyzed. For experiments 1 and 2, the effect of time and transition on each region of interest (ROI) was analyzed with separate two-way repeated-measures ANOVA with Greenhouse-Geisser corrections (8 time bins × 2 transitions). ROIs were selected to cover frontal, central, parietal, temporal, and occipital regions of the visuomotor system. Each ROI consisted of an average cluster of three electrodes (Fig. 1C). The two levels of transitions were the fixation and memory transitions in experiment 1 and the fixation and gain transitions in experiment 2 across the eight 100-ms time bins (Fig. 1D). Each significant time × transition interaction was followed with individual t-tests and corrected for multiple comparisons with Bonferroni corrections. For each significant interaction, eight t-tests were conducted and considered significant with P value < 0.00625. Electrophysiological results are reported in terms of positive or negative polarities, but no inferences are made regarding the nature of the polarities, i.e., the structure and orientation of dipole(s) or the type of postsynaptic cells (excitatory or inhibitory).
Source analysis.
To further understand the spatial pattern of brain activity during visually guided to memory-guided force control, we performed source localization on the time bins that were 1) significantly different in the electrophysiological analysis and 2) observed prior to behavioral changes in force output (experiment 1). Hence, low-resolution electromagnetic tomographic analysis (LORETA) was applied at each 100-ms time interval from 300 to 600 ms after the memory transition. The difference wave obtained by subtracting the grand-averaged event-related response during the memory transition from the fixation transition was used to compute three-dimensional linear solutions to the inverse problem within the constraints of a realistic finite-element modeling (FEM) of an average brain (EMSE Suite). FEM is a volume-based modeling technique that considers individual anisotropic conductivities of each tissue type (skin, skull, and brain/CSF) to determine the solutions to the forward model. The distribution of neuronal generators as a current density value at each voxel and spatial resolution of 5 mm was determined. LORETA solutions are characterized with the assumption that there is highly synchronized activity among neighboring neurons and that the smoothest of all possible distributions is the most plausible to explain the data (Pascual-Marqui et al. 2002). Human Motor Area Template and prior fMRI studies from our laboratory were used to identify brain regions from the LORETA solutions (Mayka et al. 2006; Vaillancourt et al. 2003).
RESULTS
Experiment 1: behavioral results.
The target force level across subjects ranged from 3.9 to 12.75 N with the mean target force = 7.23 N (SD = 2.32). The analyses examined the dependent measures in consecutive 100-ms time bins from 200 ms before to 800 ms after visual feedback was removed in the memory transitions. Repeated-measures ANOVA for mean force output was not significantly different, indicating that mean force did not change across time [F(9,99) = 1.65, P = 0.22]. SD and CV of force were also not significantly different across time [F(9,99) = 0.96, P = 0.44; F(9,99) = 0.934, P = 0.45]. These results indicate that force variability did not change over the examined time bins. However, the RMSE of force production was significantly different across time [F(9,99) = 18.53, P = 0.000025]. Tukey's HSD test was subsequently used to detect the earliest time bin that was significantly different from 200 ms before visual feedback was removed. A significant increase in force error was detected at 600 ms after visual feedback was removed. Thus subjects were able to maintain force accuracy for at least 600 ms after the removal of visual feedback.
Experiment 1: electrophysiological results.
Figure 2 illustrates the grand-averaged waveforms of all recorded electrodes during memory and fixation transitions. The ROIs that were further analyzed are labeled and highlighted with dashed circles. Figure 3 represents the topography map of the grand-averaged waveforms projected onto a standardized head shape. The visual stimulus (i.e., disappearing white force bar) initiated during both the fixation and memory transitions resulted in a similar pattern of visual ERP components between 0 and 300 ms. Next, a centrally distributed positivity was observed between 300 and 400 ms in the memory transition but not the fixation transition. This was followed by a prefrontal positivity between 400 and 600 ms in the memory transition. A centrally distributed negativity could also be seen between 400 and 600 ms in both transitions. Finally, a frontal negativity and parietal positivity could be observed in both memory and fixation transitions, with the negative component appearing earlier between 400 and 500 ms, followed by the positive component between 500 and 600 ms. This frontal-negative and parietal-positive pattern persisted for the remainder of the examined time bins in both transitions.
Fig. 2.
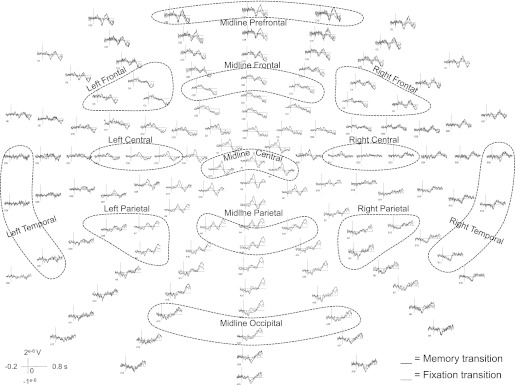
Experiment 1: grand-averaged waveforms elicited during memory transition (black) and fixation transition (gray) across all recorded electrodes. ROIs are labeled and highlighted with dashed circles.
Fig. 3.
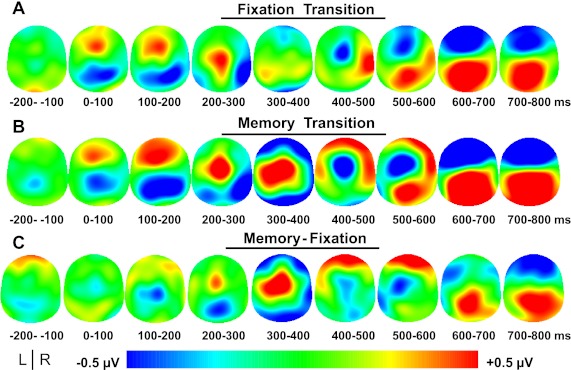
Experiment 1: grand-averaged event-related topography in 100-ms time bins from −200 ms to 800 ms after fixation transition (A), memory transition (B), and memory-fixation transitions (C). The potential distribution is projected onto a standardized head shape. L, left; R, right.
Significant time × transition interactions were found in 7 of the 13 ROIs (i.e., midline prefrontal, left frontal, right frontal, left central, midline central, left parietal, and midline parietal channel groups) (Table 1), followed by significant t-tests in 6 of the 7 significant interactions after Bonferroni corrections (i.e., midline prefrontal, left frontal, right frontal, left central, left parietal, and midline parietal channel groups) (Fig. 4). This suggests that ERPs, relating to the maintenance of force production and not the visual stimulus, were detected in these regions. Detailed results of the statistical analyses can be found in Table 1. Post hoc t-tests revealed that significant differences occurred as early as 300 ms after the removal of visual feedback. The green box in Fig. 4 shows that the first region with significant changes in ERPs was detected over the left central channel group contralateral to the hand producing force (also see Table 1). By 400 ms after visual feedback was removed, significant differences were found in the midline prefrontal channel group, and this is highlighted in the yellow box in Fig. 4. The prefrontal activity along with the left central channel group were simultaneously significant from 500 to 600 ms after visual feedback was removed. These contralateral central and midline prefrontal changes in ERPs all occurred before a significant change in behavior was detected. Finally, a significant change in ERPs occurred across bilateral frontal and left parietal cortices (left frontal, midline frontal, right frontal, left parietal, and midline parietal channel groups) at 700 ms after visual feedback was removed. As shown in the red boxes in Fig. 4, this simultaneous frontal negativity and parietal positivity can be observed more prominently during the memory transition than the fixation transition (also see Fig. 3).
Table 1.
Experiment 1 ANOVA results for condition by time interaction and follow-up t-tests
| Epoch, ms |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–100 |
100–200 |
200–300 |
300–400 |
400–500 |
500–600 |
600–700 |
700–800 |
|||||||||||
| Location of Channel Groups | F-Value F(7,77) | P | t | P | t | P | t | P | t | P | t | P | t | P | t | P | t | P |
| Midline prefrontal | 4.52 | 0.009 | −0.17 | 0.86 | 0.79 | 0.44 | −0.33 | 0.75 | −1.56 | 0.15 | 3.60 | 0.004 | 4.57 | 8E-04 | 0.004 | 0.99 | −2.29 | 0.04 |
| Left frontal | 4.84 | 0.003 | 0.72 | 0.49 | 2.03 | 0.07 | 0.05 | 0.96 | −1.90 | 0.08 | 2.13 | 0.06 | 0.94 | 0.37 | −2.97 | 0.01 | −3.69 | 0.004 |
| Midline frontal | 2.46 | 0.070 | ||||||||||||||||
| Right frontal | 6.80 | 4E-04 | 1.31 | 0.22 | 2.70 | 0.02 | −0.16 | 0.87 | −2.50 | 0.03 | 1.82 | 0.10 | 2.61 | 0.02 | −1.81 | 0.10 | −4.26 | 0.001 |
| Left central | 5.52 | 0.002 | −0.10 | 0.93 | −1.27 | 0.23 | 0.61 | 0.55 | 4.66 | 7E-04 | 0.43 | 0.68 | −3.73 | 0.003 | −1.20 | 0.26 | −0.32 | 0.76 |
| Midline central | 5.10 | 0.008 | −1.14 | 0.28 | −1.02 | 0.33 | 2.85 | 0.02 | 2.91 | 0.01 | −2.23 | 0.05 | −2.05 | 0.07 | 0.66 | 0.52 | 0.10 | 0.92 |
| Right central | 0.40 | 0.765 | ||||||||||||||||
| Left parietal | 4.94 | 0.003 | −0.76 | 0.46 | −3.09 | 0.01 | −1.08 | 0.30 | 1.72 | 0.11 | 0.53 | 0.61 | −1.50 | 0.16 | 2.63 | 0.02 | 5.63 | 2.E-04 |
| Midline parietal | 4.97 | 0.005 | −0.51 | 0.62 | −3.10 | 0.01 | −1.45 | 0.17 | 1.63 | 0.13 | −1.78 | 0.10 | −0.72 | 0.49 | 2.56 | 0.03 | 3.49 | 0.005 |
| Right parietal | 2.23 | 0.095 | ||||||||||||||||
| Left temporal | 1.20 | 0.330 | ||||||||||||||||
| Right temporal | 1.20 | 0.320 | ||||||||||||||||
| Midline occipital | 2.14 | 0.120 | ||||||||||||||||
Two-way repeated-measures ANOVA (8 time bins × 2 transitions) was performed. Corresponding F values and Greenhouse-Geisser-corrected P values are shown. Each significant interaction was followed up with individual t-tests and considered significant with a Bonferroni-corrected P value <0.00625. Corresponding t-values and P values are shown. Regions of interest (ROIs) with significant interactions followed by significant t-tests are highlighted in bold. Significant P values are highlighted in bold.
Fig. 4.
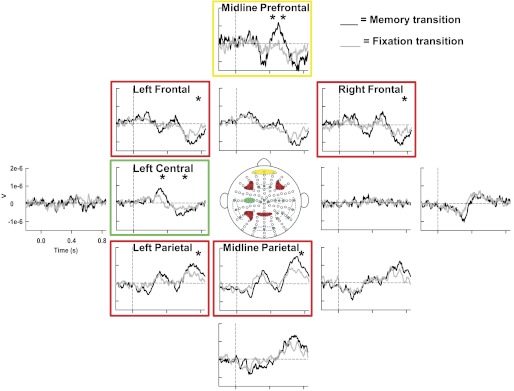
Experiment 1: grand-averaged event-related potentials (ERPs) of ROIs in the memory transition (black) and fixation transition (gray). Statistically significant ROIs are highlighted chronologically from earliest to latest time of significance (i.e., green to yellow to red). *Significant time bins within each ROI.
Experiment 2: behavioral results.
The target force level across subjects ranged from 4.2 to 12.0 N with mean target force = 8.25 N (SD = 2.42). Repeated-measures ANOVA for mean force output was not significantly different, indicating that mean force did not change across time [F(9,90) = 0.51, P = 0.51]. SD of force was very close to significance [F(9,90) = 3.13, P = 0.06], and CV of force was significantly different across time [F(9,90) = 4.24, P = 0.02]. Tukey's HSD test was performed on the CV of force. Significant increases in force variability occurred between 500 and 700 ms after high to low visual gain changes. The RMSE of force production was also significantly different across time [F(9,90) = 25.47, P = 0.000097]. Tukey's HSD test detected a significant increase in force error at 400 ms after changes in visual gain. Thus subjects were able to maintain force accuracy for at least 400 ms after the decrease in visual gain.
Experiment 2: electrophysiological results.
Figure 5 illustrates the grand-averaged waveforms of all recorded electrodes during gain and fixation transitions. The ROIs that were further analyzed are labeled and highlighted with dashed circles. Figure 6 represents the topography map of the grand-averaged waveforms projected onto a standardized head shape. First, a frontal-central positivity and posterior-occipital negativity was observed between 200 and 500 ms in the gain transition. This was followed by a frontal negativity and parietal positivity in the gain transition and to a lesser degree in the fixation transition, between 500 and 800 ms.
Fig. 5.
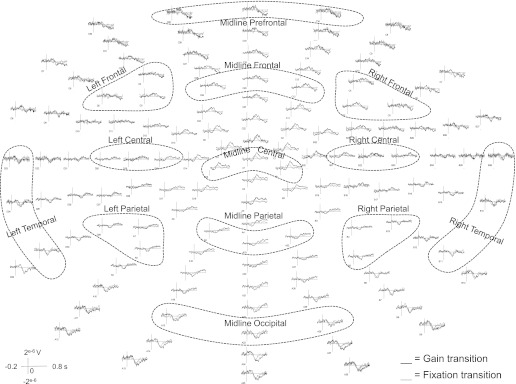
Experiment 2: grand-averaged waveforms elicited during gain transition (black) and fixation transition (gray) across all recorded electrodes. ROIs are labeled and highlighted with dashed circles.
Fig. 6.
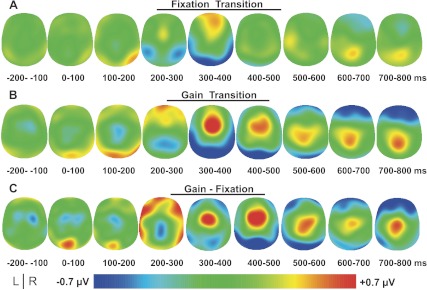
Experiment 2: grand-averaged event-related topography in 100 ms time bins from −200 ms to 800 ms after fixation transition (A), gain transition (B), and gain-fixation transitions (C). The potential distribution is projected onto a standardized head shape. L, left; R, right.
Significant time × transition interactions were found in 5 of the 13 ROIs (i.e., midline prefrontal, right frontal, midline central, midline parietal, and midline occipital channel groups) (Table 2), followed by significant t-tests in 3 of the 5 significant interactions after Bonferroni corrections (i.e., midline prefrontal, midline central, and midline occipital channel groups) (Fig. 7). This suggests that ERPs, relating to the maintenance of force production and not the visual stimulus, were detected in these regions. Detailed results of the statistical analyses can be found in Table 2. Post hoc t-tests revealed that significant differences occurred as early as 300 ms after the gain change. The green box in Fig. 7 shows that the first region with significant changes in ERPs was detected over the midline central channel group (also see Table 2). At 400 ms after visual feedback was removed significant differences were found in the midline occipital channel group, and this is highlighted in the yellow box in Fig. 7. Finally, significant changes in prefrontal activity were detected from 600 to 800 ms after gain changes as shown in the red box in Fig. 7, along with the midline central channel group, being simultaneously significant between 700 to 800 ms after gain changes. In contrast to experiment 1, these prefrontal and central changes in ERPs were observed after significant changes in behavior occurred. In addition, a pattern of early prefrontal positivity was observed in experiment 1, while a later prefrontal negativity was observed in experiment 2.
Table 2.
Experiment 2 ANOVA results for condition by time interaction and follow-up t-tests
| Epoch, ms |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–100 |
100–200 |
200–300 |
300–400 |
400–500 |
500–600 |
600–700 |
700–800 |
|||||||||||
| Location of Channel Groups | F Value F(7,70) | P | t | P | t | P | t | P | t | P | t | P | t | P | t | P | t | P |
| Midline prefrontal | 4.25 | 0.012 | 1.93 | 0.08 | 1.52 | 0.16 | 1.44 | 0.18 | −1.71 | 0.12 | −0.28 | 0.78 | −1.46 | 0.18 | −4.13 | 0.002 | −3.69 | 0.004 |
| Left frontal | 3.10 | 0.061 | ||||||||||||||||
| Midline frontal | 2.44 | 0.080 | ||||||||||||||||
| Right Frontal | 3.02 | 0.040 | −0.23 | 0.82 | 2.80 | 0.02 | 1.99 | 0.07 | −1.49 | 0.17 | 0.69 | 0.51 | 0.44 | 0.67 | 1.59 | 0.14 | −3.01 | 0.01 |
| Left Central | 1.72 | 0.185 | ||||||||||||||||
| Midline central | 6.68 | 0.002 | −1.91 | 0.09 | −1.92 | 0.08 | −0.54 | 0.60 | 4.47 | 0.001 | 3.41 | 0.007 | 2.65 | 0.02 | 2.35 | 0.04 | 5.29 | 4.E-04 |
| Right central | 2.15 | 0.125 | ||||||||||||||||
| Left parietal | 1.04 | 0.393 | ||||||||||||||||
| Midline parietal | 4.72 | 0.014 | −1.66 | 0.13 | −1.18 | 0.26 | −1.83 | 0.10 | 0.20 | 0.85 | 1.15 | 0.28 | 2.07 | 0.06 | 1.68 | 0.12 | 2.74 | 0.02 |
| Right parietal | 0.75 | 0.492 | ||||||||||||||||
| Left temporal | 1.19 | 0.329 | ||||||||||||||||
| Right temporal | 1.09 | 0.350 | ||||||||||||||||
| Midline occipital | 4.84 | 0.004 | 2.79 | 0.02 | 1.38 | 0.2 | −0.49 | 0.64 | −2.35 | 0.04 | −4.12 | 0.002 | −2.64 | 0.02 | −1.04 | 0.32 | −0.57 | 0.58 |
Two-way repeated-measures ANOVA (8 time bins × 2 transitions) was performed. Corresponding F values and Greenhouse-Geisser-corrected P values are shown. Each significant interaction was followed up with individual t-tests and considered significant with a Bonferroni-corrected P value <0.00625. Corresponding t-values and P values are shown. ROIs with significant interactions followed by significant t-tests are highlighted in bold. Significant P values are highlighted in bold.
Fig. 7.
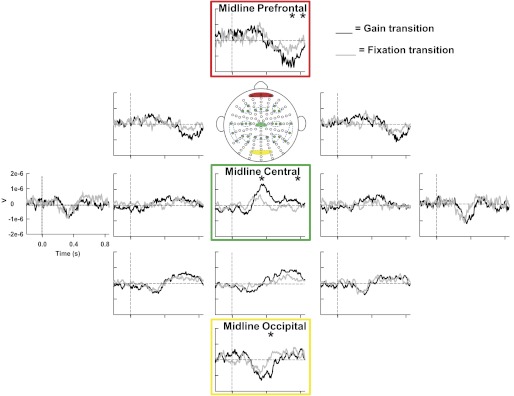
Experiment 2: grand-averaged ERPs of ROIs in the gain transition (black) and fixation transition (gray). Statistically significant ROIs are highlighted chronologically from earliest to latest time of significance (i.e., green to yellow to red). *Significant time bins within each ROI.
Source estimation.
The results of the source analysis for experiment 1 can be seen in Fig. 8. Specific brain regions corresponding to the observed solutions are overlaid onto an average brain included in the EMSE suite and distributed with SPM (Statistical Parametric Mapping). The intense red color indicates the most prominent source of activation from the three-dimensional distributed LORETA solution. Figure 8 shows the unthresholded current density values from the LORETA source analysis. The solution illustrated the strongest focus of activity in the left ventral premotor cortex from 300 to 400 ms after memory transition with the maximum current density values at X = −54, Y = 4, Z = 9. Also, there were strong sources identified in bilateral visual cortex and left motor cortex from 300 to 400 ms. From 400 to 500 ms after visual feedback was removed, left ventral premotor cortex was the strongest source at X = −54, Y = 8, Z = 11. While the left ventral premotor cortex had the highest current density values from 400 to 500 ms (Fig. 8), the two-dimensional voltage map showed the highest voltage in the anterior frontal cortex between 400 and 500 ms (Fig. 3). While there was positive voltage on the left side of the two-dimensional cortical voltage map between 400 and 500 ms that is consistent with left ventral premotor cortex (Fig. 3), the voltage was not as high as what we observed in the frontal area of Fig. 3. Finally, maximum activation from 500 to 600 ms after visual feedback removal was localized to the right ventral prefrontal cortex at X = 24, Y = 48, Z = −6 corresponding to BA10/11, although strong source activity can also be seen in the left ventral prefrontal cortex.
Fig. 8.
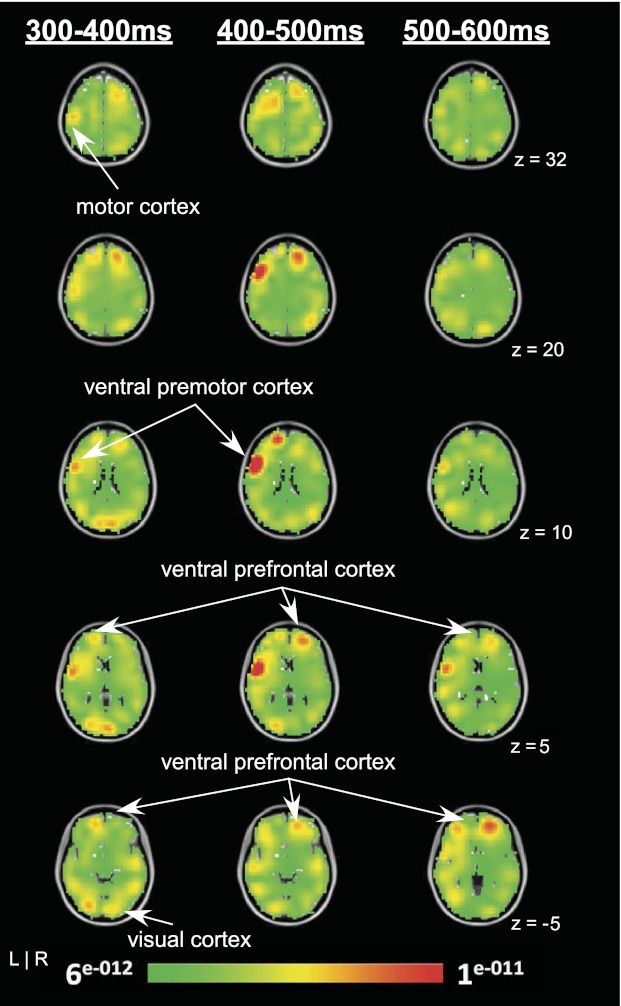
Grand-averaged ERP difference results of the LORETA analysis showing current density solutions from 300–400 ms (left), 400–500 ms (middle), and 500–600 ms time bins (right). Each map consists of axial slices showing the unthresholded source activation for each time period. L, left; R, right.
DISCUSSION
Experiment 1 of this study demonstrated the spatiotemporal pattern of brain activity during the transition from a visually guided to a memory-guided force control task. The primary finding of this study is that early event-related changes in activity of the left central and midline prefrontal regions are associated with memory-guided continuous force production. These changes occurred before any changes in behavior were detected. Further topographical analysis (LORETA) confirmed that the most prominent source of activity was localized to the left ventral premotor cortex (BA6) and right ventral prefrontal cortex (BA10/11). Decreasing the spatial gain of visual feedback in experiment 2 did not produce the same spatiotemporal pattern of brain activity as removing visual feedback, which supports our findings that subjects rely on early and rapid sensorimotor memory processes involving the ventral premotor cortex and ventral prefrontal cortex.
One of the novel findings in this study is that the first event-related activity was observed as early as 300 ms in the left central region after visual feedback was removed (experiment 1). Cortical localization (LORETA) identified the left ventral premotor cortex (BA6) as the brain region primarily contributing to the observed event-related activity between 300 and 400 ms after visual feedback was removed. Figure 8 also indicates source activity in the left motor cortex and bilateral visual cortex from 300 to 400 ms. It is possible that the memory transition effects could be related to a change in sustained cortical activity just preceding behavioral change, which would influence source localization. In addition to the well-established role of premotor cortex in motor function, this region has been associated with working memory processes (D'Esposito et al. 1998; Jonides et al. 1993; Owen et al. 2005). Premotor activation has been recorded during delayed-response memory tasks in humans (Baker et al. 1996; Jonides et al. 1993; Mecklinger et al. 2002). These prior studies have associated premotor activation with motor preparation and attention toward the target during a delayed-response memory condition. Another fMRI study was able to show ventral premotor activation contralateral to the dominant hand while retaining information about objects that require future motor actions (Mecklinger et al. 2004). Our result extends previous evidence to ventral premotor cortex involvement during grip force tasks in the active maintenance of motor requirements from memory.
The observed left-lateralized central positivity during memory-guided force control can also be related to the P300 component. The act of switching from a visually guided task to a memory-guided task could be related to the classic central-parietal P300 component that was proposed to reflect context updating and monitoring of working memory (Donchin 1981; Polich and Donchin 1988). A variety of studies have demonstrated changes in the topography of the P300 depending on the type of information processed in working memory (Lang et al. 1992; Mecklinger and Pfeifer 1996; Ruchkin et al. 1992) and changes in the amplitude of the P300 depending on the amount of information retained in working memory (Ruchkin et al. 1992). The observed scalp topography of a left-lateralized central P300 component in the present study is likely due to the fact that we studied a continuous motor memory task using the right hand, whereas prior studies that observed a P300 component in the central-parietal cortex focused on visual or auditory detection tasks (Picton 1992). Hence, our findings extend the previous evidence by showing a left-lateralized change in the P300 during the transition from visually guided to memory-guided force production.
Following the change in activity in the left central region, changes in the prefrontal cortex were identified as early as 400 ms into the memory-guided task. There was also considerable source activity in the anterior ventral prefrontal cortex, which was consistent with the voltage map in Fig. 3. We also observed that LORETA identified the strongest solution between 400 and 500 ms in left ventral premotor cortex (Fig. 8). This area was active between 400 and 500 ms but did not have the greatest voltage as shown in Fig. 3. Caution should be used when comparing voltage levels in two dimensions to the current density values in three dimensions. This issue was evident for the left ventral premotor cortex between 400 and 500 ms, where the correspondence between the peak activations in sensor space and source space were not the same. It is important to note that LORETA solutions are estimates and many factors and assumptions can affect the outcome of the LORETA solutions (Michel et al. 2004; Pizzagalli 2007). Some of these factors include the number of electrodes, whether the template was from individual subjects or a normalized template, and which anatomical constraints were placed on the solutions. The present study used a finite-element model, the template was MNI152, and LORETA solutions were constrained to the cortex.
From 500 to 600 ms, LORETA solutions identified the strongest focus of brain activity from the ventral prefrontal cortex (BA10/11). One of the early observations in monkeys was the sustained neural activity within the prefrontal cortex during the delay period of a delayed-response reaching task (Fuster 1973; Fuster and Alexander 1971). This prefrontal activity is also consistent with what has been previously identified in fMRI and PET studies during internally generated movements (Jenkins et al. 2000; Vaillancourt et al. 2003). Involvement of the prefrontal cortex has been shown in studies describing the prominent role of this region in various forms of working memory (i.e., visual, auditory, and tactile memory) (Curtis and D'Esposito 2003; Gallace and Spence 2009; Postle 2006). LORETA solutions are in line with the results of neuroimaging and neurophysiological studies indicating the specific activation of ventral prefrontal cortex during working memory tasks (Inoue et al. 2004; Owen et al. 2005; Rosenkilde et al. 1981; Wager and Smith 2003). Other support for ventral prefrontal involvement during working memory processes comes from evidence that shows that delayed-response memory performance is impaired in individuals with ventral prefrontal lesions (Meunier et al. 1997; Oscar-Berman 1975). Barbey and colleagues (Barbey et al. 2011) recently demonstrated the critical role of the ventral prefrontal cortex during working memory tasks that require multiple higher-order cognitive processing such as the n-back task. Because subjects in the present study had to actively maintain and monitor the isometric force demands during the transition from visually guided to memory-guided force control, our findings are consistent with Barbey and colleagues' study showing ventral prefrontal activity during tasks that require additional higher-order cognitive demands.
The unique spatiotemporal pattern of brain activity observed in this study provides support for a network extending across the frontal regions, including the ventral prefrontal cortex and ventral premotor cortex. These cortical areas could constitute part of a network that mediates motor memory processes. We further confirm the association of prefrontal event-related activity with memory-guided processes (experiment 1) because the same pattern of brain activity was not observed during reduced gain of visual feedback (experiment 2). These results support previously identified connections of ventral prefrontal and premotor cortices with the dorsolateral prefrontal cortex and posterior parietal cortex during tasks requiring memory in nonhuman primates (Goldman-Rakic 1988; Selemon and Goldman-Rakic 1988). Although changes in parietal activity were not observed before behavioral changes occurred, we did observe changes in parietal activity in experiment 1 by 700 ms after visual feedback was removed. This is in agreement with the strong basis of support for the dynamics between frontal and parietal cortices during memory-guided tasks as shown through single-unit recordings in primates (Chafee and Goldman-Rakic 2000; Nieder and Miller 2004). Chafee and Goldman-Rakic (2000) confirmed the reciprocal projections between prefrontal and parietal regions through cortical cooling in one region and single-unit recording of the other region during a visuomotor working memory task. Prefrontal and parietal cooling led to a significant impact on the neuronal activity of parietal and prefrontal regions, respectively. The temporal order of prefrontal activity followed by parietal activity has been demonstrated in nonhuman (Tomita et al. 1999) and human (Brass et al. 2005; Bunge et al. 2002) studies.
In summary, the high temporal resolution of EEG measures in combination with source localization provided novel insights into the spatiotemporal pattern of motor memory processing. This study demonstrates that subjects rely on sensorimotor memory processes during the absence of visual feedback and these processes correspond with changes in activity in ventral premotor cortex and ventral prefrontal cortex. These changes in ventral premotor and ventral prefrontal activity occurred prior to changes in behavioral force error and prior to any changes in the mean force output. These findings suggest that when subjects maintain force at a steady level shortly after the removal of visual feedback, they begin to rely upon specific neural processes in the premotor and prefrontal cortex. Since the premotor cortex and prefrontal cortex are affected both by aging and by specific neurological disorders, the present findings may provide insight into the brain mechanisms that underlie memory-guided motor output in health and in disease.
GRANTS
This research was supported in part by National Institute of Neurological Disorders and Stroke Grants R01-NS-58487, R01-NS-52318, and R01-NS-28127.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.P., S.A.C., and D.E.V. conception and design of research; C.P. and L.G.C.-C. performed experiments; C.P. analyzed data; C.P. and D.E.V. interpreted results of experiments; C.P. prepared figures; C.P. drafted manuscript; C.P., L.G.C.-C., S.A.C., D.M.C., and D.E.V. edited and revised manuscript; C.P., L.G.C.-C., S.A.C., D.M.C., and D.E.V. approved final version of manuscript.
REFERENCES
- Baker S, Frith C, Frackowiak R, Dolan R. Active representation of shape and spatial location in man. Cereb Cortex 6: 612–619, 1996 [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Orbitofrontal contributions to human working memory. Cereb Cortex 21: 789–795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender S, Behringer S, Freitag CM, Resch F, Weisbrod M. Transmodal comparison of auditory, motor, and visual post-processing with and without intentional short-term memory maintenance. Clin Neurophysiol 121: 2044–2064, 2010 [DOI] [PubMed] [Google Scholar]
- Binsted G, Rolheiser TM, Chua R. Decay in visuomotor representations during manual aiming. J Mot Behav 38: 82–87, 2006 [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci 17: 1367–1375, 2005 [DOI] [PubMed] [Google Scholar]
- Brignani D, Bortoletto M, Miniussi C, Maioli C. The when and where of spatial storage in memory-guided saccades. Neuroimage 52: 1611–1620, 2010 [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571, 2002 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol 83: 1550–1566, 2000 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature 386: 604–608, 1997 [DOI] [PubMed] [Google Scholar]
- Coombes S, Corcos D, Sprute L, Vaillancourt D. Selective regions of the visuomotor system are related to gain-induced changes in force error. J Neurophysiol 103: 2114–2123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci 4: 528–539, 2004 [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423, 2003 [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre G, Zarahn E, Ballard D, Shin R, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res 7: 1–13, 1998 [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs. external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19: 764–776, 2003 [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology 18: 493–513, 1981 [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 85: 2613–2623, 2001 [DOI] [PubMed] [Google Scholar]
- Elliott D, Madalena J. The influence of premovement visual information on manual aiming. Q J Exp Psychol A 39: 541–559, 1987 [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol 36: 61–78, 1973 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971 [DOI] [PubMed] [Google Scholar]
- Gallace A, Spence C. The cognitive and neural correlates of tactile memory. Psychol Bull 135: 380–406, 2009 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci 11: 137–156, 1988 [DOI] [PubMed] [Google Scholar]
- Grol MJ, Majdandzić J, Stephan KE, Verhagen L, Dijkerman HC, Bekkering H, Verstraten FA, Toni I. Parieto-frontal connectivity during visually guided grasping. J Neurosci 27: 11877–11887, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating the role of prefrontal and parietal cortices in memory-guided response with repetitive transcranial magnetic stimulation. Neuropsychologia 47: 295–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cereb Cortex 14: 106–119, 2004 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins I, Brown R, Marsden C, Passingham R, Brooks D. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain 118: 913–933, 1995 [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib M, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: 314–320, 1995 [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123: 1216–1228, 2000 [DOI] [PubMed] [Google Scholar]
- Johnston J, Rearick M, Slobounov S. Movement-related cortical potentials associated with progressive muscle fatigue in a grasping task. Clin Neurophysiol 112: 68–77, 2001 [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith E, Koeppe R, Awh E, Minoshima S, Mintun M. Spatial working-memory in humans as revealed by PET. Nature 363: 623–625, 1993 [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol 34: 337–347, 1971 [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci 14: 382–390, 2001 [DOI] [PubMed] [Google Scholar]
- Lang W, Starr A, Lang V, Lindinger G, Deecke L. Cortical DC potential shifts accompanying auditory and visual short-term memory. Electroencephalogr Clin Neurophysiol 82: 285–295, 1992 [DOI] [PubMed] [Google Scholar]
- Mayka M, Corcos D, Leurgans S, Vaillancourt D. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage 31: 1453–1474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex 6: 600–611, 1996 [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Gruenewald C, Besson M, Magnie M, Von Cramon D. Separable neuronal circuitries for manipulable and non-manipulable objects in working memory. Cereb Cortex 12: 1115–1123, 2002 [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Gruenewald C, Weiskopf N, Doeller C. Motor affordance and its role for visual working memory: evidence from fMRI studies. Exp Psychol 51: 258–269, 2004 [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Pfeifer E. Event-related potentials reveal topographical and temporal distinct neuronal activation patterns for spatial and object working memory. Brain Res Cogn Brain Res 4: 211–224, 1996 [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia 35: 999–1015, 1997 [DOI] [PubMed] [Google Scholar]
- Michel C, Murray M, Lantz G, Gonzalez S, Spinelli L, de Peralta R. EEG source imaging. Clin Neurophysiol 115: 2195–2222, 2004 [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex 12: 369–375, 2002 [DOI] [PubMed] [Google Scholar]
- Mueller VA, Brass M, Waszak F, Prinz W. The role of the preSMA and the rostral cingulate zone in internally selected actions. Neuroimage 37: 1354–1361, 2007 [DOI] [PubMed] [Google Scholar]
- Müller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience 139: 51–58, 2006 [DOI] [PubMed] [Google Scholar]
- Nieder A, Miller EK. A parieto-frontal network for visual numerical information in the monkey. Proc Natl Acad Sci USA 101: 7457–7462, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Turriziani P, Carlesimo GA, Koch G, Tomaiuolo F, Panella M, Caltagirone C. Parieto-frontal interactions in visual-object and visual-spatial working memory: evidence from transcranial magnetic stimulation. Cereb Cortex 11: 606–618, 2001 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. The effects of dorsolateral-frontal and ventrolateral-orbitofrontal lesions on spatial discrimination learning and delayed response in two modalities. Neuropsychologia 13: 237–246, 1975 [DOI] [PubMed] [Google Scholar]
- Owen A, McMillan K, Laird A, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging. Hum Brain Mapp 25: 46–59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol 24, Suppl C: 91–95, 2002 [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol 9: 456–479, 1992 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Handbook of Psychophysiology (3rd ed.), edited by Cacioppo JT. Cambridge, UK: Cambridge Univ. Press, 2007, p. 56–84 [Google Scholar]
- Polich J, Donchin E. P300 and the word frequency effect. Electroencephalogr Clin Neurophysiol 70: 33–45, 1988 [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 139: 23–38, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci 18: 1712–1722, 2006 [DOI] [PubMed] [Google Scholar]
- Rosenkilde CE, Bauer RH, Fuster JM. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res 209: 375–394, 1981 [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Grafman J, Canoune H, Ritter W. Distinctions and similarities among working memory processes: an event-related potential study. Brain Res Cogn Brain Res 1: 53–66, 1992 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber W, Doppelmayr M, Stadler W, Schabus M. The interplay between theta and alpha oscillations in the human electroencephalogram reflects the transfer of information between memory systems. Neurosci Lett 324: 121–124, 2002 [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin A, Vaillancourt D, Newell K. Intermittency in the control of continuous force production. J Neurophysiol 84: 1708–1718, 2000 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez P, Silberstein R. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng 45: 814–826, 1998 [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401: 699–703, 1999 [DOI] [PubMed] [Google Scholar]
- Vaillancourt D, Haibach P, Newell K. Visual angle is the critical variable mediating gain-related effects in manual control. Exp Brain Res 173: 742–750, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt D, Russell D. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res 145: 275–285, 2002 [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol 90: 3330–3340, 2003 [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3: 255–274, 2003 [DOI] [PubMed] [Google Scholar]


