Abstract
In vivo recordings from postsynaptic neurons in the medial nucleus of the trapezoid body (MNTB), an auditory brain stem nucleus, show that acoustic stimulation produces a burst of spikes followed by a period of hyperpolarization and suppressed spiking activity. The underlying mechanism for this hyperpolarization and reduced spiking is unknown. Furthermore, the mechanisms that control excitability and resting membrane potential are not fully determined for these MNTB neurons. In this study we investigated the excitability of principal neurons from the MNTB after high-frequency afferent fiber stimulation, using whole cell recordings from postnatal day 15–17 rat brain stem slices. We found that Na+-K+-ATPase activity mediates a progressive hyperpolarization during a prolonged tetanic train and a posttetanic hyperpolarization (PTH) at the end of the train, when postsynaptic action potentials failed to fire. Raising the temperature to more physiological levels (from 22 to 35°C) depolarized the resting membrane potential of both presynaptic and postsynaptic cells and decreased the latency of action potential firing during PTH. Higher temperatures also reduced the presynaptic calyx action potential failure rates by 50% during presynaptic PTH, thus increasing the safety-factor for presynaptic spiking. The effect of temperature on hyperpolarization-activated cation current (Ih) is reflected in the resting potential at both pre- and postsynaptic neurons. We thus propose that temperature-sensitive Na+-K+-ATPase activity and Ih contribute to set the resting membrane potential and produce a brief period of suppressed spiking (or action potential failures) after a prolonged high-frequency afferent tetanus.
Keywords: rat, medial nucleus of the trapezoid body principal cells, calyx of Held synapse, mammalian body temperature, resting membrane potential
the response of the medial nucleus of the trapezoid body (MNTB) principal cells to a sound stimulus is a burst of action potentials (APs) whose frequency increases with sound intensity (Guinan and Li 1990; Sommer et al. 1993; Spirou et al. 1990). Interestingly, an in vivo study of adult rat MNTB principal cells using intracellular sharp electrode recordings showed that the resting membrane potential shifted toward negative values during the sound stimuli and remained hyperpolarized for 100–200 ms after sound offset (Sommer et al. 1993). In addition, spontaneous spiking activity was suppressed during this hyperpolarization (see also Kopp-Scheinpflug et al. 2008). Moreover, a recent study of rat MNTB neurons showed that the duration of this period of suppressed spiking depends on sound stimuli duration, as well as on stimulus intensity (Kadner et al. 2006). However, the underlying mechanism of this suppression of spikes remains uncertain. Determining the mechanisms responsible for this membrane hyperpolarization and spike suppression period will ultimately be important for understanding how auditory signals are processed in the brain stem, where the precise timing of AP firing is thought to be critical for computing sound localization (Carr et al. 2001; Oertel 1999; Scott et al. 2005).
To reproduce in vivo bursts of spikes under ex vivo conditions, we performed brain stem slice patch-clamp recordings and stimulated afferent fibers of MNTB neurons at high frequencies. Recently, we found posttetanic hyperpolarization (PTH) with a fast and large amplitude in the calyx of Held terminal, where we observed failures of presynaptic APs shortly after a train of stimuli at room temperatures (Kim et al. 2007). However, the properties of postsynaptic PTH (post-PTH) in MNTB principal cells have not been studied to date. To mimic in vivo conditions as much as possible, we studied presynaptic PTH (pre-PTH) and post-PTH at more physiological temperatures (35°C) and in more mature animals [juvenile rats of hearing age: postnatal day 15–17 (P15–17)].
We observed a shift of postsynaptic membrane potential toward negative values during a high-frequency stimulation train at 35°C and a 5- to 10-mV post-PTH after stimulus offset. The progressive postsynaptic hyperpolarizations during the stimulus train and the post-PTH were due to increasing Na+-K+-ATPase (NKA) activity during the tetanus, whereas recovery from post-PTH was mediated by an Ih current (Banks et al. 1993). Importantly, we observed that raising temperature from 22 to 35°C depolarized both the calyx terminal and postsynaptic neuron. This may help to explain the large (150%) increase in miniature excitatory postsynaptic current (mEPSC) rates observed previously at 35°C as being due in part to this presynaptic depolarization (Kushmerick et al. 2006). We conclude that temperature-sensitive NKA and Ih regulate the resting membrane potential of MNTB principal cells and their excitability. Together with several leak potassium channels, whose identities have not yet been fully determined (Johnston et al. 2010; Klug and Trussell 2006), we propose that these temperature-sensitive factors (namely, NKA, Ih, and K+-selective conductances) set resting membrane potentials throughout the auditory system. Our results thus suggest that post-PTH contributes to induce the silencing of MNTB neuronal discharges shortly after a period of intense sound stimulation.
MATERIALS AND METHODS
Slice preparation.
Transverse brain stem slices (200 μm thick) were prepared from Sprague-Dawley rat pups of P15–17 (hearing onset occurs at P12). All procedures followed approved Oregon Health & Science University animal care protocols. After rapid decapitation, the brain stem was quickly removed from the skull and immersed in ice-cold low-calcium artificial cerebrospinal fluid (aCSF) containing the following (in mM): 125 NaCl, 2.5 KCl, 3 MgCl2, 0.1 CaCl2, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid, 3 myo-inositol, and 2 Na-pyruvate, pH 7.3–7.4 when bubbled with carbogen (95% O2, 5% CO2; osmolarity of 310–320 mosmol/l). After being cut in a vibratome slicer (VT1000; Leica, Bannockburn, IL), the slices were transferred to an incubation chamber containing normal aCSF bubbled with carbogen and maintained at 35°C for 30–45 min and thereafter at room temperature (RT). The normal aCSF was the same as the slicing aCSF, but with 1 mM MgCl2 and 2 mM CaCl2.
Electrophysiology.
Whole cell patch-clamp recordings were performed in normal aCSF at room temperature (22–24°C) except in specific experiments where the aCSF had a more physiological temperature of 35°C (Warner Instruments). Slices were perfused at 2 ml/min and visualized using an infrared differential interference contrast microscope (Axioskop FS1; Zeiss, Oberkochen, Germany) and a ×40 water-immersion objective coupled to a ×2 premagnification (Optovar; Zeiss) and a charge-coupled device camera (C73; Sony, Tokyo, Japan) with contrast enhancement controller (Hamamatsu Photonics, Hamamatsu, Japan). Pre- and postsynaptic AP recordings used a pipette solution containing (in mM) 130 K-gluconate, 20 KCl, 5 Na2-phosphocreatine, 10 HEPES, 4 Mg-ATP, and 0.3 GTP, pH adjusted to 7.3 with KOH. In addition, calyx recordings used 0.2 mM EGTA, whereas postsynaptic recordings used 5 mM EGTA. Pipettes were pulled from borosilicate glass (World Precision Instruments) with a Sutter P-97 electrode puller (Sutter Instruments, Novato, CA) to open tip resistances of 1.8–2.5 MΩ for postsynaptic recordings and 3–4 MΩ for presynaptic recordings. Access resistance (Rs) was ≤6 MΩ for postsynaptic recordings and ≤20 MΩ for presynaptic recordings. Rs was compensated >85% for postsynaptic recordings and ∼50% for presynaptic recordings. Presynaptic APs were recorded in normal aCSF, using the fast current-clamp mode of the EPC-9 after adjusting the fast capacitance cancellation while in cell-attached mode. Current-clamp recordings were continued only if the initial uncompensated Rs was <20 MΩ. In our solution composition (extracellular K+ concentration of 2.5 mM and intracellular K+ concentration of 150 mM), the calculated equilibrium potential for potassium is around −106 mV, and liquid junction potential is 11 mV. Membrane potentials were not corrected for this constant liquid junction potential between the extracellular and pipette solution, except in the experiment of Fig. 3. Presynaptic APs were elicited with a bipolar platinum-iridium electrode (Frederick Haer, Bowdoinham, ME) placed near the midline spanning the afferent fiber tract of the MNTB. An Iso-Flex stimulator delivered 100-ms pulses of <15 V (direct current at constant voltage) and was driven by a Master 8 pulse generator (A.M.P.I., Jerusalem, Israel) triggered by a Macintosh computer. Data were acquired at a 10- to 50-ms sampling rate using an EPC-9 amplifier (HEKA Electronik, Lambrecht/Pfalz, Germany) controlled by Pulse 8.4 HEKA software and filtered online at 2.9 kHz. Data were analyzed off-line and are presented using Igor Pro (Wavemetrics, Lake Oswego, OR). Differences were considered statistically significant when P values were <0.05 by the Student's t-test.
Fig. 3.
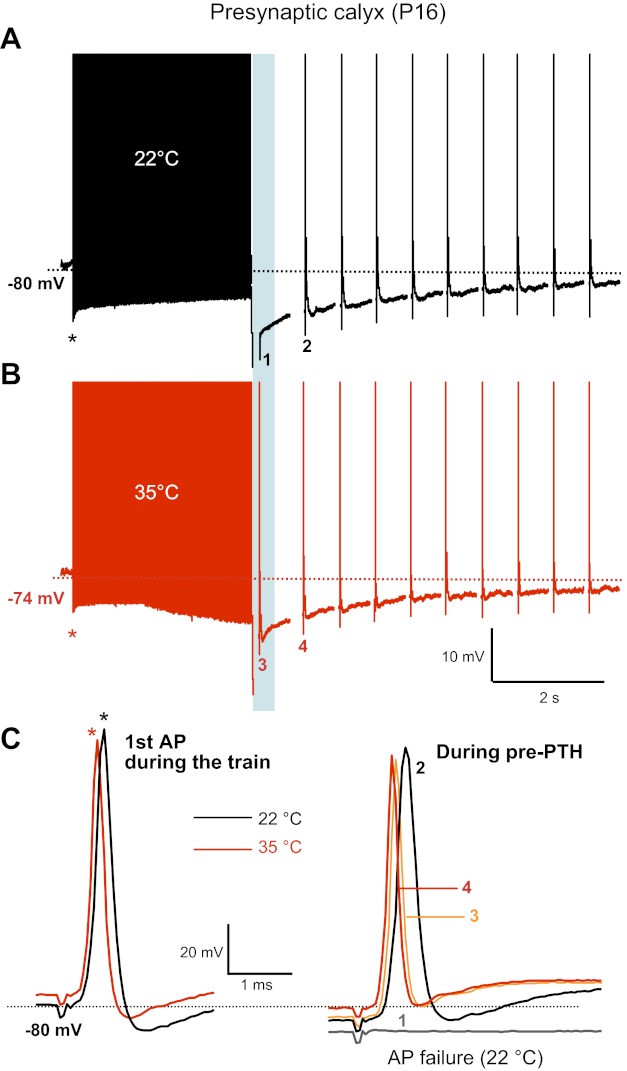
Raising the temperature reduces the calyx AP failure rate during pre-PTH and also reduces the AP latency. A and B: presynaptic calyx APs from a P16 rat brain stem slice were evoked by afferent fiber stimulation (100-Hz tetanus followed by single APs at 2 Hz) at 22°C (A, black trace) and 35°C (B, red trace). A liquid junction potential correction (−11 mV) was performed after the recording. Failure of AP firing occurred during PTH at 22°C (1) but not at 35°C (3). C: superimposed 1st presynaptic APs of the tetanic train (left; asterisks) and APs during the pre-PTH after the tetanus (100 Hz; right) at 22°C (black traces) and 35°C (red traces) shown in A. Note that the 1st single APs showed a larger afterhyperpolarization than those APs during pre-PTH.
RESULTS
Firing precision after the tetanus is regulated by temperature.
To study changes in postsynaptic excitability during prolonged stimulation, we recorded postsynaptic APs evoked by afferent fiber stimulation in P16 rat MNTB principal neurons. Cells were held at a −70-mV resting membrane potential. Compared with immature neurons at P6–8, these more mature neurons at P15–16 fired postsynaptic AP trains without AP failure at 100 Hz for 3 s and showed a reduced plateau depolarization (Fig. 1, black trace), in part due to a decreased expression of NMDA receptors (Futai et al. 2001; Taschenberger and von Gersdorff 2000). During the AP train, the resting membrane potential progressively shifted to negative potentials, resulting in a reduction of the depolarization plateau at the end of the train. On average, the resting membrane potential shifted downward by 5.0 ± 2.6 mV (n = 5) between the first and the last AP of the AP train (300 APs). In addition, at the end of the AP train, a PTH of 5.2 ± 3.1 mV was observed (i.e., membrane potential reached a value of about −75 mV; n = 11, black trace in Fig. 1). This is similar to the hyperpolarization observed by in vivo recordings of MNTB principal neurons during AP bursts, where the membrane potential shifted to negative potentials by about 4 mV during sound stimulation (Sommer et al. 1993). However, this post-PTH amplitude was much smaller than the pre-PTH of the calyx terminal (around 25 mV at P9–10; Kim et al. 2007).
Fig. 1.
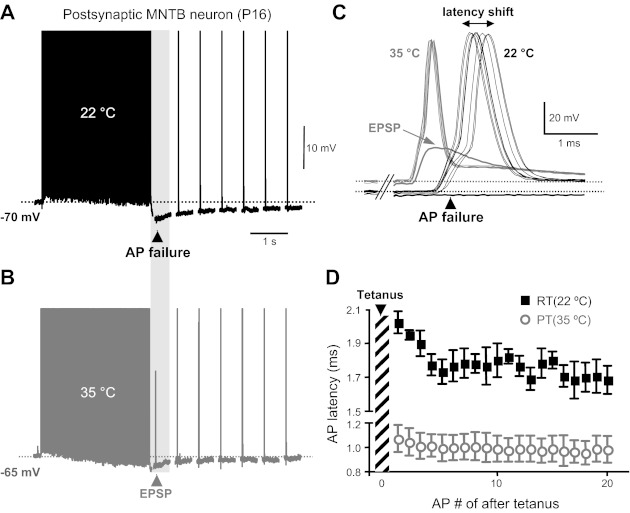
Failure of postsynaptic action potential (AP) firing during postsynaptic posttetanic hyperpolarization (post-PTH) occurs at both room temperature (RT) and at a more physiological temperature (PT). A and B: postsynaptic APs evoked by afferent fiber stimulation (100 Hz, 3-s duration) at RT (22°C; A, black trace) and at PT (35°C; B, gray trace) in a postnatal day 16 (P16) medial nucleus of the trapezoid body (MNTB) principle neuron. Stimulus artifacts were removed for clarity. One AP failure occurred during the PTH period at 22°C. At 35°C, the same postsynaptic neuron had an excitatory postsynaptic potential (EPSP) during the PTH, but this EPSP failed to fire a postsynaptic AP. No failures occurred during the tetanus at 22 or 35°C. The top of the AP train was truncated to better visualize the post-PTH. C: superimposed postsynaptic APs (elicited at 2 Hz) during the post-PTH after the tetanus (100 Hz) shown in A and B at 22 and 35°C. Note that postsynaptic APs showed a larger latency shift at 22°C than at 35°C. Horizontal dashed lines indicate the resting membrane potential at 22 and 35°C. Note the greatly reduced latency shift at 35°C (gray). D: change in the latency of postsynaptic APs during post-PTH at 22 and 35°C (n = 3).
What is the underlying mechanism for the shift of membrane potential toward negative values during the stimulus train and for post-PTH that finally leads to this suppression period (Sommer et al. 1993)? Our previous work showed that a high density of NKA contributes to generate a presynaptic PTH at the calyx of Held, and MNTB principal neurons also express the NKA α3-subunit (Kim et al. 2007). We tested the effect of ouabain, a specific blocker of NKA, on the negative shift of membrane potential during stimulus train and on post-PTH. Ouabain (100 μM) depolarized the postsynaptic neuron from −70 to −55 mV and abolished the negative shift of resting membrane potential during the AP train, and it completely inhibited the post-PTH (n = 4, gray trace, Fig. 2). These data suggest that increased postsynaptic NKA activity mediates the progressive hyperpolarization during the tetanus and generates the post-PTH after the tetanus, as well as setting the resting membrane potential.
Fig. 2.
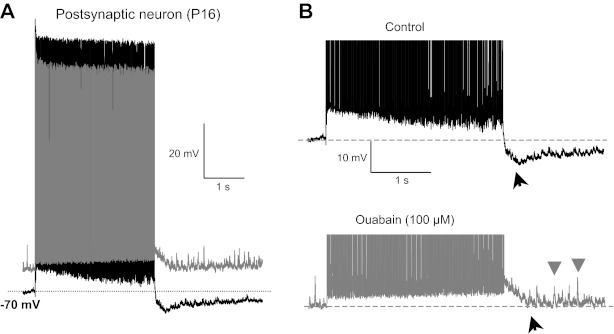
Postsynaptic Na+-K+-ATPase pump activity mediates post-PTH. A: postsynaptic APs evoked by afferent fiber stimulation (100 Hz, 3-s duration) in control (black) and in the presence of ouabain (100 μM, gray; a blocker of Na+/K+-ATPase) in a P16 rat MNTB principle neuron. B: expanded voltage scale for the same post-PTH shown in A. Horizontal lines indicate the resting membrane potential. Ouabain depolarized the postsynaptic neuron from −70 to −55 mV. Note that the postsynaptic neuron showed a progressive hyperpolarization during the AP train (control), which was blocked by ouabain. Note also that application of ouabain increased the number of spontaneous postsynaptic potentials after the AP train (gray arrowheads). Black arrowheads indicate the post-PTH.
This post-PTH also induced a period of reduced postsynaptic excitability and suppression of spikes (or failure of action potentials), which mimics the results observed in a previous in vivo study (Sommer et al. 1993). Fairly mature P16 postsynaptic principal cells could reliably generate APs during a 100-Hz stimulus train of 3 s (with no AP failures), and this tetanic train was followed by single stimuli at a rate of 2 Hz (Fig. 1A). All neurons recorded at this age faithfully fired APs during the tetanus, but 6 of 11 neurons failed to fire an AP during the post-PTH period after the tetanus (failure rate: 55%) at RT. The activity of the calyx of Held NKA, which helps to set its resting membrane potential and generates the pre-PTH period, is very dependent on temperature and ATP supply (Kim et al. 2007). Thus we tested whether changes in temperature also alter postsynaptic excitability by changing the resting membrane potential and AP firing during prolonged stimulation and during the post-PTH period. With the use of P16–17 rat brain stem slices, postsynaptic AP trains were evoked by afferent fiber stimulation (100 Hz, 3-s duration) at RT (22°C) and at physiological temperature (PT, 35°C; Fig. 1B). When the bath temperature was raised from RT (22–24°C) to 35°C, the postsynaptic MNTB principal cell resting membrane potential depolarized from −71 ± 0.4 to −60 ± 2.2 mV (n = 6, see Fig. 1 and Fig. 3), and the amplitude and half-width of the postsynaptic AP were reduced (amplitude: from 99.8 ± 7.1 to 86.3 ± 3.8 mV; half-width: from 0.65 ± 0.11 to 0.33 ± 0.06 ms; n = 5). Raising temperature to 35°C also reduced the postsynaptic AP latency (measured from the end of the tetanus) during PTH from 1.7 ± 0.1 to 0.98 ± 0.09 ms (n = 5). In addition, the timing of the postsynaptic AP peak became more accurate and had a reduced “jitter” during post-PTH at 35°C (jitter or latency shift: 0.1 ms; Fig. 1, C and D). At 35°C, 4 of 6 tested neurons still failed to fire APs during the post-PTH period (failure rate: 67%). However, as shown in Fig. 1C, raising the temperature could result in the triggering of subthreshold excitatory postsynaptic potential (EPSP) after the tetanus. Interestingly, even though the peak value of this EPSP reached the original threshold for firing an AP, the neuron could not fire an AP, presumably because the absolute AP threshold was shifted to more positive voltages after the temperature was raised. These data show that higher, or more physiological, temperatures can still produce postsynaptic AP failure during post-PTH. Therefore, higher temperatures cannot eliminate AP failures entirely during post-PTH. This suggests that even though the presynaptic AP successfully fired during pre-PTH, the resulting EPSP did not have sufficiently large amplitude to reach the threshold for firing a postsynaptic AP.
Presynaptic AP failure during pre-PTH is temperature dependent.
To directly assess the effect of raising temperature to more physiological levels on the excitability of the presynaptic calyx nerve terminal during pre-PTH, presynaptic APs were generated by afferent fiber stimulation at 100 Hz for 3 s in P16 calyces. At RT in 4 of 5 recordings from P16 calyces, APs failed to fire near the peak of pre-PTHs whose amplitude was about 25 mV (time to peak: 20 ms; Fig. 3A). At PT (35°C), AP failure still occurred during pre-PTH periods in 50% of our presynaptic recordings (data not shown; n = 4). However, in the other 50% of our recordings, an increase in bath solution temperature from 24 to 35°C triggered a presynaptic AP where one had previously failed during pre-PTH (Fig. 3B). This result suggests that raising the temperature can help the calyx terminal to fire with higher accuracy and probability during pre-PTH. In addition, raising temperature from 24 to 35°C depolarized P16 calyces by 8 mV (see Figs. 3A and 5A) and resulted in a reduction of AP amplitude and half-width (Fig. 3B; see also Kushmerick et al. 2006). Thus the reduction of failure rate at 35°C might be due to a depolarization of resting membrane potential (from −80 ± 1.7 to −72 ± 2.1 mV, n = 4; see Figs. 3C and 5) and a reduced threshold for AP firing. Note also the decrease in AP latency from 0.75 to 0.55 ms in Fig. 3C when temperature was raised.
Fig. 5.
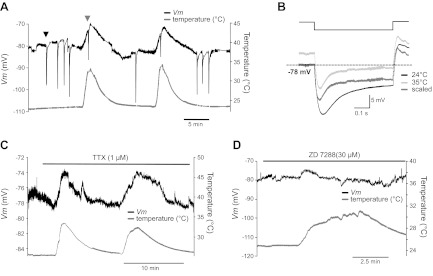
Effect of blocking presynaptic Na+ current and Ih on the temperature-dependent depolarization of the calyx of Held nerve terminal. A: change in the resting membrane potential of the presynaptic calyx terminal as the temperature was raised from 24 to 35°C. Arrowheads indicate pre-PTH after tetanic stimulation at 24°C (black) and 35°C (gray). B: membrane potential responses to hyperpolarizing current injection (−50 pA) at 24°C (black) and 35°C (light gray), and a scaled trace (dark gray). C and D: change in resting membrane potential produced by raising the temperature from 24 to 35°C at the presynaptic calyx terminal in the presence of TTX (1 μM; C) and ZD7288 (30 μM; D).
We also note that the membrane potential progressively hyperpolarized during the calyx AP train at raised temperatures (Fig. 3B). This was probably due to the increased activity of NKA at 35°C. The failure of postsynaptic APs during post-PTH is thus dependent on the failure of presynaptic APs as well as postsynaptic membrane excitability and short-term synaptic depression. Moreover, these failure rates are regulated by temperature. These results suggest that raising temperature increases presynaptic terminal excitability and regulates the timing of pre- and postsynaptic APs.
A temperature-sensitive postsynaptic Ih regulates resting membrane potentials.
NKA plays a key role in maintaining the resting membrane potential of MNTB neurons. Although the increased NKA activity at a raised temperature (35°C) could be expected to allow postsynaptic MNTB neurons to hyperpolarize, since NKA is an electrogenic pump, raising temperature from 24 to 35°C actually depolarized the postsynaptic neuron (Figs. 1 and 4). What is the underlying mechanism of this postsynaptic depolarization at higher temperatures? Ih, which has been characterized in adult rat MNTB neurons (Banks et al. 1993), can play a counterbalancing role for NKA in setting the membrane potential (Kang et al. 2004; Kim et al. 2007). We examined the function of Ih in setting membrane potential at RT (24°C) and at PT (35°C). Bath application of 50 μM ZD7288 or 1 mM CsCl (potent blockers of Ih; see Rodrigues and Oertel 2006) hyperpolarized principal neurons from −70 ± 0.4 to −88 ± 1.9 mV (n = 4, Fig. 4, A and B). This large effect of 1 mM CsCl was fully reversible, and membrane potential recovered to resting levels when CsCl was removed. By contrast, the bath application of 8-bromoadenosine-3-cyclic monophosphate (8-Br-cAMP; 50 μM), known as an agonist of Ih, depolarized the MNTB neuron from −73 to −61 mV (Fig. 4B). To examine the conductance change in the presence of CsCl or 8-Br-cAMP, we recorded membrane potential response to hyperpolarizing current injection (−50 or −100 pA). The bath application of CsCl increased the input resistance from 137 ± 9 to 191 ± 34 MΩ at a holding potential of −70 mV. By contrast, the bath application of 8-Br-cAMP reduced the input resistance by 34 MΩ. Thus the inhibition of Ih with CsCl decreased membrane conductance and the activation of Ih with 8-Br-cAMP increased it in postsynaptic MNTB neurons. In addition, CsCl significantly prolonged the recovery from post-PTH without significant effect on post-PTH amplitude (data not shown). Our results indicate that postsynaptic Ih contributes to maintain the resting membrane potential and the recovery from post-PTH as a counterbalance to the action of NKA.
Fig. 4.
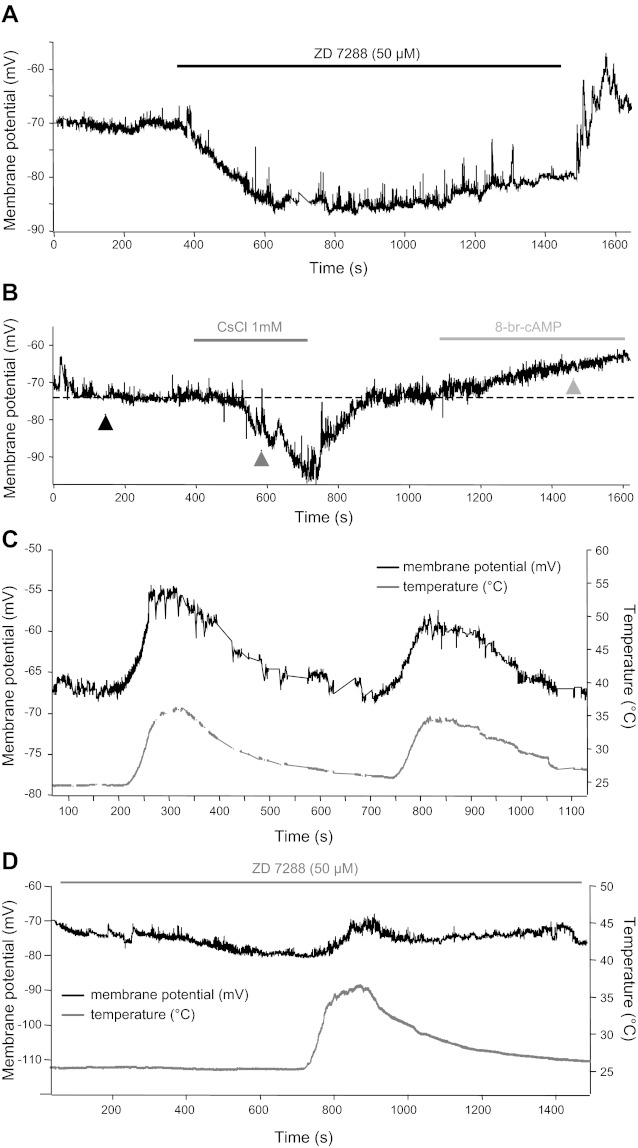
Temperature-sensitive hyperpolarization-activated cation current (Ih) contributes to set the resting membrane potential of postsynaptic MNTB neurons. A and B: example recordings of the resting membrane potential of a postsynaptic MNTB principal neuron are shown in the presence of external ZD7288 (50 μM; A) and in the presence of CsCl (1 mM) or 8-bromoadenosine-3-cyclic monophosphate (8-Br-cAMP, 50 μM; B). C: change in the resting potential of the postsynaptic neuron as the temperature is raised from 24 to 35°C. D: the addition of 30 μM ZD7822 hyperpolarized the cell by ∼9 mV. In the continuous presence of inhibition of Ih with ZD7822 (30 μM), the change in the resting membrane potential caused by a temperature increase was much smaller than in control conditions (compare C and D).
Next, we tested whether Ih currents contribute to temperature dependent changes in membrane potential of MNTB neurons. Raising temperature from 24 to 35°C depolarized the postsynaptic neurons from −71 ± 0.4 to −60 ± 2.2 mV (n = 6; Fig. 4C). However, the resting membrane potential stayed at −72 ± 3 mV at 35°C in the presence of ZD7288 (30 μM). Raising the temperature changed the resting membrane potential from −82 ± 0.5 to −73 ± 3.1 mV in the presence of 30 μM ZD7288 or 1 mM CsCl (n = 5, Fig. 4D). The range of depolarization induced by raising the temperature was reduced by the inhibition of Ih with ZD7288. Increased Ih as temperature is raised critically affects temperature-dependent depolarization. This result suggests that the increase of Ih with rising temperature contributes to depolarization at higher temperature in the MNTB neurons.
Raising temperature from 24 to 35°C depolarized the presynaptic calyx from −80 ± 1.7 to −72 ± 2.1 mV (n = 4, Fig. 5A) and reduced the input resistance measured by voltage response to injecting hyperpolarizing currents (from 160 to 110 MΩ), suggesting that raising temperature increased the membrane conductance (Fig. 5B). In the calyx terminal, persistent Na+ currents contribute in part to set the resting membrane potential (Huang et al. 2009; Kim et al. 2010). We tested whether persistent Na+ current contributes to temperature-dependent depolarization at higher temperature at the presynaptic terminal. The inhibition of persistent Na+ currents with tetrodotoxin (TTX; 1 μM) hyperpolarized the calyx from −76 to −79 mV, which is similar to the value reported by Huang et al. (2008) and Kim et al. (2010). However, raising the temperature still depolarized the calyx from −79 ± 0.6 to −73 ± 0.9 mV (n = 3, Fig. 5C). This response is reproducible to repeated temperature changes. Next, we tested whether presynaptic Ih also contributes to temperature-dependent change in presynaptic excitability in the calyx terminal. In the presence of ZD7288 (30 μM), the effect of raising temperature on presynaptic membrane potential was slightly reduced (from −80 ± 0.3 to −75 ± 1.3 mV, n = 3, Fig. 5D). Thus presynaptic Ih contributes to the temperature-dependent change in presynaptic excitability (Cuttle et al. 2001).
DISCUSSION
The mechanisms responsible for in vivo posttetanic spike suppression (Sommer et al. 1993) are not clear and have been thought to be mainly due to short-term depression of the EPSP. We propose that the intrinsic excitability of the nerve terminal and postsynaptic cell should also be considered as contributing to possible failures in postsynaptic spike generation.
The suppression of spikes after strong sound stimulation or after tetanic stimulation.
In vivo studies of the MNTB have reported that the presynaptic calyceal input can sometimes fail to evoke a postsynaptic AP during and after a prolonged bout of high-frequency firing (Guinan and Li 1990; Kopp-Scheinpflug et al. 2003). However, in vitro studies of MNTB principal neurons have shown that after periods of inactivity, they can fire AP trains of 50 APs at 600 Hz without failures already at P14 (Hermann et al. 2007; Taschenberger and von Gersdorff 2000; for adult mouse studies, see Wu and Kelly 1993). This high rate of firing has also been observed in vivo for sound-evoked spikes (Guinan and Li 1990; Spirou et al. 1990). In the present study, with in vitro recordings at room temperature and at 35°C, we have shown that during the peak hyperpolarization period of the post-PTH, APs can fail with higher probability (Fig. 1, A and B). The in vitro suppression of APs during PTH we observed thus mimics results obtained in vivo with the use of conventional sharp electrode intracellular recordings from principal cells of the adult rat MNTB, which show a shift of the membrane potential toward negative values during strong sound stimulation (i.e., 60–70 dB SPL) and, in addition, a suppression of spontaneous spikes after the sound stimulus offset period (Sommer et al. 1993). Recently, a complete suppression of spikes for about 40 ms after a stimulus train offset was also observed with extracellular single-unit spike recordings (Kadner et al. 2006).
The suppression of spikes during post-PTH may have a presynaptic or a postsynaptic origin. First, postsynaptic AP failure may occur because of the failure of a presynaptic AP during pre-PTH mediated by presynaptic NKA (Fig. 3A). Interestingly, a release-independent component of short-term depression causes failures in AP initiation at the hippocampus and is mediated by NKA (Muñoz-Cuevas et al. 2004). Second, even if the presynaptic AP fires during pre-PTH (Fig. 3B), the postsynaptic AP may still fail because the size of the EPSP is not large enough to trigger a spike during the NKA-mediated post-PTH (Fig. 1, B and C). Our results thus indicate that both the pre- and postsynaptic NKAs play a critical role in determining posttetanic short-term plasticity and excitability at mammalian auditory calyx-type synapses. Extensive studies in leech neurons also show that NKA activity controls neuronal adaptation and spiking properties during AP bursts (Arganda et al. 2007; Jansen and Nicholls 1973; Scuri et al. 2007; Van Essen 1973). Likewise, Aplysia interneurons can exhibit a prolonged inhibitory synaptic potential that is mediated by an electrogenic sodium pump (Pinsker and Kandel 1969).
What is the physiological relevance of the suppression of spike transmission during PTH in calyx synapses? First, in general terms, changes in the excitability and plasticity of MNTB brain stem synapses will strongly influence the processing of sound signals throughout the higher auditory pathways because the MNTB projects to several auditory nuclei (Carr et al. 2001; Moor and Caspary 1983; Oertel 1999). Hence, our data suggest that the suppression of spikes after high-intensity sound stimuli in the MNTB may regulate the summation of signals in the lateral superior olive (LSO), medial superior olive (MSO), and the superior paraolivary nucleus (SPON), three target nuclei that receive glycinergic input from MNTB, and this inhibition probably plays a critical role in the precise localization of low- and high-frequency sounds (Kadner et al. 2006; Kopp-Scheinpflug et al. 2011a, 2011b). Second, this suppression of spikes during post-PTH may play a role in encoding sound intensity and duration, because louder sounds (Sommer et al. 1993) and longer duration sounds cause more extended periods of spike suppression (Kadner et al. 2006).
Comparison of post-PTH with pre-PTH.
Post-PTH was similar to pre-PTH in the mechanism of its generation and decay, but the amplitude of post-PTH (5–10 mV) was two- to fivefold smaller than that of pre-PTH (Kim et al. 2007). Why is the post-PTH so much smaller than the pre-PTH? One reason may be that there is a larger density of sodium pumps in the calyx, and the calyx may also have a higher Na+ influx during the tetanus. Another partial explanation is the difference in surface-to-volume ratio. Although the calyx and postsynaptic cell at P9 have roughly the same surface area (calyx: 2,500 μm2 with axon <30 μm; Borst and Sakmann 1998; principal cell: 2,400 μm2, or about 25 pF of resting membrane capacitance), they have very different volumes (calyx: 480 μm3; principal cell: 3,400 μm3; Sätzler et al. 2002). Therefore, the surface-to-volume ratio for the calyx is 5.2 and that for the principal cell is 0.7. This sevenfold difference in surface-to-volume ratio between the calyx and its principal cell partner suggests that a fixed amount of Na+ influx will attain a higher concentration in the calyx. This higher Na+ concentration will drive the calyx NKA more strongly, thus producing a larger pre-PTH.
Temperature and membrane excitability during PTH.
How does temperature change the ability to fire APs of mammalian nerve terminals and postsynaptic neurons during PTH? Spiking probability depends highly on the resting membrane potential. In this study we unexpectedly found that raising the temperature depolarized the calyx of Held and shortened the latency of AP spiking. This depolarization will increase calcium levels in the calyx (Awatramani et al. 2005) and thus trigger a greater frequency of mEPSCs in the MNTB principal cell (Kushmerick et al. 2006), which will also tend to depolarize its resting potential. These significant changes in resting membrane potential and excitability at higher temperature may reduce the failure rate of pre- and postsynaptic AP spiking and also may increase AP fidelity during pre-PTH (Fig. 3). Consistent with our results, raising the temperature depolarizes the resting membrane potential of octopus cells in the cochlear nucleus (Cao and Oertel 2005) and increases neural activity in the hippocampus (Klyachko and Stevens 2006). Recent studies have suggested that physiological temperatures produce larger quantal amplitudes, faster AMPA receptor kinetics, and faster endocytosis and refilling of depleted synaptic vesicle pools, leading to increased synaptic strength during prolonged high-frequency firing (Kushmerick et al. 2006; Postlethwaite et al. 2007; Pyott and Rosenmun 2002; Renden and von Gersdorff 2007). However, although physiological temperatures produced an increased synaptic strength, postsynaptic AP failures still occurred during post-PTH because the EPSP was not sufficiently large to overcome the hyperpolarization of the membrane potential during PTH (Fig. 1, B and C). This might also be due in part to the synaptic depression of the EPSCs after the prolonged stimulus at high frequencies, and a subsequent posttetanic augmentation may be one mechanism for overcoming this synaptic depression (Habets and Borst 2005; Korogod et al. 2005).
Temperature critically affects the resting membrane potential of auditory neurons.
We have determined three manipulations that dramatically alter resting membrane potentials of brain stem auditory neurons: Cs+-induced block of Ih, ouabain block of NKA, and changes in temperature (Cao and Oertel 2005). Our pre- and postsynaptic membrane potential recordings show that a reversible depolarization of the calyx of Held nerve terminal and MNTB principal neuron can be induced when temperature is increased to 35°C (Figs. 4 and 5). Various ion channels such as HCN, Na+ channels, KCNQ, and NKA are involved in setting the resting membrane potential of the calyx of Held terminals (Huang et al. 2008, 2011; Kim et al. 2007). Alterations in temperature affect the kinetics of voltage-activated ion channels such as Kv1, Na+ channels, and HCN (Cao and Oertel 2005). At rest, we found that a temperature-sensitive Ih is already activated and contributes to setting the resting membrane potential in both the postsynaptic MNTB neuron and the presynaptic calyx of Held. However, there are also other factors involved in the temperature-dependent excitability change in pre- or postsynaptic neurons, because blocking Ih could not completely inhibit the temperature-dependent depolarization (Figs. 4D and 5D).
One possible mechanism that may contribute to the temperature-sensitive resting membrane potential is the presence of temperature- and pH-sensitive two-pore-domain potassium channels such as TREK and TASK. These channels are voltage insensitive and potassium selective, thereby contributing “leak” currents that drive the resting membrane potential toward the equilibrium potential for potassium. TASK-1 and TASK-3 are widely expressed throughout the brain, but with moderate expression in the auditory brain stem (Karschin et al. 2001; Talley et al. 2001). A recent study showed that there was a large sustained decrease in the expression of TASK-5, a subunit that is predominantly expressed in auditory brain stem neurons, following deafness, suggesting that these channels might play a role in deafness-associated changes in the excitability of cochlear nucleus neurons (Holt et al. 2006). We found that the application of BaCl2, a general inhibitor of TASK, had no effect on membrane potential changes induced by rising temperature (data not shown). Another possibility is that this temperature-dependent depolarization could be due to temperature-sensitive TRP (transient receptor potential) channels. A recent study showed that a TRP receptor current induced a temperature-dependent depolarization in cultured spinal neurons (Wang and Poo 2005), and a hippocampal neuron study suggested that thermosensitive TRPV receptor regulates the resting membrane potential (Shibasaki et al. 2007). However, we found that there was no significant effect of SKF96365 ruthenium red, a TRP channel blocker, on the temperature-sensitive depolarization in the calyx of Held (data not shown). Thus the underlying mechanism that causes depolarization at increased temperatures requires further study, and perhaps more specific pharmacological compounds, to block the putative “leak” channels.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants R01 DC04274 (to H. von Gersdorff) and R03 DC011140 (to J. H. Kim).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H.K. and H.v.G. conception and design of research; J.H.K. performed experiments; J.H.K. and H.v.G. analyzed data; J.H.K. and H.v.G. interpreted results of experiments; J.H.K. prepared figures; J.H.K. and H.v.G. drafted manuscript; J.H.K. and H.v.G. edited and revised manuscript; J.H.K. and H.v.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert Renden, Geetha Srinivasan, and Christopher Kushmerick for discussions.
REFERENCES
- Arganda S, Guantes R, de Polavieja GG. Sodium pumps adapt spike bursting to stimulus statistics. Nat Neurosci 10: 1467–1473, 2007 [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron 48: 109–121, 2005 [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol 70: 1420–1432, 1993 [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol 506: 143–157, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Temperature affects voltage-sensitive conductances differentially in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 94: 821–832, 2005 [DOI] [PubMed] [Google Scholar]
- Carr CE, Soares D, Parameshwaran S, Perney T. Evolution and development of time coding systems. Curr Opin Neurobiol 11: 727–733, 2001 [DOI] [PubMed] [Google Scholar]
- Cuttle MF, Rusznák Z, Wong AY, Owens S, Forsythe ID. Modulation of a presynaptic hyperpolarization-activated cationic current Ih at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol 534: 733–744, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse. J Neurosci 21: 3342–3349, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Li RY. Signal processing in brainstem auditory neurons which receive giant endings (calyces of Held) in the medial nucleus of the trapezoid body of the cat. Hear Res 49: 321–334, 1990 [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol 564: 173–187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol 98: 807–820, 2007 [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Duncan RK, Lomax CA, Juiz JM, Altschuler RA. Deafness associated changes in expression of two-pore domain potassium channels in the rat cochlear nucleus. Hear Res 216–217: 146–153, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Trussell LO. Control of presynaptic function by a persistent Na+ current. Neuron 60: 975–979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci 14: 840–847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JKS, Nicholls JG. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurons following impulses. J Physiol 229: 635–655, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Forsythe ID, Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J Physiol 588: 3187–3200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner A, Kulesza RJ, Jr, Berrebi AS. Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. J Neurophysiol 95: 1499–1508, 2006 [DOI] [PubMed] [Google Scholar]
- Kang Y, Notomi T, Saito M, Zhang W, Shigemoto R. Bidirectional interactions between h-channels and Na+-K+ pumps in mesencephalic trigeminal neurons. J Neurosci 24: 3694–3702, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Müller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci 18: 632–648, 2001 [DOI] [PubMed] [Google Scholar]
- Kim JH, Kushmerick C, von Gersdorff H. Presynaptic resurgent Na+ currents sculpt the action potential waveform and increase firing reliability at a CNS nerve terminal. J Neurosci 30: 15479–15490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Sizov I, Dobretsov M, von Gersdorff H. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the α3 Na+/K+-ATPase. Nat Neurosci 10: 196–205, 2007 [DOI] [PubMed] [Google Scholar]
- Klug A, Trussell LO. Activation and deactivation of voltage-dependent K+ channels during synaptically driven action potentials in the MNTB. J Neurophysiol 96: 1547–1555, 2006 [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF. Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses. J Neurosci 26: 6945–6957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in KCNA1-null mice: an electrophysiological study in vivo. J Neurosci 23: 9199–9207, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tolnai S, Malmierca MS, Rubsamen R. The medial nucleus of the trapezoid body: Comparative physiology. Neuroscience 154: 160–170, 2008 [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tozer AJ, Robinson SW, Tempel BL, Hennig MH, Forsythe ID. The sound of silence: ionic mechanisms encoding sound termination. Neuron 71: 911–925, 2011a [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Steinert JR, Forsythe ID. Modulation and control of synaptic transmission across the MNTB. Hear Res 279: 22–31, 2011b [DOI] [PubMed] [Google Scholar]
- Korogod N, Lou X, Schneggenburger R. Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held. J Neurosci 25: 5127–5137, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci 26: 1366–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci 3: 237–242, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cuevas J, Vara H, Colino A. Characterization of release-independent short-term depression in the juvenile rat hippocampus. J Physiol 558: 527–548, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brainstem auditory nuclei of vertebrates. Annu Rev Physiol 61: 497–519, 1999 [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kandel ER. Synaptic activation of an electrogenic sodium pump. Science 163: 931–935, 1969 [DOI] [PubMed] [Google Scholar]
- Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol 579: 69–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol 539: 523–535, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol 98: 3349–3359, 2007 [DOI] [PubMed] [Google Scholar]
- Rodrigues ARA, Oertel D. Hyperpolarization-activated currents regulate excitability in stellate cells of the mammalian ventral cochlear nucleus. J Neurophysiol 95: 76–87, 2006 [DOI] [PubMed] [Google Scholar]
- Sätzler K, Söhl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lübke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci 22: 10567–10579, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LL, Mathews PJ, Golding NL. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J Neurosci 25: 7887–7895, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuri R, Lombardo P, Cataldo E, Ristori C, Brunelli M. Inhibition of Na+/K+ ATPase potentiates synaptic transmission in tactile sensory neurons of the leech. Eur J Neurosci 25: 159–167, 2007 [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 27: 1566–1575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Lingenhohl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res 95: 223–239, 1993 [DOI] [PubMed] [Google Scholar]
- Spirou GA, Brownell WE, Zidanic M. Recording from the cat trapezoid body and HRP labeling of globular bushy cell axons. J Neurophysiol 63: 1169–1190, 1990 [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol 230: 509–534, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434: 898–904, 2005 [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hear Res 68: 189–201, 1993 [DOI] [PubMed] [Google Scholar]


