Abstract
Although the protective cellular immune response to Mycobacterium tuberculosis requires recruitment of macrophages and T lymphocytes to the site of infection, the signals that regulate this trafficking have not been defined. We investigated the role of C-C chemokine receptor 2 (CCR2)-dependent cell recruitment in the protective response to M. tuberculosis. CCR2−/− mice died early after infection and had 100-fold more bacteria in their lungs than did CCR2+/+ mice. CCR2−/− mice exhibited an early defect in macrophage recruitment to the lung and a later defect in recruitment of dendritic cells and T cells to the lung. CCR2−/− mice also had fewer macrophages and dendritic cells recruited to the mediastinal lymph node (MLN) after infection. T cell migration through the MLN was similar in CCR2−/− and CCR2+/+ mice. However, T cell priming was delayed in the MLNs of the CCR2−/− mice, and fewer CD4+ and CD8+ T cells primed to produce IFN-γ accumulated in the lungs of the CCR2−/− mice. These data demonstrate that cellular responses mediated by activation of CCR2 are essential in the initial immune response and control of infection with M. tuberculosis.
The control of Mycobacterium tuberculosis infection requires the coordinated interaction of macrophages, dendritic cells (DCs), and T cells, acting through essential effectors that include IFN-γ, reactive nitrogen intermediates, and perforin (reviewed in ref. 1). These interactions require specific cell trafficking and activation, but the mediators controlling these actions have not been defined.
Chemokines are low-molecular-mass chemotactic cytokines that mediate the recruitment of leukocytes to inflammatory sites, the constitutive trafficking of cells through the secondary lymphoid organs, and the modulation of T cell proliferation and cytokine production (2–6). The monocyte chemoattractant proteins (MCPs) 1–5 are potent chemoattractants for monocytes (7–9), memory T cells (10), natural killer cells (11, 12), and immature DCs (13). Chemokines exert their effects through G-protein-coupled receptors. C-C chemokine receptor 2 (CCR2) is the receptor for the MCPs.
Mice deficient in CCR2 exhibit defects in monocyte/macrophage trafficking to sites of inflammation (14–17) and have transiently reduced IFN-γ responses (14, 17–19). CCR2 is present on murine immature DCs (20), and Langerhans cells from CCR2−/− mice traffic abnormally from the skin to the draining lymph nodes (21). In this study, we used CCR2−/− mice to investigate the role of CCR2 in M. tuberculosis infection. We report that CCR2−/− mice infected with M. tuberculosis recruit fewer macrophages to the lung and fewer macrophages and DCs to the mediastinal lymph node (MLN) (1). CCR2−/− mice have a delay in the development of T cells primed to secrete IFN-γ in the MLN, have reduced numbers of IFN-γ-producing T cells in their lungs, and die rapidly of overwhelming infection.
Materials and Methods
Mice.
CCR2−/− mice were generated as described (14) and backcrossed nine times with C57BL/6 mice (The Jackson Laboratory). CCR2+/+ and CCR2−/− littermates were then bred to generate the mice used in the experiments. Mice were maintained in specific-pathogen-free conditions and were studied at 6–12 weeks of age.
Infection.
All experiments used M. tuberculosis H37Rv from the same frozen stock. Mice were infected by tail vein injection, with 3.3–8.0 × 105 colony-forming units in 200 μl of PBS/0.05% Tween 80. Mice were weighed on the day of infection and were euthanized and analyzed on predesignated days after infection or when they had lost ≥20% of their original weight and lacked mobility. Quantitative mycobacterial cultures were performed on the right middle lung lobe of each mouse by plating serial 10-fold dilutions of tissue homogenates on Middlebrook 7H11 agar. Colonies were counted 3 weeks later.
Histology of Infected Lungs.
Lungs were fixed with 4% paraformaldehyde in PBS and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin. Ziehl–Neelsen staining was used to visualize M. tuberculosis.
Cell Culture and Activation.
To prepare single-cell suspensions for cytokine analysis, MLNs were passed through cell strainers (70 μm; Falcon Becton Dickinson), and RBCs were removed by hypotonic lysis at room temperature. Cells (2.5 × 106 per ml) were cultured in RPMI medium 1640 supplemented with heat-inactivated FCS (10%, vol/vol), penicillin (100 units/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), Hepes (10 mM), nonessential amino acids (100 μM each), sodium pyruvate (1 mM), and 2-mercapto-ethanol (50 μM) (all from Life Technologies, Gaithersburg, MD). The cells were restimulated in vitro with purified protein derivative (PPD) (1) of M. tuberculosis (10 μg/ml; Ministry of Agriculture Fisheries and Food, Addlestone, Surrey, U.K.). Supernatants were collected 48 h later and stored at −80°C.
IFN-γ ELISA.
Supernatants from stimulated cells were assayed in an IFN-γ sandwich ELISA as described (14), except that primary and secondary antibodies were from R & D Systems.
Isolation of Leukocytes.
Lung and MLN leukocytes were isolated as described (19). Single-cell suspensions were prepared by passing collagenase-digested fragments through a cell strainer. Cell viability was assessed by trypan blue exclusion, and live cells were counted with a hemocytometer.
Phenotyping and Quantitation of Leukocytes.
Lung and MLN leukocytes were stained with the following monoclonal antibodies: anti-F4/80-R-phycoerythrin (PE) (Caltag, South San Francisco, CA), anti-CD11b-PE or biotin plus streptavidin-FITC, anti-CD11c-PE, anti-Gr-1-PE, anti-CD4-FITC, and anti-CD8-FITC (PharMingen). The cells were collected with a FACScan (Becton Dickinson) and analyzed with CELLQuest software.
RNA Isolation and Analysis.
RNA was isolated from snap-frozen lung tissue with TRIzol (Life Technologies, Grand Island, NY). Gene expression was quantitated by RNase protection assay (PharMingen) as described (22) with templates from PharMingen.
Intracellular IFN-γ Analysis.
Lung leukocytes were stimulated briefly (5 h) (to specifically stimulate previously primed cells) with PPD (10 μg/ml) or anti-CD3 and anti-CD28 (0.1 and 1 μg/ml, respectively) in the presence of brefeldin A (Sigma). The cells were then washed with fluorescence-activated cell sorter buffer, stained with anti-CD4 and anti-CD8 antibodies, and fixed in 1% paraformaldehyde overnight at 4°C. The next day the cells were washed and permeabilized with Perm/Wash buffer (PharMingen) and stained with anti-IFN-γ antibody (XMG1.2). All antibodies were from PharMingen. Fluorescence data were collected with a FACScan (Becton Dickinson) and analyzed with cellquest software.
Statistical Analysis.
The statistical significance of differences in bacterial loads was determined with the t test. The Mann–Whitney U test was used for all other statistical analyses. P values ≤ 0.05 were considered significant.
Results
CCR2−/− Mice Succumb Rapidly to M. tuberculosis Infection.
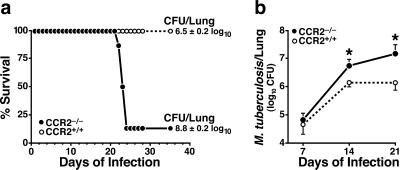
CCR2−/− mice had a rapidly progressive course of disease after infection with M. tuberculosis. The mice appeared ill and had lost weight by day 20 and were moribund or dead by day 22–24 (Fig. 1a). The control CCR2+/+ mice all appeared healthy and survived to day 40, when the experiment was terminated. This rapid disease progression was reflected by >100-fold higher bacterial titers cultured from the lungs of the moribund CCR2−/− mice as compared with wild-type mice killed concurrently (Fig. 1a). Bacteria loads were also slightly higher in the spleens, but not in the livers, of CCR2−/− mice than of CCR2+/+ mice (data not shown).
Figure 1.
CCR2−/− mice exhibit a rapidly progressive course of disease after infection with M. tuberculosis. (a) Survival curve of mice infected with M. tuberculosis (4.5 × 105 colony forming units per mouse). Mice were euthanized when they appeared moribund, as defined by ≥20% weight loss and lack of normal mobility. Results of quantitative M. tuberculosis cultures from the lungs of the CCR2−/− (n = 6) and concurrently euthanized CCR2+/+ (n = 8) mice are shown. The remainder of the CCR2+/+ mice and one surviving CCR2−/− mouse were allowed to survive for an additional 2 weeks, and appeared healthy at the end of the experiment. Similar results were obtained in another independent experiment. (b) Time course of M. tuberculosis growth in the lungs of CCR2+/+ and CCR2−/− mice. Mice were infected on day 0, and four mice of each genotype were analyzed at each time point. Results shown are from one of three experiments that yielded similar results (*, P ≤ 0.05).
To determine when the absence of CCR2 began to affect the course of the infection, we analyzed the bacterial load in the lungs at weekly intervals. At day 7, there was no difference in the lungs of the two strains of mice. However, by 14 days, CCR2−/− mice had 4-fold more bacilli in their lungs than did the CCR2+/+ mice, and 10-fold more at 21 days (Fig. 1b).
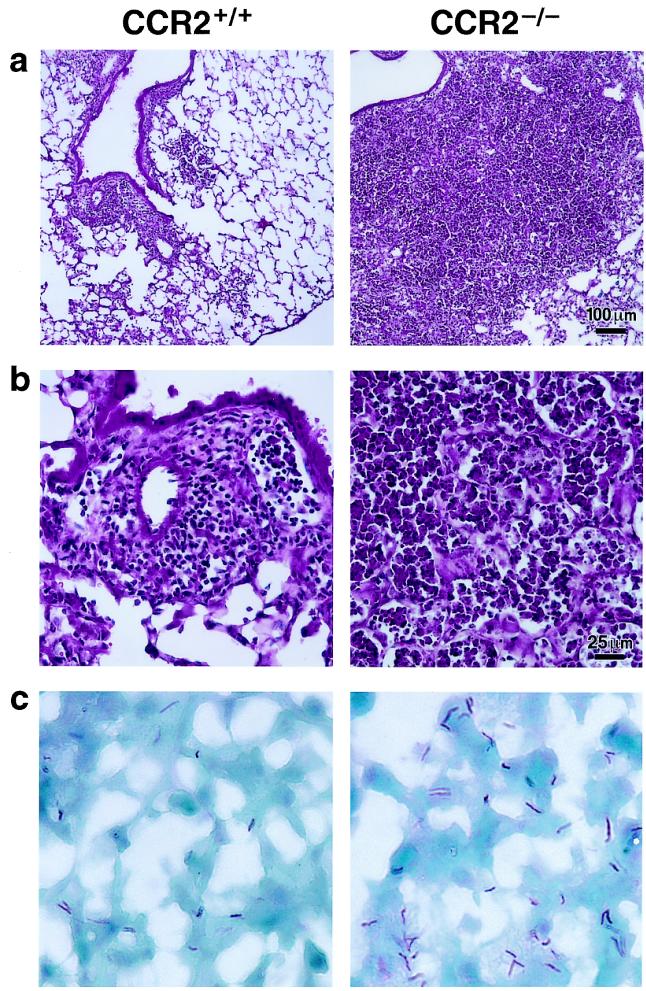
Histopathologic analysis of the lungs 16 days after infection showed interstitial infiltrates consisting of macrophages and lymphocytes adjacent to small airways and in peripheral areas of CCR2+/+ lungs (Fig. 2 a and b). At the same time, the lungs of CCR2−/− mice showed much larger areas of cellular infiltration, including macrophages, lymphocytes, and neutrophils (Fig. 2 a and b). Cellular infiltrates were largely confined to the interstitium of CCR2+/+ mice, although some mononuclear cells were present in the alveoli. In contrast, cellular infiltrates filled the alveolar air spaces as well as the interstitium in CCR2−/− mice. No eosinophils or plasma cells were seen in either strain of mice. Also at day 16, the lungs of CCR2−/− mice exhibited massive neutrophil infiltration, and cellular infiltrates occupied the majority of the lung volume in moribund animals (Fig. 2 a and b). Acid-fast staining of lung sections showed that cellular infiltrates in CCR2−/− lungs contained more infected cells and more bacteria per infected cell than CCR2+/+ mice lungs. All bacteria were cell-associated in both strains of mice (Fig. 2c).
Figure 2.
Lung sections of CCR2+/+ and CCR2−/− mice 16 days after infection with M. tuberculosis. (a and b) Hematoxylin and eosin stain at low (a) and high (b) power. (c) Ziehl-Neelsen stain for acid-fast bacilli. (×500.)
CCR2−/− Macrophages Exhibit Early Recruitment Defects to the Lungs.
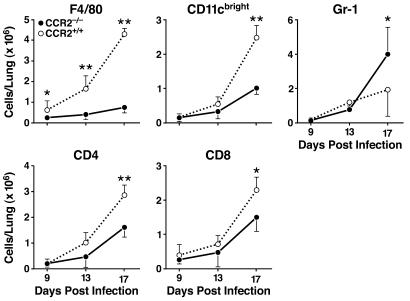
Because CCR2−/− mice have defects in macrophage recruitment into inflammatory sites (14, 15, 17, 18), we asked whether defective cell recruitment could account for the inability of CCR2−/− mice to control bacterial replication in the lungs. There was no significant difference in the number of resident macrophages in lungs of uninfected CCR2−/− and CCR2+/+ mice (data not shown). During the course of infection, the number of (F4/80+) macrophages isolated from the lungs progressively increased in the CCR2+/+ mice, although significantly fewer macrophages were recruited to the lungs of CCR2−/− mice (Fig. 3). Other macrophage markers (CD11b+Gr-1− and CD11cdim) were also analyzed, and yielded similar results (data not shown). At early time points in infection (days 9 and 13), macrophages were the only cells that differed quantitatively in the lungs of CCR2+/+ versus CCR2−/− mice (Fig. 3). A second population of F4/80+ cells was also seen in the lungs, and although their numbers increased during infection, there were no differences between the CCR2+/+ and CCR2−/− mice (data not shown). Later in the course of infection (day 17), in addition to containing fewer F4/80+ macrophages, CCR2−/− lungs also contained fewer CD11cbright DCs and fewer CD4 and CD8 T cells than CCR2+/+ lungs. The increased lung infiltration by neutrophils seen by histopathology was confirmed by flow cytometry (Fig. 3). Cytokines in the lungs were assayed by RNase protection, and revealed no differences for tumor necrosis factor-α, IL-1α, IL-1β, and IL-12 (data not shown).
Figure 3.
Lung leukocyte analysis after infection with M. tuberculosis. Lung leukocytes from CCR2−/− and CCR2+/+ mice were isolated and analyzed by flow cytometry on days 9, 13, and 17 after infection. The cells were stained with fluorescent antibodies to F4/80 (macrophages), CD11c (bright; DCs), Gr-1 (neutrophils), CD4, and CD8. Shown are the mean cell numbers for each cell type, for six CCR2−/− and six CCR2+/+ mice on each day. One of three similar experiments is shown. Error bars represent the standard deviation from the mean (*, P ≤ 0.05; **, P ≤ 0.01). Analysis of macrophages by two other markers (CD11b+Gr-1− and CD11cdim) yielded results similar to those obtained with F4/80 (data not shown).
CCR2−/− Mice Exhibit Early Defects in Macrophage and DC Recruitment to the MLN.
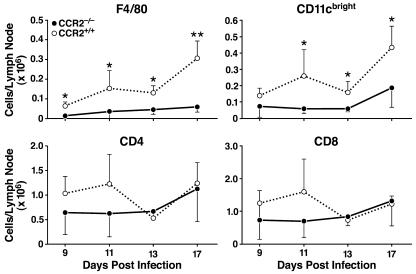
Because macrophages have been implicated in transport of phagocytosed particles from the lung to the MLN (23), a defect in macrophage recruitment to the lungs might result in the migration of fewer macrophages bearing M. tuberculosis antigens to the MLN. Examination of the cell phenotypes in the MLN at 9, 11, 13, and 17 days after infection showed fewer F4/80+ macrophages in the CCR2−/− mice than in CCR2+/+ mice (Fig. 4). A small number of F4/80dim cells were also present, but did not increase in number over time and were not significantly different between the CCR2+/+ and CCR2−/− mice at any time point after infection (data not shown). These cells may represent a resident macrophage population within the MLN. At 11, 13, and 17 days after infection, there were also fewer CD11cbright DCs in the MLN of CCR2−/− mice. However, CD4 and CD8 T cells were present in similar numbers in the MLNs of both genotypes at all time points after infection.
Figure 4.
MLN leukocyte analysis after infection with M. tuberculosis. Leukocytes from MLNs were isolated from CCR2−/− and CCR2+/+ mice and analyzed by flow cytometry on days 9, 11, 13, and 17 after infection. The cells were stained with fluorescent antibodies to F4/80 (macrophages), CD11c (bright; DCs), CD4, and CD8. Shown are the mean cell numbers for each cell type for four to six CCR2−/− and CCR2+/+ mice on each day. Error bars represent the standard deviation from the mean (*, P ≤ 0.05; **, P ≤ 0.01). Analysis of another macrophage marker (CD11b+Gr-1−) yielded results similar to those obtained with F4/80 (data not shown).
CCR2−/− Mice Exhibit Delays in the Priming of T Cells in MLNs.
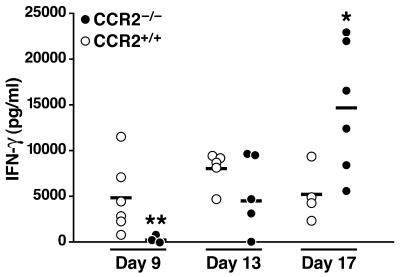
Cells from draining lymph nodes of CCR2−/− mice exhibit acute IFN-γ defects after antigen administration (14, 17–19). Because migration of antigen-presenting cells from tissues to secondary lymphoid organs is necessary for generation of a cellular immune response, and because fewer macrophages and DCs are recruited to MLNs of CCR2−/− mice during M. tuberculosis infection, we considered the possibility that this resulted in defective priming of naïve T cells in the MLNs of CCR2−/− mice. Therefore, we examined the time course and extent of IFN-γ production by T cells isolated from the MLNs of CCR2−/− and CCR2+/+ mice after infection. Before day 9 of infection, neither strain of mice exhibited evidence of primed T cells in MLNs, as there was little IFN-γ secretion after ex vivo restimulation with PPD (data not shown). By day 9, MLN cells of CCR2+/+ secreted considerable amounts of IFN-γ in response to ex vivo restimulation with PPD, although MLN cells from CCR2−/− mice produced much less (Fig. 5). However, by day 13, CCR2−/− and CCR2+/+ MLN cells produced similar amounts of IFN-γ, and by day 17, CCR2−/− MLN cells actually produced more IFN-γ than did CCR2+/+ mice (Fig. 5).
Figure 5.
IFN-γ produced by MLN cells after infection with M. tuberculosis. MLN cells from CCR2−/− and CCR2+/+ mice were isolated and re-stimulated in vitro with PPD (10 μg/ml) on days 9, 13, and 17 after infection. After 48 h, the supernatants were assayed for IFN-γ by ELISA. The individual values for CCR2−/− and CCR2+/+ mice from one of three similar experiments (*, P ≤ 0.05; **, P ≤ 0.01) are shown.
Fewer CD4+ and CD8+ T Cells Primed to Produce IFN-γ Are Recruited to CCR2−/− Lungs.
To determine whether the delay in T cell priming in the MLNs was associated with a delay in a protective T cell response in the lungs, we analyzed the number of IFN-γ-producing CD4+ and CD8+ T cells by intracellular IFN-γ staining after brief (5 h) ex vivo restimulation with PPD. Nine days after infection, neither strain of mice contained a significant number of lung T cells capable of responding to ex vivo restimulation with PPD. By days 11 and 13, the lungs of CCR2−/− and CCR2+/+ mice contained low, but detectable numbers of CD4+ T cells primed to produce IFN-γ (Fig. 6). However, by day 17, lungs of CCR2+/+ mice contained more primed CD4+ cells than mice at earlier time points in infection and significantly more than in the lungs of CCR2−/− mice (Fig. 6). Because restimulation in vitro with PPD does not allow antigens to gain access to the class I antigen processing/presentation pathway, no detectable responses of CD8+ T cells to PPD were observed. To determine responses of CD8+ as well as CD4+ T cells, we briefly stimulated the pulmonary T cells with anti-CD3 and CD28 antibodies, a treatment that activates primed, but not naïve, T cells to produce IFN-γ (24). When isolated lung leukocytes from infected mice were restimulated ex vivo with anti-CD3/CD28, measurable responses were detected by intracellular staining for IFN-γ in CD4+ T cells from CCR2+/+ mice by day 11 (Fig. 6). Significantly greater numbers of IFN-γ+ CD4+ cells were present in CCR2+/+ mice than in CCR2−/− mice on days 11, 13, and 17. Responses of CD8+ T cells to anti-CD3/CD28 stimulation were less marked than those of CD4+ T cells, but were significantly greater in CCR2+/+ mice than in CCR2−/− mice on day 17 (Fig. 6), indicating that CD8+ T cells, as well as CD4+ T cells, were primed in vivo.
Figure 6.
Intracellular IFN-γ produced by T cells in the lungs after infection with M. tuberculosis. Lung leukocytes were isolated and restimulated with PPD (10 μg/ml) or anti-CD3/CD28 (0.1 and 1 μg/ml, respectively) for 5 h. The numbers of IFN-γ-producing CD4 and CD8 T cells were quantitated by flow cytometry on days 9, 11, 13, and 17 after infection. The mean numbers of IFN-γ-positive CD4 and CD8 T cells from four to six CCR2−/− and CCR2+/+ mice at each time point are shown. Error bars represent the standard deviations from the mean (*, P ≤ 0.05).
All Known Mouse CCR2 Ligands Are Expressed in the Infected Tuberculosis Lungs.
In contrast to the findings reported here for mice lacking CCR2 (the receptor for MCP-1), MCP-1−/− mice are resistant to tuberculosis (25). Because MCP-3 and MCP-5 are also ligands for CCR2, we considered the possibility that the discordant results between MCP-1−/− and CCR2−/− mice might be due to induction of these other CCR2 ligands. RNase protection assays revealed that all three CCR2 ligands were induced in the lungs of M. tuberculosis-infected mice by day 9 of infection (Fig. 7).
Figure 7.
CCR2 ligands produced in the lungs of CCR2−/− and CCR2+/+ mice after infection with M. tuberculosis. Total RNA was isolated from lungs of three CCR2−/− and three CCR2+/+ mice on days 9, 13, and 17, and the relative levels of expression of MCP-1, -3, and -5 were measured by RNase protection assay compared with the housekeeping gene L32. Error bars represent the standard deviations from the mean. No significant differences were observed between the levels of mRNA encoding these chemokines in CCR2−/− and CCR2+/+ mice.
Discussion
The major finding of this study is that CCR2−/− mice have a rapidly progressive and fatal course after infection with M. tuberculosis. The absence of CCR2 causes an early and persistent defect in macrophage recruitment to the lungs, reduced numbers of macrophages and DCs in the MLN, delayed priming of T lymphocytes in the MLN, and deficient accumulation of CD4+ and CD8+ T cells in the lungs. These defects in cell recruitment lead to the inability to control the growth of M. tuberculosis in the lung. These findings establish an essential and nonredundant role for the chemokine receptor CCR2 in initiating a protective immune response to infection with M. tuberculosis.
Defective recruitment of macrophages to the lungs of infected CCR2−/− mice was not a surprise because CCR2 is expressed on macrophage precursors (monocytes), and CCR2−/− mice have defects in macrophage recruitment to other sites of inflammation, including atherosclerotic plaques (15, 16, 26, 27). However, the profound inability to control the growth of M. tuberculosis was unexpected, and it prompted further investigation to understand how impaired trafficking of macrophages or other leukocytes could account for a fulminant course of tuberculosis.
Macrophages serve multiple roles in tuberculosis. First, they are the principal in vivo cellular hosts for M. tuberculosis and provide an intracellular site for persistence and replication (28). However, M. tuberculosis is not an obligate intracellular parasite, and the highest bacterial densities of M. tuberculosis in human patients are actually found in an extracellular state in cavitary lung lesions (29). We considered the possibility that a deficiency of macrophage recruitment to the lungs promoted extracellular replication of the bacteria. However, review of multiple sections of lungs of CCR2−/− mice revealed that all acid-fast bacteria were cell associated and were not found in dense extracellular accumulations. Second, macrophages infected with M. tuberculosis produce multiple proinflammatory cytokines, such as tumor necrosis factor-α, IL-1α, IL-1β, and IL-12, all of which are essential for a protective immune response to M. tuberculosis in mice (30–33), and might have been expected to be present at reduced levels in mice with fewer macrophages in the lungs. However, the expression of these cytokines was not deficient in the lungs of infected CCR2−/− mice. The finding that expression of mRNA encoding these cytokines was not deficient in the lungs of CCR2−/− mice despite a profound lack of macrophages implies that the macrophages that are present compensate by producing more cytokine mRNA per cell, or that other cells express these cytokine genes in an attempt to compensate for the deficiency of macrophages. Third, macrophages may serve as antigen-presenting cells in tuberculosis. We therefore investigated the possibility that an early deficiency of macrophage recruitment to the lungs results in a deficiency of antigen-presenting cells in the MLN and, thus, reduced activation of T lymphocytes.
Quantitation of macrophages and DCs in the MLN revealed that both macrophages (F4/80+) and DCs (CD11cbright) increased in number in the MLN during the course of infection and that CCR2−/− mice exhibited a deficiency in both of these types of antigen-presenting cells after infection. The temporal course of the deficiency of macrophage recruitment to the MLNs of CCR2−/− mice resembled that seen in the lungs. That is, macrophages were present in reduced numbers in both the lungs and MLN of CCR2−/− mice at all time points examined after infection, and particularly at the early time points at which IFN-γ production was reduced in the CCR2−/− mice. This was not the case for DCs. Fewer CD11cbright DCs were present in CCR2−/− than CCR2+/+ MLNs at days 11, 13, and 17 after infection; however there was no difference in the number of DCs in the lungs until late in infection (day 17).
Several mechanisms might account for a deficiency of DCs in the MLN without a concurrent deficiency of DCs in the lungs. First, subsets of DC may traffic differently to the lung, and CCR2 may be important in migration to the MLN. Second, DC turnover rates may differ between the lung and the MLN. If DCs are short-lived in the MLN, defective DC recruitment is likely to be detected sooner. Third, because monocytes can acquire DC markers and characteristics after migrating to lymph nodes (34), it is possible that defective recruitment of monocytes to the lungs of CCR2−/− mice results in a deficiency of cells capable of acquiring DC characteristics in the MLN. Further investigation will be necessary to determine which of these potential mechanisms contributes to the early deficiency of DCs in the MLN of mice infected with M. tuberculosis.
When we assayed responses of the MLN T cells to ex vivo restimulation with PPD, we found that CCR2−/− mice exhibit a delay in appearance of MLN T cells capable of responding to PPD by secreting IFN-γ. Consistent with studies that reported acute IFN-γ defects in the CCR2−/− mice (14, 17–19), we found that early in infection CCR2−/− mice had defective IFN-γ responses. However, later in infection, CCR2−/− mice were capable of generating an IFN-γ response at least equivalent to that of CCR2+/+ mice, indicating that CCR2−/− mice do not have an intrinsic inability of their T cells to express or secrete IFN-γ. This is consistent with our recent finding that splenocytes from naïve CCR2−/− mice secrete equal amounts of IFN-γ in response to anti-CD3/CD28 stimulation (17). This is also consistent with the findings of a recent report showing that the early defect in IFN-γ production in CCR2−/− mice infected with Leishmania major is overcome later in infection (18).
It is unclear why the IFN-γ responses are similar in the CCR2−/− and CCR2+/+ MLNs at the late time point, when the numbers of antigen-presenting cells are still reduced. However, because the bacterial load is significantly higher in the CCR2−/− lungs at the late time point, it is possible that the antigen-presenting cells in the MLN contain a higher antigen load, resulting in increased T cell activation. Although these experiments do not reveal the relative roles of macrophages and DCs in priming naïve T cells in the MLN during tuberculosis, they indicate that defective recruitment of one or more types of antigen-presenting cell can profoundly affect the course of the infection.
The early defect in T cell priming in MLNs of CCR2−/− mice was accompanied by a deficiency of CD4+ and CD8+ T cells in the lungs. Because the bacteria were administered intravenously in these experiments, it is likely that T cells were primed, not only in the MLN, but also in the spleen. Regardless of the secondary lymphoid tissue site of T cell priming, defective generation of memory/effector T cells is likely to result in defective accumulation of T cells in infected and inflamed tissues, as we observed in these experiments. It is also possible that the deficiency of T lymphocytes in the lungs of M. tuberculosis-infected CCR2−/− mice reflects a requirement for CCR2 expression on T lymphocytes themselves. Further experiments are needed to determine whether a requirement for CCR2 on T lymphocytes contributes directly to the deficiency of CD4+ and CD8+ T cells in the lungs of M. tuberculosis-infected CCR2−/− mice. It will also be interesting to determine whether the deficiency of macrophages in the lung diminishes the frequency of activation of CD4+ and CD8+ T lymphocytes that are recruited to the lungs of M. tuberculosis-infected CCR2−/− mice. Taken together, our observations provide support for a general model in which defective recruitment of monocytes and macrophages to the lungs of M. tuberculosis-infected CCR2−/− mice results in defective trafficking of antigen-presenting cells (macrophages and DCs) to draining lymph nodes, which, in turn, causes defective priming of T cells.
Rhoades et al. (35) examined lungs of mice infected with several strains of M. tuberculosis by the aerosol route and did not detect expression of chemokines (including MCP-1) until late in the infection. In contrast to those findings, we detected induction of MCP-1, MCP-3, and MCP-5 by day 9 of infection, the earliest time point examined, and the same time as the number of macrophages increased in the lungs of the CCR2+ /+ mice.
In contrast to CCR2−/− mice, MCP-1−/− mice have been reported to control M. tuberculosis infection as well as do wild-type mice (25). Our finding that M. tuberculosis infection induces expression of all three known murine ligands for CCR2 suggests that MCP-3 and MCP-5, which are potent agonists for murine CCR2, might compensate for the lack of MCP-1. Unlike CCR2−/− mice, MCP-1−/− mice make normal amounts of IFN-γ (25, 36) and this may explain why they are not as susceptible to tuberculosis as CCR2−/− mice. The reason for the disparity in cytokine production between the CCR2−/− mice and MCP-1−/− mice is not yet known. It is interesting to note that Rutledge et al. (37) found that transgenic mice constitutively overexpressing MCP-1 were less able to control M. tuberculosis than controls. Overexpression of MCP-1 in these transgenic mice might down-regulate CCR2, making them hyporesponsive to activation by MCPs.
The i.v. route of infection with M. tuberculosis delivers the bacteria to the lungs and other tissues through the capillary bed, and inhalation of bacteria in droplet nuclei delivers them to the alveolar epithelium. However, histopathologic observations indicate that both routes of infection of mice result in infiltration of the lungs with macrophages and T lymphocytes (38). Our findings, which indicate that CCR2 is essential for recruitment of macrophages to the lungs early after i.v. infection, suggest that it will be worthwhile to determine whether the mechanisms of macrophage recruitment to the lung differ, depending on the route of infection. On this point, it is worth noting that abundant quantities of MCP-1 have been detected in bronchoalveolar lavage fluid of people with active tuberculosis (39) and that human tuberculosis is characterized by an influx of monocytes during the course of infection (40). Taken together with these data from human patients, the findings in this paper provide strong evidence for a chemokine- and, more specifically, a CCR2-dependent mechanism for macrophage recruitment in resistance to tuberculosis.
The finding that CCR2 is required for control of M. tuberculosis infection in CCR2−/− mice may have important implications for human disease. First, efforts are underway to develop antagonists to CCR2 for use in chronic inflammatory diseases, such as rheumatoid arthritis and atherosclerosis. Our results suggest that prolonged use of such antagonists may be complicated by increased susceptibility to intracellular pathogens, such as M. tuberculosis, as has been discovered for therapeutic antagonists of tumor necrosis factor-α (41). Second, the CCR2 gene is polymorphic, and at least one allelic variant (at codon 64) has an apparent functional effect, manifest as a 2- to 4-year slower progression of HIV infection to AIDS (42). The presence of this and other known polymorphisms of human CCR2, together with our finding that a CCR2 deficiency profoundly affects the outcome of M. tuberculosis infection in mice, suggests that variants of CCR2 may partially account for differences in susceptibility of humans to tuberculosis (43).
In conclusion, these data support an essential role for CCR2 in the control of M. tuberculosis. A full understanding of the regulation of cell trafficking and cytokine production in resistance to tuberculosis awaits further experimentation. However, our results indicate that CCR2-dependent cellular responses are essential early in the immune response to M. tuberculosis and that activation of CCR2 is an essential mechanism for macrophage recruitment to the lung after infection with M. tuberculosis.
Acknowledgments
We thank Mr. Carl Ng for technical assistance, Dr. David Sanan, Mr. Dale Newland, and Ms. Shelley Mettler for histology and microscopy assistance, Mr. John Carroll and Mr. Jack Hall for preparation of the figures, Mr. Stephen Ordway and Dr. Gary Howard for editorial assistance, Ms. Naima Contos for manuscript preparation, and Dr. Richard Locksley for comments on the manuscript. This research was supported by National Institutes of Health Grants HL51992 (to J.D.E.) and HL52773 (to I.F.C.) and a grant from the Sandler Family Foundation (to J.D.E.). J.D.E. and I.F.C. dedicate this paper to the memory of Ira M. Goldstein, M.D., a good friend and mentor.
Abbreviations
- DC
dendritic cell
- CCR
C-C chemokine receptor
- MCP
monocyte chemoattractant protein
- MLN
mediastinal lymph node
- PPD
purified protein derivative
References
- 1.Flynn J L, Ernst J D. Curr Opin Immunol. 2000;12:432–436. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 2.Rossi D, Zlotnik A. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Mackay C R, Lanzavecchia A. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 4.Bacon K B, Premack B A, Gardner P, Schall T J. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 5.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 6.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 7.Valente A J, Graves D T, Vialle-Valentin C E, Delgado R, Schwartz C J. Biochemistry. 1988;27:4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura T, Robinson E A, Tanaka S, Appella E, Kuratsu J-I, Leonard E J. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushima K, Larsen C G, DuBois G C, Oppenheim J J. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr M W, Roth S J, Luther E, Rose S S, Springer T A. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allavena P, Bianchi G, Zhou D, van Damme J, Jílek P, Sozzani S, Mantovani A. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 12.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- 13.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay C R, Qin S, Lanzavecchia A. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Boring L, Gosling J, Chensue S W, Kunkel S L, Farese R V, Jr, Broxmeyer H E, Charo I F. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurihara T, Warr G, Loy J, Bravo R. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuziel W A, Morgan S J, Dawson T C, Griffin S, Smithies O, Ley K, Maeda N. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters W, Dupuis M, Charo I F. J Immunol. 2000;165:7072–7077. doi: 10.4049/jimmunol.165.12.7072. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Kuziel W A, Melby P C, Reddick R L, Kostecki V, Zhao W, Maeda N, Ahuja S K, Ahuja S S. J Immunol. 1999;163:5519–5525. [PubMed] [Google Scholar]
- 19.Traynor T R, Kuziel W A, Toews G B, Huffnagle G B. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 20.Vecchi A, Massimiliano L, Ramponi S, Luini W, Bernasconi S, Bonecchi R, Allavena P, Parmentier M, Mantovani A, Sozzani S. J Leukoc Biol. 1999;66:489–494. doi: 10.1002/jlb.66.3.489. [DOI] [PubMed] [Google Scholar]
- 21.Sato N, Ahuja S K, Quinones M, Kostecki V, Reddick R L, Melby P C, Kuziel W A, Ahuja S S. J Exp Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanga C A, Mohan V P, Yu K, Joseph H, Tanaka K, Chan J, Flynn J L. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmsen A G, Muggenburg B A, Snipes M B, Bice D E. Science. 1985;230:1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- 24.Serbina N V, Flynn J L. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu B, Rutledge B J, Gu L, Fiorillo J, Lukacs N W, Kunkel S L, North R, Gerard C, Rollins B J. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boring L, Gosling J, Cleary M, Charo I F. Nature (London) 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 27.Dawson T C, Kuziel W A, Osahar T A, Maeda N. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 28.McNeil W R, Besra G S, Brennan P J. In: Tuberculosis. Rom W N, Garay S M, editors. Brown, Boston: Little; 1996. pp. 171–186. [Google Scholar]
- 29.Canetti G. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 30.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 32.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Lab Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 33.Cooper A M, Magram J, Ferrante J, Orme I M. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph G J, Inaba K, Robbiani D F, Steinman R M, Muller W A. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 35.Rhoades E R, Cooper A M, Orme I M. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu L, Tseng S, Horner R M, Tam C, Loda M, Rollins B J. Nature (London) 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 37.Rutledge B J, Rayburn H, Rosenberg R, North R J, Gladue R P, Corless C L, Rollins B J. J Immunol. 1995;155:4838–4843. [PubMed] [Google Scholar]
- 38.Cardona P-J, Cooper A, Luquín M, Ariza A, Filipo F, Orme I M, Ausina V. Scand J Immunol. 1999;49:362–366. doi: 10.1046/j.1365-3083.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 39.Sadek M I, Sada E, Toossi Z, Schwander S K, Rich E A. Am J Respir Cell Mol Biol. 1998;19:513–521. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 40.Schwander S K, Sada E, Torres M, Escobedo D, Sierra J G, Alt S, Rich E A. J Infect Dis. 1996;173:1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 41.Maini R, St. Clair E W, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 42.Smith M W, Dean M, Carrington M, Winkler C, Huttley G A, Lomb D A, Goedert J J, O'Brien T R, Jacobson L P, Kaslow R, et al. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 43.Hill A V S. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]