Abstract
In the vertebrate nose, increasing air speed tends to increase the magnitude of odor-evoked activity in olfactory receptor neurons (ORNs), given constant odor concentration and duration. It is often assumed that the same is true of insect olfactory organs, but this has not been directly tested. In this study, we examined the effect of air speed on ORN responses in Drosophila melanogaster. We constructed an odor delivery device that allowed us to independently vary concentration and air speed, and we used a fast photoionization detector to precisely measure the actual odor concentration at the antenna while simultaneously recording spikes from ORNs in vivo. Our results demonstrate that Drosophila ORN odor responses are invariant to air speed, as long as odor concentration is kept constant. This finding was true across a >100-fold range of air speeds. Because odor hydrophobicity has been proposed to affect the air speed dependence of olfactory transduction, we tested a >1,000-fold range of hydrophobicity values and found that ORN responses are invariant to air speed across this full range. These results have implications for the mechanisms of odor delivery to Drosophila ORNs. Our findings are also significant because flies have a limited ability to control air flow across their antennae, unlike terrestrial vertebrates, which can control air flow within their nasal cavity. Thus, for the fly, invariance to air speed may be adaptive because it confers robustness to changing wind conditions.
Keywords: odor, insect, flux, velocity, hydrophobicity
for all terrestrial animals, the sense of smell is directly connected to the movement of air. Terrestrial vertebrates draw air into their nose with active sniffing behaviors, and air speed within the nose has been shown to be a critical variable in determining the magnitude of odor responses in olfactory receptor neurons (ORNs). Specifically, ORN response magnitudes tend to increase with increasing air speed, given a fixed odor concentration and odor pulse duration (Doving 1987; Mozell et al. 1991a, 1991b; Scott et al. 2006; Sobel and Tank 1993). Accordingly, the perceived odor intensity of a fixed odor concentration in humans can grow with increasing air speed through the nose (Le Magnen 1944; Rehn 1978; Schneider et al. 1963). Olfactory performance in both humans and rodents can depend on sniff rate (Kepecs et al. 2007; Laing 1983), a phenomenon that may be mediated by the effect of air speed on ORN responses.
What are the reasons why air speed might affect olfactory transduction? Four explanations have been proposed on the basis of previous studies (Fig. 1).
Fig. 1.
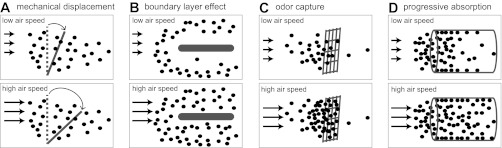
Proposed mechanisms of air speed dependence in olfactory transduction. Arrows indicate the direction and magnitude of the air velocity, and the density of black dots indicates relative odor concentration. A: increases in air speed can exert forces on olfactory receptor neurons (ORNs), thereby leading to displacements that activate intrinsically mechanosensitive conductances in ORNs. Increasing air speeds would produce larger displacements. Note that this is the only mechanism that does not invoke a spatial nonuniformity in odor concentration. B: a boundary layer of air can form around the olfactory organ where odor concentration is lower than the concentration outside this layer. Because the layer should become thinner with increasing air speed, its effect is diminished as air speed increases. C: the olfactory organ might act as a sieve that captures odor molecules. If capture were essentially irreversible, then the rate of capture (and thus local odor concentration) would grow with increasing air speed. D: in the vertebrate nasal cavity, odorized air is drawn over a large absorptive surface that can progressively deplete odor from the air, forming a gradient of odor concentration through the length of the cavity. The steepness of the gradient should decrease with increasing air speed, and so increasing air speed should increase the odor concentrations that are delivered to downwind sites in the cavity. For ORNs that are located downwind, this would increase odor responses.
Mechanosensitivity.
ORNs may be intrinsically responsive to mechanical stimuli. In particular, odorant receptor proteins have been proposed to be force activated as well as ligand activated. This conclusion was suggested by the finding that the responses of mouse ORNs in vitro can grow with increasing delivery pressure of Ringer solution (Grosmaitre et al. 2007). Given this, increasing air speed might be expected to increase ORN responses.
Boundary layer thinning.
At low air speeds, an object will be surrounded by a layer of slow-moving air (the “boundary layer”) (Koehl 2006; Moore et al. 1989). This boundary layer slows the movement of odor molecules to the olfactory organ, lowering the effective concentration of odor at the receptors. Increasing air speed decreases the thickness of the boundary layer. This creates better penetration of odor molecules into the surface of the olfactory organ, e.g., into crevices of the nasal cavity (Mozell et al. 1991b) or gaps between hairs on the surface of insect antennae. Similarly, at high water flow rates, aqueous odor penetrates more deeply between hairs on crustacean antennules (Koehl 2006). As a result, increasing air speed can increase odor concentration at the surface of the olfactory organ.
Increased odor capture.
This model treats the olfactory organ as a molecular “sieve” that captures much of the odor in its vicinity and makes the odor available to ORNs (Kaissling 1971, 1986). The rate of odor delivery into the sieve will be proportional to air speed. If the probability of an incoming odor molecule being captured by the sieve is independent of air speed, then the local odor concentration will increase with air speed. For this to be true, it is also important that the rate of removal of odor from the sieve does not keep pace with the increasing rate of odor delivery. Evidence for this model comes from measurements showing that about a third of radioactive pheromone molecules passing over a moth antenna are absorbed and not readily released (Kanaujia and Kaissling 1985). The finding that some ORN responses far outlast the duration of the nominal stimulus has been cited as further evidence that captured odor is not readily removed (Kaissling 1971). How this process might work on a microscopic level is not known.
Decreased preabsorption.
In the vertebrate nose, odor enters at the nostrils and moves through the long, closed path of the nasal cavity. At each location in this path, some odor is absorbed into the mucosa, and some of this absorbed odor may be actively removed (e.g., by diffusing into capillaries) rather than returning to the air. This effect can create a gradient of odor concentration along the nasal cavity, with lower concentrations at locations more distal to the nostrils. If increasing air speed decreases the probability of an odor molecule being absorbed at any location in the path (because its dwell time at that location decreases), then increasing air speed will make the gradient more shallow. This means that distal ORNs will be exposed to higher odor concentrations. This effect should be largest for odors that are most readily absorbed into mucus, i.e., hydrophilic odors (Kent et al. 1996; Mozell and Jagodowicz 1973; Mozell et al. 1991a, 1991b; Schoenfeld and Cleland 2005; Scott et al. 2006).
In thinking about the effects of air speed on olfaction, it is worth thinking about whether the organism actively controls air speed. Whereas vertebrates control the flow of air through their nose, many insects have comparatively little control over air flow across their olfactory organs. Much of the air movement across insect olfactory organs is driven by wind in the environment, although wing and antennal movements can play a role (Dethier 1987; Loudon and Koehl 2000; Mamiya et al. 2011). Because insects cannot fully control this stimulus parameter, it is important to understand whether it might confound insect olfactory transduction.
The first three mechanisms described above might plausibly apply to insect olfactory organs. (The fourth mechanism would not apply, because unlike air moving through the vertebrate nasal cavity, air moving across an insect antenna is not confined to a long, closed path.) No previous studies have directly measured whether air speed affects olfactory transduction in insects. Nevertheless, many theoretical studies and review articles have proposed or assumed that olfactory transduction in insects grows with increasing air speed (Kaissling 1971, 1986, 1998, 2001; Kaissling and Rospars 2004; Lansky and Rospars 1998; Rospars et al. 2000).
It is of particular interest to know whether olfactory transduction in Drosophila depends on air speed because of the general interest in exploiting the genetic toolbox of Drosophila to study olfactory transduction, processing, and learning (Davis 2011; Hallem and Carlson 2004; Masse et al. 2009; Olsen and Wilson 2008; Ramdya and Benton 2010). Like most insect ORNs, Drosophila ORNs are housed in hairlike structures (called sensilla) on the surface of the antenna (Keil 1999). By inserting a fine electrode into a single sensillum, one can record from individual ORNs in vivo (de Bruyne et al. 1999, 2001). An experimental virtue of this preparation is the ability to unambiguously identify different ORN types in these recordings, where a “type” is defined by the odorant receptor that an ORN expresses (Couto et al. 2005; Fishilevich and Vosshall 2005).
In this study, we constructed and validated an odor delivery device designed to independently control odor concentration and air speed. We used this device to test whether air speed affects olfactory transduction in two different types of Drosophila ORNs in vivo. Given that the dependence of transduction on air speed has been proposed to be related to the hydrophobicity of the odor, we used three different odors with widely varying hydrophobicity. Our results argue that olfactory transduction in Drosophila is invariant to air speed, at least within the parameter space we have explored. This has implications for the mechanisms of odor delivery from the perireceptor space in Drosophila ORNs. It also implies that an organism that cannot fully control air flow over its olfactory organ is capable of evolving air speed-invariant mechanisms of olfactory transduction. This stands in contrast to vertebrate olfactory systems, where air speed is both critical to transduction and under the control of the organism.
MATERIALS AND METHODS
Odor delivery.
We designed a custom odor delivery device to allow independent control over air speed and odor concentration (Fig. 2A). A continuous stream of charcoal-filtered air was fed into two adjustable flowmeters set to the same flow rate. Depending on the range of air speeds that was desired in the experiment, we used a different pair of matched flowmeters (127657-1, 234509-1, or 277577-1 from Cole-Parmer), permitting maximum flow rates of 300 ml/min, 2.5 l/min, or 10 l/min (indicated in black, light gray, and dark gray in Fig. 3B). By controlling the flow rate through these flowmeters, we could control the speed of the final odorized air stream. The output of one of the two flowmeters was sent to a large gas-washing bottle (the “bubbler” in Fig. 2A; either Ace Glass 7538-29 or, preferably, Corning 31770-500C). This device forced air through a fritted glass diffuser and up through a large column of pure liquid odorant to produce saturated (or nearly saturated) vapor in the head space of the bubbler. This odorized air stream and the matched clean air stream were each delivered to a three-way Teflon solenoid valve (STV-3-1/4 UKG 24VDC, Clark Solutions). These two valves were controlled via a microcontroller platform (Arduino Nano, Arduino Software) and custom routines written in MATLAB. The two valves were always held in opposite states, such that at any moment one line would be vented while the other line would be passed to an odor/air mixing chamber. The two valves were programmed to alternate between the vent and the mixing chamber with a period of 1 s. By varying the duty cycle of this switching, we could vary the ratio of odorized air to clean air that was delivered to the mixing chamber and thus the equilibrium odor concentration in the mixing chamber. The mixing chamber was a 500-ml glass Erlenmeyer flask. We allowed 5 min to elapse after any change in the duty cycle to permit the odor concentration in the flask to reequilibrate before odor was delivered to the fly. The output of the mixing flask was delivered to a third and final solenoid valve that could be switched between a vent and the fly. This last valve allowed us to control the duration of the odor pulse. All odor stimuli were 5 s in duration and are reported as nominal percentages of saturated vapor. All the odor vents in the system were positioned near a vacuum tube but were not connected to this tube, and thus there was essentially no negative pressure on the vents. The final odor tube had an inner diameter of 3 mm and terminated <1 mm away from the fly (Fig. 2B). The water solubility values for dibutyl sebacate and 1-propanol are taken from Yalkowsky et al. (2010), and the water solubility of linalool oxide was estimated with the U.S. Environmental Protection Agency's EPI Suite software (v. 4.10).
Fig. 2.
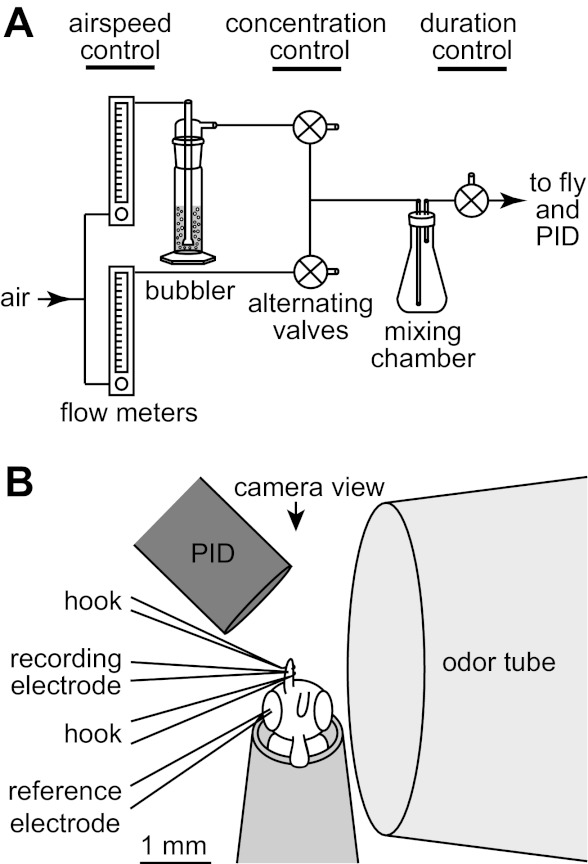
Experimental setup. A: schematic of the odor delivery device. Air speed was controlled by changing the flow through 2 matched flowmeters that were set to the same flow rate. The output of 1 flowmeter was sent through a large column of pure liquid odorant, producing saturated (or nearly saturated) vapor. The odorized air stream and its matched clean air stream were each sent to a 3-way valve. These 2 valves were always held in opposite states so that only 1 would be passed to the mixing chamber at any given time while the other was vented. The concentration of the final odor pulse was controlled by altering the duty cycle of switching between the valves. The timing of the final odor pulse was controlled by a valve near the fly. PID, photoionization detector. B: scale diagram of the recording configuration, as seen from above, through the microscope objective. The fly was placed in as close as possible to the odor tube and the PID. A miniature video camera near the fly's head permitted precise positioning of the fly. One antenna was lifted off the fly's head and stabilized with a pair of fine glass hooks. The recording electrode was inserted into a sensillum on this antenna, and the ground electrode was inserted into an eye.
Fig. 3.
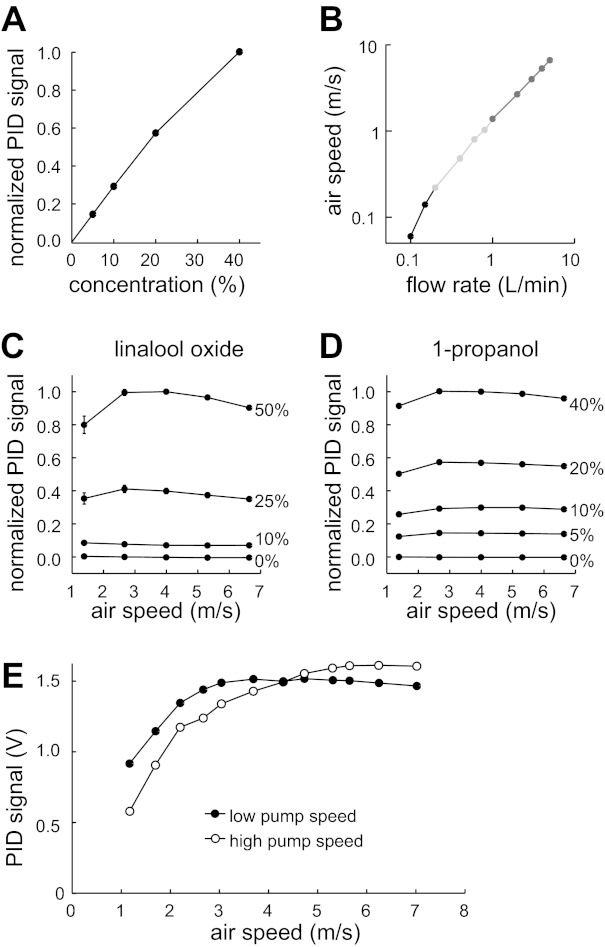
Validation of odor delivery device with PID measurements. A: the normalized PID voltage (which should be proportional to odor concentration at the device outlet) depended nearly linearly on the duty cycle of valve switching (which should be proportional to the concentration in the mixing chamber, reported here as % of saturated vapor). Data were averaged over 11 experiments using linalool oxide at an air speed of 5.3 m/s. B: air speed (as measured by the anemometer) depended linearly on the nominal flow rates delivered through the device. Note log-log axes. Data were collected with 3 different sets of matched flowmeters, labeled here in different shades of gray (see materials and methods). Odor is linalool oxide. C: concentration of linalool oxide delivered to the PID was independent of air speed (mean ± SE, n = 11; some error bars are obscured by markers). D: concentration of 1-propanol delivered to the PID was independent of air speed (mean ± SE; n = 20). E: fall-off in PID signals at low air speeds is more pronounced when the PID pump speed is high (i.e., when the PID is exerting a large negative pressure). This implies that the fall-off is an artifact of the fact that when the PID pump is not completely matched by the odor delivery device outflow, the PID will draw in clean air. Low pump speed is 970 ml/min; high pump speed is 1,652 ml/min. Odor is linalool oxide.
In this study, we generally used duty cycles of 0–50%, corresponding to 0–50% saturated vapor. In principle, the 0% condition should deliver no odor to the fly, and it was included as a negative control. In practice, we often observed that this stimulus elicited a small response. This disappeared every time we replaced all the components of the olfactometer, and so it likely reflects odor contamination of the apparatus. For this reason, whenever we switched odors we replaced all components except the bubbler, which we washed with water and ethanol.
Note that the odor pulse duration was constant for all air speeds, meaning that the total number of delivered odor molecules per odor pulse grew proportionately with increasing air speed. Some authors have pointed out that it can be useful to keep the number of delivered molecules constant (Mozell et al. 1991b), especially when olfactory transduction depends on air speed. However, when transduction depends only on odor concentration (as it does in our results), keeping odor pulse duration constant does not introduce any confounds in interpretation.
We noted that as air speeds increased above 5 m/s the measured odor concentration at the outlet of the device showed a small systematic decrease (≤10% of maximum; Fig. 3). This is likely due to the fact that high flow rates cause high pressures in the system, which can cause odor vapor to leak out prior to mixing. The leak occurred at the glass-glass junction between the two parts of the gas-washing bottle, and although the junction was wrapped tightly with Parafilm (Pechiney Plastic Packaging), we were not able to completely eliminate the leak at very high pressures. This effect was often statistically significant: when we ran separate linear regressions of measured odor concentration versus air speed for each combination of odor and duty cycle, we often noted a statistically significant negative linear correlation between these values. This likely explains why we noted a nonsignificant trend toward decreased ORN firing rates with increasing air speeds for certain odor and duty cycle settings. This phenomenon did not appear to significantly influence our ORN recordings (see below), probably because it is relatively small in magnitude.
Photoionization and anemometer measurements.
We used a photoionization detector (PID; 200A miniPID, Aurora Scientific) to measure the magnitude and time course of the odor pulse at the output of the last valve. The magnitude of the PID signal is proportional to odor concentration, with the proportionality constant depending on the odor composition. The PID is capable of reporting concentration fluctuations at speeds of up to 330 Hz, according to the manufacturer. The PID inlet was positioned 1 mm from the fly, downwind from the valve, and was used to measure the output of the device for all experiments using air speeds >1 m/s (Fig. 2B). The PID was operated on the low flow rate setting (970 ml/min), and we verified that ORN responses were the same regardless of whether the PID was turned on or off for all experimental conditions where the PID was used (i.e., for air speeds >1 m/s). The glass bulb inside the PID head was cleaned periodically to remove accumulated residue that diminished PID sensitivity. Despite this, the PID sensitivity drifted slowly over the time course of days, and therefore PID values were normalized to a within-experiment measurement before they were averaged across experiments (see Data analysis). The accuracy of the PID was diminished at flow rates below 2.0 l/min (corresponding to 2.67 m/s at the outlet of our final valve) because the negative pressure exerted by the PID pump was not fully balanced by the positive pressure provided by the air stream. For this reason, we did not measure PID values for the lowest range of flow rates/air speeds in our study. We measured PID responses for two of the three odors we used in this study (linalool oxide and 1-propanol) but not for the third odor (dibutyl sebacate), because it did not elicit a measureable PID signal. To measure air speed, we used a hot wire anemometer (Anemomaster A004, Kanomax) positioned at the location of the fly. According to the manufacturer's specifications, the anemometer does not provide accurate readings below 0.1 m/s, and therefore the reading of 0.06 m/s (see Fig. 8B) should be regarded with caution. We were not able to obtain stable readings below 0.06 m/s, and so we did not investigate air speeds below this value in this study. In addition, at air speeds lower than this value, odor delivery tends to become turbulent.
Fig. 8.
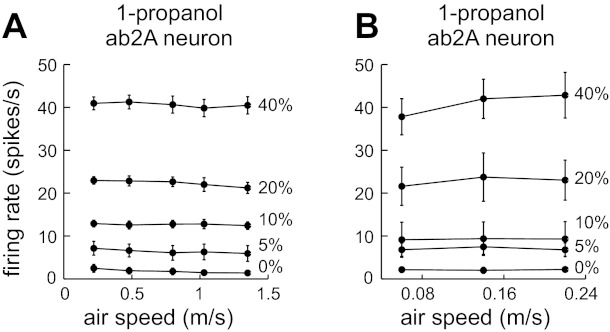
ORN responses to 1-propanol are invariant to air speed at a range of low air speeds associated with natural Drosophila flight. A: average ab2A firing rates evoked by 1-propanol, with air speeds in the range of those experienced by Drosophila flying in still air (n = 5). B: average ab2A firing rates evoked by 1-propanol, with even lower air speeds than those shown in A (n = 7). Error bars are SE and are sometimes obscured by markers.
Electrophysiology.
Flies were reared at room temperature on conventional cornmeal agar medium. All ORN recordings were performed on adult female flies from the wild-type strain w1118, 2–5 days after they eclosed from their pupal cases. Flies were cold-anesthetized and wedged into the trimmed end of a 200-μl plastic pipette tip. Each fly was secured by waxing the head and proboscis to the end of the pipette tip. The fly was then placed under an upright compound microscope (Olympus BX51) with a ×50 air objective. A video camera pointed at the head of the fly (Unibrain Fire-I BBW 1.3 Camera, equipped with an 8-mm telephoto lens; 1394Store.com) allowed the fly to be positioned precisely relative to the odor tube and the PID. The antenna was stabilized by two pulled glass capillaries fashioned with small hooks at the ends. The recording and reference electrodes were silver chloride wires inserted into saline-filled glass electrodes. The recording electrode was inserted into a single antennal sensillum, while the reference electrode was inserted into the eye (see Fig. 2B). Sensillum types were identified based on their size, the spike waveforms and spontaneous firing rates of the neurons in the sensillum, and the responses of the neurons to a panel of odors (de Bruyne et al. 2001). Voltage signals were acquired with an A-M Systems model 2400 amplifier and low-pass filtered at 2 kHz with a LPF202A signal conditioner (Warner Instruments) before digitization at 10 kHz. Digitized signals were acquired with custom routines written in IgorPro (Wavemetrics) through a PCI-6251 data acquisition board (National Instruments). The sample trace shown in Fig. 5A was high-pass filtered at 15 Hz after digitization to remove the slow local field potential component of the response.
Fig. 5.
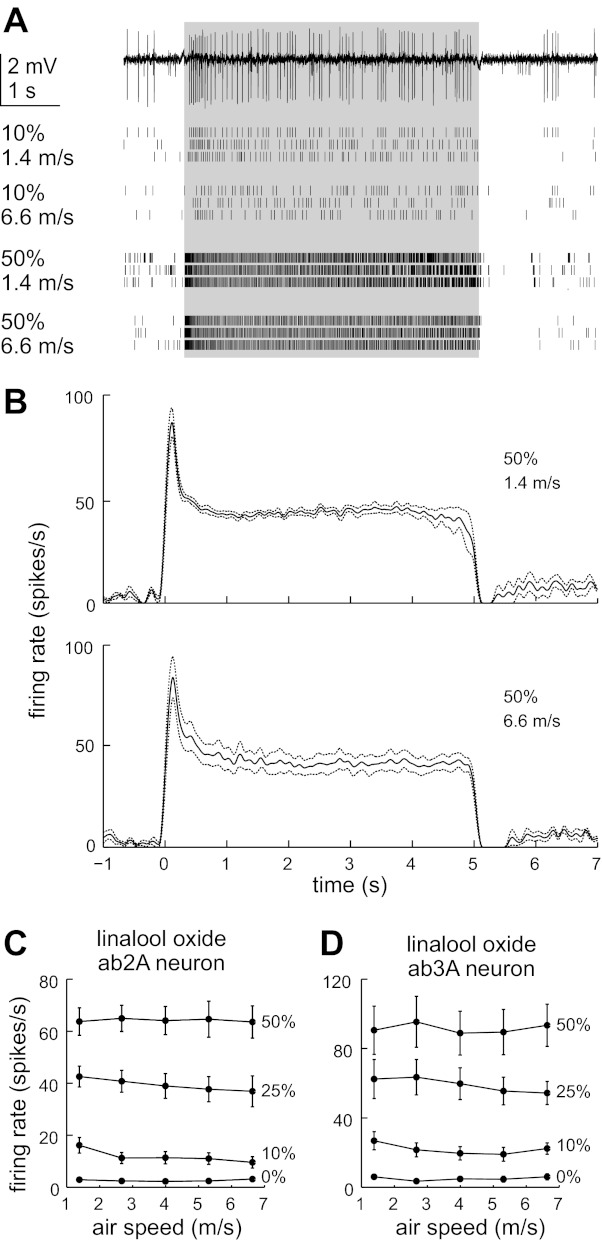
ORN responses to linalool oxide depend on concentration but not air speed. A: sample raw single-sensillum recording (top) showing the responses of the ab2A neuron to 10% linalool oxide at an air speed of 1.4 m/s. In the raw trace, the ab2A neuron corresponds to the large spike waveform (de Bruyne et al. 2001). The stimulus artifact at odor onset and offset is clipped for clarity. Rasters (bottom) show spiking responses at different concentrations and air speeds, with 3 trials per condition. Odor pulse duration is in gray. B: average ORN firing rates (±SE) plotted over time for a low and a high air speed condition (50% linaool oxide, n = 7). C: average ab2A firing rates evoked by linalool oxide (n = 7). D: average ab3A firing rates evoked by linalool oxide (n = 4). Error bars are SE and are sometimes obscured by markers.
Data analysis.
Spikes were identified with custom routines written in IgorPro (Wavemetrics) that filtered, differentiated, and thresholded the raw signal. Statistics were computed in MATLAB (MathWorks). Baseline (pre-odor) firing rates were not subtracted from the measured firing rate during odor presentation. Except in Fig. 4 and Fig. 5, A and B, all firing rates and PID measurements were averaged over the entire 5-s duration of the odor pulse. Except in Fig. 3E and Fig. 4A, each PID value was normalized to the value measured in that experiment at an air speed of 4.0 m/s at the highest duty cycle and then averaged across all trials and experiments. This corrects for the fact that the absolute sensitivity of the PID can drift slowly on a time scale of days. In Figs. 5–8, firing rates were first averaged across three trials using the same stimulus in the same experiment; these values were then averaged across experiments, and Figs 5–8 report means ± SE across experiments. Peristimulus time histograms in Fig. 5B were calculated by accumulating spikes across trials within an experiment, convolving spike times with a Hanning window (200 ms), and then averaging the resulting histogram across experiments.
Fig. 4.
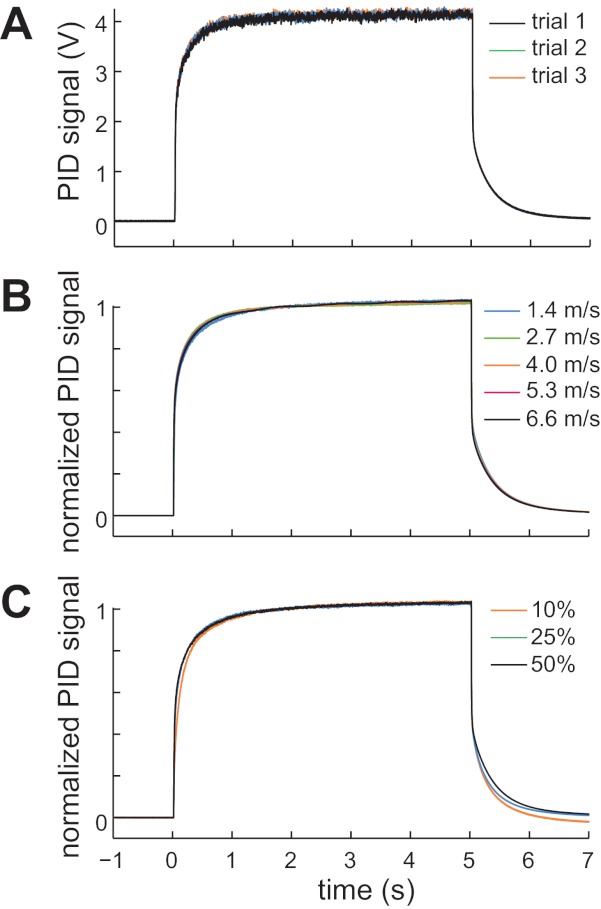
Consistency of the odor pulse delivered to the PID. A: consistent raw PID voltages elicited by 3 successive stimulus presentations (10% linalool oxide at an air speed of 2.7 m/s). B: consistent dynamics of normalized PID responses to the same odor at different air speeds (10% linalool oxide; n = 11). C: consistent dynamics of normalized PID responses to the same odor at different concentrations (linalool oxide at an air speed of 2.7 m/s; n = 11). Traces in B and C were normalized to their maximum value and then averaged across all trials and experiments. Note that ORN firing rates in Figs. 5–8 were measured over the time window from 0 to 5 s.
Fig. 6.
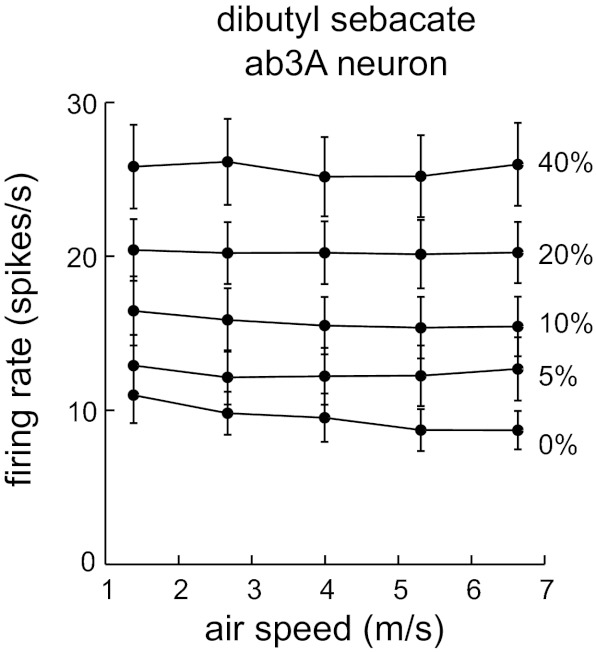
ORN responses to dibutyl sebacate depend on concentration but not air speed: average ab3A firing rates evoked by dibutyl sebacate (n = 6). Responses of ab2A neurons to this odor were inhibitory, and so were not investigated. Error bars are SE.
Fig. 7.
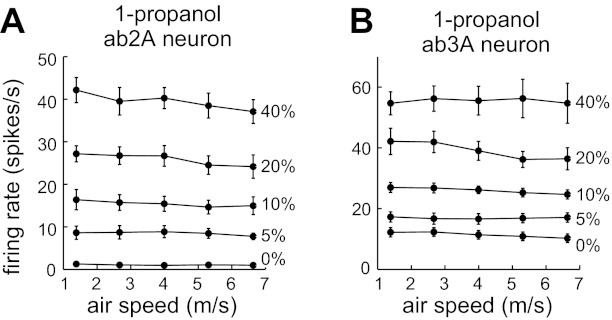
ORN responses to 1-propanol depend on concentration but not air speed. A: average ab2A firing rates evoked by 1-propanol (n = 9). B: average ab3A firing rates evoked by 1-propanol (n = 10). Error bars are SE and are sometimes obscured by markers.
To assess whether firing rate exhibited any statistically significant dependence on either concentration or air speed, we performed a three-step statistical procedure. First, we performed a repeated-measures two-way ANOVA test corresponding to each condition (where a “condition” is defined as specific ORN type, odor, and set of flowmeters). In other words, we performed a separate ANOVA test for each of the panels in Figs. 5, C and D, 6, 7, A and B, and 8, A and B. Second, in the event that we observed a significant effect of air speed for a given condition, we then performed post hoc paired t-tests for all possible pairwise comparisons between air speeds for each odor concentration tested under that condition. For example, for the ab2A ORN and linalool oxide (see Fig. 5C), we performed a total of 30 pairwise comparisons (10 comparisons each for 10%, 25%, and 50%). The results of each of these tests were subjected to a Bonferroni correction, and the P values reported in the text reflect this correction. Third, in the event that any of these corrected values indicated a significant difference between the firing rates measured at different air speeds, we then asked whether there was a statistically significant linear correlation between measured odor concentration (i.e., PID voltage) and air speed for that particular set of experiments. If so, then this would be evidence that we had failed to actually keep concentration constant in these experiments.
RESULTS
Independent control of air speed and odor concentration.
To assess whether olfactory transduction in Drosophila is dependent on air speed, we needed to be able to control odor concentration independent of the air speed (and thus flow rate) through the device. This is difficult to achieve in a conventional odor delivery device for two reasons. First, a device with a limited head space of odor vapor is depleted at a rate that depends on the rate of flow through the system. As a result of this, changing the flow rate will also tend to change odor concentration. Second, most conventional devices vary odor concentration by diluting odor in a quasi-odorless liquid solvent, such as paraffin oil. However, many solute-solvent pairs deviate from ideal solution assumptions (Raoult's law) and thus yield vapor mixtures where the ratio of solute to solvent differs from the ratio in the liquid phase. For example, if the ratio of odor to solvent is higher in the vapor phase than in the liquid phase, then as vapor is removed from the head space the odor will be progressively removed from the container more quickly than the solvent is removed. As a consequence, odor concentration will run down over time at a rate that increases with increasing flow rate. Both these problems can be solved by using a large head space in the container of odor and by varying odor concentration via vapor-phase dilutions rather than liquid dilutions.
For these reasons, we designed and constructed an air dilution odor delivery device with a large head space (Fig. 2A, also see materials and methods). All measurements were taken as close as possible to the final outlet of the device (Fig. 2B). We varied the nominal odor concentration from 0% to 50% saturated vapor and verified that this produces a linear increase in the odor concentration at the output of the device, as measured by a PID (Fig. 3A). We also varied the flow rate through the system from 0.1 to 5.0 l/min and verified that this produces a linear increase in the air speed at the output of the device, as measured by an anemometer (Fig. 3B).
Importantly, this device allowed independent control of air speed and concentration. Over the range of air speeds over which the PID can operate, we confirmed that changing the air speed causes only small variations in measured odor concentration (Fig. 3, C and D). The small variations are attributable to two phenomena. The first phenomenon is that, at high air speeds (>5 m/s), the measured concentration showed a small systematic decrease (up to 10% of maximum), which is likely due to odor vapor leaking out of the system prior to mixing. The magnitude of this phenomenon was small, and it did not appear to influence most of our recordings, except in a few cases (see below).
A second phenomenon is that, at low air speeds (<2 m/s), the measured PID values also fall off. This is likely due to an artifact of the way in which PID samples air. That is, when the negative flow rate exerted by the PID is faster than the positive flow rate of the odor delivery device, the PID draws in clean air in addition to the odorized air, and this produces an artifactual drop in the measured concentration. Consistent with this, the threshold air speed for this fall-off depends on the negative flow rate of the PID, with high negative flows producing steeper fall-off (Fig. 3E). Because this phenomenon is an artifact, it does not indicate a true fall-off in the odor concentration delivered to the fly, and, as expected, it did not significantly affect our ORN recordings (see below).
We also verified that the odor pulse produced by this device shows low trial-to-trial variability in its magnitude and dynamics (Fig. 4A). This implies that the composition of the mixing chamber is constant across trials. In addition, the dynamics of the odor pulse are similar across air speeds (Fig. 4B) and odor concentrations (Fig. 4C).
Effect of air speed on olfactory receptor neuron responses.
We delivered odor pulses of varying concentration and air speed to the Drosophila antenna while we made extracellular recordings of spikes from ORNs. To probe the generality of our results, we made recordings from two different ORN types, ab2A and ab3A (de Bruyne et al. 2001). The ab2A ORN expresses the odorant receptor Or59b, and the ab3A ORN expresses the odorant receptors Or22a/22b (Couto et al. 2005; Dobritsa et al. 2003; Hallem et al. 2004). We selected these ORNs because they are among the easiest to record from and their spike waveforms are easily identifiable (Fig. 5A; also see materials and methods).
In vertebrates, the degree to which ORN responses depend on air speed can vary with odor hydrophobicity (Kent et al. 1996; Mozell et al. 1991a, 1991b; Schoenfeld and Cleland 2005; Scott et al. 2006). Also, the evidence for odor capture by insect antennae (which could in theory produce air speed dependence) derives from experiments that used extremely hydrophobic odors (Kaissling 1971, 1986; Kanaujia and Kaissling 1985). In this study, we therefore used three different odors that collectively span a wide range of hydrophobicity values. We also deliberately used odors that produced only moderate (submaximal) ORN responses, in order to avoid saturating transduction.
We selected linalool oxide as our first odor because it has moderate hydrophobicity (water solubility 1.0 × 10−2 mol/l), it evokes a measureable signal in the PID, and it drives moderate excitatory responses in both ORN types we recorded from (ab3A and ab2A). Increasing the concentration of this odor increased the evoked firing rate of both ORN types (Fig. 5, A–D). However, increasing the air speed (from 1.4 m/s to 6.6 m/s) had no substantial effect on firing rate (Fig. 5, A–D). Changing air speed over this range also had little effect on the dynamics of the ORN response (Fig. 5, A and B).
To test whether there was any statistically significant effect of either stimulus parameter (concentration or air speed) on firing rate, we performed repeated-measures two-way ANOVA tests. For both types of ORNs, we found a highly significant effect of concentration (P = 5 × 10−9 for ab2A, P = 5 × 10−4 for ab3A). For ab3A, there was no significant effect of air speed (P = 0.32). For ab2A, we did uncover a significant effect of air speed (P = 0.02), although the magnitude of this effect is modest. To determine which air speed conditions differed significantly from each other, we performed all possible pairwise comparisons between air speeds for each concentration in Fig. 5C. None of these comparisons yielded significant differences, except the comparison between the lowest air speed and the highest air speed at the 10% concentration level (P = 0.02). However, in this set of experiments, we found that the PID voltage showed a significant negative correlation with air speed (p = 0.01), indicating that the actual odor concentration delivered to the ORNs was falling as air speed was increasing. Thus the modest decline in firing rate in this particular set of experiments is likely due to a drop in odor concentration resulting from slight odor leak from the odor delivery device at high pressure, and not a true dependence of ORN firing rate on air speed. Overall, these analyses indicate that there is no significant effect of air speed as long as odor concentration is kept constant.
Next, we repeated these experiments with a highly hydrophobic odor, dibutyl sebacate (water solubility 1.6 × 10−4 mol/l). Part of the motivation for this is the fact that moth antennae are reportedly capable of capturing pheromone molecules, and these pheromones are likely to be highly hydrophobic (Kaissling 1971, 1986; Kanaujia and Kaissling 1985). Although we could not use insect pheromones in our odor delivery device, because of our need for large liquid odor volumes and the high cost of pure pheromones, dibutyl sebacate is an 18-carbon long-chain hydrocarbon that has a hydrophobicity similar to pheromones like bombykol. Moreover, of the many long-chain hydrocarbons we tested in pilot experiments, it was the only one that evoked even a moderate excitatory response in the ab3A ORNs. We did not investigate responses to dibutyl sebacate in the ab2A ORNs because it induced inhibition in these neurons, not excitation.
We systematically varied both odor concentration and air speed while recording spikes from ab3A ORNs. We observed that increasing odor concentration increased ORN firing rates, as expected, but increasing air speed did not produce any clear changes (Fig. 6). Accordingly, a repeated-measures two-way ANOVA showed a highly significant effect of concentration (Fig. 6; P = 9 × 10−8) but no significant effect of air speed (P = 0.11).
We then repeated these experiments with a highly hydrophilic odor, 1-propanol (water solubility 3.1 mol/l). As before, increasing odor concentration increased firing rates, but there was again no systematic effect of increasing air speed (Fig. 7). A repeated-measures two-way ANOVA showed a highly significant effect of concentration for both ORN types (P = 1 × 10−16 for ab2A, P = 9 × 10−11 for ab3A). For ab3A, there was no significant effect of air speed (P = 0.08). For ab2A, we did observe a significant effect of air speed (P = 3 × 10−7), although the magnitude of this effect is small. Post hoc paired t-tests revealed no significant differences between air speeds for any concentration condition, except a marginal effect for the 40% condition (P = 0.048), and in this particular set of experiments the PID values showed a highly significant negative correlation with air speed (P = 2 × 10−5), indicating that the actual odor concentration delivered to the ORNs was dropping as air speed increased. As before, these analyses indicate overall that there is no significant effect of air speed as long as odor concentration is kept constant.
Any boundary-layer effects (Fig. 1B) will be largest in the low-air speed regime (Koehl 2006). Therefore, in a final set of experiments, we investigated regimes of even lower air speeds. An additional motivation for these experiments is that the mean flight speed of Drosophila is in the range of 0.5–1.0 m/s (Marden et al. 1997), which is near the lower bound of the range that we had used thus far. We therefore explored two additional low-air speed regimes: a range of speeds associated with natural flight (0.22–1.35 m/s; Fig. 8A) and an even lower air speed regime that reaches the limits of our instrumentation (see materials and methods; 0.06–0.22 m/s, Fig. 8B). (Because each of these two regimes required installing new flowmeters in our odor delivery device, they were investigated in separate experiments, and the ORN firing rates we measured in these experiments were not precisely the same as those we measured previously at the same nominal air speeds and concentrations.) As before, we found that varying concentration had a highly significant effect on the firing rate of ab2A for both the intermediate air speed regime (Fig. 8A; P = 1 × 10−11) and the lowest air speed regime (Fig. 8B; P = 3 × 10−7, repeated-measures 2-way ANOVAs). Varying air speed produced no clear changes in firing rate by visual inspection (Fig. 8, A and B), and although ANOVAs examining the effect of air speed did reach the level of statistical significance (P = 0.02 for both Fig. 8, A and B), post hoc paired t-tests did not reveal any significant differences between air speeds. Thus, even in the lowest ranges of air speeds, firing rate does not appear to vary substantially with air speed.
DISCUSSION
In this study, we were able to achieve an unprecedented level of independent control and validation for two key parameters of olfactory stimuli: odor concentration and air speed. This degree of control allowed us to test rigorously whether transduction in Drosophila depends on air speed, as it does on concentration. Our experiments revealed that there was no significant effect of air speed on ORN odor responses as long as odor concentration was held constant while air speed was varied. This finding was consistent across a >100-fold range of air speeds, as well as a >1,000-fold range of odor hydrophobicity values. The same result was observed for two different types of ORNs.
A technical caveat with our approach is that we sometimes observed small drops in odor concentration at high air speeds. We have noted this in the text where it was a factor in our experiments. This observation highlights the importance of actually measuring the odor delivered to the ORNs in every experiment.
Of course, it is possible that olfactory transduction in other insects might depend on air speed. For example, the moth antenna might differ from the Drosophila antenna in this respect, given the difference in the morphology of the antenna in moths versus flies. Whereas the Drosophila antenna is a stubby clublike structure, the moth antenna resembles a feather. Also, whereas Drosophila sensilla are <25 μm long, sensilla in some other insects can be 600 μm in length (Keil 1999), and this might magnify boundary-layer effects. We also cannot exclude the idea that Drosophila ORNs might show air speed-dependent responses to odors that we did not investigate (e.g., pheromones, which we could not test in our experimental setup). There is evidence that pheromones are delivered to odorant receptors by odorant binding proteins (Xu et al. 2005) and chaperone proteins (Benton et al. 2007), and these cofactors could potentially affect the answer to this question. Nevertheless, our results are likely to generalize to most odors and ORN types in Drosophila.
The key finding of this study—that Drosophila ORN responses are generally independent of air speed—has implications for the mechanisms of olfactory transduction in this organism. First, it implies that Drosophila ORNs are not intrinsically mechanosensitive, at least not in the regime of mechanical forces that we tested. In this respect, Drosophila ORNs may differ from vertebrate ORNs (Grosmaitre et al. 2007).
Second, our results do not indicate a role for boundary-layer effects, at least on the timescales that we could resolve in this study. The thickness of the boundary layer around the Drosophila antenna may simply remain constant over the range of air speeds we have explored. Alternatively, the boundary layer may change thickness, but the rate of diffusion through the layer may not be rate limiting on the timescales we could resolve. It is important in this regard to recognize that boundary-layer effects should not change steady-state odor concentrations—only the kinetics of the approach to steady state. In this study, the timescales where we could potentially resolve any boundary-layer effects are limited by the variations in latency from the final valve click to the arrival of odor at the fly. We estimate this latency at ∼5 ms at our fastest air speeds and ∼500 ms at our slowest air speeds (given a 3-cm distance from the valve to the fly). This means we could not resolve any boundary-layer effects that occur on timescales less than ∼500 ms. We did not observe any air speed dependence of ORN responses on timescales longer than this, and so we do not need to invoke boundary-layer effects to explain any of our results.
Third, our results argue that the Drosophila antenna does not capture odor molecules with a probability that is invariant to air speed. If the probability of an odor molecule being captured were invariant to air speed, then the rate of odor capture should be proportional to air speed, and unless some process of odor destruction or removal was also accelerating equally fast, then the local concentration of odor in the antenna should rise with increasing air speed. This would make ORN firing rates grow with increasing air speed, which we do not observe. The idea that the antenna captures odor molecules and does not readily release or destroy them has been suggested by the observation that some moth ORN responses far outlast the duration of the nominal stimulus (Kaissling 1971), and this has been cited as evidence for the “sieve” model. Such “supersustained” ORN responses can also occur in Drosophila ORNs (Montague et al. 2011). In the course of this study, we too observed this type of supersustained response (data not shown). The incidence of supersustained responses increased with odor concentration. For example, we observed supersustained responses in 2 of 11 recordings where we used 50% linalool oxide and in 7 of 9 recordings where we used 100% linalool oxide (all in ab3A neurons), but never at lower concentrations. Nevertheless, although supersustained responses were correlated with odor concentration, we found no correlation with air speed. In this set of experiments, supersustained responses occurred at both low air speeds (<2 m/s, 4 of 9 cases) and high air speeds (>2 m/s, 5 of 9 cases). Thus supersustained responses appear to be caused by exposure to high odor concentrations, not high air speeds.
Finally, our results imply that preabsorption phenomena are unlikely to occur in the Drosophila antenna. This is hardly surprising, because the Drosophila antenna is exposed to ambient air over its entire surface, and so absorption at one end of the antenna should not reduce the concentration delivered to the other end. This stands in contrast to the vertebrate nasal cavity, which forms a long, closed path over which odor can be progressively absorbed.
Invariance to air speed may be adaptive in an organism that has little control over air flow across its antennae. Viewed from this perspective, invariance to air speed can be seen as a feature that should make Drosophila olfaction robust to shifting wind conditions. Of course, changes in the wind will also change the structure of turbulent odor plumes (Murlis et al. 1992), and thus olfaction will be indirectly affected. But the intrinsic invariance of this process to air speed may be an advantage to the fly. In contrast to this, it has been suggested that vertebrates actively exploit the dependence of olfactory transduction on air speed, by manipulating sniff dynamics and thereby manipulating the gradient of odor concentration through the nasal cavity (Schoenfeld and Cleland 2005). These considerations may be relevant not only to the comparative ecology of olfaction but also to the design of so-called “electronic noses” (Wilson and Baietto 2011), where the regulation of air across the sensor is potentially an important design choice.
GRANTS
This work was funded by Hertz and NDSEG predoctoral fellowships (to Y. Zhou) and a research project grant from the National Institute on Deafness and Other Communication Disorders (R01 DC-008174). R. I. Wilson is an HHMI Early Career Scientist.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z. and R.I.W. conception and design of research; Y.Z. performed experiments; Y.Z. analyzed data; Y.Z. and R.I.W. interpreted results of experiments; Y.Z. and R.I.W. prepared figures; Y.Z. and R.I.W. drafted manuscript; Y.Z. and R.I.W. edited and revised manuscript; Y.Z. and R.I.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Joseph S. Bell and Mehmet Fisek contributed to the design of the odor delivery device. We thank Bob Datta, Bernardo Sabatini, and Gary Yellen for helpful conversations. Members of the Wilson lab provided useful feedback on the manuscript.
REFERENCES
- Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450: 289–293, 2007 [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 15: 1535–1547, 2005 [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron 70: 8–19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci 19: 4520–4532, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron 30: 537–552, 2001 [DOI] [PubMed] [Google Scholar]
- Dethier VG. Sniff, flick, and pulse: an appreciation of interruption. Proc Am Philos Soc 131: 159–176, 1987 [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37: 827–841, 2003 [DOI] [PubMed] [Google Scholar]
- Doving KB. Response properties of neurones in the rat olfactory bulb to various parameters of odour stimulation. Acta Physiol Scand 130: 285–298, 1987 [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol 15: 1548–1553, 2005 [DOI] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci 10: 348–354, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet 20: 453–459, 2004 [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell 117: 965–979, 2004 [DOI] [PubMed] [Google Scholar]
- Kaissling KE. Insect olfaction. In: Handbook of Sensory Physiology: Olfaction, edited by Beidler LM. Heidelberg, Germany: Springer, 1971, p. 351–431 [Google Scholar]
- Kaissling KE. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci 9: 121–145, 1986 [DOI] [PubMed] [Google Scholar]
- Kaissling KE. Flux detectors versus concentration detectors: two types of chemoreceptors. Chem Senses 23: 99–111, 1998 [DOI] [PubMed] [Google Scholar]
- Kaissling KE. Olfactory perireceptor and receptor events in moths: a kinetic model. Chem Senses 26: 125–150, 2001 [DOI] [PubMed] [Google Scholar]
- Kaissling KE, Rospars JP. Dose-response relationships in an olfactory flux detector model revisited. Chem Senses 29: 529–531, 2004 [DOI] [PubMed] [Google Scholar]
- Kanaujia S, Kaissling KE. Interactions of pheromone with moth antennae: adsorption, desorption and transport. J Insect Physiol 31: 71–81, 1985 [Google Scholar]
- Keil TA. Morphology and development of the peripheral olfactory organs. In: Insect Olfaction, edited by Hansson BS. Berlin: Springer, 1999, p. 6–47 [Google Scholar]
- Kent PF, Mozell MM, Murphy SJ, Hornung DE. The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. J Neurosci 16: 345–353, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol 98: 205–213, 2007 [DOI] [PubMed] [Google Scholar]
- Koehl MA. The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses 31: 93–105, 2006 [DOI] [PubMed] [Google Scholar]
- Laing DG. Natural sniffing gives optimum odour perception for humans. Perception 12: 99–117, 1983 [DOI] [PubMed] [Google Scholar]
- Lansky P, Rospars JP. Odorant concentration and receptor potential in olfactory sensory neurons. Biosystems 48: 131–138, 1998 [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Étude des facteurs dynamiques de l'excitation olfactive. Annee Psychol 45: 77–89, 1944 [Google Scholar]
- Loudon C, Koehl MA. Sniffing by a silkworm moth: wing fanning enhances air penetration through and pheromone interception by antennae. J Exp Biol 203: 2977–2990, 2000 [DOI] [PubMed] [Google Scholar]
- Mamiya A, Straw AD, Tomasson E, Dickinson MH. Active and passive antennal movements during visually guided steering in flying Drosophila. J Neurosci 31: 6900–6914, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH, Wolf MR, Weber KE. Aerial performance of Drosophila melanogaster from populations selected for upwind flight ability. J Exp Biol 200: 2747–2755, 1997 [DOI] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol 19: R700–R713, 2009 [DOI] [PubMed] [Google Scholar]
- Montague SA, Mathew D, Carlson JR. Similar odorants elicit different behavioral and physiological responses, some supersustained. J Neurosci 31: 7891–7899, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA, Gerhardt GA, Atema J. High resolution spatio-temporal analysis of aquatic chemical signals using microelectrochemical electrodes. Chem Senses 14: 829–840, 1989 [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science 181: 1247–1249, 1973 [DOI] [PubMed] [Google Scholar]
- Mozell MM, Kent PF, Murphy SJ. The effect of flow rate upon the magnitude of the olfactory response differs for different odorants. Chem Senses 16: 631–649, 1991a [Google Scholar]
- Mozell MM, Kent PF, Scherer PW, Hornung DE, Murphy SJ. Nasal airflow. In: Smell and Taste in Health and Disease, edited by Getchell TV, Doty RL, Bartoshuk LM, Snow JBJ. New York: Raven, 1991b [Google Scholar]
- Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu Rev Entomol 37: 505–532, 1992 [Google Scholar]
- Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci 31: 512–520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdya P, Benton R. Evolving olfactory systems on the fly. Trends Genet 26: 307–316, 2010 [DOI] [PubMed] [Google Scholar]
- Rehn T. Perceived odor intensity as a function of air flow through the nose. Sens Processes 2: 198–205, 1978 [PubMed] [Google Scholar]
- Rospars JP, Krivan V, Lansky P. Perireceptor and receptor events in olfaction. Comparison of concentration and flux detectors: a modeling study. Chem Senses 25: 293–311, 2000 [DOI] [PubMed] [Google Scholar]
- Schneider RA, Costiloe JP, Vega A, Wolf S. Olfactory threshold technique with nitrogen dilution of n-butane and gas chromatography. J Appl Physiol 18: 414–417, 1963 [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci 28: 620–627, 2005 [DOI] [PubMed] [Google Scholar]
- Scott JW, Acevedo HP, Sherrill L. Effects of concentration and sniff flow rate on the rat electroolfactogram. Chem Senses 31: 581–593, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel EC, Tank DW. Timing of odor stimulation does not alter patterning of olfactory bulb unit activity in freely breathing rats. J Neurophysiol 69: 1331–1337, 1993 [DOI] [PubMed] [Google Scholar]
- Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors (Basel) 11: 1105–1176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45: 193–200, 2005 [DOI] [PubMed] [Google Scholar]
- Yalkowsky SH, He Y, Jain P. Handbook of Aqueous Solubility. Boca Raton, FL: CRC, 2010 [Google Scholar]


