Abstract
Previous findings have linked lower socioeconomic status (SES) with elevated morbidity and mortality. Leukocyte telomere length (LTL), which also has been associated with age-related disease morbidity and mortality, is a marker of aging at the cellular level, making it a valuable early biomarker of risk and an indicator of biological age. It is hypothesized that SES will be associated with LTL, indicating that SES influences disease risk by accelerating biological aging. In the present sample we test for associations of childhood SES and adult SES (i.e. education, income, home ownership) with LTL, and examine whether these associations vary by racial/ethnic group. Analyses on 963 subjects (18.7% White, 53% Hispanics, and 28.5% African American) from the Stress ancillary study of the Multi-Ethnic Study of Atherosclerosis revealed a significant difference in LTL between home owners and renters in Hispanic and White participants (p < .05), but not amongst African Americans (p = .98). There were no linear associations of adult education or family income with LTL, however, there was an inverse association between father’s education and LTL (p = .03). These findings suggest that for Whites and Hispanics renting vs. owning a home is associated with an older biological age; however we did not replicate previous findings linking education with LTL.
Keywords: Telomere length, childhood SES, socioeconomic status, home ownership, wealth, parental education, cellular aging, biological aging, ethnicity
1.0 Introduction
A substantial body of literature has demonstrated that individuals with lower socioeconomic status (SES) are at increased risk for morbidity and mortality from numerous diseases of aging (Adler & Ostrove, 1999; Haan et al., 1987; Yen & Kaplan, 1999; Yen et al., 2009). Within the United States, these findings are present in Whites and Blacks, as well as acculturated Hispanic populations, although the strength of these effects often vary depending on the measure of SES used (Adler & Ostrove, 1999; Gallo et al., 2009a, 2009b; Lutsey et al., 2008). Although methodological complexities make identifying the contributions of different types of factors to the socioeconomic gradient difficult, it has been argued that access to healthcare and poorer health behaviors, although important contributors, do not fully account for this association. Given that the magnitude and quantity of chronic stressors increases with decreasing socioeconomic status (Steptoe & Feldman, 2001; Baum et al., 1999), it has been hypothesized that stress and related psychosocial processes may cumulatively influence the health of lower SES individuals and contributes to the SES patterning of many health outcomes (Baum et al., 1999; Yen & Syme, 1999).
Stressors are thought to cause cumulative biological wear and tear through repeated and prolonged perturbations of the stress response systems, and ultimately contribute to premature aging, increases in multiple biological risk factors, and higher disease morbidity and mortality (Seeman et al., 2010; McEwen, 2000). Several of these biological processes affected by stress, including excess oxidative stress, increases in inflammation, disruptions in restorative processes such as sleep and repair of damage to DNA, and dysregulation of cardiovascular, immune, and metabolic systems, may in turn have consequences for cellular aging and disease risk, including cardiovascular disease and cancer (Epel et al., 2004, 2006; Seeman et al., 2010; von Zglinicki, 2002). Telomere length has recently emerged as a marker of aging at the cellular level. The length of this tandem repeat of DNA at the end of chromosomes within leukocytes has been inversely associated with greater age, presence of subclinical cardiovascular disease, risk factors for disease, and age-related disease morbidity and mortality (e.g. cardiovascular disease and cancer) (Bakaysa et al., 2007; Brouilette et al., 2003; Ehrlenbach et al., 2009; Fitzpatrick et al., 2007; Farzaneh-Far et al., 2008; Willeit et al., 2010). Telomere shortening is thought to reflect cell replication history and cumulative oxidative stress burden within the progenitor cells and history of exposures and replication in the circulating cells (von Zglinicki, 2002). One driver of cell replication in the immune system is inflammation, which has been linked to shorter telomere length (Aviv et al, 2006; Bekaert et al., 2007; Fitzpatrick et al., 2007; O’Donovan et al., 2011; Shiels et al., 2011) and is thought to be a contributor to telomere loss. More rapid attrition indicates greater biological aging and may thus be a useful tool in examining the impact of the social environment and stress on health, premature aging, and disease vulnerability.
Modest associations of adult objective markers of SES with leukocyte telomere length (LTL) have been reported (Adler et al., 2012; Cherkas et al., 2006; Parks et al., 2009; Steptoe et al., 2011; Robertson et al., 2012; Pearce et al., 2012; Sheils et al., 2011), associations that were largely independent of health behaviors and disease risk factors. Several studies have reported associations between educational attainment and LTL, with higher levels of education associated with longer LTL in mid- to late- life male and female Caucasian samples (Parks et al., 2009; Pearce et al., 2012; Robertson et al., 2012; Steptoe et al., 2011) and one study in a multi-ethnic sample (Adler et al., 2012). However, null (Adams et al., 2007; Batty et al., 2009) and inverse associations have also been reported (Woo et al., 2009). Interestingly, Adler and colleagues (2012) report, in a sample of older adults (ages 70–79), racial differences in the strength of the association between education and LTL, such that Blacks show greater difference in LTL by education than Caucasians.
In addition to the findings linking personal educational attainment or social class to LTL, two studies examined associations of home ownership (an indicator of wealth) with LTL (Shiels et al., 2011; Robertson et al., 2012). In the West of Scotland Twenty-07 cohorts study, modest differences in LTL between home owners and renters were observed in the youngest cohort (Roberston et al., 2012), while in the psychological, social, and biological determinants of ill health (pSoBid) study steeper declines in LTL with age were present in those renting vs. owning a home (Shiels et al., 2011). These findings are of particular interest given findings that identify wealth, in terms of total assets, as one of the strongest SES predictors of health outcomes (Adams et al., 2003).
Childhood SES has also been linked to LTL, with positive associations between LTL and parental educational attainment observed in both children (Needham et al., 2012) and adult subjects (Robertson et al., 2012), while others report no associations between various measures of childhood social status and LTL in adulthood (Adams et al., 2007).
Racial/ethnic differences in telomere length have been reported, with finding from the present MESA cohort showing greater inverse associations of age with telomere length in Blacks and Hispanics compared to Whites (Diez-Roux et al., 2009), and similar findings suggesting more rapid telomere shortening with age in African Americans compared to Whites in the Family Heart Study and the Bogalusa Heart Study (Hunt et al., 2008).
In our analyses we sought to extend existing work examining associations between SES and LTL, which have predominantly occurred in European and Caucasian samples, by examining these associations in a multi-ethnic sample from the United States. The present analyses add to the literature in this field in several key ways. First, we examine the associations of LTL with indicators of both childhood (i.e. parental education) and adult SES (i.e. education, income, home ownership) in an African American, Caucasian, and Hispanic sample, which is unique to the existing literature. Secondly, we examine associations of LTL with home ownership (a marker of adult wealth) across a broad age range of community participants in the United States, an association that has yet to be tested in a US sample. Furthermore, we sought to build upon our prior work (Diez-Roux et al., 2009) reporting stronger cross-sectional associations of LTL with age in African-American and Hispanic participants compared to Whites by examining whether the childhood and adult SES-telomere associations vary by racial/ethnic group.
Our hypotheses were as follows:
Lower childhood SES and lower adult SES will be related to more rapid cellular aging, i.e. shorter LTL. These associations will remain after controlling for age, gender, biomedical factors (i.e. BMI, systolic and diastolic blood pressure, medication use, inflammation, diabetic status), and health behaviors that have been previously liked to telomere length (i.e. physical activity, smoking, and diet).
As prior analyses (Diez-Roux et al., 2009) reported differences in the associations of age with telomere length by race/ethnicity, we hypothesized that associations of SES with LTL would be more pronounced in Hispanics and African Americans compared to Whites.
2.0 Methods
2.1 Study population
The Multi-ethnic Study of Atherosclerosis (MESA) is a multi-site, longitudinal study of subclinical and clinical cardiovascular disease risk in the United States of America (Bild, 2002). A total of 6,814 men and women (ages 45–84 years old) free of clinical cardiovascular disease were recruited from six US communities (New York City, NY, Los Angeles County, CA, Baltimore, MD, Chicago, IL, Forsyth County, NC, St. Paul, MN) between July 2000 and August 2002. Analyses focus on a subset of the overall MESA cohort who participated in an ancillary study designed to examine the influence of stress on cardiovascular disease (the MESA Stress Study) initiated at Exam 2. For that subset of subjects, funding was obtained to allow assessment of LTL, using DNA previously collected and preserved from Exam 1. In total, 978 participants were recruited from New York and Los Angeles sites (Diez-Roux et al., 2009). 975 telomere length values were within <3 SD of the mean. Fourteen subjects were missing smoking history data and one was missing physical activity data. Analyses include 963 subjects with complete covariate data. 1 The racial/ethnic distribution of this sample of 963 is as follows: 180 (18.7%) White, 509 (53%) Hispanics, and 274 (28.5%) African American (the ancillary study did not enroll any Asian Americans). The present analyses only include variables obtained at Exam 1 as the LTL measure is based on DNA from Exam 1 blood samples; no data collected from subsequent MESA or MESA Stress exams are used since they post-date the LTL outcome assessments. Informed consent was obtained from all participants included in the present analyses and the study was approved by Institutional Review Boards of each participating institution.
2.2 Demographics, biomedical, and lifestyle risk factors
Demographic factors, including age, gender, and racial/ethnic identity was reported by participants. Biomedical factors found to be associated with LTL in previous reports were derived from the initial MESA exam, and include body mass index (BMI) = kg/m2) (Aviv et al., 2009; Cherkas et al., 2006), diabetes status (defined as > or equal to 126 ng/dL fasting glucose or use of diabetic medications) (Fitzpatrick et al., 2007), systolic and diastolic blood pressure (mmHg) (Jeanclos et al., 2000), use of cholesterol and/or blood pressure lowering medications, C-Reactive Protein (CRP), LDL, and HDL cholesterol. Secondary analyses adjusting for lifestyle risk factors associated with LTL included physical activity (Metabolic Equivalent (MET)-min/week of moderate and vigorous physical activity) (Cherkas et al., 2008), smoking history (pack-years smoked), and consumption of processed meats (Nettleton et al., 2008). A detailed description of these procedures has been reported previously (Bild, 2002; Golden et al.; Nettleton et al., 2008; Ranjit et al., 2007a) Missing dietary data (n = 99) was replaced with sample mean to prevent sample size changes in the final model.
2.3 Childhood and Adult SES
Parental education was determined through self-report of mother’s and father’s highest level of educational attainment. Both were used as predictors to determine the independent influence of mother’s vs. father’s education on LTL. The categorization for education was selected based on previous work (Lemelin et al., 2009) reflecting the truncated distribution of education in parents. Categories were as follows: less than high school, completed high school/some college, or college degree.
Adult level SES indicators, which have been included in previous MESA analyses (Diez-Roux et al., 2005; Lemelin et al., 2009; Lutsey et al., 2008; Ranjit et al, 2007b), included individual educational attainment (same classification as parental education) and current family income. Estimated family income was first reported as falling within an average annual range (e.g. $12,000–15,999, $16,000–19,999, $20,000–24,999, $25,000–29,999, $30,000–34,999, etc). We assigned the median dollar amount for that category range and then divided by the number of people in the household with equivalence scale using Oxford method of adjustments by weighting head of house hold (1), successive adults (.7), and children (.5) (OECD (1982), The OECD List of Social Indicators, Paris). Each was then assigned a category based on estimated per capita annual family income creating eight groups (1 = $0–9,999, 2 = $10–19,999, 3 = $20–29,999, 4 = $30,000–39,999, 5 = $40–49,999, 6 = $50–59,999, 7 = $60–69,999, 8 = $70,000 or more per capita). Distributions can be seen in Table 1. In addition to these measures of SES, our analyses included a marker of family wealth, home ownership (owner = 1) previously included in a composite measure of adult SES from the larger MESA cohort (Lemelin et al., 2009). This indicator of wealth was included as existing work indicates wealth, in terms of family assets, is a strong predictor of biological risk and health outcomes (Adams et al., 2003; Carroll et al., 2012; Hajat et al., 2011) and has recently been related to LTL in European samples (Shiels et al., 2011; Robertson et al., 2012).
Table 1.
Descriptive Statistics for Indices of Socioeconomic Status(SES) Within Race/Ethnic Group
| Whites | African-American | Hispanic | |
|---|---|---|---|
| Age | 62.5 (10.3) | 60.9 (10.1) | 61.4 (9.7) |
| Gender (% Male) | 48.3% | 44.9% | 48.5% |
| Father’s Education | |||
| Less than HS | Diploma 33.3% | 54.9% | 80.4% |
| HS Diploma, Some college | 34.5% | 34.8% | 16.9% |
| Completed College Degree | 32.2% | 10.3% | 2.6% |
| Mother’s Education | |||
| Less than HS Diploma | 34.1% | 54.9% | 82.3% |
| HS Diploma, Some college | 46.9% | 37.2% | 15.7% |
| Completed College Degree | 19% | 7.9% | 2.0% |
| Adult Education | |||
| Less than HS Diploma | 5% | 9.9% | 43.2% |
| HS Diploma, Some college | 33.9% | 67.2% | 47% |
| Completed College Degree | 61.1% | 23% | 9.8% |
| Annual Family Income Per Capita | |||
| $0–9,999 | 2.3% | 8.6% | 30.5% |
| $10–19,999 | 9.9% | 28% | 37.8% |
| $20–29,999 | 16.9% | 22.4% | 14.9% |
| $30–39,999 | 13.4% | 17.2% | 6.6% |
| $40–49,999 | 25% | 12.7% | 5.8% |
| $50–59,999 | 10.5% | 4.1% | 2.8% |
| $60–69,999 | 16.3% | 6.7% | 1.2% |
| $70,000 and greater | 5.8% | .4% | .4% |
| Home Ownership | |||
| Owned Home | 58.5% | 34.2% | 43.5% |
| Rented Home | 41.5% | 65.8% | 56.5% |
2.4 Blood LTL procedures
A detailed description of the telomere length procedures in MESA have been reported previously (Diez-Roux et al., 2009). Briefly, quantitative PCR (Q-PCR) assessment of LTL (Cawthon, 2002) was performed on isolated DNA from peripheral blood leukocytes. This method amplifies the telomeric (T) DNA and a single copy (S) control gene (36B4) used to normalize values. Cycle threshold (CT) values are converted to nanograms of DNA using a standard curve of serial dilutions. Using these values a relative measure of LTL is computed (T/S ratio). Intra- and inter-assay coefficients of variation for this assay were 6 and 7%, respectively.
2.5 Analyses
Participant summary descriptive statistics of primary predictors are reported for mean or percentage within each racial category. Each variable was assessed for outliers and normal distributions. Unadjusted and age-adjusted regression coefficient for demographic covariates, biomedical factors (BMI, diabetes, systolic and diastolic blood pressure (SBP; DBP), LDL and HDL cholesterol, and blood pressure and lipid lowering medication use), and lifestyle factors (smoking, physical activity, and dietary intake of processed meats) with LTL were first examined. Next, linear regression analyses were employed to examine associations of childhood SES, adult education, income, and home ownership with LTL, with the initial model adjusted for confounders (age, gender, race/ethnicity), and the second model adjusting for biomedical risk factors (BMI, blood pressure, diabetes status, cholesterol levels, and medication use). In the third model, further adjustments were made for lifestyle factors. Given previous findings in this data set, interactions of age by race/ethnicity and age by gender were also included as covariates in these initial models. To test for heterogeneity of SES associations across each racial/ethnic category, interaction analyses were performed, and significant interactions were followed with analyses on each group separately.
3.0 Results
3.1 Descriptive Statistics and Bivariate Associations
Of the 963 participants with ages ranging from 45–84 (mean age 61), 48% were male, and 18.5% White, 53% Hispanics, and 28.5% African American. Distribution of socioeconomic status across each racial/ethnic group is reported in Table 1. White participants were more likely to be in the higher SES categories than Hispanics and African American participants. As an example, Whites are more likely to have obtained higher education than Hispanics or African Americans (i.e. 61% having a college degree among Whites vs. 9.8% for Hispanics and 23% among African Americans).
Table 2 presents unadjusted and age-adjusted regression coefficients for associations of demographic, biomedical, and lifestyle risk factors with LTL. Age was significantly inversely associated with LTL (p < .001). Similarly, being male, diabetic, or taking medications to lower blood pressure or cholesterol were associated with shorter LTL. Age- and gender-adjusted LTL was inversely associated with pack-years of cigarette smoking and consumption of processed meats, but no longer associated with diabetic status or medication use.
Table 2.
Unadjusted and age/gender-adjusted mean differences (standard error) in T/S ratio by age, gender, biomedical, and lifestyle factors
| Unadjusted | Age/Gender-Adjusted | |||
|---|---|---|---|---|
|
| ||||
| Mean difference (SE) | p | Mean difference (SE) | p | |
| Demographics | ||||
| Age (per year) | −.005(.001) | .000 | ||
| Gender (Male=1) | −.05(.01) | .000 | ||
| Biomedical Factors | ||||
| Diabetic (Yes=1) | −.03(.01) | .04 | −.003(.01) | .77 |
| Body Mass Index (kg)/(m^2) | .001(.001) | .13 | .000(.001) | .88 |
| Systolic Blood Pressure (mmHg) | .000(.00) | .20 | .001(.000) | .04 |
| Diastolic Blood Pressure (mmHg) | .000(.001) | .67 | .000(.001) | .53 |
| Blood Pressure Medication (Yes=1) | −.022(.012) | .053 | −.001(.01) | .94 |
| Cholesterol Lowering Medication (Yes=1) | −.031(.015) | .04 | −.004(.01) | .78 |
| LDL Cholesterol (mg/dL) | −.0001(.00) | .74 | .000(.000) | .42 |
| HDL Cholesterol (mg/dL) | .0001(.000) | .88 | .0001(.000) | .84 |
| C-Reactive Protein (mg/L) | .000(.001) | .64 | −.001(.001) | .62 |
| Lifestyle | ||||
| Pack-Years Cigarette Smoking | −.002(.00) | .000 | −.001(.00) | .01 |
| Consumption of Processed Meats | −.06(.02) | .01 | −.06(.02) | .01 |
| Moderate & Vigorous Physical Activity (MET min/day standardized) | .01(.005) | .06 | .004 | .44 |
3.2 Education, Income, and LTL
No significant associations of individual educational attainment, or family income, with LTL were observed in the full sample (See Table 3). These findings did not change after controlling for biomedical and lifestyle factors. However, there was a significant inverse association of father’s education with LTL, an association that remained significant after entering biomedical and lifestyle factors in the model (R2 = .007, p = .02). The interaction of racial/ethnic category with mother’s/father’s education, adult education, or family income in the prediction of LTL were all not significant (all p’s > .15).
Table 3.
Mean differences (standard error) in leukocyte telomere length (T/S ratio) by unit change in the predictor after adjustments by covariates
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictors | B(SE) | p value | B(SE) | p value | B(SE) | p value |
| Father’s Education (n = 926) | ||||||
| Less than high school | .03(.01) | .04 | .03(.01) | .03 | .03(.01) | .03 |
| HS Diploma, some college | Reference | Reference | Reference | |||
| Completed college degree | −.01(.02) | .56 | −.02(.02) | .38 | −.01(.02) | .53 |
| Mother’s Education (n = 947) | ||||||
| Less than high school | .01(.01) | .62 | .01(.01) | .62 | .01(.01) | .47 |
| HS Diploma, some college | Reference | Reference | Reference | |||
| Completed college degree | −.02(.02) | .39 | −.03(.02) | .27 | −.02(.02) | .47 |
| Annual Family Income | −.002(.003) | .47 | −.002(.003) | .59 | −.002(.003) | .59 |
| Adjusted for Household Size (Per $10,000 increment; n = 938) | ||||||
| Adult Education (n = 952) | ||||||
| Less than high school | .03(.02) | .07 | .03(.02) | .09 | .03(.02) | .09 |
| HS Diploma, some college | .02(.01) | .13 | .02(.01) | .12 | .02(.01) | .10 |
| Completed college degree | Reference | Reference | Reference | |||
| Home Ownership (Own=1) (n = 920) | .05(.01) | .000 | .05(.01) | .000 | .04(.01) | .000 |
Model 1: Adjusted for age, gender, age X gender, age X race
Model 2: Adjusted for age, gender, age X gender, age X race, BMI, SBP, DBP, diabetes, HDL, LDL, CRP, medication use
Model 3: Adjusted for age, gender, age X gender, age X race, BMI, SBP, DBP, diabetes, HDL, LDL, CRP, medication use, smoking, physical activity, diet intake of processed meats
3.3 Home Ownership and LTL
As can be seen in Table 3, owning a home was significantly associated with longer LTL, an association that remained after adjustments for biomedical factors and lifestyle variables (R2 = .014, p < .001). Interaction analyses revealed a significant interaction of race/ethnicity (p = .01). Race/ethnicity stratified analyses, adjusting for demographic, age X gender, age X race/ethnicity, biomedical factors, and lifestyle variables, showed that the association between home ownership and LTL was present for Whites and Hispanics (R2 = .024, p = .03; R2 = .025, p < .001), but not for African Americans (R2 = .00, p = .98). As can be seen in Figure 1, there are no difference in LTL by home ownership among African Americans, with lower average LTL among African Americans regardless of home ownership. By contrast, among both Whites and Hispanics, those who own their home exhibit significantly higher LTL.
Figure 1. Estimated marginal mean leuckocyte telomere length by home ownership status.a.
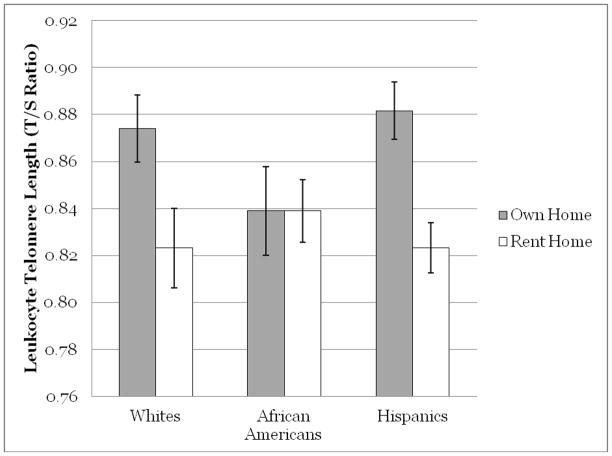
a.Estimated means adjusting for age, gender, age X gender, BMI, diabetes, pulse pressure, physical activity, and smoking
4.0 Discussion
4.1 Primary Results
In the present analyses of a racially/ethnically diverse population sample, renting one’s home (as opposed to owning) was associated with shorter LTL, independent of age, gender, and biomedical and lifestyle risk factors. When stratified analyses were performed by racial/ethnic group, the associations were no longer present in the African American sample; however, associations remained in the Hispanic and White groups. These findings suggest that for Whites and Hispanics limited wealth (as indexed by renting vs. owning a home) may increase the rate of biological aging, consistent with the theory that lower socioeconomic status may affect health through accelerated aging at the cellular level, evidenced by greater leukocyte telomere erosion (Adams, 2004, Bird et al., 2010).
Contrary to our hypothesis that racial/ethnic disadvantage would strengthen the associations of SES factors with LTL, associations of home ownership with leukocyte telomere length were weaker or absent in the African American sample. These analyses should be taken with caution however, given limited subgroup sample size, and different distributions of SES across race/ethnic groups, which means that the range of exposures across which associations could be examined differed by group. In addition, the extent to which a variable such as home ownership appropriately proxies the set of wealth-related exposures that could affect health could vary across race/ethnic groups, possibly explaining the heterogeneity in associations of wealth with TL in Blacks compared to other groups. However these results need to be replicated in other samples before any definitive conclusions can be drawn.
Interestingly, across the entire sample neither income or education indicators of socioeconomic status were associated with LTL, a finding that is in contrast to other reports of positive associations of LTL with occupational level (Cherkas et al., 2006) and educational attainment (Adler et al., 2012; Parks et al., 2009; Pearce et al., 2012; Robertson et al., 2012; Steptoe et al., 2011), but similar to others reports of little or no association (Batty et al., 2009; Adams et al., 2007).
Our findings suggesting LTL is longer amongst those owning a home is consistent with other recent reports (Robertson et al., 2012; Shiels et al., 2011), and is the first study to test for racial/ethnic differences in the association of home ownership with LTL. For the MESA Stress ancillary study cohort of adults aged 45–84, home ownership (as opposed to education or income) may provide a more valid measure of SES. Here, older adults may have a more diversified income, which when used to estimate SES is limited in its ability to capture other socioeconomic resource such as accumulated wealth, partially indexed by owning a home. The present findings suggest home ownership is associated with longer LTL, and may thus confer more protection from rates of biological aging. An alternative explanation is that renting a home is an indicator of neighborhood quality, and thus is associated with shorter LTL only as it is correlated with a poorer quality place of residence. Future analyses may want to consider how neighborhood characteristics may impact LTL, and consider improved measurement of wealth using additional indicators over the lifetime.
Surprisingly, there was a modest inverse association of father’s education with LTL, which remained significant after adjustments for lifestyle variables. Further secondary analyses (not shown) suggest that this inverse association was only present in the Hispanic sample, which included a high percentage of non-US born participants (50%). Other studies have suggested that the socioeconomic patterning of health may be different in immigrants than in the native born (Gallo et al., 2009a). Thus the sample composition may have affected our ability to fully examine associations of parental education with LTL. Likewise, although parental education is less vulnerable to recall bias than other childhood SES measures, it does not necessarily serve as a comprehensive measure of socioeconomic status in childhood as it fails to capture other dimensions of childhood socioeconomic position including wealth and income and occupation of the parents. Future work should consider inclusion of a childhood SES measure that captures aspects of family wealth (Carroll et al., 2011) and more comprehensive assessment of educationnal attainment of the primary caregivers.
An important limitation of the present analyses is their cross-sectional nature. Ideally investigations of predictors of LTL should examine associations with changes over time in leukocyte telomeres. Predictors and covariates were measured at a single point in time, although cumulative exposures over the life course are likely to be the most relevant for LTL. Similarly, as our sample includes participants with ages up to 84 years old, the associations between SES and TL may be buffered among health “survivors” into old age. It is also possible that the association of home ownership with LTL may be due to unmeasured or residual confounds related to individual or environmental factors (e.g. personality, pollution, neighborhood quality). In this regard, we noted differences in the percentage of home owners by recruitment site, with 70% of participants from UCLA reporting home ownership while only 19% from New York. This difference may be due to both differences in racial/ethnic recruitment strategies by site and variations in site specific population rates of home ownership (e.g. US Census reports New York rates of 19–27% while Los Angeles has 39–51% home owner occupied dwelling). The associations of home ownership with TL may be confounded by environmental factors correlated with site. In that site is strongly associated with home ownership it is difficult to estimate their independent effects, and is a limitation to the current analyses. Moreover, we cannot rule out the possibility that LTL differences were due to differences in circulating counts of white blood cells, which may contribute to differences in total white blood cell TL estimates (Weng, 2001). Strengths of the present analyses include the diverse population sample with detailed risk factor measurement and the availability of multiple measures of individual SES.
4.2 Mechanisms
There are a number of mechanisms through which economic resources could affect LTL. Health related behaviors such as smoking, diet, and physical activity have been linked to both SES and LTL. In our analyses associations persisted after adjustment for these factors although limited accuracy in these measures likely may have limited our ability to properly adjust for these variables. Alternatively, the associations we observed may be partially mediated through psychobiological pathways--causing repeated and prolonged neuroendocrine disruptions that have a cumulative toll on several regulatory systems gradually contributing to physiologically and clinically significant dysregulation (Seeman et al., 2010). Indeed, recent evidence reports increases in allostatic load among those of lower socioeconomic backgrounds and those living in neighborhoods of lower socioeconomic position (Bird et al., 2010; Merkin et al., 2009; Seeman et al., 2010; Gruenewald et al., 2012; Karlamangla et al., 2005).
These cumulative exposures to economic burdens, interpersonal chronic stressors, and an often concomitant lack of resources, chronically elicits a neuroendocrine response that disrupts normal homeostasis in cardiovascular, neuroendocrine, immune, and metabolic regulatory systems. The telomere, whose length is affected by exposure to oxidative stress and inflammatory activity, may be partially capturing this history of chronic wear and tear (i.e. allostatic load) as perturbations in these regulatory systems increase inflammation, oxidative stress, and decreases cellular capacity to repair damage (see (Robles & Carroll, 2011 for review). Prolonged and repeated disruptions in these systems are thought to contribute to age related disease, including cardiovascular disease, as well as accelerate biological aging. Although the single time point measures of inflammation (CRP), cholesterol, and blood pressure were not related to age-adjusted LTL in our cross-sectional analyses, it remains possible that repeated elevations in these biomarkers may contribute to telomere erosion over longer periods and be a pathway to accelerated aging and age-related disease initiation and progression. Longitudinal analyses characterizing the role of these biomarkers and telomere length in human disease and longevity are necessary before such conclusions can be drawn (Aviv, 2004). Further research is necessary to both determine the extent to which LTL captures allostatic load and to identify the mechanisms through which chronic environmental and psychological stress contributes to leukocyte telomere attrition.
4.3 Summary
Paralleling existing literature linking wealth indicators of socioeconomic status to health and disease, the present findings suggest that for Whites and Hispanics, increased wealth as indexed by home ownership may contribute to better health outcomes by slowing the rate at which the body ages, independent of health behaviors. Whereas chronological age accounts for ~9% of the variance in LTL, home ownership accounts for an additional 1.5%. Based on estimates within the current sample that the average LTL loss for each year of chronological age is .005 (T/S), the regression coefficient estimate for home ownership of .04 indicates an average shorter LTL by .04 (T/S) in renters vs. homeowners, and can be translated to be roughly equivalent to 8 years of chronological aging on LTL (.04/.005=8). The true size of the association may be stronger than these current estimates given the present analyses does not capture lifetime history of wealth. However, given the lack of consistency of associations of LTL with other SES measures (education and family income) our findings need to be replicated in other samples before firm conclusions can be drawn. Longitudinal studies of the links between cumulative socioeconomic, psychosocial, and environmental exposures, and leukocyte telomere length changes are necessary to confirm the presence and magnitudes of these associations.
Highlights.
Home ownership is associated with longer leukocyte telomere length in White and Hispanic participants.
Acknowledgments
This study was supported by grants R01 HL101161 (A.V.D-R.) and N01-HC 95159 through N01-HC 95169 from the National Heart, Lung, and Blood Institute. The manuscript preparation was supported by grant T32-MH19925 from the National Institute of Mental Health, and the Cousins Center for Psychoneuroimmunology, UCLA (J.E.C.). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- SES
Socioeconomic status
- LTL
leukocyte telomere length
- BMI
body mass index
Footnotes
Sample size in regression models vary depending on SES predictor as each has missing data. Sample sizes for each predictor reported in table: Income is missing 25, education 11, father’s education 37, mother’s education 16, home ownership 43.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams P, Hurd MD, McFadden D, Merrill A, Ribeiro T. Healthy, wealthy, and wise? Tests for direct causal paths between health and socioeconomic status. Journal of Econometrics. 2003;112(1):3–56. [Google Scholar]
- Adams JM. Biological ageing: A fundamental, biological link between socio-economic status and health? The European Journal of Public Health. 2004;14(3):331–334. doi: 10.1093/eurpub/14.3.331. [DOI] [PubMed] [Google Scholar]
- Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6:125–128. doi: 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Annals of the New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Adler N, Pantell M, O’Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, et al. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain, behavior, and immunity. 2012 doi: 10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A. Telomeres and human aging: facts and fibs. Science of aging knowledge environment: SAGE KE. 2004;2004(51):pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. American Journal of Epidemiology. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, Samani NJ, Ford I. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. Journal of Epidemiology and Community Health. 2009;63:839–841. doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Annals of the New York Academy of Sciences. 1999;896:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging cell. 2007;6(5):639–47. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bird CE, Seeman T, Escarce JJ, Basurto-Dávila R, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M, et al. Neighbourhood socioeconomic status and biological “wear and tear” in a nationally representative sample of US adults. Journal of Epidemiology and Community Health. 2010;64:860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain, Behavior, and Immunity. 2011;25:1468–74. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:47e–47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Archives of Internal Medicine. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8:251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstätter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. International Journal of Epidemiology. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow J, Cawthon R. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Science. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Gallo LC, de Los Monteros KE, Allison M, Roux AD, Polak JF, Watson KE, Morales LS. Do socioeconomic gradients in subclinical atherosclerosis vary according to acculturation level? Analyses of Mexican-Americans in the multi-ethnic study of atherosclerosis. Psychosomatic Medicine. 2009a;71:756–762. doi: 10.1097/PSY.0b013e3181b0d2b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? Journal of personality. 2009b;77:1707–1746. doi: 10.1111/j.1467-6494.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Golden SH, Lee HB, Schreiner PJ, Roux AD, Fitzpatrick AL, Szklo M, Lyketsos C. Depression and type 2 diabetes mellitus: the multiethnic study of atherosclerosis. Psychosomatic medicine. 69:529–536. doi: 10.1097/PSY.0b013e3180f61c5c. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Social science & medicine. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan M, Kaplan GA, Camacho T. Poverty and health. Prospective evidence from the Alameda County Study. American journal of epidemiology. 1987;125:989–998. doi: 10.1093/oxfordjournals.aje.a114637. [DOI] [PubMed] [Google Scholar]
- Hajat A, Kaufman JS, Rose KM, Siddiqi A, Thomas JC. Long-term effects of wealth on mortality and self-rated health status. American journal of epidemiology. 2011;173(2):192–200. doi: 10.1093/aje/kwq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging cell. 2008;7(4):451–8. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, HJ, Aviv A, Skurnick JH. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Williams DR, Schwartz JE, Matthews Ka, Kiefe CI, Seeman TE. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA Study (USA) Social Science & Medicine. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Lemelin ET, Diez Roux AV, Franklin TG, Carnethon M, Lutsey PL, Ni H, O’Meara E, et al. Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Social science & medicine (1982) 2009;68(3):444–51. doi: 10.1016/j.socscimed.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsey PL, Diez Roux AV, Jacobs DR, Burke GL, Harman J, Shea S, Folsom AR. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. American journal of public health. 2008;98(11):1963–70. doi: 10.2105/AJPH.2007.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce J, Seeman T. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Annals of Epidemiology. 2009;19:194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) American Journal of Clinical Nutrition. 2008;88:1405–12. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS one. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MS, Mann KD, Martin-Ruiz C, Parker L, White M, von Zglinicki T, Adams J. Childhood growth, IQ and education as predictors of white blood cell telomere length at age 49–51 years: the Newcastle Thousand Families Study. PloS one. 2012;7(7):e40116. doi: 10.1371/journal.pone.0040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007a;116(21):2383–90. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez Roux AV, Shea S, Cushman M, Seeman T, Jackson S, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Archives Internal Medicine. 2007b;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- Robertson T, Batty GD, Der G, Green MJ, McGlynn LM, McIntyre A, Shiels PG, et al. Is Telomere Length Socially Patterned? Evidence from the West of Scotland Twenty-07 Study. PLoS ONE. 2012;7(7):e41805. doi: 10.1371/journal.pone.0041805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Carroll JE. Restorative biological processes and health. Social and Personality Psychology Compass. 2011;5:518–537. doi: 10.1111/j.1751-9004.2011.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, Macintyre A, Johnson PCD, Batty GD, Burns H, Cavanagh J, et al. Accelerated Telomere Attrition Is Associated with Relative Household Income, Diet and Inflammation in the pSoBid Cohort. PloS one. 2011;6(7):e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: Development of a measure of neighborhood problems, and associations with socioeconomic status and health. Annals of Behavioral Medicine. 2001;23:177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimäki M, Marmot M, Blackburn E, Erusalimsky JD. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain, Behavior, and Immunity. 2011;25:1292–8. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Weng N. Interplay between telomere length and telomerase in human leukocyte differentiation and aging Abstract: Blood leukocytes derive from bone. Journal of Leukocyte Biology. 2001;70:861–867. [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Woo J, Suen EWC, Leung JCS, Tang NLS, Ebrahim S. Older men with higher self-rated socioeconomic status have shorter telomeres. Age and Ageing. 2009;38:553–558. doi: 10.1093/ageing/afp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen IH, Kaplan Ga. Poverty area residence and changes in depression and perceived health status: evidence from the Alameda County Study. International Journal of Epidemiology. 1999;28:90–94. doi: 10.1093/ije/28.1.90. [DOI] [PubMed] [Google Scholar]
- Yen IH, Michael YL, Perdue L. Neighborhood environment in studies of health of older adults: a systematic review. American Journal of Preventive Medicine. 2009;37:455–463. doi: 10.1016/j.amepre.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annual Review of Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]


