Abstract
Independent control of finger movements characterizes skilled motor behaviors such as tool use and musical performance. The purpose of the present study was to identify the effect of movement frequency (tempo) on individuated finger movements in piano playing. Joint motion at the digits was recorded while 5 expert pianists were playing 30 excerpts from musical pieces with different fingering and key locations either at a predetermined normal tempo or as fast as possible. Principal component analysis and cluster analysis using an expectation-maximization algorithm determined three distinct patterns of finger movement coordination for a keypress with each of the index, middle, ring, and little fingers at each of the two tempi. The finger kinematics of each coordination pattern was overall similar across the tempi. Tone sequences assigned into each cluster were also similar for both tempi. A linear regression analysis determined no apparent difference in the amount of movement covariation between the striking and nonstriking fingers at both metacarpo-phalangeal and proximal-interphalangeal joints across the two tempi, which indicated no effect of tempo on independent finger movements in piano playing. In addition, the standard deviation of interkeystroke interval across strokes did not differ between the two tempi, indicating maintenance of rhythmic accuracy of keystrokes. Strong temporal constraints on finger movements during piano playing may underlie the maintained independent control of fingers over a wider range of tempi, a feature being likely to be specific to skilled pianists.
Keywords: individuated finger movements, synergy, multijoint movements, principal component analysis, cluster analysis
musical performance with accuracy in rhythm and articulation requires independent control of finger movements. In piano playing, for example, expert pianists are capable of moving a striking finger independently of nonstriking fingers over a wide variety of tone sequences (Furuya et al. 2011). However, independent control of fingers challenges against innate constraints on the hand at neurophysiological (Keen and Fuglevand 2004; Kilbreath and Gandevia 1994; Schieber and Hibbard 1993) and biomechanical (Lang and Schieber 2004; Leijnse 1997; Leijnse et al. 1993; Tubiana 1981; Watson 2006) levels, which yields covariation of motion across fingers (Schieber and Santello 2004). A superior independence of finger movements for expert pianists over untrained individuals (Aoki et al. 2005; Parlitz et al. 1998) suggested that extensive piano training allowed for overcoming these constraints. This may include functional reorganization of cortical regions responsible for dexterous finger movements (Gentner et al. 2010; Jäncke et al. 2000; Slobounov et al. 2002a) and anatomical changes that can lower biomechanical constraints on individual fingers (Smahel and Klimová 2004). However, it remains unknown whether the independent control of finger movements in piano playing is maintained over a wide range of movement rates (i.e., tempo). The answer to this question may provide the basis for studying not only neural mechanisms responsible for production of a rich repertoire of skilled movements but also neurological disorders such as focal dystonia (Altenmüller and Jabusch 2010), in light of anecdotal observations that dystonic symptoms in musicians emerge particularly when they are playing fast.
Studies comparing the organization of limb movements across different speeds have demonstrated variations in movement pattern with speed in computer typing (Angelaki and Soechting 1993), circle drawing (Pfann et al. 2002), and ball throwing (Hirashima et al. 2007; Hore et al. 2005). In cyclical finger movements, the amount of movement covariation between one particular finger and the other fingers became larger when they were moving at a faster rate, which indicated a decrease in independence of finger movements (Häger-Ross and Schieber 2000). It is therefore reasonable to postulate less individuated finger movements while playing the piano at faster tempo. By contrast, some researchers who investigated targeted reaching found no variation of movement patterns in relation to speed, defined as “speed invariance” (Atkeson and Hollerbach 1985; Hollerbach and Flash 1982; Nishikawa et al. 1999; Soechting and Lacquaniti 1981). Thus an alternative hypothesis in piano playing with both spatial and temporal constraints is invariance of individuated finger movements across different tempi.
To test these hypotheses, the present study aimed to assess similarity and differences in patterns of hand movements in piano playing between two different tempi. Principal component (PC) and cluster analyses were performed to identify a set of fundamental patterns of finger movement at each tempo, and the amount of covariation of motion between the striking and nonstriking fingers was compared between the tempi for each of the patterns. In addition, the effect of tempo on rhythmic consistency of the keystrokes was assessed.
METHODS
Participants
Five highly skilled pianists (two women, three men; 33 ± 8 yr, all right-handed) participated in our experiment after giving informed consent for the study. All of the participants had won prizes at international and/or national piano competitions. To minimize the possibility that any movement features in the keystroke motions could reflect a specific technique that had been intensively taught at a certain piano school, participants who had been taught to play the piano by different instructors and had piano education in different countries were selected. The age at which they started to play was 4.8 ± 2.2 yr, they had been playing the piano for 27.8 ± 8.5 yr, and their daily practice duration was 3.5 ± 1.6 h. The experimental protocol was approved by the University of Minnesota's Institutional Review Board.
Experimental Design
We asked pianists to play several measures of 11 musical pieces requiring use of the right hand. The selections chosen were 30 short excerpts ranging from 9 to 24 notes (mean ± SD = 13.5 ± 2.8) from 11 pieces, which were Études Op. 10, Nos. 1, 4, and 8 and Op. 25, Nos. 11 and 12 by Frédéric Chopin; Das wohltemperierte Klavier, Vol. 1, No. 15 and Vol. 2, Nos. 1, 2, 10, and 15 by Johann Sebastian Bach; and 15 Études, Op. 72, No. 6 by Moritz Moszkowski. Examples were shown in our previous paper (see Fig. 1 in Furuya et al. 2011). These pieces were chosen so that all possible combination of fingerings should be used. That is, they included 1) fingerings in which preceding and subsequent keystrokes of the keypress with one digit were performed by one of the other four digits and 2) several different key positions played with the same fingering. None of the sequences involved repetitive use of the same digit, which would require lifting a digit prior to and following a keypress. All notes were sixteenth notes, and chords were eliminated. The fingering was specified on each score presented so that all pianists would use the same finger patterns for the same pieces of music.
Fig. 1.
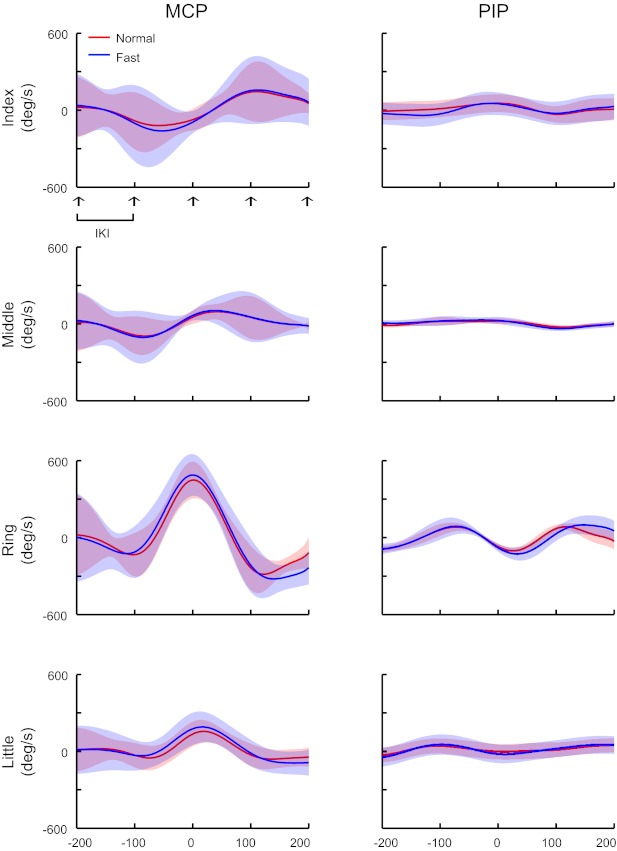
Average joint velocity waveforms across all tone sequences having a keypress with the ring finger at the center of the sequence in 1 pianist at normal (red line) and fast (blue line) tempi (pianist 5). Shaded areas indicate standard deviation across tone sequences. Left: metacarpo-phalangeal (MCP) joint. Right: proximal-interphalangeal (PIP) joint. Each row represents each of the 4 fingers. Arrows indicate moment of each of 5 keypresses (= when a key started to descend). IKI indicates 1 interkeystroke interval (= 100 time points on x-axis).
The pianists played a digital piano (Roland ep-5, 61 keys) connected to a Windows computer (SONY VAIO VGN-Z90PS) via a MIDI interface (Roland EDIROL UA-4FX). They were provided with the score for each of the pieces on a computer monitor located in front of the piano and were allowed practice to familiarize themselves with the piano and with the musical selection.
Pianists were asked to play each of the 30 excerpts for 10 successful trials at a certain tempo [interkeystroke interval (IKI) = 125 ms] and at the fastest tempo (IKI = ∼95 ms; see Fig. 4, left). When pianists were playing at a certain tempo, the tempo was provided by a metronome and pianists played in synchrony with the metronome. All pieces were played with legato touch, meaning that a key was not released until the next key was depressed. Pianists were asked to play at the loudness of 100 MIDI velocity.
Fig. 4.
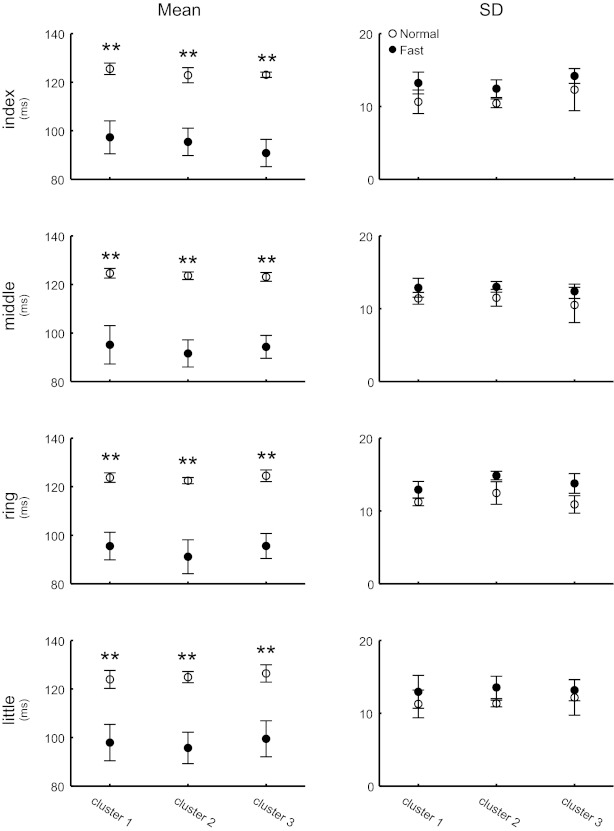
Combined results from all pianists summarize the similarities and differences in playing tempo and rhythmic accuracy of strokes between normal and fast tempi. Mean (left) and SD (right) of the IKI across 5 successive strokes of each tone sequence were averaged across all tone sequences. ○, Normal tempo; ●, fastest tempo. Two-way ANOVA confirmed a significant difference in the mean IKI (playing tempo) but not in the SD of IKI (rhythmic accuracy) between the 2 tempi. **P < 0.01 by Tukey post hoc test, which was performed after 2-way repeated-measures ANOVA.
Data Acquisition
We recorded the pianists' hand postures dynamically with sensors embedded in a right-handed glove (CyberGlove, Virtual Technologies, Palo Alto, CA). The glove fit tightly but was thin and flexible and open at the fingertips. We recorded the motions at 17 degrees of freedom with angular resolution <0.5°, at 12-ms intervals (Furuya et al. 2011; Santello et al. 2002). The measured angles were the metacarpo-phalangeal (MCP) and proximal-interphalangeal (PIP) joint angles of the four fingers as well as the angles of abduction (abd) between adjacent fingers. For the thumb the MCP, abd, and interphalangeal (IP) angles were measured, as was the angle of thumb rotation (ROT) about an axis passing through the trapeziometacarpal joint of the thumb and index MCP joint. Two additional degrees of freedom defined wrist rotation, which was not considered in our analysis. Flexion and abduction were defined as positive; the MCP and PIP angles were defined as zero when the finger was straight and in the plane of the palm.
We also recorded MIDI data from the keyboard by using a custom-made script in LabVIEW (National Instruments), running at 200 Hz in synchronization with the CyberGlove (Furuya and Soechting 2010). From MIDI data, we derived information on the velocity with which each key was depressed (loudness) and on the time the key was depressed (Engel et al. 1997). Using this information, each IKI was time normalized as 100 samples, so as to minimize intertrial and intersubject variability in timing. Consequently, the normalized times of keypress occur at times −200, −100, 0, +100, and +200 (see Fig. 1).
Data Analysis
We analyzed a sequence of five successive strokes having a particular keypress with each of the four fingers at the center of the sequence. From 30 music excerpts, we obtained in total 64, 49, 56, and 51 patterns of tone sequences played with a variety of key positions requiring different fingerings for the keypress with the index, middle, ring, and little fingers.
Principal component analysis.
To characterize patterns of coordination among various degrees of freedom at the hand, we performed PC analysis. The PC analysis identified patterns of covariation of time-varying joint kinematics across tone sequences, which enabled us to determine the movement templates to reconstruct a variety of finger movements.
The PC analysis used was one that was developed in previous studies (Furuya et al. 2011; Santello et al. 2002). The input to our time-varying synergy PC analysis was the averaged angular velocity for each joint, for each tone sequence, during the time interval of five successive strokes (2 keypresses before and after the particular keystroke with each finger). Each of the tone sequence vectors consisted of a series of 15 joint velocity waveforms. We did a separate analysis for the tone sequences centered on each of the four fingers, for each of the five subjects, for each of the two tempi.
The PC analysis in the present study results in n basic PC waveforms, computed from the n × n covariance matrix of the n tone sequence vectors (n ranging from 49 to 64). The covariance calculation removes the mean from each of the n columns of the input matrix. Thus the angular velocity waveforms at each joint for each tone sequence (400 time units for each joint) could be perfectly reconstructed as the average angular velocity at a jth joint for an ith tone sequence (mean θ̇ij) plus a weighted sum of the n PC waveforms (PC1…nj) at a jth joint:
| (1) |
where W1i–Wni are the weighting coefficients for an ith tone sequence. The PCs are ranked such that PC1 accounts for the largest portion of the variance.
We then quantitatively determined the correspondence of PCs across pianists based on the technique that we used previously (Furuya et al. 2011).
Cluster analysis.
Using the weighting coefficients (Wni) derived from the time-varying synergy PC analysis, we performed a cluster analysis using an expectation-maximization (EM) algorithm (Dempster et al. 1977) for the data sets centered on the keypress for each of four fingers for each pianist at each of the tempi separately (see details in Furuya et al. 2011). The aim of this analysis was to determine whether or not a few patterns were adequate to describe coordination of finger movements during the playing of a wide variety of sequences. We used the Wni of the first four PCs at all tone sequences in the cluster analysis, those PCs accounting for >60% of the total variance for all digits at both of the two tempi (for a keypress with each of the index, middle, ring, and little fingers, the group mean of variance accounted for by the first 4 PCs was 66.7 ± 2.1, 65.6 ± 3.8, 65.2 ± 3.7, and 61.8 ± 4.7 at the normal tempo and 68.6 ± 4.7, 68.2 ± 5.1, 66.9 ± 6.8, and 61.3 ± 6.3% at the fast tempo, respectively).
The number of clusters set for the EM algorithm was two to six, and for each of the numbers the sum of variance of the weighting coefficients within each cluster (the sum of within-cluster variance) was computed as follows:
| (2) |
where c is the number of clusters (c = 1, 2,…, 6), i is the number of a sequence belonging to that cluster, n is the total number of sequences, and X is the vector consisting of the Wni of the first four PCs. The value σwc2 was computed for each pianist and for the keystroke with each of four fingers separately and then averaged across pianists. The average value was plotted relative to the number of clusters used for the EM. A breakpoint of the plotted curve was used to determine an optimal number of clusters for further analysis.
To further ascertain the optimal number of clusters, we also computed the silhouette value for each number of clusters, using the weighting coefficients (Rousseeuw 1987). The value becomes larger as the within-cluster variance and between-cluster variance become smaller and larger, respectively. The silhouette value ranges from −1 to 1, depending on whether the sample has been assigned to an appropriate cluster or misclassified. We therefore reasoned that the mean silhouette value across pianists should be largest at the optimal cluster number.
Statistics
To assess the amount of covariation of joint motion across fingers, a linear regression analysis was performed for motions at the MCP and PIP joints between the striking finger and each of the nonstriking fingers at each tone sequence for each of the index, middle, ring, and little finger keystrokes. The derived r2 value was averaged across tone sequences belonging to each of the clusters at each of the two tempi. To evaluate differences in the amount of the movement covariation across fingers, two-way analysis of variance (ANOVA) with repeated measures was performed by using tempo and finger combination as independent variables and z-transformed r2 value as a dependent variable. Tukey post hoc tests were performed where appropriate to correct for multiple comparisons.
RESULTS
We analyzed the motions of each of the fingers by initially separating the data according to the finger that was used to press a particular key and then time-normalizing the data so that the interval between successive keypresses was constant and equal to 100 units. Our analysis focused on the time interval that spanned ±2 keypresses around the keypress of interest. This analysis yielded a set of waveforms at the 15 degrees of freedom of the hand, representing between 49 and 64 tone sequences, depending on the central digit.
We begin by describing the similarity of the patterns of joint motions during keypresses across tempi. The average and variability of joint motion across all tone sequences showed that each joint moved in a similar manner irrespective of tempo. Figure 1 shows the mean and standard deviation of joint velocity waveforms across all tone sequences having a keypress with the ring finger at the center of the sequence in one pianist at the normal and fast tempi. Overall, the waveform at each joint was considerably similar across the tempi, but more or less inconsistent across sequences. This tendency was commonly observed at the other joints, fingers used for the keypress, and pianists.
To isolate fundamental patterns of movement covariation, a cluster analysis using the EM algorithm was performed for each of the two tempi. The cluster analysis yielded similar results of classification of tone sequences across tempi. The rates at which a given tone sequence was categorized into the same cluster for the two tempi were 82.8 ± 4.9%, 84.9 ± 5.5%, 82.5 ± 3.4%, and 84.7 ± 7.3% for the index, middle, ring, and little finger keypress, respectively (mean ± SD across pianists). This indicates that >80% of tone sequences were assigned into the same cluster between the two tempi. Here, the number of clusters was set as three for each of the four fingers at both tempi according to the breakpoint of the total within-cluster variance of the weighting coefficients of the first four PCs. A computation of silhouette value also showed the largest value when the number of clusters was three for a keypress with each of the four fingers at both tempi (Table 1). These results are in agreement with our previous finding that a variety of hand movements during piano playing were categorized into three distinct clusters (Furuya et al. 2011).
Table 1.
Silhouette value for each number of clusters at normal and fast tempi
| Number of Clusters |
|||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| Normal tempo | |||||
| Index | |||||
| Mean | 0.290 | 0.305 | 0.253 | 0.177 | 0.174 |
| SE | (0.028) | (0.035) | (0.022) | (0.030) | (0.025) |
| Middle | |||||
| Mean | 0.263 | 0.264 | 0.184 | 0.202 | 0.124 |
| SE | (0.014) | (0.017) | (0.025) | (0.030) | (0.034) |
| Ring | |||||
| Mean | 0.245 | 0.270 | 0.196 | 0.173 | 0.157 |
| SE | (0.011) | (0.028) | (0.028) | (0.035) | (0.032) |
| Little | |||||
| Mean | 0.262 | 0.298 | 0.207 | 0.171 | 0.143 |
| SE | (0.022) | (0.026) | (0.023) | (0.012) | (0.025) |
| Fast tempo | |||||
| Index | |||||
| Mean | 0.272 | 0.283 | 0.182 | 0.244 | 0.175 |
| SE | (0.019) | (0.013) | (0.020) | (0.039) | (0.023) |
| Middle | |||||
| Mean | 0.207 | 0.285 | 0.196 | 0.145 | 0.176 |
| SE | (0.015) | (0.009) | (0.015) | (0.008) | (0.039) |
| Ring | |||||
| Mean | 0.248 | 0.272 | 0.143 | 0.154 | 0.147 |
| SE | (0.014) | (0.017) | (0.026) | (0.014) | (0.029) |
| Little | |||||
| Mean | 0.252 | 0.284 | 0.180 | 0.195 | 0.163 |
| SE | (0.011) | (0.031) | (0.022) | (0.026) | (0.040) |
Number in bold indicates the number of clusters with the largest silhouette value.
To further assess similarity of waveforms of joint motion across tempi, a cluster analysis was performed for the data sets from both tempi. If the waveform was not different across the tempi, the number of tone sequences assigned to each cluster should be the same between the two tempi. The ratio of the number of tone sequences between the two tempi was therefore computed for each of the clusters during a keypress with each of the digits and then was averaged across clusters. The mean and standard deviation across participants were 1.01 ± 0.09, 1.01 ± 0.02, 1.10 ± 0.11, 1.10 ± 0.07, and 1.02 ± 0.03 for the thumb, index, middle, ring, and little finger keypress, respectively. This indicated that the cluster analysis failed to separate tone sequences according to tempo, confirming similarity of the waveform of joint kinematics across the normal and fast tempi.
Figure 2 illustrates the average velocity waveforms at the MCP and PIP joints for each of the three clusters at the normal and fast tempi for sequences centered on the index and ring keystrokes by one pianist. For both the MCP and PIP joints, the overall waveform of joint rotation looked similar across tempi. The velocity at the MCP joint of the finger being used for the keypress (“striking finger”) was generally characterized by a triphasic pattern that formed an initial extension, followed by flexion with a peak at or slightly preceding the keypress, followed by extension. This pattern was consistent across clusters and fingers used for the keypress. In contrast, movement patterns of the nonstriking fingers considerably differed both from the striking finger and across the clusters. For example, during the index finger keypress, the index MCP joint moved for flexion while the nonstriking fingers were extending in clusters 2 and 3 (Fig. 2A). For cluster 1, the index MCP extension following the keystroke occurred along with flexion of the nonstriking fingers. The independent motions between the striking and nonstriking fingers were similarly observed for all pianists.
Fig. 2.
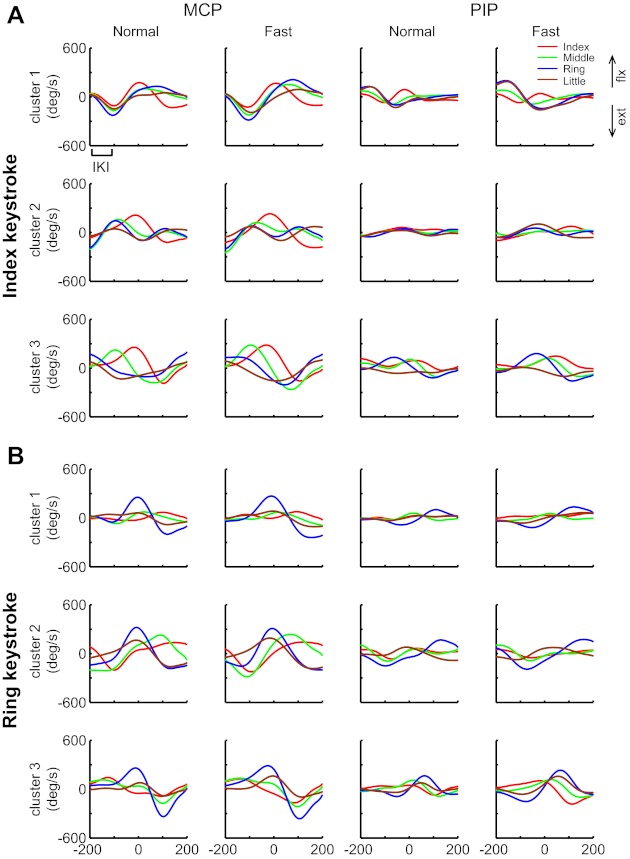
Average velocity waveforms at the MCP (left) and PIP (right) joints for each of the 3 clusters for sequences centered on the index (A) and ring (B) keystrokes. All data are reconstructed from the first 4 principal components (PCs) of pianist 4. IKI indicates 1 interkeystroke interval (= 100 time points on x-axis).
To quantitatively evaluate differences in the amount of covariation of movements across fingers between the two tempi for each of the clusters, we performed a linear regression analysis. For each cluster and for keystrokes with each of the fingers, we compared the waveform of the MCP joint velocity at the striking finger to the waveforms of the MCP of the adjacent fingers and the PIP of all fingers. Figure 3 shows the mean r2 value derived from the regression analysis at the MCP joint across pianists at the normal and fast tempi. A two-way repeated-measures ANOVA using tempo and finger as independent variables revealed that the z-transformed r2 value did not differ between the tempi for both MCP and PIP joints, except only for the middle and ring MCP in cluster 3 [tempo effect, F(1,4) = 24.3] during the index keypress (P < 0.01). At the PIP joint, ANOVA found that none of any pair of fingers/joints showed significant difference in the r2 value between the two tempi (data not shown). These results indicate that the amount of movement covariation between the striking and nonstriking fingers did not depend on tempo.
Fig. 3.
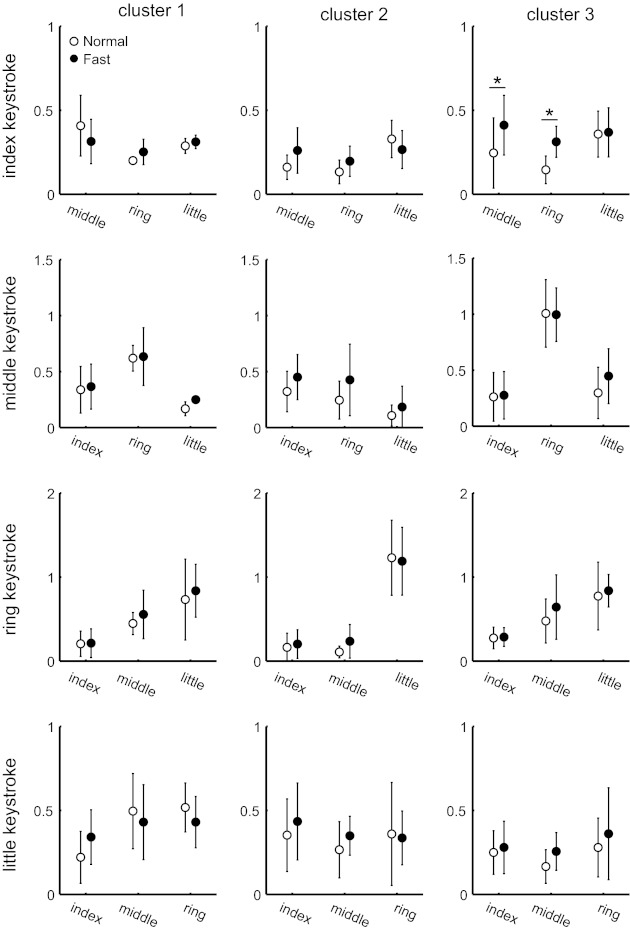
Combined results from all pianists summarize the similarities in covariation of joint movements across the 2 tempi: average of z-transformed r2 value between the MCP joint of the striking finger and the remaining MCP joints of the nonstriking fingers (x-axis). Data points are averages across pianists for each of 3 clusters during the keystroke with the index, middle, ring or little finger. ○, Normal tempo; ●, fast tempo. Error bars indicate 1 SD (n = 5). *P < 0.05 by Tukey post hoc test, which was performed after 2-way repeated-measures ANOVA.
To assess differences in the rhythmic accuracy of keypresses between the two tempi, the standard deviation of the IKI across five successive strokes was computed at each tone sequence and then averaged across sequences (Fig. 4, right). For each of three clusters during a keypress executed by each of the four fingers, a two-way repeated-measures ANOVA using tempo and cluster as independent variables confirmed no significant difference in the interstroke variability of IKI between the normal and fast tempi (P value for tempo effect was 0.07, 0.11, 0.06, and 0.08 at the index, middle, ring, and little fingers, respectively), which indicated maintenance of rhythmic accuracy of keystrokes across the tempi. In contrast, the mean IKI value was significantly smaller at the fast tempo than at the normal tempo (Fig. 4, left) (P < 0.001), which confirmed a faster rate of keystrokes at the former tempo condition.
The standard deviation of the MIDI velocity (= keystroke velocity) across five successive strokes was also computed at each tone sequence and then averaged across sequences and clusters. For a keypress executed by each of the index, middle, ring, and little fingers, the mean and standard deviation across pianists were 9.0 ± 1.0, 9.5 ± 1.2, 9.5 ± 1.1, and 9.8 ± 1.3 units at the normal tempo and 9.3 ± 1.5, 9.9 ± 1.6, 9.8 ± 1.5, and 9.9 ± 1.4 units at the fast tempo, respectively. A two-way repeated-measures ANOVA using tempo and finger used for a keypress confirmed no significant difference between the two tempi (P value for tempo effect was 0.50, 0.68, 0.49, and 0.42 at the index, middle, ring, and little fingers, respectively), indicating tempo invariance in accuracy of the keystroke velocity (i.e., articulation).
DISCUSSION
The present study aimed to identify the effect of tempo on independent control of fingers in piano playing by highly skilled individuals. Our results demonstrated a lack of apparent differences in the amount of covariation of joint motion across fingers between the normal and fast tempi over a wide variety of tone sequences for a keystroke with each of the four fingers. This confirmed maintenance of independence of finger movements across tempi for expert pianists. However, the result is in contrast with a finding from musically untrained individuals who performed cyclical and individuated finger movements at ∼2–3 Hz (Häger-Ross and Schieber 2000). Häger-Ross and Schieber found that motion at a finger covaried more with the remaining fingers at faster movement tempo, even though the movement tempo was much slower than the present piano playing (∼8–10.5 Hz). The finding indicates increased demands for independent control of fingers when moving faster. A study that examined static force production with a single finger by nonmusicians also demonstrated a larger spillover of force exerted by one finger to the adjacent fingers when eliciting larger force (Slobounov et al. 2002b), which was again inconsistent with our observation. This is because although the invariant joint velocity waveforms at faster tempo (Fig. 2) indicated production of higher acceleration and thereby larger muscular force, this did not result in less independent finger movements. A discrepancy between the present and previous findings may be due to the temporal constraints specifically imposed not only on the striking finger but also on the remaining fingers in piano playing. During a keypress with one finger, the remaining fingers also moved simultaneously to prepare for the upcoming keypresses (Engel et al. 1997; Furuya et al. 2011). Thus a decrease in individuation of movements across fingers can disrupt these preparatory finger motions, possibly lowering rhythmic accuracy in successive keystrokes. However, the present study failed to observe deficits in rhythmic accuracy of keystrokes even when the experts were playing faster, which suggested that tempo-invariant independence of finger movements is an outcome of strong temporal constraints on all fingers in piano playing.
Possible constraints influencing independent control of fingers include anatomical and neurophysiological coupling across fingers (Schieber and Santello 2004; van Duinen and Gandevia 2011). For example, extrinsic finger muscles are anatomically connected with multiple fingers and various tendons are interconnected (Leijnse 1997; Tubiana 1981). Thus production of force at a certain finger involves pulling the other anatomically connected fingers simultaneously. In addition, motor cortical representations of the muscles moving different fingers overlap (Sanes et al. 1995; Schieber and Hibbard 1993). Hence, individuated finger movements require force production not only at muscles to move a particular finger but also at muscles to keep the remaining fingers unmoved (Fuglevand 2011; Schieber 1995). The observation of a larger amount of movement covariation across fingers when moving a finger faster (Häger-Ross and Schieber 2000) may therefore result from less insufficient muscular compensation for the force spillover to stabilize the fingers at faster movement tempo. This can be associated with greater spillover of force to the anatomically connected fingers during larger force production (Slobounov et al. 2002b; Zatsiorsky et al. 2000) and/or activation of a larger cortical area responsible for finger movements to move fingers at faster tempo (Sadato et al. 1996; Schlaug et al. 1996). In contrast, the lack of stronger covariation of movements from the striking finger to nonstriking fingers when pianists played faster suggested accurate compensation for the tempo-dependent variation of the spillover. The short IKI (∼100 ms) indicated that this compensation was made in a predictive manner.
Maintenance of independent control of finger movements across a wide range of tempi and various tone sequences by pianists can emerge as a consequence of extensive piano practice (Jäncke 2009; Münte et al. 2002). A PET study using repetitive finger movements revealed that more individuated finger movements elicited larger activation of motor-related cortical regions (Remy et al. 1994). Also, improvement of independent control of the ring finger following extensive training resulted in larger movement-related cortical activity (Chiang et al. 2004). Greater neural resources are likely to be needed for independent finger movements. Increased demands for independent control of fingers when playing at faster tempo might be fulfilled by expanded motor cortical and subcortical regions observed in pianists compared with nonmusicians (Amunts et al. 1997; Gaser and Schlaug 2003) and after long-term piano training (Hyde et al. 2009).
We found invariance of accuracy in both rhythm and velocity of strokes across the tempi, inconsistent with a trade-off between speed and accuracy in movements (Fitts 1954). Besides the maintained independent control of fingers across tempi, a lack of trade-off can be attributed to increases in joint stiffness during faster playing. During repetitive and simultaneous piano keystrokes with the thumb and little finger, an increase in striking tempo increased muscular coactivation at extrinsic finger muscles, indicating an increase in stiffness of extrinsic finger muscles (Furuya et al. 2012). Also, typing on a computer keyboard at faster tempo yielded larger activities at both the extrinsic finger flexor and extensor muscles (Gerard et al. 2002). Since augmented stiffness ensures mechanical robustness against spontaneous variability of motor commands (Osu et al. 2004), an increased finger muscular coactivation may enable maintained rhythmic accuracy of keystrokes across tempi.
A limitation of the present study would be submaximal force production during the keypresses. To control the loudness of tones across tone sequences and tempi, the pianists were asked to play with a certain keystroke force, which was lower than the maximum (100 compared with 127 MIDI velocity). A previous study reported lack of independent finger control particularly when exerting the maximum force (Zatsiorsky et al. 2000). Thus relatively low force might allow for maintenance of independent control of finger movements between the two tempi, which should be clarified in future studies by examining keystrokes with a wide range of force production.
In conclusion, by using PC and cluster analyses, the present study identified three fundamental patterns of the hand movements in piano playing at each of the normal and fast tempi. The independence of finger movements between striking and nonstriking fingers was assessed by a regression analysis and compared across the tempi. Overall, we found no apparent difference in the amount of movement covariation across fingers between the tempi, confirming maintenance of independent finger movements across tempi. In addition, the rhythmic accuracy of keypresses was unchanged across the tempi, being inconsistent with speed-accuracy trade-off (Fitts 1954). The contrasting observations between the previous and present studies are likely to reflect the superior dexterity of the fingers in the expert pianists.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke R01 NS-015018.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.F. and J.F.S. conception and design of research; S.F. performed experiments; S.F. analyzed data; S.F. and J.F.S. interpreted results of experiments; S.F. prepared figures; S.F. drafted manuscript; S.F. and J.F.S. edited and revised manuscript; S.F. and J.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Martha Flanders for suggestions and support for this work.
REFERENCES
- Altenmüller E, Jabusch HC. Focal dystonia in musicians: phenomenology, pathophysiology and triggering factors. Eur J Neurol 17, Suppl 1: 31–36, 2010 [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp 5: 206–215, 1997 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Soechting JF. Non-uniform temporal scaling of hand and finger kinematics during typing. Exp Brain Res 95: 319–329, 1993 [DOI] [PubMed] [Google Scholar]
- Aoki T, Furuya S, Kinoshita H. Finger-tapping ability in male and female pianists and nonmusician controls. Motor Control 9: 23–39, 2005 [DOI] [PubMed] [Google Scholar]
- Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci 5: 2318–2330, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H, Slobounov SM, Ray W. Practice-related modulations of force enslaving and cortical activity as revealed by EEG. Clin Neurophysiol 115: 1033–1043, 2004 [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Ser B 39: 1–38, 1977 [Google Scholar]
- Engel KC, Flanders M, Soechting JF. Anticipatory and sequential motor control in piano playing. Exp Brain Res 113: 189–199, 1997 [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954 [PubMed] [Google Scholar]
- Fuglevand AJ. Mechanical properties and neural control of human hand motor units. J Physiol 589: 5595–5602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Aoki T, Nakahara H, Kinoshita H. Individual differences in the biomechanical effect of loudness and tempo on upper-limb movements during repetitive piano keystrokes. Hum Mov Sci 31: 26–39, 2012 [DOI] [PubMed] [Google Scholar]
- Furuya S, Flanders M, Soechting JF. Hand kinematics of piano playing. J Neurophysiol 106: 2849–2864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Soechting JF. Role of auditory feedback in the control of successive keystrokes during piano playing. Exp Brain Res 204: 223–237, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci 23: 9240–9245, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol 20: 1869–1874, 2010 [DOI] [PubMed] [Google Scholar]
- Gerard MJ, Armstrong TJ, Martin BJ, Rampel DA. The effects of work pace on within-participant and between-participant keying force, electromyography, and fatigue. Hum Factors 44: 51–61, 2002 [DOI] [PubMed] [Google Scholar]
- Häger-Ross C, Schieber MH. Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. J Neurosci 20: 8542–8550, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M, Kudo K, Watarai K, Ohtsuki T. Control of 3D limb dynamics in unconstrained overarm throws of different speeds performed by skilled baseball players. J Neurophysiol 97: 680–691, 2007 [DOI] [PubMed] [Google Scholar]
- Hollerbach MJ, Flash T. Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44: 67–77, 1982 [DOI] [PubMed] [Google Scholar]
- Hore J, O'Brien M, Watts S. Control of joint rotations in overarm throws of different speeds made by dominant and nondominant arms. J Neurophysiol 94: 3975–3986, 2005 [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. Musical training shapes structural brain development. J Neurosci 29: 3019–3025, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L. The plastic human brain. Restor Neurol Neurosci 27: 521–538, 2009 [DOI] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res 10: 177–183, 2000 [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 57–62, 2004 [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol 479: 487–497, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol 92: 2802–2810, 2004 [DOI] [PubMed] [Google Scholar]
- Leijnse JN. Anatomical factors predisposing to focal dystonia in the musician's hand—principles, theoretical examples, clinical significance. J Biomech 30: 659–669, 1997 [DOI] [PubMed] [Google Scholar]
- Leijnse JN, Snijders CJ, Bonte JE, Landsmeer JM, Kalker JJ, Van der Meulen JC, Sonneveld GJ, Hovius SE. The hand of the musician: the kinematics of the bidigital finger system with anatomical restrictions. J Biomech 26: 1169–1179, 1993 [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L. The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473–478, 2002 [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, Murray ST, Flanders M. Do arm postures vary with the speed of reaching? J Neurophysiol 81: 2582–2586, 1999 [DOI] [PubMed] [Google Scholar]
- Osu R, Kamimura N, Iwasaki H, Nakano E, Harris CM, Wada Y, Kawato M. Optimal impedance control for task achievement in the presence of signal-dependent noise. J Neurophysiol 92: 1199–1215, 2004 [DOI] [PubMed] [Google Scholar]
- Parlitz D, Peschel T, Altenmüller E. Assessment of dynamic finger forces in pianists: effects of training and expertise. J Biomech 31: 1063–1067, 1998 [DOI] [PubMed] [Google Scholar]
- Pfann KD, Corcos DM, Moore CG, Hasan Z. Circle-drawing movements at different speeds: role of inertial anisotropy. J Neurophysiol 88: 2399–2407, 2002 [DOI] [PubMed] [Google Scholar]
- Remy P, Zilbovicius M, Leroy-Willig A, Syrota A, Samson Y. Movement- and task-related activations of motor cortical areas: a positron emission tomographic study. Ann Neurol 36: 19–26, 1994 [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20: 53–65, 1987 [Google Scholar]
- Sadato N, Ibanez V, Deiber MP, Campbell G, Leonardo M, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab 16: 23–33, 1996 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science 268: 1775–1777, 1995 [DOI] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Patterns of hand motion during grasping and the influence of sensory guidance. J Neurosci 22: 1426–1435, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: the roles of extrinsic finger muscles. J Neurosci 15: 284–297, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science 261: 489–492, 1993 [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol 96: 2293–2300, 2004 [DOI] [PubMed] [Google Scholar]
- Schlaug G, Sanes JN, Thangaraj V, Darby DG, Jancke L, Edelman RR, Warach S. Cerebral activation covaries with movement rate. Neuroreport 7: 879–883, 1996 [DOI] [PubMed] [Google Scholar]
- Slobounov S, Chiang H, Johnston J, Ray W. Modulated cortical control of individual fingers in experienced musicians: an EEG study. Clin Neurophysiol 113: 2013–2024, 2002a [DOI] [PubMed] [Google Scholar]
- Slobounov S, Johnston J, Chiang H, Ray W. The role of sub-maximal force production in the enslaving phenomenon. Brain Res 954: 212–219, 2002b [DOI] [PubMed] [Google Scholar]
- Smahel Z, Klimová A. The influence of age and exercise on the mobility of hand joints. 1. Metacarpophalangeal joints of the three-phalangeal fingers. Acta Chir Plast 46: 81–88, 2004 [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Invariant characteristics of a pointing movement in man. J Neurosci 1: 710–720, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana R. Architecture and functions of the hand. Hand 1: 19–93, 1981 [Google Scholar]
- van Duinen H, Gandevia SC. Constraints for control of the human hand. J Physiol 589: 5583–5593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AH. What can studying musicians tell us about motor control of the hand? J Anat 208: 527–542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res 131: 187–195, 2000 [DOI] [PubMed] [Google Scholar]


