Abstract
Epidemiological studies have demonstrated that heavy drinking and alcohol abuse and dependence peak during the transition between late adolescence and early adulthood. The objective of the present study was to determine whether a model of early onset adolescent ethanol drinking exposure that is followed by an ethanol vapor regimen during late adolescence and young adulthood leads to an increase in drinking in adulthood. In this model, initiation of voluntary ethanol drinking in adolescence, using a sweetened solution, was followed by an 8-wk intermittent ethanol vapor regimen in Wistar rats. A limited-access two-bottle choice paradigm was then used to measure intake of a 10% (w/v) ethanol solution. No differences in water intake (g/kg), total fluid intake (ml/kg) and body weight (g) were observed between air-exposed and ethanol-vapor exposed groups during the pre-vapor and post-vapor phases. The eight wks of ethanol vapor exposure was found to produce only a modest, but statistically significant, elevation of ethanol intake during the protracted withdrawal period, compared to air-exposed rats. A significant increase in ethanol preference ratio was also observed in ethanol-vapor exposed rats during the sucrose-fading phase, but not during the protracted withdrawal period. The findings from the present study suggest that in addition to alcohol exposure, environmental variables that impact appetitive as well as consumptive behaviors may be important in developing robust drinking effects that model, in animals, the increased risk for alcohol dependence seen in some human adolescents who begin drinking at an early age.
Keywords: Adolescence, Dependence, Ethanol Consumption, Ethanol Vapor, Ethanol Intake, Protracted Withdrawal
1. Introduction
Adolescence is a critical time period for brain development when cognitive, emotional and social maturation occur (see Dahl and Spear, 2004). The 2007 National Survey on Drug Abuse and Health has reported that approximately 16% of teens between the age of 12 and 17 were current users of ethanol, with 10% of these individuals classified as binge drinkers (U.S. Department of Health and Human Services, 2008). Additionally, underage college students have been shown to be more likely to drink to excess, when they drank, than their older peers (Wechsler et al., 2002). Evidence from the Monitoring the Future (MTF) study showed that 30-day prevalence and heavy drinking in men peaks at ages 21-22 and then declines linearly into adulthood (Bachman et al., 1997). Consistent with these findings, Grant and colleagues (Grant et al., 2004) reported that individuals within the ages 18-29 exhibit the highest rates of past-year ethanol abuse and dependence.
For some individuals ethanol use during early adolescence is clearly a risk factor for the later development of alcohol dependence (Ehlers et al., 2006; Grant, 1998; Grant and Dawson, 1997; Hicks et al., 2010; Hingson et al., 2008). How early adolescent drinking causes an increased risk for alcohol dependence in some individuals is not known. One hypothesis posits that early heavy drinking can disrupt the normal course of social and intellectual development leading to an increased risk for a number of social and psychological pathologies including drug addictions (DeWit et al., 2000; York, 1999). An alternate hypothesis is that some individuals who initiate drinking during early adolescence may be more likely to have an underlying predisposition to disinhibitory behavior and psychopathology that drives their early drinking (Iacono et al., 2002; Jessor and Jessor, 1977). These hypotheses are difficult to disentangle in human studies; however, the development of an animal model in order to study the effects of adolescent ethanol exposure on drinking behaviors in adulthood could be useful in the understanding of the brain mechanisms underlying the effects of early adolescent drinking.
The adolescent period in rodents has many similarities to the human condition making it a good model to study the short- and long-term consequences of adolescent ethanol exposure (Spear, 2000b, 2000c; Spear and Varlinskaya, 2005). Variables that have been used to investigate adolescent drinking patterns in animal models include sex, age, ethanol fluid concentration, isolate-housing and use of different sipper-tube types in paradigms with 24-hr access to ethanol solutions (Bell et al., 2006; Brunell and Spear, 2005; Doremus et al., 2005; Ehlers et al., 2007; Fullgrabe et al., 2007; Lancaster et al., 1996; Siciliano and Smith, 2001). Studies characterizing developmental differences in drinking patterns indicate that adolescent rats show greater levels of ethanol intake than adult rats (Brunell and Spear, 2005; Doremus et al., 2005; Spear, 2004, 2007; Vetter et al., 2007; Vetter-O'Hagen et al., 2009). In studies of alcohol preferring rats (AA) male AA rats were shown to decrease their ethanol consumption with age (Sarviharju et al., 2001).
Studies using animal models have also indicated that voluntary ethanol drinking during adolescence can, in some models, be shown to facilitate the acquisition of alcohol self-administration, increase craving behavior, and increase the probability of relapse in adults (see McBride et al., 2005; Spear, 2000a; Gilpin et al., 2012). However, other studies have shown that ethanol exposure during adolescence has no effect on subsequent ethanol consumption in adulthood. For instance, Vetter et al. (2007) found that adult rats trained to drink ethanol during adolescence showed no differences in ethanol drinking when compared to a control group not exposed to ethanol during adolescence. Additionally, Siegmund et al., (2005) have shown that Wistar rats that initiated alcohol consumption during adolescence, when not exposed to stress, actually consumed less alcohol and showed lower preference than rats who were initiated into drinking as adults. The reason that disparate findings have been obtained between studies is at this point is not clear. Potentially important factors include: the strain of the rats, whether the alcohol exposure during adolescence was voluntary, the length of alcohol exposure, the effects of stress, and the dose of alcohol.
Since in humans drinking typically begins during adolescence but then continues during young adulthood and then declines into later adulthood, studying a model that assesses the consequences of ethanol dependence during a period that includes late adolescence and young adulthood could provide insight into the importance of this transition period on the maintenance of ethanol drinking patterns in adulthood. One potential approach to developing such an animal model is based on experiments in adult animals that assess whether alcohol exposure, that is high enough to induce symptoms associated with withdrawal, is important in the development and maintenance of subsequent ethanol addiction (see Koob and LeMoal, 2008). Typically such a model uses intermittent exposure to ethanol vapor to study the increase in voluntary consumption and self-administration of ethanol following periods of abstinence in animals with a history of prolonged vapor exposure (see Heilig et al., 2010). Findings from studies in adult rats have shown that chronic intermittent ethanol vapor exposure produces a robust increase in voluntary ethanol consumption (Rimondini et al., 2002a, 2002b, 2003; Sommer et al., 2008; Thorsell et al., 2005a) and operant ethanol self-administration (O'Dell et al., 2004; Roberts et al., 1996, 2000; Thorsell et al., 2005b; Walker and Koob, 2007). However, studies evaluating the effects of exposure to 2 wks of alcohol vapor in male Sprague-Dawley rats during adolescence on subsequent drinking behavior revealed that the alcohol exposed animals did not show increases in drinking in adulthood, even after noise stress (Slawecki and Betancourt, 2002). It was suggested by the authors that forced exposure to ethanol vapor during adolescence does not seem to be sufficient to alter the initiation or maintenance of ethanol self-administration (Slawecki and Betancourt, 2002).
There is evidence to suggest that intermittent ethanol vapor requires a minimum duration of ethanol exposure in order to produce a lasting upregulation of ethanol preference in adult rats. For instance, studies have shown that a minimum duration of intermittent ethanol vapor exposure is needed to produce a lasting increase of ethanol preference in adult rats (e.g., O'Dell et al., 2004; Rimondini et al., 2002a; Slawecki and Betancourt, 2002; Sommer et al., 2008). Taken together these findings suggest that (1) a longer duration of ethanol vapor exposure may be required in adolescent rats to produce long-lasting effects in ethanol drinking and (2) that it may be necessary to initiate voluntary drinking in rats prior to vapor exposure during adolescence in order to see subsequence increases in drinking in adulthood. Therefore, the present study evaluated a model of early onset ethanol drinking that is followed by an ethanol vapor exposure regimen during late adolescent/young adulthood designed to resemble the pattern of heavy drinking and alcohol-related problems and dependence shown to peak during late adolescence and early adulthood and to decline linearly into adulthood (Bachman et al., 1997; Baer, 1993; Grant et al., 2004; Johnston et al., 2001a, 2001b). Rats in the present study were exposed to an 8-wk intermittent ethanol vapor regimen shown to produce behavioral, neurophysiological and neurochemical changes that persist into the late protracted withdrawal period in adult Sprague-Dawley and Wistar rats (Criado and Ehlers, 2010; Criado et al., 2008a, 2008b, 2011; Slawecki, 2002; Slawecki et al., 2001).
The present report is part of a larger study characterizing the risks and consequences of adolescent ethanol drinking in animal models and humans (Criado et al., 2008b; Ehlers et al., 2006; Pian et al., 2008a, 2008b, 2010; Slawecki et al., 2001; Walker et al., 2008). These experiments were designed to investigate whether initiation of voluntary drinking followed by prolonged exposure to ethanol vapor during late adolescence/young adulthood increases or decreases adult ethanol intake during protracted withdrawal relative to: (1) pre-exposure ethanol drinking levels; and (2) to an air-exposed control group. The working hypothesis of this study is that initiation of voluntary ethanol drinking in adolescence followed by an 8-wk intermittent ethanol vapor regimen during late adolescence and young adulthood would produced a significant increase in ethanol intake in adult Wistar rats during protracted withdrawal.
2. Materials and methods
2.1. Subjects
Thirty-six male Wistar rats (Charles River, Wilmington, MA) were used in the present study and were 23 days of age (P23) on arrival. The animals were pair housed in standard home cages measuring 25 cm wide × 20 cm high × 45 cm long in a temperature-controlled room maintaining a 12 hr light/dark cycle (lights on at 6am). Upon arrival, animals were weighed and handled daily. Ad libitum food and water were provided for the duration of the experiment, except during the 30-min limited-access sessions. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute's Institutional Animal Care and Use Committee.
2.2. General Procedure
All limited-access drinking tests occurred two hrs after the onset of the light phase of the light/dark cycle, which has previously been shown to promote enhanced ethanol consumption in adolescent animals (Walker et al., 2008). Animals were weighed and transported to the testing room 30 min prior to the initiation of the two-bottle choice sessions and 10 min prior to the start of the session were transferred to plastic cages [(25 (w) × 20 (h) × 45 cm (l)] with wire bar cage tops that were separated into two equal-sized compartments using a Plexiglas divider. Solutions were presented using 100 ml graduated cylinders (Nalgene Labware, Rochester, NY) fitted with curved ball-point sipper tubes (Ancare, Bellmore, NY). Animals were given 30 min of access to the solutions without food availability, after which the cages were cleaned with 70% ethanol. Following the 30-min limited-access sessions, animals were returned to the vivarium.
2.3. Acquisition of Sweetened Ethanol Drinking
Acquisition of ethanol drinking took place during adolescence (P25-P43) and was based on an adaptation of Samson's sweetened fading procedure (Samson, 1986). Two bottles were presented: one contained water and the other contained a sucrose solution (w/v) comprised of 10% sucrose (10S) and tap water. Ethanol (95%; Gold Shield Chemicals; Hayward, CA) was added to the sucrose solution until the appropriate concentration (w/v) was reached. The order and number of consumption sessions was as follows: 10S (2 days), 10S + 1% ethanol (10S1E, 1 day), 10S + 2.5% ethanol (10S2.5E, 1 day), 10S + 5% ethanol (10S5E ethanol, 4 days) and 10S + 10% ethanol (10S10E, 5 days). Figure 1 shows a diagram of the experimental design. Bottle position alternated daily to avoid position preference. After the 5 days of 10S10E access, the animals were subjected to 8 wks of intermittent ethanol vapor exposure.
Figure 1. Diagram of the experimental design.
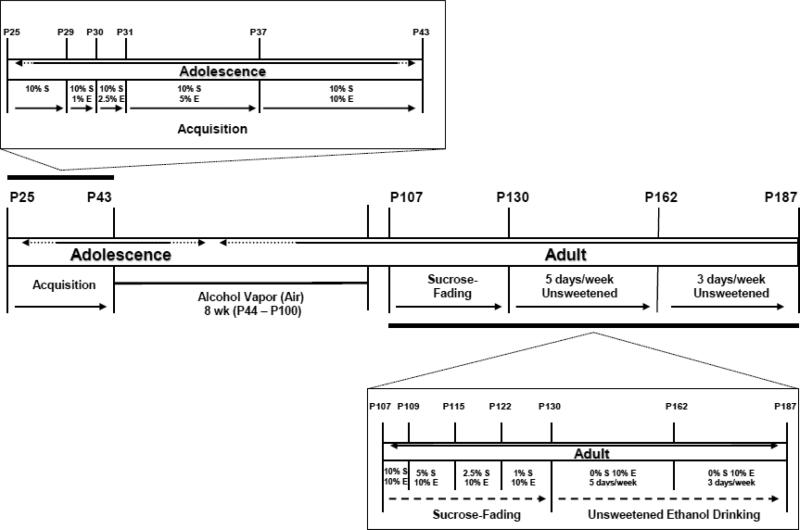
The model consists of an acquisition phase characterized by an early onset ethanol drinking during adolescence (P25 – P43). This is followed by exposure to an 8-wk intermittent ethanol vapor regime (P44 – P100) during late adolescent/young adulthood (control rats were exposed to air). Rats were then assessed in the Post-Ethanol Vapor phase during adulthood in three different stages: Sucrose-Fading (P107-P127), 5 days/wk Unsweetened Ethanol Drinking (P130-P158) and 3 days/wk Unsweetened Ethanol Drinking (P162-P187).
2.4. Intermittent Ethanol Vapor Exposure
Ethanol vapor exposure has been shown to reliably allow for the titration of blood ethanol concentrations (BEC) that are sufficient for inducing behavioral changes that are reflective of ethanol withdrawal-like symptoms (e.g., O'Dell et al., 2004; Roberts et al.,1996; Walker et al., 2008). In this paradigm, BECs can be easily titrated by the experimenter to fit established criterion and the animals show normal weight gain and are freely moving (Rogers et al., 1979). Standard rat cages were sealed and ethanol vapor or air was pumped through the chambers. Ethanol vapor was created by dripping 95% ethanol into 2000ml Erlenmeyer flasks that remained at 50°C due to a warming tray. Air (11L/min) was passed over the bottom of the flask so that when the ethanol hits the warm glass and was vaporized, the air carried it into the vapor chamber. Alteration of the ethanol vapor concentration was accomplished by modulating the air flow carrying the ethanol vapor into the chamber. The animals were subjected to intermittent vapor exposure (14 hrs on /10 hrs off) over the course of 8 wks (from P44 – P100) (Figure 1). Target BECs were 175-250 mg% across the 8-wk exposure period and were determined by sampling blood collected from the tail (0.5 ml) weekly, generating a mean BEC for the 8-wk exposure period. Following centrifugation, plasma ethanol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
2.5. Post-Vapor Ethanol Drinking
Following the 8-wk vapor exposure period, the animals were again allowed daily 30 min limited-access sessions (Mon- Fri) as described above. However, to investigate the effect of vapor exposure on protracted abstinence, the limited-access sessions were initiated one wk after the ethanol-vapor exposure terminated. Thus, the animals never experienced ethanol drinking during acute withdrawal (i.e., within the first 48 hrs after the vapor exposure was completed). Initially, the solutions provided to the animals during the limited-access sessions were sweetened ethanol solutions. Over the course of 15 sessions, the sweetener was slowly faded out according to the following schedule: 10S10E (2 days), 5S10E (4 days), 2.5S10E (5 days) and 1S10E (4 days) (Figure 1).Subsequent to the removal of the sweetener, the animals were allowed to drink 10% ethanol five days per wk (Mon. – Fri.) for 20 sessions during 30 min limited-access sessions. Next, the animals were allowed to drink 10% ethanol three days per wk (Mon., Wed. and Fri.) for an additional nine sessions (Figure 1).
2.6. Data Analysis
Statistical analyses were performed by using SPSS (SPSS, Inc., Chicago, IL). Values are mean ± standard error of the mean (SEM). Data analyses were performed independently on body weight (g), ethanol intake (g/kg), water intake (g/kg), total fluid intake (ml/kg; water intake (ml) + ethanol solution intake (ml)/body weight (kg)) and ethanol preference ratio (ethanol intake/ethanol intake + water intake). The study was divided into two phases: Pre-Ethanol Vapor and Post-Ethanol Vapor (Figure 1). Body weight, ethanol preference and consumption levels in the Pre-Ethanol Vapor phase were determined in the following groups (and age): 10S1E (P29), 10S2.5E (P30), 10S5E (P31-P33, P36) and 10S10E (P37-P40, P43). Dependent variables in the Post-Ethanol Vapor phase were further divided into three different stages: Sucrose-Fading (P107-P127), 5 days/wk Unsweetened Ethanol Drinking (P130-P158) and 3 days/wk Unsweetened Ethanol Drinking (P162-P187). Groups consisting of more than one session were averaged in the Pre-Ethanol Vapor and in the Post-Ethanol Vapor phase. In both Unsweetened Ethanol Drinking stages of the Post-Ethanol Vapor phase, sessions were averaged by wk.
A mixed-model two-way analysis of variance (ANOVA) was used to assess body weight, ethanol intake, water intake, total fluid intake and ethanol preference during both Pre- and Post-Ethanol Vapor phases. Treatment (ethanol vapor or air) was assessed as a between subject variable. Session was assessed as within subject repeated measures. If violation of sphericity in Mauchly's test was found Greenhouse-Geisser corrected p-values were reported for all repeated measures analyses. Post hoc analysis of two-way ANOVA utilized independent one-way ANOVA. For these analyses, p-value was set at p < 0.05 to determine the levels of statistical significance.
A two-way ANOVA (treatment (ethanol vapor or air) × time period (pre or post ethanol vapor)) was used to compare the levels of ethanol intake, water intake, total fluid intake and ethanol preference before and after ethanol vapor exposure. Baseline levels of ethanol intake were obtained in each group by averaging the final 2 days of ethanol intake prior to the initiation of ethanol vapor exposure (10S10E). Post-vapor ethanol intake levels were determined in each group by averaging the first 2 days of ethanol intake (10S10E). Greenhouse-Geisser corrected p-values were reported. Post hoc analysis of two-way ANOVA utilized independent one-way ANOVA by comparing baseline vs. post-ethanol vapor values in each group. If comparisons between baseline and post-vapor levels show statistically significant differences in both air-exposed and ethanol vapor-exposed groups, the percent change in the ethanol preference ratio from baseline following their respective treatments was used to compare both groups with an independent one-way ANOVA. An independent one-way ANOVA was also used to determine whether the reduction (percent change) in ethanol intake following sucrose removal from solution was significantly different between groups. For these analyses, p-value was set at p < 0.05 to determine the levels of statistical significance.
3. Results
3.1. Blood Ethanol Concentrations (BECs)
The BECs during the 8-wk exposure period were maintained with the required sustained BECs (>175 mg/dl) and a mean BEC of 208 ± 6 mg% (n = 14). Determination of BEC during the intermittent ethanol vapor regimen showed the following levels: day 6 (P49) = 119 ± 13 mg/dl; day 13 (P56) = 177 ± 16 mg/dl; day 20 (P63) = 198 ± 14 mg/dl; day 25 (P68) = 204 ± 16 mg/dl; day 30 (P73) = 238 ± 19 mg/dl; day 38 (P81) = 240 ± 12 mg/dl; day 42 (P85) = 243 ± 24 mg/dl; day 49 (P92) = 242 ± 16 mg/dl; day 55 (P98) = 212 ± 12 mg/dl. Due to a technical problem with a single ethanol vapor chamber, four animals from the vapor-exposed group had to be removed from the study.
3.2. Body weight
During the acquisition of ethanol drinking the two-way ANOVA revealed no significant differences between treatment groups (Table 1). The effects of intermittent ethanol vapor on body weight were also assessed at 2 wks (P57) and 6 wks (P86) during the vapor regimen and 2 days after completion of the vapor regimen (P102). Independent one-way ANOVAs showed no significant differences (F's(1,32) < 1.7; p's > 0.05) in body weight between control and ethanol vapor-exposed rats at P57 (control: 308 ± 6; ethanol: 298 ± 9), P86 (control: 438 ± 8; ethanol: 419 ± 12) and P102 (control: 478 ± 10; ethanol: 474 ± 13).
Table 1.
Mean body weight (g ± SEM).
| Exposed | Stage Ethanol Vapor- | Session | Air-Exposed | |
|---|---|---|---|---|
| Pre-Ethanol Vapor Phase | Acquisition of Sweetened Ethanol Drinking | 10S1E | 89 ± 2 | |
| 90 ± 3 | ||||
| 10S2.5E | 96 ± 3 | |||
| 97 ± 3 | F(1.1,34.1) = 0.7, p > 0.05 | |||
| 10S5E | 122 ± 3 | |||
| 123 ± 4 | ||||
| 10S10E | 175 ± 4 | |||
| 178 ± 5 | ||||
| Post-Ethanol Vapor Phase | Ethanol Drinking Sucrose-Fading | 10S10E | 500 ± 11 | |
| 496 ± 13 | ||||
| 5S10E | 507 ± 11 | |||
| 504 ± 13 | F(1.3,42.8) = 1.6, p > 0.05 | |||
| 2.5S10E | 518 ± 11 | |||
| 517 ± 13 | ||||
| 1S10E | 531 ± 12 | |||
| 531 ± 14 | ||||
| Unsweetened: Ethanol Drinking 5 Days/wk | 0S10E WK 1 | 545 ± 12 | ||
| 546 ± 14 | ||||
| 0S10E WK 2 | 560 ± 12 | |||
| 566 ± 15 | F(1.3,41.9) = 6.2, p < 0.05 | |||
| 0S10E WK 3 | 570 ± 13 | |||
| 579 ± 15 | ||||
| 0S10E WK 4 | 579 ± 13 | |||
| 588 ± 16 | ||||
| Unsweetened: Ethanol Drinking 3 Days/wk | 0S10E 3X WK 1 | 590 ± 13 | ||
| 596 ± 16 | ||||
| 0S10E 3X WK2 | 597 ± 14 | |||
| 606 ± 16 | F(1.3,42.5) = 1.5, p > 0.05 | |||
| 0S10E 3X WK3 | 604 ± 14 | |||
| 614 ± 17 | ||||
10S1E = 10% Sucrose + 1% Ethanol. 10S2.5E = 10% Sucrose + 2.5% Ethanol. 10S5E = 10% Sucrose + 5% Ethanol. 10S10E = 10% Sucrose + 10% Ethanol. 5S10E = 5% Sucrose + 10% Ethanol. 2.5S10E = 2.5% Sucrose + 10% Ethanol. 1S10E = 1% Sucrose + 10% Ethanol. 0S10E = 0% Sucrose + 10% Ethanol. Air-Exposed (Pre-Vapor, n = 20; Post-Vapor, n = 18). Ethanol Vapor-Exposed (Pre- and Post-Vapor, n = 14).
A mixed-model two-way ANOVA conducted on body weight showed a significant session × treatment interaction during the 5 days/wk Unsweetened Ethanol Drinking stage (Table 1). However, post hoc assessment revealed no significant differences between control and ethanol vapor-exposed rats. During the Sucrose-Fading and 3 days/wk Unsweetened Ethanol Drinking the two-way ANOVA revealed no significant differences between treatment groups (Table 1).
3.3. Ethanol intake
Means and SEM for the four groups of sessions (10S1E, 10S2.5E, 10S5E, 10S10E) during the acquisition of sweetened ethanol drinking in the Pre-Ethanol Vapor phase of the study are shown in Figure 2A. The two-way ANOVA conducted on ethanol intake (g/kg) revealed no significant interaction of session × treatment (F(3, 96) = 0.5; p > 0.05; Figure 2A) and no main effect of treatment (F(1,32) = 0.2; p > 0.05). However, two-way ANOVA showed a main effect main effect of session (F(3,96) = 60.8; p < 0.001), which indicate that consumption changed over time.
Figure 2.
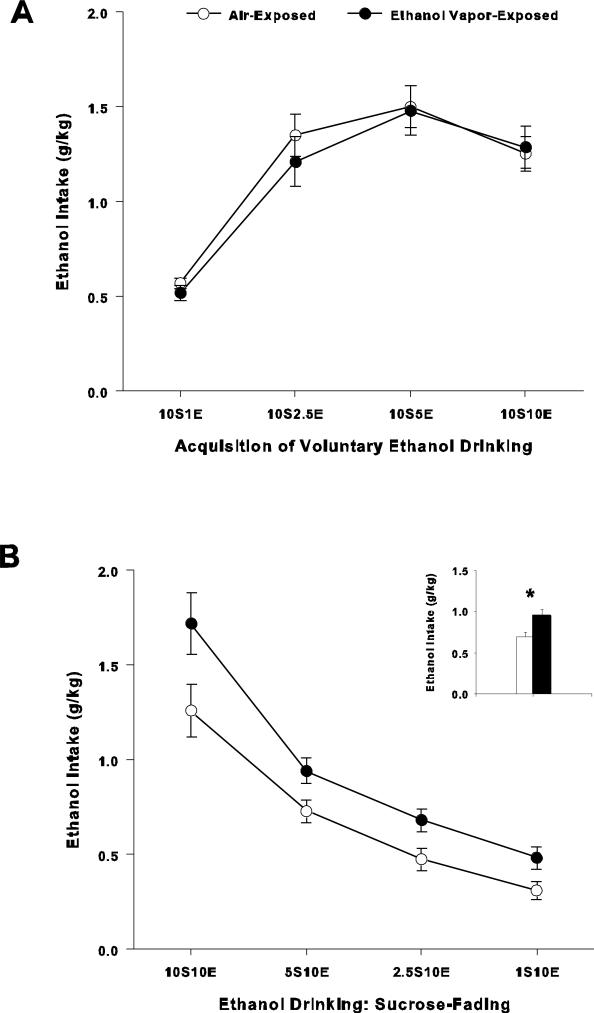
Ethanol intake during Pre-Vapor and Sucrose-Fading phases. A. Acquisition of sweetened ethanol drinking during the pre-vapor phase. Mean (+/- S.E.M.) ethanol intake (g/kg) while consuming a sweetened ethanol solution preceding ethanol vapor-exposure. Concentrations of the solutions for each of the 11 sessions (30-min per session) are as follows: 10S1E (1 session), 10S2.5E (1 session), 10S5E (4 sessions) and 10S10E (5 sessions). Air-Exposed (n = 20); Ethanol Vapor-Exposed (n = 14). Inset: Mean ± S.E.M. ethanol intake (g/kg) of the 11 sessions prior to the 8-wk ethanol vapor or air exposure periods. B. Sweetened ethanol consumption patterns during the “fade-out” of the sweetener following ethanol-vapor withdrawal. Sweetened ethanol intake (g/kg) following 8-wk of intermittent ethanol vapor-exposure. Concentrations of the solutions for each of the 15 sessions (30-min per session) are as follows: 10S10E (2 sessions), 5S10E (4 sessions), 2.5S10E (5 sessions) and 1S10E (last 4 sessions). Air-Exposed (n = 18); Ethanol Vapor-Exposed (n = 14). Inset: Mean ± S.E.M. ethanol intake (g/kg) of the 15 sessions following the 8-wk ethanol vapor or air exposure periods (* = p < 0.05).
Figure 2B displays the consumption patterns during the Sucrose-Fading stage that occurred one wk following cessation of ethanol vapor exposure. The two-way ANOVA indicated no significant session × treatment interaction (F(1.3, 38.9) = 1.8; p > 0.05). However, statistical analyses showed significant main effects of session (F(1.3,38.9) = 91.2; p < 0.001; Figure 2B) and treatment (F(1, 30) = 7.9; p < 0.01; Figure 2B). The significant effect of treatment indicates that rats exposed to ethanol vapor consumed larger amounts of ethanol than control rats exposed to air. These results also showed that although the consumption pattern changed over time comparably for both groups, air-exposed and ethanol vapor-exposed showed statistically significant differences during this stage.
Figure 3A shows five days per wk consumption of 10% ethanol (5 days/wk Unsweetened Ethanol Drinking). The reduction in ethanol consumption between termination of the Sucrose-Fading stage (1S10E) and 5 days/wk Unsweetened Ethanol Drinking (0S10E: Week 1) were similar between groups (control: -31.6 ± 5%; ethanol: -33 ± 6%; F(1,31) = 0.04, p > 0.05). While the two-way ANOVA revealed no session × treatment interaction (F(3, 90) = 0.6; p > 0.05), it showed a significant main effect of treatment (F(1, 30) = 4.7; p < 0.05; Figure 3A). Moreover, statistical analyses showed a significant main effect of session (F(3, 90) = 4.1; p < 0.01). These results indicate that the escalated intake patterns observed during the Sucrose-Fading stage and increase in ethanol intake in rats exposed to ethanol vapor were maintained once 10% ethanol alone was provided for voluntary drinking.
Figure 3.
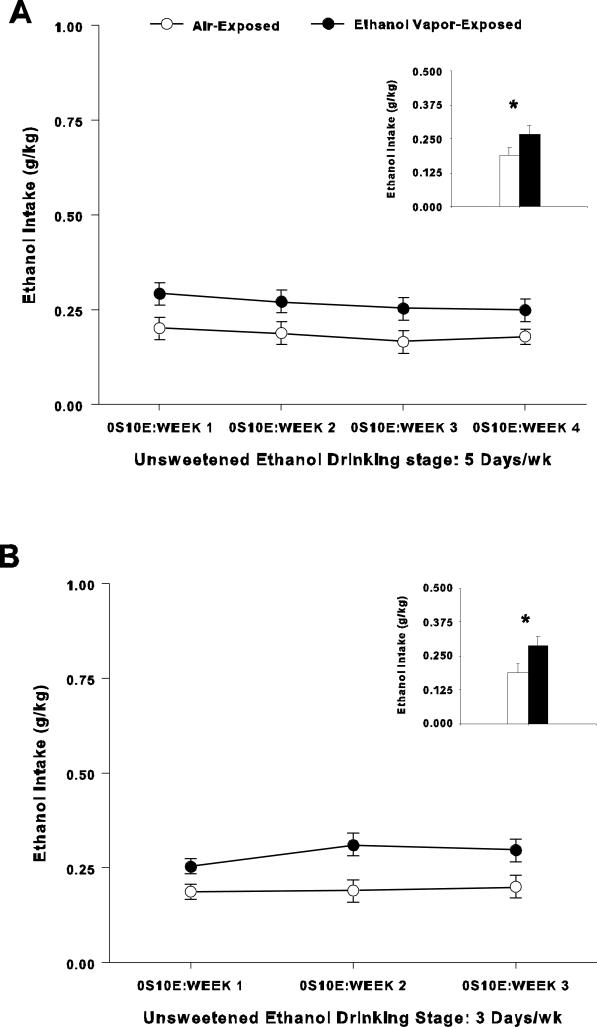
Ethanol intake during 5 days/wk and 3 days/wk Unsweetened Ethanol Drinking phases. A. Unsweetened ethanol drinking during late protracted withdrawal when tested five days per wk. Unsweetened 10% ethanol intake (g/kg) over 4 wks (20 sessions, 30-min per session; mean ± SEM are weekly averages) following ethanol-vapor withdrawal. Air-Exposed (n = 18); Ethanol Vapor-Exposed (n = 14). Inset: Mean ± S.E.M. ethanol intake (g/kg) of the 20 sessions following the 8-wk ethanol vapor or air exposure periods (* = p < 0.05). B. Unsweetened ethanol drinking during late protracted withdrawal when tested three days per wk. Unsweetened 10% ethanol intake (g/kg) over three wks (9 sessions, 30-min per session, mean ± SEM are weekly averages) following ethanol-vapor withdrawal. Air-Exposed (n = 18); Ethanol Vapor-Exposed (n = 14). Inset: Mean ± S.E.M. ethanol intake (g/kg) of the 9 sessions following the 8-wk ethanol vapor or air exposure periods (* = p < 0.05).
Results for the last stage of the Post-Ethanol Vapor phase of the experiment (3 days/wk Unsweetened Ethanol Drinking) are shown in Figure 3B. The two-way ANOVA showed no session × treatment interaction (F(2, 60) = 2.6; p > 0.05). However, a significant main effect of treatment was found indicating that rats exposed to ethanol vapor consumed larger amounts of ethanol than control rats (F(1, 30) = 6.0; p < 0.05; Figure 3B). Statistical analyses also showed a significant main effect of session (F(2, 60) = 4.5; p < 0.05). These findings are consistent with results in earlier stages of the Post-Ethanol Vapor phase. These results also show that group differences can persist for up to 10 wks into protracted abstinence following intermittent ethanol vapor exposure.
Baseline consumption of sweetened ethanol (10S10E) during the final two sessions prior to vapor exposure averaged 1.3 ± 0.1 g/kg in air exposed control rats and 1.5 ± 0.2 g/kg in ethanol vapor-exposed rats. Post-vapor sweetened ethanol (10S10E) intake levels averaged 1.3 ± 0.1 g/kg in air exposed control rats and 1.7 ± 0.2 g/kg in ethanol vapor-exposed rats. Analysis of the levels of ethanol intake (10S10E) between air-exposed and ethanol vapor-exposed groups before and after ethanol vapor exposure showed no significant interaction of treatment × time period (F(1,30) = 0.9; p > 0.05).
3.4. Ethanol preference ratio
Two-way ANOVA conducted on ethanol preference ratios during the Pre-Vapor phase in air-exposed (n = 20) and ethanol vapor-exposed (n = 14) groups revealed no significant interaction of session × treatment (F(1.7,55.1) = 2.2, p > 0.05) and no main effect of treatment (F(1,32) = 1.3, p > 0.05). Statistical analyses during the Post-Vapor phase (air-exposed, n = 18; and, ethanol vapor-exposed, n = 14) showed no significant session × treatment interaction during the Sucrose-Fading stage (F(1.8, 54.4) = 1.4; p > 0.05). However, a significant main effect of treatment was found indicating that rats exposed to ethanol vapor showed greater ethanol preference ratios than control rats exposed to air (control: 0.62 ± 0.03; ethanol: 0.73 ± 0.03; F(1, 30) = 6.0; p < 0.05). In contrast, statistical analyses showed no significant session × treatment interaction during the last two stages following intermittent ethanol vapor exposure: 5 days/wk Unsweetened Ethanol Drinking (F(2.5,73.7) = 0.7, p > 0.05) and 3 days/wk Unsweetened Ethanol Drinking (F(2,60) = 0.2, p > 0.05). Consistent with these findings, no main effect of treatment was observed during these stages (F's(1,30) < 1.3; p's > 0.05).
Analysis of ethanol preference ratios in control and ethanol-exposed groups before and after vapor exposure showed a significant interaction of treatment × time period (F(1,30) = 4.7; p < 0.05). Post hoc analyses revealed that control rats showed an increase in the ethanol preference ratio following air vapor exposure, compared to the baseline ethanol preference ratio (baseline: 0.76 ± 0.02; post air vapor: 0.85 ± 0.01; F(1, 17) = 30.8; p < 0.0001). Post hoc analyses also showed that ethanol vapor-exposed rats had an increase in the ethanol preference ratio following vapor exposure, compared to the baseline ethanol preference ratio (baseline: 0.76 ± 0.02; post air vapor: 0.90 ± 0.02; F(1, 13) = 57.0; p < 0.0001). Comparison of the percent change between both groups suggests a trend toward an increase in the ethanol preference ratio in ethanol vapor-exposed rats in comparison to air-exposed rats (ethanol vapor-exposed: 20.2 ± 3.2%; post air vapor: 12.8 ± 2.4%; F(1, 30) = 3.5; 0.05 < p < 0.10).
3.5. Water and Total Fluid intakes
Two-way ANOVAs were conducted on water intake (g/kg) during the Pre-Vapor phases revealed no significant differences between treatment groups (Table 2). Analysis of the levels of water intake between air-exposed and ethanol vapor-exposed groups before and after ethanol vapor exposure showed no significant interaction of treatment × time period (F(1,32) = 1.3; p > 0.05).
Table 2.
Mean water intakes (g/kg ± SEM)
| Exposed | Stage Ethanol Vapor- | Session | Air-Exposed | |
|---|---|---|---|---|
| Pre-Ethanol Vapor Phase | Acquisition of Sweetened Ethanol Drinking | 10S1E | 7.6 ± 3.8 | |
| 9.0 ± 0.7 | ||||
| 10S2.5E | 6.0 ± 0.4 | |||
| 6.0 ± 0.5 | F(2.0,62.0) = 0.9, p > 0.05 | |||
| 10S5E | 5.5 ± 0.3 | |||
| 5.7 ± 0.3 | ||||
| 10S10E | 4.5 ± 0.6 | |||
| 5.2 ± 0.7 | ||||
| Post-Ethanol Vapor Phase | Ethanol Drinking Sucrose-Fading | 10S10E | 1.6 ± 0.1 | |
| 1.5 ± 0.1 | ||||
| 5S10E | 3.3 ± 0.5 | |||
| 2.0 ± 0.5 | F(1.9,56.2) = 2.7, p > 0.05 | |||
| 2.5S10E | 5.2 ± 0.8 | |||
| 3.6 ± 0.9 | ||||
| 1S10E | 4.7 ± 0.8 | |||
| 5.5 ± 0.9 | ||||
| Unsweetened: Ethanol Drinking 5 Days/wk | 0S10E WK 1 | 4.7 ± 0.9 | ||
| 5.9 ± 1.0 | ||||
| 0S10E WK 2 | 4.2 ± 0.7 | |||
| 4.4 ± 0.8 | F(2.4,72.8) = 1.6, p > 0.05 | |||
| 0S10E WK 3 | 3.2 ± 0.7 | |||
| 4.4 ± 0.8 | ||||
| 0S10E WK 4 | 3.3 ± 0.8 | |||
| 5.1 ± 0.9 | ||||
| Unsweetened: Ethanol Drinking 3 Days/wk | 0S10E WK 1 | 2.9 ± 0.6 | ||
| 3.6 ± 0.7 | ||||
| 0S10E WK2 | 2.9 ± 0.6 | |||
| 3.7 ± 0.6 | F(2,60) = 0.2, p > 0.05 | |||
| 0S10E WK3 | 2.7 ± 0.6 | |||
| 3.7 ± 0.6 | ||||
10S1E = 10% Sucrose + 1% Ethanol. 10S2.5E = 10% Sucrose + 2.5% Ethanol. 10S5E = 10% Sucrose + 5% Ethanol. 10S10E = 10% Sucrose + 10% Ethanol. 5S10E = 5% Sucrose + 10% Ethanol. 2.5S10E = 2.5% Sucrose + 10% Ethanol. 1S10E = 1% Sucrose + 10% Ethanol. 0S10E = 0% Sucrose + 10% Ethanol. Air-Exposed (Pre-Vapor, n = 20; Post-Vapor, n = 18). Ethanol Vapor-Exposed (Pre- and Post-Vapor, n = 14).
Statistical analyses conducted on total fluid intake during the Pre-Vapor phases revealed no significant differences between treatment groups (Table 3). Analysis of the levels of water intake between air-exposed and ethanol vapor-exposed groups before and after ethanol vapor exposure showed no significant interaction of treatment × time period (F(1,32) = 0.7; p > 0.05).
Table 3.
Mean total fluid intake (ml/kg ± SEM)
| Exposed | Stage Ethanol Vapor- | Session | Air-Exposed | |
|---|---|---|---|---|
| Pre-Ethanol Vapor Phase | Acquisition of Sweetened Ethanol Drinking | 10S1E | 65.2 ± 4.3 | |
| 68.8 ± 5.2 | ||||
| 10S2.5E | 61.3 ± 4.4 | |||
| 55.2 ± 5.3 | F(2.2,70.5) = 0.8, p > 0.05 | |||
| 10S5E | 36.1 ± 2.1 | |||
| 36.0 ± 2.6 | ||||
| 10S10E | 17.3 ± 1.3 | |||
| 18.3 ± 1.6 | ||||
| Post-Ethanol Vapor Phase | Ethanol Drinking Sucrose-Fading | 10S10E | 14.8 ± 1.4 | |
| 18.6 ± 1.6 | ||||
| 5S10E | 10.7 ± 0.7 | |||
| 11.4 ± 0.8 | F(1.9,59.9) = 2.4, p > 0.05 | |||
| 2.5S10E | 10.4 ± 0.7 | |||
| 10.4 ± 0.9 | ||||
| 1S10E | 8.2 ± 0.8 | |||
| 10.3 ± 0.9 | ||||
| Unsweetened: Ethanol Drinking 5 Days/wk | 0S10E WK 1 | 7.5 ± 0.8 | ||
| 8.9 ± 0.9 | ||||
| 0S10E WK 2 | 6.4 ± 0.7 | |||
| 7.1 ± 0.8 | F(2.4,77.8) = 1.6, p > 0.05 | |||
| 0S10E WK 3 | 5.0 ± 0.6 | |||
| 6.9 ± 0.8 | ||||
| 0S10E WK 4 | 5.4 ± 0.8 | |||
| 7.6 ± 0.9 | ||||
| Unsweetened: Ethanol Drinking 3 Days/wk | 0S10E 3X WK 1 | 5.5 ± 0.6 | ||
| 6.4 ± 0.7 | ||||
| 0S10E 3X WK2 | 5.2 ± 0.6 | |||
| 6.9 ± 0.7 | F(2,64) = 0.7, p > 0.05 | |||
| 0S10E 3X WK3 | 5.5 ± 0.7 | |||
| 6.7 ± 0.8 | ||||
10S1E = 10% Sucrose + 1% Ethanol. 10S2.5E = 10% Sucrose + 2.5% Ethanol. 10S5E = 10% Sucrose + 5% Ethanol. 10S10E = 10% Sucrose + 10% Ethanol. 5S10E = 5% Sucrose + 10% Ethanol. 2.5S10E = 2.5% Sucrose + 10% Ethanol. 1S10E = 1% Sucrose + 10% Ethanol. 0S10E = 0% Sucrose + 10% Ethanol. Air-Exposed (Pre-Vapor, n = 20; Post-Vapor, n = 18). Ethanol Vapor-Exposed (Pre- and Post-Vapor, n = 14).
4. Discussion
The present study used a limited-access two-bottle consumption model to measure ethanol intake in male Wistar rats following initiation of drinking and an exposure to an 8-wk chronic intermittent ethanol vapor regimen. Rats exposed to ethanol vapor showed higher amounts of ethanol intake and an increase in ethanol preference ratio than air-exposed control rats during the Sucrose-Fading stage of protracted ethanol withdrawal. Following complete “fade-out” of the sweetener, adult rats exposed to chronic ethanol vapor during late adolescence (P44-P55) and adulthood (P56-P100) maintained significantly higher levels of ethanol drinking than air-exposed rats for up to 10 wks into the ethanol protracted withdrawal. However, 5 days/wk ethanol intake (10E) in rats exposed to ethanol vapor was lower (0.27 ± 0.03 g/kg) than the levels found in our previous studies in adult rats exposed to an 8-wk intermittent ethanol vapor regimen during adulthood using a one-bottle limited access paradigm (0.84 ± 0.7 g/kg) (Thorsell et al., 2005b). These findings suggest that exposure to an 8-wk ethanol vapor regimen increased sweetened and unsweetened ethanol intake in adult Wistar rats during the protracted withdrawal period. However, exposure to ethanol vapor during the adolescence-adult transition period does not produce more robust increases in ethanol intake than what is seen in rats exposed to ethanol solely during adulthood..
We recently assessed the impact of appetitive motivational engagement with ethanol during adolescence on adult ethanol drinking. We trained adolescent rats to traverse an operant runway to drink a sweetened 10% ethanol solution (w/v) during 30 min two-bottle limited access sessions and assessed their ethanol intake during adulthood (Walker and Ehlers, 2009a). Our study found that adult rats allowed to traverse the runway during adolescence did not display an increase in ethanol consumption during the fade procedure when compared to the yoked-control group. However, once the sweetener had been removed from the 10E solution, the experimental group of rats significantly drank more 10E solution, compared to the yoked-control group (Walker and Ehlers, 2009b). The lack of difference between groups during the fading procedure was attributed to consumption patterns that were driven more by the sweetened solution than the ethanol itself (Walker and Ehlers, 2009a). In contrast, animals that had control over their ethanol consumption during adolescence showed a selective enhancement of adult ethanol intake when the sweetener was removed from the ethanol solution, compared to yoked-control group that consumed similar amounts of ethanol during adolescence (Walker and Ehlers, 2009a).
Comparisons between this previous study (Walker and Ehlers, 2009a) with findings from the present study could provide insight into factors that may play a role increasing adult ethanol intake in rats with a history of ethanol exposure during late adolescence/early adulthood. Given that the adolescent sweetened alcohol consumption was comparable between the studies, and since both studies initiated testing during protracted withdrawal, the possible explanation for the increase in adult sweetened and unsweetened ethanol intake found in the present study during the fading stage may be attributed to the consequences of the intermittent ethanol vapor regimen, whereas the increase observed in Walker and Ehlers (2009b) was specific to unsweetened ethanol consumption and attributed to the fact that the animals had an appetitive experience associated with their ethanol consumption during adolescence.
The model of early onset ethanol drinking followed by late adolescent/young adulthood ethanol dependence assessed in the present study was designed to resemble a pattern of heavy drinking and alcohol-related problems and dependence shown to peak during late adolescence and early adulthood and to decline linearly into adulthood (Bachman et al., 1997; Baer, 1993; Grant et al., 2004; Johnston et al., 2001a, 2001b). Studies have shown that prolonged exposure to intermittent ethanol vapor exposure in adult rats produces a lasting increase of ethanol preference (e.g., O'Dell et al., 2004; Rimondini et al., 2002a; Slawecki and Betancourt, 2002; Sommer et al., 2008). Rats in the present study were exposed to an 8-wk intermittent ethanol vapor regimen shown to produce long lasting behavioral and physiological effects that persist into the late protracted withdrawal period in adult Sprague-Dawley and Wistar rats (Criado and Ehlers, 2010; Criado et al., 2008a, 2008b, 2011; Slawecki, 2002; Slawecki et al., 2001). Findings from studies in animal models with a history of adult ethanol dependence suggest that subtle neuroadaptations during protracted abstinence may also be associated with increases in voluntary ethanol intake (Heilig et al., 2010). In view of the evidence of the long-term cellular and molecular consequences produced by the intermittent ethanol vapor exposure in animals, and studies demonstrating the impact of early drinking in humans, we expected to see higher levels of ethanol intake following ethanol vapor exposure during adolescence/young adulthood in our model.
One potential explanation may be that adolescents/young adults exposed to ethanol vapor may experience a qualitative or quantitative difference in withdrawal symptoms than adults, that could impact their drinking following vapor exposure. There have been several studies indicating that overall adolescent rats may have a quantitatively similar severity of withdrawal but may have a qualitatively different experience of withdrawal (Doremus-Fitzwater and Spear 2007; Slawecki and Roth, 2004; Slawecki et al., 2006; Wills et al., 2009, 2010). The 8-wk intermittent ethanol vapor regimen used in the present study have been shown to produce electrophysiological and neurochemical changes that persist into the late protracted withdrawal period in adult Sprague-Dawley and Wistar rats (Criado and Ehlers, 2010; Criado et al., 2008a, 2008b, 2011; Slawecki, 2002; Slawecki et al., 2001). Previous studies from our laboratory used this intermittent ethanol vapor regimen to evaluate electroencephalographic (EEG) sleep parameters following ethanol vapor exposure during adolescents as compared to adults. The results revealed that the long term effects of ethanol exposure on slow wave sleep amplitude was significantly more robust in adults than in adolescent exposed animals (Criado et al., 2008a; Ehlers and Slawecki, 2000). In view of the evidence demonstrating a strong association between sleep disturbances and relapse to ethanol drinking in clinical studies of adults (Brower et al., 1998; Clark et al., 1998, 1999; Drummond et al., 1998; Foster and Peters, 1999; Gillin et al., 1994) if adolescents and young adults are less impacted by ethanol-induced sleep disturbances they may drink less or be at less risk for relapse during protracted withdrawal.
The BECs during the 8-wk exposure period were maintained above the 175 mg/dl target level (mean BEC of 208 ± 6 mg%). However, mean BECs were initially around 119 mg/dl during the first wk, reaching the target level above 175 mg/dl between the first and second wks of ethanol vapor exposure. This gradual increase in BECs is a standard procedure that is part of the intermittent ethanol vapor regimen used in this laboratory, which allows animals to gradually acquire tolerance to ethanol and to maintain normal weight gain and motor activity. This ethanol vapor regimen takes in consideration individual differences in body weight, body gain, and sensitivity/tolerance to ethanol during the 8-wk vapor exposure period. While the BECs of 119 mg/dl observed after one wk of ethanol vapor exposure were lower than our overall target level of 175 mg/dl, we have shown that this ethanol vapor regimen and its gradual increase in BECs produces long lasting changes in NMDAR subunit expression in adolescent rats (e.g., Pian et al., 2010).
Findings from the present study showed that the average levels of unsweetened ethanol consumed in adult rats during the protracted withdrawal periods (5 days/wk and 3 days/wk stages) were 0.19 ± 0.01 g/kg (control group) and 0.27 ± 0.02 g/kg (experimental group). Previous studies have shown that following 30 min operant sessions or 30 min post-gavage administration, lower doses of ethanol are highly correlated with BECs in Wistar rats (Richardson, Lee, O'Dell, Koob, & Rivier, 2008; Walker & Ehlers, 2009b), with levels of 0.5 g/kg and 0.75 g/kg reliably resulting in BECs of ~0.05 and 0.075 g%. Therefore, in the present study, one could infer that the control and experimental levels of intake would have resulted in BECs of approximately 0.019 g% and 0.027 g%, respectively. However, BECs were not directly measured during the ethanol drinking phases and the possibility exists that there could be a pharmacokinetic explanation for the differential intake between air- and vapor-treated animals.
It is important to consider that preingestive factors may have also contributed to the higher amounts of ethanol intake observed in adult rats exposed to ethanol vapor during the Sucrose-Fading stage and maintained for up to 10 wks into ethanol protracted withdrawal. For instance, preingestive factors such as changes in ethanol taste following ethanol vapor exposure may have influenced the higher amounts of ethanol intake in ethanol-exposed rats. Studies in B6 mice have shown that the large amounts of ethanol intake were not mediated by its pharmacological properties, but rather by their inability to discriminate ethanol taste and/or their high rate of ethanol metabolism (Dole et al., 1988). The present study did not assess the consequences of ethanol vapor exposure on ethanol taste discrimination and metabolism. However, studies have shown that exposure to this intermittent ethanol vapor regimen (2 wk) in adolescent rats had no effect on sucrose intake and preference 48 hr after ethanol withdrawal (Slawecki and Roth, 2004). Moreover, while findings from the present study showed that the levels of ethanol intake were greater in ethanol-exposed than in air-exposed rats, the levels of ethanol intake in ethanol-exposed rats were lower than the levels found in mice (e.g., Dole, 1986) and than the levels found in our previous studies in adult rats exposed to an 8-wk intermittent ethanol vapor regimen during adulthood (Thorsell et al., 2005b). Future studies are needed to determine whether this intermittent ethanol vapor exposure paradigm altered ethanol taste.
In summary, this study demonstrated that the initiation of voluntary ethanol drinking followed by an 8-wk intermittent ethanol vapor exposure during late adolescent/early adulthood significantly increased sweetened and unsweetened ethanol drinking in adult rats during protracted withdrawal. However, the increases in drinking were not as robust as that seen in rats exposed to ethanol vapor during adulthood. These findings suggest that the effects of ethanol withdrawal may not be as motivating to drink during adolescent/ young adult alcohol exposure as in adults. Secondly, in light of the results of our previous studies demonstrating that robust increases in drinking can be induced in adolescents exposed to an appetitively motivated drinking paradigm (Walker and Ehlers 2009a), it may be that important environmental and or social associations with drinking are necessary in order to produce enhanced drinking in adulthood. Findings from the present study provide the basis for further development of future studies characterizing the neural and cellular mechanisms implicated in the development of protracted withdrawal symptoms and increased risk of ethanol dependence in adult rats exposed to ethanol during the transition between late adolescence and young adulthood.
Highlights.
Ethanol exposure during early adolescence increases risk of dependence in adults
Ethanol-intake was measured in adult rats after initiation of drinking in adolescence followed by ethanol vapor exposure
Adolescent ethanol vapor exposure modestly increased adult ethanol intake
Adolescent vapor exposure may not be as potent in increasing drinking in adults as adult vapor exposure
Acknowledgments
This study was supported by the National Institutes of Health (NIH) and the National Institute on Alcoholism and Alcohol Abuse grants AA006059 and AA019969 awarded to CLE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachman JG, Wadsworth KN, O'Malley PM, Johnston LD, Schulenberg JG. Smoking, Drinking, and Drug Use in Young Adulthood: The Impacts of New Freedoms and New Responsibilities. Lawrence Elrlbaum; Mahwah, NJ: 1997. [Google Scholar]
- Baer JS. Etiology and secondary prevention of alcohol problems with young adults. In: Baer JS, Marlatt GA, McMahon RJ, editors. Addictive Behaviors Across the Life Span: Prevention, Treatment, and Policy Issues. Sage; Thousand Oaks, CA: 1993. pp. 111–137. [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–71. [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, et al. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43:601–7. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, et al. Polysomnography and depressive symptoms in primary alcoholics with and without a lifetime diagnosis of secondary depression and in patients with primary major depression. J Affect Disord. 1999;52:177–85. doi: 10.1016/s0165-0327(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res. 2010;210:164–70. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008a;42:631–9. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of Dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008b;32:1752–62. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Liu T, Ehlers CL, Mathe AA. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats. Pharmacol Biochem Behav. 2011;99:104–11. doi: 10.1016/j.pbb.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. The New York Academy of Sciences; New York, N.Y: 2004. [DOI] [PubMed] [Google Scholar]
- De Wit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dole VP, Ho A, Gentry RT, Chin A. Toward an analogue of alcoholism in mice: analysis of nongenetic variance in consumption of alcohol. Proc Natl Acad Sci USA. 1988;82:827–30. doi: 10.1073/pnas.85.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–27. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, Demodena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–9. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–65. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–9. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–51. [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–6. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–7. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–84. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: adolescent risk factors and adult outcomes. Alcohol Clin Exp Res. 2010;34:819–33. doi: 10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud Alcohol Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–7. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: a longitudinal study of youth. Academic Press; New York: 1977. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG. Monitoring the Future: National results on drug use, 1975-2000. Volume I, Secondary school students (NIH Publication No. 01-4924) National Institute on Drug Abuse; Bethesda, MD: 2001a. http://www.monitoringthefuture.org/pubs/monographs/vol1_2000.pdf. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG. Monitoring the Future: National results on drug use, 1975-2000. Volume 2, College students and adults ages 19-40 (NIH Publication No. 01-4925) National Institute on Drug Abuse; Bethesda, MD: 2001b. http://www.monitoringthefuture.org/pubs/monographs/vol2_2000.pdf. [Google Scholar]
- Koob GF, Le Moal MM. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–9. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences: studies with animal models. In: Galanter M, editor. Recent Advances in Alcoholism Research: Alcohol Problems in Adolescents and Young Adults. Kluwer Academic/Plenum; New York, NY: 2005. pp. 123–142. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008a;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2008b;32:2062–73. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–54. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampaned neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002a;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. Effects of tiagabine and diazepam on operant ethanol self-administration in the rat. J Stud Alcohol. 2002b;63:100–6. [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–9. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–98. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–86. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sarviharju M, Jaatinen P, Hyytia P, Hervonen A, Kiianmaa K. Effects of lifelong ethanol consumption on drinking behavior and motor impairment of alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol. 2001;23:157–66. doi: 10.1016/s0741-8329(01)00132-x. [DOI] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–43. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–54. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder DA. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000a;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Curr Dir Psych Sci. 2000b;9:111–4. [Google Scholar]
- Spear LP. Adolescent period: Biological basis of vulnerability to develop alcoholism and other ethanol-mediated behaviors. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000c. pp. 315–33. [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann NY Acad Sci. 2004;1021:23–6. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. The developing brain and adolescent-typical behavior patterns: an evolutionary approach. In: Romer D, Walker EF, editors. Annenberg Public Policy Center and Annenberg Foundation Trust at Sunnylands editors. Adolescent psychopathology and the developing brain: integrating brain and prevention science. Oxford University Press; New York: 2007. pp. 9–30. [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res. 2005a;29:584–90. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005b;161:133–40. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration . Results from the 2007 National Survey on Drug Use and Health: National findings. U.S. Department of Health and Human Services, Office of Applied Studies; Rockville, MD: 2008. (DHHS Publication No. SMA 08-4343, NSDUH Series H-34) http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/2k7results.cfm. [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Varlinska EI, Spear LP. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009a;123:926–35. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009b;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–9. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Nelson TF, Kuo M. Underage college students’ drinking behavior, access to alcohol, and the influence of deterrence policies. Findings from the Harvard School of Public Health College Alcohol Study. J Am Coll Health. 2002;50:223–236. doi: 10.1080/07448480209595714. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33:455–63. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2010;34:1603–12. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL. Clinical significance of alcohol intake parameters at initiation of drinking. Alcohol. 1999;19:97–9. doi: 10.1016/s0741-8329(99)00020-8. [DOI] [PubMed] [Google Scholar]


