Abstract
The current study examined how a randomized one-year aerobic exercise program for healthy older adults would affect serum levels of brain-derived neurotrophic factor (BDNF), insulin-like growth factor type 1 (IGF-1), and vascular endothelial growth factor (VEGF) - putative markers of exercise-induced benefits on brain function. The study also examined whether (a) change in the concentration of these growth factors was associated with alterations in functional connectivity following exercise, and (b) the extent to which pre-intervention growth factor levels were associated with training-related changes in functional connectivity. In 65 participants (mean age = 66.4), we found that although there were no group-level changes in growth factors as a function of the intervention, increased temporal lobe connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus was associated with increased BDNF, IGF-1, and VEGF for an aerobic walking group but not for a non-aerobic control group, and greater pre-intervention VEGF was associated with greater training-related increases in this functional connection. Results are consistent with animal models of exercise and the brain, but are the first to show in humans that exercise-induced increases in temporal lobe functional connectivity are associated with changes in growth factors and may be augmented by greater baseline VEGF.
Keywords: exercise, aging, functional connectivity, fMRI, default mode network, aerobic fitness, growth factors
Introduction
Aerobic exercise is beneficial for brain function in older adults (Colcombe et al., 2004; Rosano et al., 2010; Voss et al., 2010b). However, the neurobiological mechanisms for these benefits are not fully understood. Whereas animal models have identified several neurochemicals that mediate downstream effects of exercise on the brain and cognition, including brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF) (Cotman et al., 2007b), the role of these molecules in exercise-induced changes in human brain function is unknown. We have previously found that exercise training benefits functional connectivity in several brain networks (Voss et al., 2010b) that are relevant for understanding cognition and human behavior, including the Default Mode Network (DMN) and two brain networks involved in cognitive control (Fronto-parietal and Fronto-executive, also referred to as the Cingulo-opercular network) (Voss et al., 2010a). The goal of this study was to investigate the relationship between serum BDNF, IGF-1, and VEGF, and functional connectivity in healthy elderly adults following one year of exercise training.
The DMN includes the posterior cingulate, ventral and superior frontal medial cortices, and bilateral lateral occipital, middle frontal, hippocampal and parahippocampal, and middle temporal cortices, with the posterior cingulate and temporal cortex portions being most adversely affected by age and mild cognitive impairment MCI status (Buckner et al., 2008; Fox et al., 2005; Greicius et al., 2004). The DMN shows greater activity during autobiographical memory and theory of mind processes, and is less metabolically active when attention is engaged exogenously (Buckner et al., 2008). However, the extent to which different regions in the DMN co-activate at rest has also been associated with individual differences in cognitive performance, progression from MCI to Alzheimer’s Disease, and other psychiatric disorders (Andrews-Hanna et al., 2007; Khamsi, 2012; Voss et al., 2010a). We have previously reported that one year of moderate intensity aerobic exercise (walking) increases task-independent functional coactivation of the hippocampus with the middle temporal gyrus and the lateral parieto-occipital cortex, as well as the middle temporal gyrus with the left middle frontal gyrus (Voss et al., 2010b). Given the links between the DMN, cognitive aging, and progression of MCI to AD, in conjunction with links between exercise and reduced risk of MCI and AD (Larson et al., 2006), these results suggest one pathway for the benefits of exercise are through improved DMN function. However the neurobiological mechanisms for improved DMN function remain unknown.
The fronto-executive network includes the anterior prefrontal cortex, insular and frontal operculum cortices, the temporo-parietal junction, and the dorsal posterior and anterior cingulate gyri and is involved in sustained task-set maintenance and error feedback for tuning top-down control (Dosenbach et al., 2006; Rushworth et al., 2004). Of all the regions in this network, aerobic exercise training was associated with increased task-independent functional connectivity of the left and right anterior prefrontal cortices in this network (Voss et al., 2010b). The fronto-parietal network includes the inferior parietal cortices, the supplementary motor and primary cortices, the frontal eye-fields, primary and extrastriate visual cortices, the inferior frontal cortex, and some overlapping portions of the temporo-parietal junction with the fronto-executive network, and is involved in rapid engagement and tuning of goal-directed attention (Dosenbach et al., 2006). In our previous study we found that a stretching and toning intervention benefited this network after 6 months of training (Voss et al., 2010b). These results are exciting in that moderate aerobic exercise can benefit functional brain networks in regions that typically degrade with aging and onset of disease. However, the mechanisms for how aerobic exercise confers such benefits in humans are relatively unknown. The present study seeks to further examine the potential mechanisms through which exercise exerts it benefits on functional brain connectivity in late life.
BDNF, IGF-1, and VEGF likely play complementary roles in explaining how exercise impacts brain networks. Central BDNF, and its receptor tyrosine kinase (TrkB), are highly concentrated in the hippocampus, but are also distributed throughout the brain (Murer et al., 1999), and mediate the effects of exercise on synaptogenesis, synaptic plasticity, and enhanced learning and memory (Christie et al., 2008). Consistent with this, in humans, circulating levels of BDNF have been linked to greater hippocampal volume and spatial memory performance (Erickson et al., 2010), and exercise-induced change in hippocampal volume (Erickson et al., 2012). Decreased BDNF plasma and serum levels have also been associated with behavioral and cognitive symptoms of clinical depression, Alzheimer’s disease, and psychiatric disorders such as schizophrenia and autism (for reviews, see Erickson et al., 2012; Sen et al., 2008). These studies suggest that plasma and serum BDNF may in part reflect BDNF released from the brain and may be viable biomarkers for age- and clinically- relevant brain dysfunction.
However, BDNF is also produced by a number of organs and tissues in the peripheral nervous system, including the heart and lungs (Scarisbrick et al., 1993; Timmusk et al., 1993), and is stored and released from blood platelets and immune cells (Yamamoto et al., 1990; Gielen et al., 2003; Kerschensteiner et al., 1999). Thus an important area of future research is to improve the understanding of the relationship between human peripheral and brain BDNF. This area of future research is challenging however, if not impossible, given the inherent limitations in acquiring brain biopsies from living humans. A promising alternative approach is to conduct longitudinal studies that manipulate a variable expected to modulate growth factor levels in the brain, such as exercise, and examine the covariance of individual differences in serum BDNF and brain-related biomarkers hypothesized to be functionally related to BDNF expression. A cross-sectional study with a similar approach found that serum BDNF was positively associated with a marker of neuronal integrity and metabolism in the neocortex (Lang et al., 2007), which parallels an animal study finding high correlation between peripheral and cortical BDNF (Karege et al., 2002). Previous studies have found support for the idea that aerobic exercise training increases serum BDNF, however findings are mixed overall with some studies not finding reliable increases in BDNF post exercise (for review, see Coelho et al., 2012; Erickson et al., 2012; Knaepen et al., 2010). Though, many of these (human) studies do not look at how individual differences in change in BDNF relate to other brain-based outcomes known to be associated with aerobic training, such as structural and functional integrity of the hippocampus, which may have better sensitivity to shared variance between brain-derived BDNF in the periphery and direct effects of BDNF in the brain. The current study examined both mean-level change in serum BDNF following chronic aerobic training, and covariance of individual differences in fitness gains with a measure of functional network integrity (functional connectivity) that has previously been related to increased availability of central BDNF based on genetic variation of the val66met polymorphism (Thomason et al., 2009).
More consistent findings have shown that training-induced neuroprotective effects of IGF-1 may stem from increased brain uptake of peripheral (primarily liver-derived) IGF-1 during exercise, particularly in the hippocampus (Carro et al., 2000). Peripheral IGF-1 is produced primarily in the liver from growth hormone stimulation (Yakar et al., 1999). Animal studies have found that IGF-1 mediates exercise-induced angiogenesis, stimulates increased central BDNF and VEGF production (Ding et al., 2006; Lopez-Lopez et al., 2004), and is necessary for exercise-induced neurogenesis (Carro et al., 2000; Trejo et al., 2001). Related to aging, studies have shown late life is accompanied by reduction in both serum IGF-1 and density of IGF-1 receptors in the hippocampus and throughout the brain (for review, see Sonntag et al., 2005). Similar to IGF-1, animal studies have found that peripheral VEGF increases during aerobic exercise and in part mediates exercise-induced angiogenesis and neurogenesis (Lopez-Lopez et al., 2004). Sources of circulating VEGF include blood platelets (Mohle et al., 1997) and skeletal muscle contractions (Kraus et al., 2004). Through peripheral-central receptor pathways, circulating VEGF may promote neurogenesis and synaptic plasticity by stimulating neural stem cell proliferation and differentiation and increases central endothelial cell and astrocytic production of VEGF, BDNF, and IGF-1 (Zacchigna et al., 2008; Ruiz de Almodovar et al., 2009). Together with some evidence that VEGF expression in the hippocampus declines with age (Shetty et al., 2005), exercise-associated increases in VEGF may play an important role in the therapeutic effects of exercise on the brain.
Given the evidence for their interdependence, we hypothesized that BDNF, IGF-1, and VEGF would increase following the aerobic exercise intervention, and that each would be associated with increased functional connectivity in the temporal and frontal cortices, where BDNF is highly concentrated and age-related brain dysfunction is greatest.
Materials and methods
Participants
Participants were recruited from the local community of Urbana-Champaign, Illinois. Eligible participants had to (1) demonstrate strong right handedness (since brain organization can vary based on handedness), with a 75% or above on the Edinburgh Handedness Questionnaire (Oldfield, 1971), (2) be between the ages of 55 and 80 years (3) score ≥ 51 on the modified Mini-Mental Status Exam (mMMSE, Stern et al., 1987)), a screening questionnaire to rule out potential neurological pathology, (4) score < 3 on the Geriatric Depression Scale (GDS) (Sheikh and Yesavage, 1986), (5) have normal color vision (6) have a corrected visual acuity of at least 20/40 and (7) sign an informed consent. In addition, participants had to report not being currently physically active, which was defined as having been physically active (i.e., walking briskly or activity that induces sweating and elevated heart rate) for 30 minutes or more no more than two times in the last six months. Further exclusionary criteria included evidence of abnormal cardiac responses or conditions during graded exercise testing and evidence for chronic inflammation (e.g., severe arthritis, psoriasis, inflammatory bowel disease, asthma, polyneuropathies, Lupus). All women were self-reported post-menopausal. Participants completed a mock magnetic resonance imaging (MRI) session, wherein they were screened for their ability to complete an experiment in an MRI scanner. Participants who passed the mock screening subsequently completed a series of structural and functional MRI scans, and a graded maximal exercise test. Prior to MR scanning all participants were tested for visual acuity and (if need be) corrective lenses were provided within the viewing goggles to ensure a corrected vision of at least 20/40 while in the scanner. Participants were compensated for their participation.
Participants were further randomized to either an aerobic walking group or a control group that participated in a stretching and toning program. The walking group included 30 participants with an average age of 67.3 (SD=5.8), of which 73% were female; mean education for the walking group was 15.9 years (SD=2.8) and their mean mMMSE score was 55.2 (SD=1.4). The flexibility, toning, and balance (FTB) group included 35 participants with an average age of 65.4 (SD=5.2), of which 71% were female; mean education for the walking group was 15.9 years (SD=2.7) and their mean mMMSE score was 54.8 (SD=1.9). The groups did not significantly differ in baseline age, aerobic fitness, mean years of education, or gender (all p>.05). Neuroimaging measures were collected as part of a larger task battery, and were originally developed to be passive viewing tasks for localizing stimulus-specific processing regions of the ventral visual cortex. Participants in this study represent a subset of a previously published investigation of age-related differences in stimulus processing specificity (Voss et al., 2008) and represent the full sample reported in a study focused on characterizing effects of exercise training on functional connectivity across multiple brain systems (Voss et al., 2010b); it is also important to point out that the sample in this study represents a subset of those that started the study with acceptable functional MRI data (N=120, see Voss et al., 2010a). Note that in Voss et al. (2010b), effects of exercise training on age-related decrements in functional connectivity were assessed by first determining regions of interest where older adults had significantly less functional connectivity than a young adult comparison group (average age of 23.9 (SD=4.4) years, see Voss et al., 2010b for more details). The young adult group did not undergo exercise training or blood draws so biomarkers are unknown in this group.
Aerobic fitness assessment
Participants were required to obtain consent from their personal physician before cardiorespiratory fitness testing was conducted. Aerobic fitness (VO2 max) was assessed by graded maximal exercise testing on a motor-driven treadmill. The protocol involved the participant walking at a speed slightly faster than their normal walking pace (approximately 30–100m per minute) with increasing grade increments of 2% every two minutes. A cardiologist and nurse continuously monitored measurements of oxygen uptake, heart rate and blood pressure. Oxygen uptake (VO2) was measured from expired air samples taken at 30-second intervals until a maximal VO2 was attained or to the point of test termination due to symptom limitation and/or volitional exhaustion. VO2 max (mL/kg/min) was defined as the highest recorded VO2 value when two of three criteria were satisfied: (1) a plateau in VO2 peak between two or more workloads; 2) a respiratory exchange ratio >1.00; and (3) a heart rate equivalent to their age predicted maximum (i.e. 220 - age). Due to scheduling difficulty, two participants in the stretching group did not have fitness assessments at the 6-month session; all participants had fitness assessments at baseline and 12-month sessions. Note that across all three of the time points, even with improvements from the intervention, both groups of participants were in the bottom 10th percentile of the population for VO2 max based on their age and gender (Whaley et al., 2006), reflecting our exclusive recruitment of currently sedentary older adults.
Exercise intervention
Older adults were randomly assigned to participate in either an aerobic walking program, or a control group that did non-aerobic flexibility, toning, and balance (FTB) exercises. The FTB control group served to match groups for social contact associated with group exercise and to determine effects on brain function specific to aerobic exercise. Both the walking and control groups met three times per week in dedicated exercise facilities. The walking group exercised on an indoor track whereas the he FTB group exercised in a multi-purpose, well-it area designed for older adults participation in yoga, flexibility, and strengthening activities. Both facilities were on a university campus, were easily accessible, and had parking provided.
For the walking program, a trained exercise leader supervised all sessions. Participants started by walking for ten minutes and increased walking duration weekly by five-minute increments until a duration of 40 minutes was achieved at week seven. Participants walked for 40 minutes per session for the remainder of the program. All walking sessions started and ended with approximately five minutes of stretching for the purpose of warming up and cooling down. Participants wore heart rate monitors and were encouraged to walk in their target heart rate zone, which was calculated using the Karvonen method (Strath et al., 2000) based on the resting and maximum heart rates achieved during the baseline maximal graded exercise test. The target heart rate zone was 50–60% of the maximum heart rate reserve for weeks one to seven and 60–75% for the remainder of the program. Participants in the walking group completed an exercise log at each exercise session. Every four weeks, participants received written feedback forms that summarized the data from their logs. Participants with low attendance and/or exercise heart rate were encouraged to improve their performance in the following month.
For the FTB program a trained exercise leader led sessions. All FTB classes started and ended with warm-up and cool-down stretches. During each class, participants engaged in four muscle toning exercises utilizing dumbbells or resistance bands, two exercises designed to improve balance, one yoga sequence, and one exercise of their choice. To maintain interest, a new group of exercises was introduced every three weeks. During the first week, participants focused on becoming familiar with the new exercises, and during the second and third weeks, they were encouraged to increase the intensity by using more weight or adding more repetitions. Participants in the FTB group also completed exercise logs at each exercise session and received monthly feedback forms. They were encouraged to exercise at an appropriate intensity (13–15 on the Borg RPE scale; (Borg, 1985) and attend as many classes as possible.
Imaging Methods
Structural MRI
For all participants, high resolution T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures, echo time (TE)=3.87 ms, repetition time (TR)=1800 ms, field of view (FOV)=256 mm, acquisition matrix 192 mm × 192 mm, slice thickness=1.3mm, and flip angle=8°. All images were collected on a 3T head-only Siemens Allegra MRI scanner.
Functional MRI
Functional MRI (fMRI) scans were acquired during three passive viewing tasks: 1) a checkerboard task comprised of luminance-matched flashing black-and-white checkerboards and flashing color checkerboards at a rate of 8 Hz, each checkerboard condition was presented in two separate 30-second blocks that alternated with 20-second blocks of fixation baseline; 2) a word viewing task comprised of 30-second blocks of words, pseudo-words, and letter strings, presented separately in two 30-second blocks that alternated with 20-second blocks of fixation baseline, each block consisted of 20 unique stimuli that were each presented for one-second with a 500-ms fixation between each word presentation; and 3) a face/building viewing task comprised of three 20-second blocks of faces and buildings that alternated with 20-second blocks of luminance matched scrambled images (taken from the face and building stimulus set) as the baseline condition, each block consisted of 20 unique black-and-white images (controlled for luminance and dimension) that were each presented for one-second. In each task participants were instructed to keep their eyes open and to pay attention to the screen.
Visual stimuli were presented with MRI-safe fiber optic goggles (Resonance Technologies, Inc.). Participants completed the passive viewing tasks as part of a larger battery of cognitive paradigms within the scanner. For the fMRI tasks, T2* weighted images were acquired using a fast echo-planar imaging (EPI) sequence with Blood Oxygenation Level Dependent (BOLD) contrast (64 × 64 matrix, 4 mm slice thickness, TR = 1500 ms, TE = 26 ms, flip angle = 60). A total of 150 volumes were acquired per participant for the checkerboard task, 220 volumes for the word task, and 180 volumes for the face/building task.
Image Analysis
Structural MRI preprocessing
Each participant’s low-resolution EPI image was registered to his or her high-resolution T1 structural image, which was subsequently registered to stereotaxic space (study-specific template generated using 152 T1 MNI as the target volume, Montreal Neurological Institute) using FLIRT 12-parameter affine linear registration (Jenkinson et al., 2002). A study-specific template was created from the baseline structural images from this sample. Functional images from six- and 12-month sessions were also registered to this study-specific template. To create the study-specific template, high-resolution structural images were first skull-stripped using BET (Smith, 2002), and manually inspected and corrected for any skull-stripping errors. Next, the structural images were registered to the 152 T1 MNI volume using FLIRT 12-parameter affine linear registration (Jenkinson et al., 2002). Finally, registered volumes were averaged to form a representative reference volume. Before group analyses, functional data were registered to stereotaxic space using transforms generated from the alignment of high-resolution T1 images.
fMRI Preprocessing
fMRI data preprocessing was carried out using FSL 4.1.4 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The following pre-statistics processing was applied: rigid body motion correction using MCFLIRT (Jenkinson et al., 2002), removal of non-brain structures using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of FWHM 6.0-mm, grand-mean intensity normalisation of the entire 4D dataset by a single multiplicative factor, and temporal filtering to restrict the bandwidth of the fMRI signal to .008 < f < .080 Hz.
Functional connectivity seeding analysis
Detailed description of the functional connectivity procedures are reported elsewhere (Voss et al., 2010b), which followed standard procedures from other studies (Andrews-Hanna et al., 2007; Fox et al., 2006). In addition to the typical nuisance regression of white matter, CSF, and global signal, to further isolate our examination to intrinsic functional connectivity, we also controlled for signal from a bilateral ROI in primary visual cortex (125 anatomical-voxel spheres centered at ±18, −98, −4, derived from the literature (Andrews-Hanna et al., 2007)). This visual cortex regressor, along with the global signal regressor, were cautionary measures to ensure our estimates of functional connectivity were not inflated due to the additive influence of synchronized task-evoked signal change. In addition to nuisance fMRI signal, six motion parameters computed by rigid body translation and rotation in preprocessing (Jenkinson et al., 2002) were included in the nuisance regression. Functional connectivity of all ROI pairs was measured as the average Fisher’s Z transform of Pearson correlation coefficients across the three passive viewing runs.
In this study we focus on the functional connections that showed sensitivity to training-induced gains in functional connectivity over a 12-month intervention for either the FTB or walking group (Voss et al., 2010b). Table 1 lists these regional connections and their standard MNI coordinates. Connections showing increased functional connectivity in favor of the walking group were three ROI-pairs in the DMN, including bilateral parahippocampus-bilateral middle temporal gyrus, bilateral parahippocampus-bilateral lateral occipital cortex, and bilateral middle temporal gyrus-left middle frontal gyrus, and one ROI-pair from the fronto-executive network, including the right and left anterior prefrontal cortices.
Table 1.
Regional functional connections that showed significant group differences over 12-month exercise intervention.
| ROI abbreviations | ROI-ROI anatomical regions | Hypothesized network | Intervention effect | |
|---|---|---|---|---|
| BMTG – BPHG | Bilateral middle temporal gyrus L: −52,−18,−18 R: 58, −10,−18 |
Bilateral parahippocampal gyrus L: −24,−26,−20 R: 24,−26,−20 |
DMN | W > S |
| BPHG - LOC | Bilateral parahippocampal gyrus L: −24,−26,−20 R: 24,−26,−20 |
Left lateral occipital/parietal cortex −44,−72,34 |
DMN | W > S |
| LMFG - BMTG | Left middle frontal gyrus −30,20,50 |
Bilateral middle temporal gyrus L: −52,−18,−18 R: 58, −10,−18 |
DMN | W > S |
| RALPFC - PFC | Right anterior lateral prefrontal cortex 32,40,28 |
Bilateral prefrontal cortex L: −36,34,28 R: 32, 42, 36 |
Fronto-executive/CO | W > S |
| RALPFC - LHC | Right anterior lateral prefrontal cortex 32,40,28 |
Left hippocampus −24,−22,−18 |
Fronto-executive/CO-AND-DMN | -W > -S |
| RFOI - RLOC | Right frontal operculum/insula 28, 26, 8 |
Right lateral occipital/parietal cortex 26,−64,54 |
Fronto-executive/CO-AND-Fronto-parietal | S > W |
All coordinates are in MNI space, L and R refer to Left and Right hemisphere, respectively. Networks, DMN=Default Mode Network, CO=cingulo-opercular network, which is another name for the fronto-executive network. Group effects, W=walking group, S=flex, tone, and balance control group.
Two other seed pairs showed changes in functional connectivity over the one-year intervention. The connectivity between the right anterior lateral prefrontal cortex and the left hippocampus was significantly reduced in the walking group compared to the FTB group, resulting in the aerobically trained older adults showing patterns of connectivity similar to that of the young adult comparison group. Since walking resulted in connectivity more similar to young adults, for this ROI pair, we interpreted an increasing negative correlation as a beneficial change for the aerobic exercise group. Lastly, connectivity between the right frontal operculum and right lateral occipital cortex increased significantly more for the FTB compared to the walking group; however, these ROIs do not typically co-occur in the same resting networks, so this functional change may reflect the cognitive demands of the FTB training (see Voss et al., 2010b for more detailed discussion).
Blood serum collection and analysis
Blood sampling for BDNF, IGF-1, and VEGF analysis was performed approximately two weeks before each MRI session (pre and post one-year intervention). Fasted subjects reported to the laboratory at 0800, at which time blood from the antecubital vein was collected in sterile serum separator tubes (Becton Dickenson, Franklin Lakes, NJ, USA). The blood samples were kept at room temperature for 15 minutes to allow for clotting, after which the samples were centrifuged at 1100 × g at 4°C for 15 minutes. Serum was then harvested, aliquoted, and stored at −80°C until analysis approximately 6 months later. Protein levels were quantified using enzyme-linked immunosorbent assays following manufacturer’s instructions (R & D Systems, Minneapolis, MN). For some subjects, blood growth factor levels could not be estimated due to values being below detection limits of our assays. Table 2 lists different sub-groupings of participants based on those with available data for each growth factor of interest. Serum (rather than plasma) was examined since it is the most commonly employed method of examining the relationship of growth factors in blood in humans to individual differences in neuropsychiatric, cognitive, and exercise factors (e.g., see reviews, Brunoni et al., 2008; Knaepen et al., 2010).
Table 2.
Summary of demographics and intervention variables for subgroups with blood data
| BDNF | IGF-1 | VEGF | ||||
|---|---|---|---|---|---|---|
| Variable | FTB Control | Walkers | FTB Control | Walkers | FTB Control | Walkers |
| N | 30 | 26 | 16 | 14 | 28 | 24 |
| Age (SD) | 66.1 (5.2) | 67.5 (5.9) | 65.6 (5.7) | 67.8 (6.3) | 65.7 (5.3) | 66.8 (5.9) |
| % Female | 70 | 69 | 75 | 86 | 64 | 71 |
| Educationa | 15.8 (2.8) | 16.0 (3.0) | 15.9 (2.9) | 16.1 (2.8) | 15.9 (2.8) | 15.7 (2.9) |
| mMMSEb | 54.7 (2.0) | 55.1 (1.5) | 54.8 (2.3) | 54.9 (1.7) | 54.8 (2.0) | 55.2 (1.5) |
| BMI | 28.3 (4.1) | 28.8 (4.4) | 28.7 (3.2) | 28.9 (4.2) | 28.5 (4.2) | 28.9 (3.9) |
| VO2 Pre | 21.31 (4.7) | 20.96 (4.1) | 21.43 (4.7) | 20.46 (2.5) | 21.93 (5.3) | 21.52 (4.5) |
| VO2 Post | 21.06 (4.4) | 22.04 (5.2) | 21.54 (4.0) | 20.80 (2.9) | 21.70 (4.5) | 22.50 (5.5) |
| VO2 Post-Prec | −.25 (2.4) | 1.08 (2.0)* | .12 (2.6) | .34 (1.8) | −.23 (2.6) | .99 (2.0)* |
| Adherence (%) | 81 | 76 | 82 | 77 | 81 | 75 |
| Baseline growth factor leveld | 23,180 (13,325) | 21,233 (11,524) | 60 (36) | 54 (54) | 215 (278)* | 446 (289)* |
Education refers to self-reported years of education;
mMMSE=modified mini-mental status exam;
VO2 (aerobic fitness, mL/kg/min) change,
indicates significant group differences in fitness change in favor of the walking group, based on a one-tailed, two-sample t-test, p<.05;
values represent medians, with inter-quartile range in parentheses; units of analysis: BDNF (pg/mL), IGF-1 (ng/mL), VEGF (pg/mL);
indicates significant group difference (p<.05) in baseline growth factor level, based on Mann-Whitney Test. No demographic variables are significantly different; continuous measures were tested with independent samples t-test and gender was tested with chi-square test. BDNF=brain-derived neurotrophic factor, IGF-1=insulin-like growth factor type 1, VEGF=vascular endothelial growth factor.
Statistical analysis
Change in functional connectivity was characterized using the difference score of pre-intervention functional connectivity subtracted from post-intervention functional connectivity. Difference scores were used since ordinary least squares regression straight-line fits to the three-time-point data were highly variable (average R2 was between 45 and 65% with an average standard deviation of 35% across participants). The method of residualized change scores was not used, since growth factor data was not normally distributed and so residuals from a linear regression would likely be unreliable, and it was preferable to examine change between functional connectivity and growth factors with analogous measures of change. For these reasons and others (Willett, 1988), difference scores were used for measuring change in functional connectivity.
As described above, blood serum measures of BDNF, IGF-1 and VEGF did not consistently approximate a normal distribution within groups at both pre-test and post-test. Therefore difference scores were used to measure change (Post – Pre intervention). Note one FTB participant’s IGF-1 change data were discarded as an extreme outlier (> 4.5 SD from mean and median). Change scores also did not approximate a normal distribution. For non-normally distributed blood measures we examined differential group change with a one-tailed Mann-Whitney test, and examined the association of change in neurobiological markers with changes in brain connectivity by conducting non-parametric Kendall’s tau (τ) correlations. In addition to zero-order correlations between change measures, follow-up analyses were conducted that controlled for variance associated with age, sex, and change in anterior hippocampal volume since these variables represent potential confounds in individual variation of change (Erickson et al., 2012).
To test for the association between pre-intervention blood growth factors and exercise-induced changes in functional connectivity, we conducted partial correlations between baseline growth factors and change in functional connectivity, while controlling for variance associated with age, sex, and change in anterior hippocampal volume.
Partial correlations were Kendall’s tau partial correlations (τr) carried out in R. All correlations were carried out within exercise group. To approximate the strength of the difference between group tau correlation values, Fisher’s r-to-z transformation was conducted to compare the magnitude of the partial correlations and we report the effect size (q) associated with the difference between correlations (Cohen, 1988). All other statistical analyses were done using PASW 18.0 for Macintosh.
Results
Change in blood levels of growth factors from exercise training
There were no group differences in change in BDNF (pg/mL) between the walking (Mdn = 693.50, IQR=11,869.25) and FTB (Mdn = 1159, IQR=5760.25) group, p >.05. In addition, a two-tailed Wilcoxen signed ranks test revealed no main effect of change from pre-intervention (Mdn=22,870, IQR=11,676) to post-intervention (Mdn=25,086, IQR=10,430), Z=1.58, p=.11. There were also no group differences in change in IGF-1 (ng/mL) between the walking (Mdn = −6.62, IQR=15.38) and FTB (Mdn = −5.18, IQR=15.23) group. However, a two-tailed Wilcoxen signed ranks test revealed a significant main effect of change (reduction in IGF-1) from pre-intervention (Mdn=57.90, IQR=46.97) to post-intervention (Mdn=52.17, IQR=38.03), Z=2.11, p<.05. Finally, there were no differences in change in VEGF (pg/mL) between the walking group (Mdn = 10.5, IQR=187.75) and FTB (Mdn = 15.35, IQR=52.5) group. In addition, a two-tailed Wilcoxen signed ranks test revealed no main effect of change from pre-intervention (Mdn=312, IQR=316.40) to post-intervention (Mdn=370, IQR=332.30), Z=1.39, p=.17.
Change in growth factors and change in functional connectivity
We examined potential mechanisms of exercise-associated changes in functional connectivity by assessing their association with change in putative markers of plasticity. Below we report results where at least one group showed a significant association, p<.05 (one-tailed).
BDNF
Change in blood serum BDNF was positively correlated with aerobic exercise-induced increases in connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus, τ = .25, p <.05, and this correlation was non-significant in the FTB group (τ = .11, p>.05). Since we have previously shown that anterior hippocampal volume increased for the walking group but not the FTB group (Erickson et al., 2011), it is possible that these results are due to increases in volume for the walking group. However, when controlling for variance associated with change in anterior hippocampal volume, the association became stronger for the walking group, τr = .29, p<.05, and remained non-significant for the FTB group (τr = .12, p>.05). When controlling for age, sex, and change in anterior hippocampal volume, the relationship also became stronger for the walking group, τr=.40, p<.05, and remained non-significant for the FTB group (τr=.11, p>.05), see Figure 1B. Therefore, it would appear that changes in hippocampal volume, or other potential confounders, cannot account for this relationship. A Fisher’s Z test on the difference in correlations was not statistically significant (p=.14), however the approximate effect size for the difference in correlations was of medium size, q=.31 (Cohen, 1988).
Figure 1.
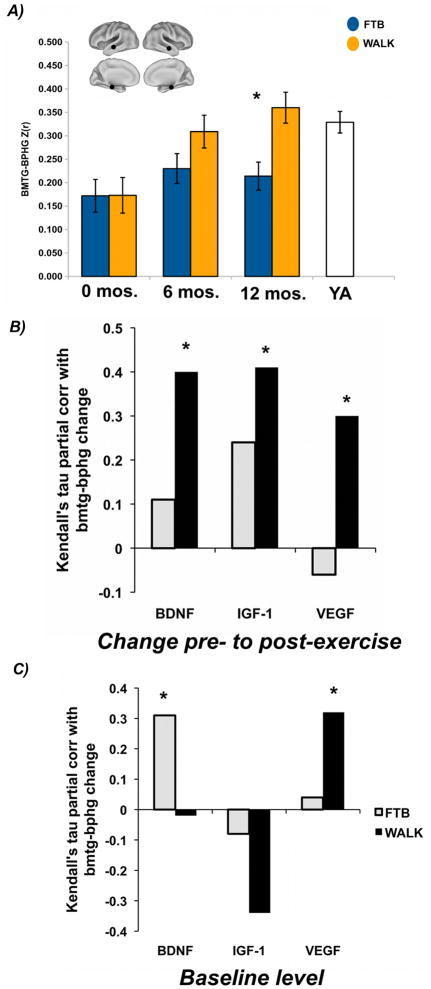
Change in temporal lobe connectivity associated with change and baseline levels of growth factors
Caption: A) figure adapted from Voss et al., 2010b, y-axis for 6 and 12 mos sessions represents marginal means from ANCOVA model that controlled for variance associated with baseline (0 mos); YA refers to Young Adult comparison group that was not recruited based on self-reported activity level; aerobic fitness was not measured for young adults (see also Voss et al., 2010b); bmtg-bphg refers to the non-directional functional connection between the bilateral middle temporal gyrus and the bilaterial parahippocampal/hippocampal gyrus, B and C) partial correlation indicates that data are plotted after controlling for variance associated with age, sex, and change in anterior hippocampal volume; *p≤.05.
*Note Figure 1A can be printed without color
IGF-1
Change in blood serum IGF-1 was also positively correlated with aerobic exercise-induced increases in connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus, τ= .34, p <.05, but was not significant (τ= .24, p>.05) in the FTB group. When controlling for variance associated with change in anterior hippocampal volume, the association remained significant for the walking group, τr = .38, p<.05, and non-significant for the FTB group (τr = .23, p >.05). When controlling for age, sex, and change in anterior hippocampal volume, the relationship again became stronger for the walking group, τr=.41, p<.05, and remained non-significant for the FTB group (τr = .24, p>.05), see Figure 1B. A Fisher’s Z test on the difference in correlations was not statistically significant (p=.32), and the approximate effect size for the difference in correlations was of small size, q=.19 (Cohen, 1988).
VEGF
There were no statistically significant zero-order associations between change in functional connectivity and change in blood serum VEGF. However, when controlling for age, sex, and change in anterior hippocampal volume, there was a significant association between change in VEGF and change in connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus for the walking group, τr = .30, p<.05, and this association was non-significant in the FTB group, τr = −.06, p>.05, see Figure 1B. A Fisher’s Z test on the difference in correlations was not statistically significant (p=.11), however the approximate effect size for the difference in correlations was of medium size, q=.37 (Cohen, 1988).
Baseline growth factors and change in functional connectivity
Age, sex, and change in anterior hippocampal volume were treated as covariates in all analyses of baseline measures’ association with change. In addition, all correlations in this section are two-tailed, since there could be cases where greater peripheral levels of growth factors are associated with less exercise-induced change.
BDNF
Greater pre-intervention baseline serum BDNF was associated with greater increases in functional connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus for the FTB group, τr =.31, p<.05, but not for the walking group, τr =−.03, p>.05, see Figure 1C. This was unexpected since the FTB group did not show significant mean-level increase in connectivity between bilateral parahippocampus and the bilateral middle temporal gyri.
IGF-1
There were no associations between pre-intervention serum IGF-1 and change in functional connectivity between any of the ROI pairs.
VEGF
Greater pre-intervention serum VEGF was associated with greater increases in functional connectivity between the bilateral parahippocampus and the bilateral middle temporal gyrus for the walking group, τr =.32, p<.05, whereas this correlation was non-significant for the FTB group, τr = .04, p>.05, see Figure 1C.
Discussion
Results are consistent with an extensive rodent literature demonstrating that BDNF, IGF-1, and VEGF are important pathways by which aerobic exercise influences brain function (Cotman et al., 2007) and recent evidence in humans that circulating BDNF levels are related to greater hippocampal volume (Erickson et al., 2010). However, this is the first evidence for an association between circulating BDNF, IGF-1, and VEGF and exercise-related functional plasticity in humans. Results suggest that the three growth factors are consistent in their associated change with functional brain connectivity in the medial and lateral temporal cortices. We also report novel evidence for an association between greater baseline circulating VEGF and increased aerobic exercise-induced benefits on functional connectivity in the temporal cortex.
That the associations between BDNF, IGF-1, and VEGF were commonly associated with increased functional connectivity between the bilateral parahippocampal gyrus and middle temporal gyrus has a number of implications. First, this suggests that circulating levels of these three molecules may provide some overlapping variance with the central mechanisms of exercise-induced changes in brain function, and that these growth factors likely interact. For instance, while evidence is strongest for the relationship between BDNF and functional connectivity, the effectiveness of BDNF may be modulated by IGF-1 and VEGF, which both stimulate the growth of endothelial cells, which in turn express nitric oxide synthase (eNOS), which is required for exercise-induced up-regulation of BDNF in the hippocampus (Chen et al., 2005; Chen et al., 2006; Christie et al., 2008). BDNF may be linked to improved functional connectivity by increasing synaptogenesis and dendritic spine density (Stranahan et al., 2007; Vaynman et al., 2006), and therefore increasing the connection capacity of neurons and synaptic plasticity in the form of long-term potentiation (LTP) (Rex et al., 2006; Schinder et al., 2000). There is also evidence that neural activity in the hippocampus is increased during aerobic exercise, based on increased c-Fos expression in the hippocampus (Carro et al., 2000; Clark et al., 2009; Clark et al., 2010) and cortex (Carro et al., 2000), which would initiate a cascade of activity-dependent changes in synaptic plasticity that could strengthen existing functional connections in the brain (Schinder et al., 2000). At a larger scale, this is consistent with the findings of Thomason et al. (2009), who found increased availability of central BDNF (based on genetic variation of the val66met polymorphism) was associated with increased functional connectivity in the BOLD signal in the DMN and an executive function network in children (Thomason et al., 2009).
More generally, healthy aging is accompanied by decreased functional connectivity in the DMN, fronto-parietal, and fronto-executive networks (Andrews-Hanna et al., 2007; Madden et al., 2010; Voss et al., 2010b), coupled with reductions in the availability of BDNF (Erickson et al., 2010; Ziegenhorn et al., 2007), IGF-1 (Markowska et al., 1998; Sonntag et al., 2005), and VEGF (Gao et al., 2009; Rivard et al., 1999; Shetty et al., 2005). However, exercise-associated up-regulation of BDNF, IGF-1, and VEGF may help to offset age-related reductions in synaptogenesis, neurogenesis, angiogenesis, synaptic plasticity, and learning and memory, providing for a more resilient brain in the face of age-related structural and functional neurodegeneration (see Cotman et al., 2002 see Cotman et al., 2007a for reviews).
Yet, the functional significance of the observed individual differences in growth factor change needs future study. In a previous study we did not find robust change in cognitive performance for the walking group compared to the FTB group (Voss et al., 2010; Voss et al., in press). Both of these papers report subsets of the full dataset in the randomized clinical trial, based on individuals that had acceptable neuroimaging data across study time-points. These subsets therefore have reduced power that may have affected the ability to detect a benefit for walking. Another possibility is there could be a lag between brain changes and benefits on cognitive aging. Follow-up assessments of cognitive performance years after the intervention will allow us to examine this question. Finally, we do not know the threshold of change in functional connectivity that would result in changes of group, mean-level cognitive performance. Given the positive correlations between change in functional connectivity and change in cognition (Voss et al., 2010) and growth factors at the level of individual differences in this study, it is possible that more intense or more frequent training per week are needed for mean-level effects with this sample size. The effect of manipulating exercise frequency and intensity on cognitive and brain outcomes is relatively unknown and ripe for future study. Alternatively, it is possible that incorporation of resistance training, which has been shown to increase circulating IGF-1 (Cassilhas et al., 2007), would have also boosted the growth factor response for the walking group and resulted in group differences and greater increases in functional connectivity. However, the effects of exercise type on circulating growth factors in healthy elderly adults are unknown, and future research is needed to confirm the likelihood of these explanations.
Secondly, the functional connection between the bilateral parahippocampus and middle temporal gyrus has significance for the cohesion of the DMN (Fransson et al., 2008; Kahn et al., 2008) and the structural atrophy of the DMN associated with progression of Alzheimer’s Disease (Li et al., 2010; Whitwell et al., 2007). Therefore, results not only inform potential converging neurobiological mechanisms for the benefits of aerobic exercise on DMN function (Voss et al., 2010b), but also support a current hypothesis that exercise-induced increase in circulating IGF-1 is a key factor in prevention or reversal of cognitive decline associated with aging and Alzheimer’s Disease (Carro et al., 2005; Carro et al., 2006). We should note, however, that there is also evidence that inhibition of IGF-1 is associated with decreased cancer risk, increasing lifespan, and slowing progression of Alzheimer’s Disease (for review, see Piriz et al., 2011). In addition, there is research to suggest that just 6 weeks of low-intensity cycling (50% of HR max) can lower IGF-1 serum levels by 9% (Nishida et al., 2010), which may offer an explanation for why both groups showed a decrease in IGF-1 from pre- to post-intervention. Thus, it is clear the role of IGF-1 in cognitive aging and exercise-induced plasticity is in need of more translational research that bridges animal and human models.
Our results also suggest that increased availability of VEGF before starting an aerobic exercise program is involved in enhancing the effects of exercise on increased functional connectivity between the parahippocampus and lateral temporal cortex. However, too much VEGF can also result in negative outcomes for stroke and tumor growth (Storkebaum et al., 2004). Nevertheless, future research would benefit from a greater understanding of lifestyle factors and behavioral interventions that affect circulating VEGF, such as diet, stress, social enrichment, or cognitive training, that could serve as added components to aerobic exercise programs for improving brain health. For example, one known factor that increases VEGF is hypoxia (Asano et al., 1998; Storkebaum et al., 2004), suggesting that readjusting to high-altitude conditions over several weeks to months before engaging in an aerobic exercise program may boost the effects of aerobic exercise on brain health.
Regarding the unexpected finding that greater baseline BDNF was associated with greater functional connectivity change in the temporal lobe for the FTB group, one interpretation of this would be that greater baseline BDNF facilitates greater learning-related or experience-dependent increases in functional connectivity. This is based on the idea that FTB training included a learning component, whereby participants were introduced to a diversity of new exercises and poses that they had to remember and implement at each new session. Another unexpected observation in this analysis (Figure 1C) was the negative correlation between baseline IGF-1 and temporal lobe functional connectivity change. The correlation was not statistically significant, but generally the differing pattern in correlations for baseline serum levels compared to change in serum (Figure 1B) suggests more study is needed on the interaction of individual differences in baseline physiological profiles and brain-based markers of response to aerobic exercise training. There may be useful biomarkers in physiological profiles that help us understand when brain health is most likely to benefit from aerobic training.
In some respects, our results may be seen as preliminary since group differences in the strength of correlation were non-significant. This may have resulted from our relatively low N, which affects the power of the Fisher’s z test for differences in correlation. For example, approximate power on the observed effect sizes was quite low at 27% (BDNF), 12% (IGF-1), and 39% (VEGF), and for the observed effects sizes for the difference in correlations to be statistically significant at a one-tailed alpha of .05, sample sizes would have had to been extremely high, with a total N of 280 for BDNF, 600 for IGF-1, and 160 for VEGF (Cohen, 1988). Another possibility is that the sensitivity of serum growth factors to changes in systems-levels changes in brain function is small. For instance, since BDNF is expressed in many peripheral tissues such as the heart and lungs (Scarisbrick et al., 1993; Timmusk et al., 1993), the extent to which changes in serum BDNF are from exercise-induced central mechanisms is unclear and this will be important to examine in future research. One recent example is a study that showed peripheral administration of serum BDNF reversed behavioral symptoms of depression and anxiety and resulted in increased survival of progenitor cells in the hippocampus and prefrontal cortex, demonstrating covariation between modulations in serum BDNF and central expression of BDNF, and in turn behavior change (Schmidt et al., 2010). Therefore peripheral and central sources of serum BDNF in response to exercise likely interact in their relationship to central expression in the cortex.
Although we found evidence for the role of BDNF, IGF-1, and VEGF in exercise-induced functional connectivity in the temporal cortex, we did not find associations between changes in these growth factors and change in any of the other functional connections examined (see Table 1). Future research will be needed to understand why this may be, but one explanation could be that we were able to detect effects only where the concentration of BDNF is greatest, and that sensitivity of circulating growth factor associations with improvements in more long-range connections may require longer adherence to an aerobic exercise program. An alternative explanation is that a separate, independent pathway mediated increased functional connectivity in the other regional connections.
This study is not without limitations. A primary limitation of the study is that the measures of growth factors were exclusively from the periphery. There is, however, currently no viable alternative for assessing individual differences in BDNF, IGF-1, and VEGF in a large sample of healthy older adults. However, one potential problem with the current study that could be improved is the consistency of blood sampling in reference to the end date of the exercise intervention. In this study, participants had their blood taken within two weeks of their baseline and post-test MRI, and it is possible that this variable time-window introduced noise in the estimates of individual change in growth factors as a function of group. We also did not assess important factors that may have affected short-term fluctuations in growth factors such as nutritional status and changes in energy balance from the exercise interventions (Estivariz & Ziegler, 1997; Monteleone et al., 2004). In addition, examining the interactive moderating effects of the changes in biomarker concentration could not be reliably examined with the sample sizes available for each growth factor, but this will be important for future research. We were also unable to examine the effects of gender on training-related changes in serum growth factors or prediction of change from baseline serum levels; previous literature supports that females have greater baseline serum BDNF (Driscoll et al., 2012), so it is possible the effects could be stronger in men. Additionally, given there were no significant effects on cognitive performance in favor of the walking group compared to the FTB group in this sub-sample of our larger study (Voss et al., 2010b), the downstream effects of individual variation in changes in growth factors on cognition was not assessed here. Finally, we did not include assessment of biomarkers that we did not expect to change as a function of aerobic exercise. Despite no “control” biomarker, there was still specificity by way of the diversity of region of interest pairs that we examined in association with growth factor change. That is, we did not expect regions that became more functionally connected for the FTB group to have correlated change with growth factors associated with response to aerobic training (see Table 1). We also did not find correlated change with region of interest pairs that did not include any region in the temporal cortex for either group (see Table 1). This provides some specificity on the brain side, that in future studies could be complemented by specificity with a biomarker control.
In sum, this study demonstrates the first link between exercise-related changes in functional connectivity in the temporal cortex and changes in three putative neurobiological mechanisms for exercise-induced benefits on brain function, including BDNF, IGF-1, and VEGF. These results lend credibility to the low frequency BOLD signal as reflective of neuronal processes, and suggest that aerobic exercise-related increases in circulating growth factors are related to temporal lobe functional brain connectivity in elderly humans. Future research is necessary to understand how exercise type, duration, and intensity, interact with changes in growth factors, as well as how exercise-related changes in growth factors are related to clinically relevant outcomes such as cognition and disease progression.
Acknowledgments
This work was supported by the National Institute on Aging at the National Institutes of Health (grant numbers 05 R37 AG025667, RO1 AG25032). We would also like to thank the Institute for the Study of Aging for support of analysis of BDNF and VEGF factors. Finally, we thank Nancy Dodge, Holly Tracy, and the Exercise Psychology Laboratory for their help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Kaneoka K, Nomura T, Asano K, Sone H, Tsurumaru K, Yamashita K, Matsuo K, Suzuki H, Okuda Y. Increase in serum vascular endothelial growth factor levels during altitude training. Acta Physiol Scand. 1998;162:455–459. doi: 10.1046/j.1365-201X.1998.0318e.x. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and bdnf levels: Implications for the role of neuroplasticity in depression. The international journal of neuropsychopharmacology. 2008;11:1169. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor i mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor i. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor i receptor in the choroid plexus originates alzheimer’s-like neuropathology in rodents: New cues into the human disease? Neurobiology of Aging. 2006;27:1618–1631. doi: 10.1016/j.neurobiolaging.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Viana VAR, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Medicine and science in sports and exercise. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Ivy AS, Russo-Neustadt AA. Nitric oxide synthesis is required for exercise-induced increases in hippocampal bdnf and phosphatidylinositol 3′ kinase expression. Brain Res Bull. 2006;68:257–268. doi: 10.1016/j.brainresbull.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Clark P, Brzezinska W, Puchalski E, Krone D, Rhodes J. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009 doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-fos induction during escalation of voluntary wheel running in c57bl/6j mice. Behavioural Brain Research. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FGdM, Gobbi S, Andreatto CAA, Corazza DI, Pedroso RV, Santos-Galduróz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (bdnf): A systematic review of experimental studies in the elderly. Archives of gerontology and geriatrics. 2012;54:348–351. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum; 1988. [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: Learning from animal models. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2007a;3:S30–37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007b;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor i interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma bdnf is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS ONE. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivariz CF, Ziegler TR. Nutrition and the Insulin-Like Growth Factor System. Endocrine. 1997;7:65–71. doi: 10.1007/BF02778066. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gao P, Shen F, Gabriel RA, Law D, Yang E, Yang G-Y, Young WL, Su H. Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke. 2009;40:3596–3600. doi: 10.1161/STROKEAHA.109.561050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased Brain-Derived Neurotrophic Factor Expression in White Blood Cells of Relapsing–Remitting Multiple Sclerosis Patients. Scandinavian journal of immunology. 2003;57(5):493–497. doi: 10.1046/j.1365-3083.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- Greicius M, Srivastava G, Reiss A, Menon V. Default-mode network activity distinguishes alzheimer’s disease from healthy aging: Evidence from functional mri. Proceedings of the National Academy of Sciences. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neuroscience Letters. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? The Journal of experimental medicine. 1999;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamsi R. Diagnosis by default. Nature Publishing Group. 2012;18:338–340. doi: 10.1038/nm0312-338. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports medicine (Auckland, NZ) 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kraus RM, Stallings HW, Yeager RC, Gavin TP. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. Journal of Applied Physiology. 2004;96(4):1445–1450. doi: 10.1152/japplphysiol.01031.2003. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biological Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Li X, Coyle D, Maguire L, Watson DR, McGinnity TM. Gray matter concentration and effective connectivity changes in alzheimer’s disease: A longitudinal structural mri study. Neuroradiology. 2010 doi: 10.1007/s00234-010-0795-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor i is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. NeuroImage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proceedings of the National Academy of Sciences. 1997;94(2):663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosomatic medicine. 2004;66(5):744–748. doi: 10.1097/01.psy.0000138119.12956.99. [DOI] [PubMed] [Google Scholar]
- Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to alzheimer’s disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Matsubara T, Tobina T, Shindo M, Tokuyama K, Tanaka K, Tanaka H. Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. International Journal of Endocrinology. 2010;2010:1–8. doi: 10.1155/2010/452820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriz J, Muller A, Trejo JL, Torres-Aleman I. Igf-i and the aging mammalian brain. In Exp Gerontol. 2011:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin C-Y, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. Journal of Neurophysiology. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, et al. Psychomotor speed and functional brain mri 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Jones EG, Isackson PJ. Coexpression of mrnas for ngf, bdnf, and nt-3 in the cardiovascular system of the pre- and postnatal rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends in Neurosciences. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral bdnf produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biological Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors fgf-2, igf-1, and vegf exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (igf-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Carmeliet P. Vegf: Once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Yoo DJ, Glover GH, Gotlib IH. Bdnf genotype modulates resting functional connectivity in children. Frontiers in human neuroscience. 2009;3:1–10. doi: 10.3389/neuro.09.055.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat bdnf gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor i mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of bdnf. Brain Research. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF. Dedifferentiation in the visual cortex: An fmri investigation of individual differences in older adults. Brain Research. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010a;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in aging neuroscience. 2010b;2:1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley MH, Brubaker PH, Otto RM. ACSM’s guidelines for exercise testing and prescription. 7. New York, NY: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR. 3D maps from multiple mri illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to alzheimer’s disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JB. Questions and answers in the measurement of change. Review of Research in Education. 1988;15:345–422. [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences. 1999;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. The Journal of neuroscience. 1990;10(11):3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung H-D, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiology of Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]


