Abstract
Objective
To evaluate whether eliciting repetitive cortical and autonomic arousals during sleep is able to induce the occurrence of periodic leg movements during sleep (PLMS).
Methods
Fifteen normal subjects underwent one night of uninterrupted and two sequential nights of experimental sleep fragmentation achieved by auditory and mechanical stimuli eliciting frequent EEG arousals. Sleep was polygraphically recorded and subsequently used to determine the frequency of arousals and occurrence of LM activity during the first (baseline) and the second fragmentation night. Also, heart rate variability parameters were obtained to assess the autonomic changes induced by the stimulation.
Results
Sleep fragmentation was associated with an increase in the arousal index, percentage of sleep stage 1, and frequency of stage shifts. In addition, there was a decrease in sleep latency and in percentage of slow-wave sleep. Moreover, a significant increase in heart rate variability and especially of its sympathetic component, was also found. In contrast, parameters of the leg movement activity showed no significant change following experimental sleep fragmentation. The lack of an increase in leg movement activity was also observed in one subject who demonstrated PLMS at baseline.
Conclusions
Experimental sleep fragmentation is not associated with an increase in PLMS in normal young adults.
Keywords: Periodic leg movement during sleep, PLMS, arousal, autonomic activation, sleep fragmentation, heart rate variability
1. Introduction
Periodic leg movements during sleep (PLMS) are a common polysomnographic phenomenon characterized by repetitive activations of the tibialis anterior muscle that are typically between 0.5 and 10s in duration and occur approximately every 10–30 s [1]. More than 80% of patients with restless legs syndrome (RLS) manifest PLMS, which may also occur in healthy elderly subjects [2] and other chronic conditions [3]. Dopamine-agonists are effective in suppressing PLMS in patients with RLS, particularly in those with a high frequency of events. PLMS are often accompanied by profound changes in heart rate and blood pressure [4–8], comparable to those observed in association with upper airway collapse in obstructive sleep apnea [9]. Because leg movements that co-occur with obstructive apneas and hypopnea are likely to be pathophysiologically distinct, they are not classified as PLMS according to the current criteria [1,10].
The significance of cortical arousals in eliciting PLMS has been a topic of significant controversy. Periodic increase in electro-cortical and sympathetic activity can occur physiologically during sleep even in the absence of overt PLMS [11–13]. In patients with RLS, the use of pharmacological interventions, such as pramipexole, can decrease the frequency of PLMS without a concomitant decrease in the frequency of arousals [14]. Conversely, reducing the number of arousals by agents such as clonazepam is not associated with a decrease of PLMS [15]. Collectively, these findings suggest that inter-relationships of cortical, motor, and autonomic events during sleep are likely complex and require further clarification.
Important insights on the association between arousals and PLMS can be gained by examining the effects of experimentally-induced arousals on PLMS in normal subjects that are free of comorbid conditions. Thus, the primary aim of the current study was to determine if an increase in the frequency of arousals and autonomic events during sleep is associated with an increase in PLMS. It was hypothesized that, in normal subjects, experimentally-induced arousals would not be associated with an increase in PLMS.
2. Methods
2.1 Study Sample
Healthy volunteers were recruited from the general community. To participate, volunteer subject had to be less than 40 years in age, have a body mass index <30 kg/m2, consume less than two alcoholic or three caffeinated beverages per day, habitually sleep for at least 7 hours/night, have a usual bedtime before midnight and not work at night or on a rotating shift schedule. Additional exclusion criteria included cigarette smoking and use of any prescription medications or non-prescription anti-inflammatory agents. Eligibility for participation also required absence of the following conditions: type 2 diabetes mellitus, angina, myocardial infarction, coronary revascularization, congestive heart failure, stroke, obstructive lung disease, renal or hepatic dysfunction, psychiatric or neurologic disease. After an initial telephone screening, eligible volunteers were required to complete a serologic screen and an overnight polysomnogram to rule out obstructive sleep apnea as previously described [16]. Usual sleep habits were also objectively assessed with a wrist activity monitor that was worn for at least five days including one weekend. A normal polysomnogram, demonstration of habitual bedtime by midnight and an average of at least seven hours of sleep on actigraphy, and normal serum chemistries were required for enrollment. Most subjects had at least a college education with the exception of one subject who had only completed high school.
After enrollment, multiple contacts were made to counsel each subject on maintaining at least seven hours of sleep per night. Ambulatory monitoring of sleep habits was repeated for three nights prior to the baseline evaluation to confirm that habitual sleep patterns remain unchanged. Volunteers were excluded from participating in the study if sleep duration on any one night was less than six hours or the average sleep duration was less than seven hours preceding admission to the clinical research unit (CRU). Female volunteers were scheduled for the study protocol during the follicular phase (day 4–10) of the menstrual cycle. The experiments were entirely conducted at the Johns Hopkins University, School of Medicine, Baltimore, MD, U.S.A. Informed consent was obtained from each volunteer and the study protocol was approved by the local institutional review board.
2.2 Study Protocol
The study protocol entailed that each subject was admitted to the CRU and had one night of uninterrupted (night 1) and two sequential nights of experimental sleep fragmentation (nights 2 and 3). Results obtained from this study protocol on several outcomes have been previously published [17,18].
2.3 Polysomnographic Recordings
The overnight recording included the following montage: EEG (4 channels, including C3, C4, O1, and O2, referred to the contralateral mastoid); bilateral electrooculograms (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to the left mastoid), electromyogram (EMG) of the submentalis muscle, ECG (CM4 derivation: anode in position V6 and cathode attached to the manubrium of the sternum), and one EMG channel combining both tibialis anterior muscles. Sleep signals were digitized using the Somnologica software (Embla Systems) and converted to European data format for further analysis.
2.4 Sleep Fragmentation
Continuous polysomnographic monitoring was performed on each of the three nights in the CRU. Lights out and morning wake times for each subject were matched to their usual bedtimes and wake times and kept constant throughout the three study nights. During the day, subjects were ambulatory in the CRU, but were not allowed to sleep. Sleep fragmentation was achieved by auditory and mechanical stimuli in anticipation of habituation that may occur with a single repeated auditory stimulus type. Auditory tones were broadcast through two speakers placed 12 inches from the head of the bed. Mechanical stimuli were administered using a commercially-available mechanical vibrator. Four such devices were placed underneath the mattress. The aim was to elicit EEG micro-arousals (>3 to <15 s), as defined by standard criteria [10], at a frequency of 30 or more events/hour using the following guidelines; following lights out, two minutes of continuous stage 2 sleep (or higher) were observed before applying the first auditory stimulus, a sine-wave auditory tone of 500 ms duration and 57 dB. If an EEG micro-arousal was not elicited, subsequent stimuli were varied by increasing tone volume in 5–10 dB increments up to a maximum of 100 dB, modulating the frequency of the auditory tone, and applying the mechanical stimulus alone or in combination with the auditory tone. Once an arousal was elicited, at least 30 seconds of stimulus free interval of sleep were required before applying a subsequent stimulus. Arousals were elicited irrespective of sleep stage.
2.5 Sleep, Arousal, and Leg Movement Activity Analysis
Sleep stages were scored using standard criteria on 30 second epochs [19]. Arousals were also scored using standard criteria [10] and the arousal index was calculated as the number of arousals/hour of sleep. Leg movements were recorded and scored on the rectified EMG signal [20] according to WASM-IRLSSG criteria [1] with visual annotation of all automatic events. Outcomes included the total LM index (total number of leg movements per hour of sleep), PLMS index (entire night and in REM and NREM sleep), as well as the total number of leg movements and number of PLMS sequences. The Periodicity index was calculated as the number of intervals belonging to sequences of at least 3 inter-LM intervals 10<i≤90s/total number of inter-LM intervals. This index can vary between 0 (absence of periodicity) to 1 (all intervals with length 10<i≤90s)[21]. The Periodicity Index is independent of the absolute number of leg movements recorded and was calculated for all the subjects. Finally, PLMS associated with arousals [1] were tabulated and the PLMS-Arousal index was determined (number/hour of sleep).
2.6 Analysis of Heart Rate Variability during NREM Sleep Stage 2
In order to study the autonomic system changes induced by the stimulation protocol we decided to analyze heart rate variability (HRV) by focusing our attention on NREM sleep stage 2 because of the extensive presence of this stage in all subjects enrolled, which allowed us to find artifact-free epochs lasting 5 minutes during the second NREM sleep cycle of each recording. During all epochs chosen for HRV analysis, subjects rested in supine or lateral positions.
In each 5 minuteepoch, the ECG signal (sampled at 200 Hz) was analyzed for automatic detection of R waves with Hypnolab 1.2 (SWS Soft, Italy) utilizing a simple threshold plus first and second derivative algorithm. R-R intervals from each epoch were calculated and their value was obtained at regular intervals of 1s by linear interpolation of the measured values, for the first 256 s which were utilized for all subsequent analysis steps.
From these intervals, a series of time-domain measures was calculated:
mean R-R value, SDNN (standard deviation of all R-R intervals),
RMSSD (the square root of the mean of the sum of the squares of differences between adjacent R-R intervals),
NN50 (number of pairs of adjacent R-R intervals differing by more than 50 ms in the entire epoch),
pNN50 (percentage of NN50 among the total R-R intervals).
Further, the interpolated R-R interval tachograms were also processed by means of a Fast-Fourier Transform (FFT) algorithm and the following spectral parameters were obtained:
VLF (power in very low-frequency range, < 0.04 Hz);
LF (power in low-frequency range, 0.04–0.15 Hz);
HF (power in high-frequency range, 0.15 – 0.4 Hz);
total power (VLF+LF+HF);
LF% (LF power in normalized units: LF/(total power – VLF) ×100;
HF% (HF power in normalized units: HF/(total power – VLF) ×100 and
LF/HF (ratio LF/HF).
2.7 Statistical analysis
Comparisons between the non-fragmented (baseline) and fragmented nights were made with the use of the nonparametric Friedman ANOVA for paired observations using the commercially available Statistica software package (StatSoft, Inc., 2001. STATISTICA data analysis software system, Version 6. www.statsoft.com) was used. A p <0.05 was used to define statistical significance.
3. Results
The study sample included 15 subjects (12 males and 3 females, mean age 24.6 years, 4.07 S.D.) that completed the study protocol. One subject was excluded from the analysis because of numerous artifacts in the leg EMG signal at baseline and another subject was analysed separately because of the presence of a PLMS at baseline. The remaining 13 subjects were included in current analysis reported herein.
Figure 1 is an example of a polysomnographic recording fragment from a night of sleep fragmentation demonstrating the differences in spontaneous and evoked EEG arousals (sound meter peaks) evoked by auditory stimuli. The increase in heart rate following an auditory stimulus was significantly higher than the increase observed with a spontaneous arousal. Table 1 includes the parameters of sleep architecture obtained from the non-fragmented (baseline) and fragmented nights. With sleep fragmentation, sleep latency decreased on night 3, the number of stage shifts and of awakenings, as well as the percentage of sleep stage 1 increased, and the percentage of slow-wave sleep was reduced. Not surprisingly, the arousal index was higher on the fragmented nights versus the non-fragmented night.
Fig. 1.
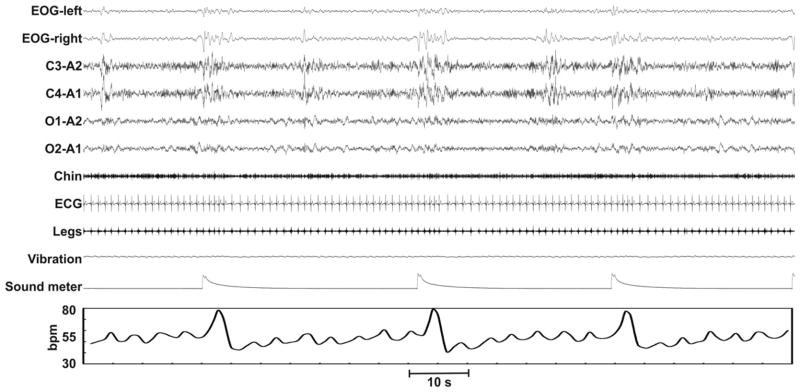
Example of a polysomnographic recording during the fragmentation night; heart rate fluctuations are also reported on the bottom. Note the different responses in spontaneous and evoked EEG activations (sound meter peaks) elicited by auditory stimuli and the subsequent rises in heart rate.
Table 1.
Sleep parameters at baseline (non-fragmented sleep, night 1) and during the 2 nights of sleep fragmentation (nights 2 and 3).
| Baseline night 1 | Fragmentation night 2 | Fragmentation night 3 | Friedman ANOVA p-value | ||||
|---|---|---|---|---|---|---|---|
| median | interquartile range | median | interquartile range | median | interquartile range | ||
| TIB, min | 480.0 | 479.5–483.0 | 480.0 | 479.0–484.0 | 479.5 | 479.0–481.5 | NS |
| SPT, min | 474.5 | 470.5–480.5 | 476.0 | 464.0–480.0 | 477.5 | 474.5–480.5 | NS |
| TST, min | 452.0 | 437.5–463.5 | 443.0 | 428.0–458.5 | 454.5 | 438.0–459.0 | NS |
| SL, min | 5.5 | 3.0–10.5 | 4.5 | 2.0–16.5 | 2.5 | 1.0–16.5 | 0.02 |
| FRL, min | 68.0 | 63.5–91.0 | 84.5 | 78.5–112.0 | 83.0 | 73.0–137.0 | NS |
| Stage shifts/hour | 11.5 | 11.3–16.8 | 21.9 | 18.8–29.5 | 22.4 | 16.1–30.9 | 0.002 |
| Awakenings/hour | 3.4 | 2.9–4.7 | 6.6 | 5.4–8.6 | 5.9 | 3.9–7.1 | 0.014 |
| SE, % | 94.3 | 88.1–96.2 | 91.7 | 89.6–95.2 | 94.5 | 88.9–95.7 | NS |
| WASO, % | 4.0 | 2.9–7.7 | 6.0 | 4.1–8.1 | 5.1 | 3.5–9.9 | NS |
| S1, % | 3.6 | 2.8–8.3 | 16.4 | 8.4–20.2 | 10.8 | 9.3–17.9 | 0.0002 |
| S2, % | 53.3 | 50.5–58.7 | 57.2 | 47.2–59.0 | 54.8 | 47.6–58.8 | NS |
| SWS, % | 11.9 | 10.8–16.2 | 2.3 | 0.9–3.3 | 2.1 | 0.9–7.7 | 0.001 |
| REM, % | 21.8 | 18.8–23.0 | 17.5 | 15.0–21.5 | 19.8 | 17.8–21.3 | NS |
| Arousal index, n/hour | 14.2 | 12.0–17.6 | 36.8 | 21.9–51.0 | 30.0 | 18.8–39.0 | 0.025 |
TIB: time in bed, SPT: sleep period time, TST: total sleep time, SL: sleep latency, FRL: first REM latency, SE: sleep efficiency.
Table 2 reports the heart rate variability parameters obtained during the three experimental nights. Sleep fragmentation was accompanied by an evident increase in heart rate variability (SDNN and total power at the spectral analysis), which was due especially to the increase of parameters related to its sympathetic component (LF and VLF bands, LF/HF ratio). This increase was evident in both fragmentation nights.
Table 2.
Heart rate variability at baseline (non-fragmented sleep, night 1) and during the 2 nights of sleep fragmentation (nights 2 and 3).
| Baseline night 1 | Fragmentation night 2 | Fragmentation night 3 | Friedman ANOVA p-value | ||||
|---|---|---|---|---|---|---|---|
| median | interquartile range | median | interquartile range | median | interquartile range | ||
| Mean R-R, s | 1.04 | 1.02–1.10 | 1.10 | 1.07–1.19 | 1.14 | 1.11–1.20 | NS |
| SDNN | 0.05 | 0.04–0.07 | 0.07 | 0.08–0.13 | 0.09 | 0.07–0.11 | 0.003 |
| RMSSD | 0.05 | 0.04–0.07 | 0.07 | 0.05–0.11 | 0.06 | 0.05–0.09 | NS |
| NN50 | 68.00 | 40.00–147.00 | 147.00 | 50.00–150.00 | 80.00 | 58.00–121.00 | NS |
| pNN50 | 26.67 | 15.69–57.65 | 57.65 | 19.61–58.82 | 31.37 | 22.75–47.45 | NS |
| VLF power | 0.07 | 0.06–0.11 | 0.11 | 0.12–0.22 | 0.17 | 0.13–0.21 | 0.00025 |
| LF power | 0.15 | 0.12–0.22 | 0.22 | 0.25–0.40 | 0.28 | 0.24–0.38 | 0.0001 |
| HF power | 0.20 | 0.11–0.28 | 0.28 | 0.17–0.39 | 0.26 | 0.18–0.40 | NS |
| Total power | 0.39 | 0.32–0.59 | 0.59 | 0.55–1.09 | 0.70 | 0.52–0.99 | 0.003 |
| LF, % | 39.91 | 37.80–44.75 | 44.75 | 45.44–58.25 | 51.44 | 49.69–57.55 | 0.00005 |
| HF, % | 60.09 | 55.25–62.20 | 62.20 | 41.75–54.56 | 48.56 | 42.45–50.31 | 0.00005 |
| LF/HF | 0.66 | 0.61–0.81 | 0.81 | 0.83–1.40 | 1.06 | 0.99–1.36 | 0.00005 |
SDNN = standard deviation of all R-R intervals; RMSSD = square root of the mean of the sum of the squares of differences between adjacent R-R intervals; NN50 = number of pairs of adjacent R-R intervals differing by more than 50 ms in the entire epoch; pNN50 = NN50%; VLF = power in very low-frequency range (<0.04 Hz); LF = power in low-frequency range (0.04–0.15 Hz); HF = power in high-frequency range (0.15–0.4 Hz); total power = VLF + LF + HF; LF% = LF power in normalized units (LF/[total power–VLF] × 100): HF% = HF power in normalized units (HF/[total power–VLF] × 100); LF/HF = ratio LF/HF.
The leg movement activity parameters from the non-fragmented (baseline) and fragmented nights are shown in Table 3. None of leg movement activity parameters tabulated were significantly different between the baseline and fragmented nights of sleep. The distributions of leg inter-movement intervals from the baseline and fragmented nights are shown in Figure 2 (top panel). A peak at approximately 4s with a decrease to 10–12s was observed for all nights. The lower panel of Figure 2 displays the distributions of total leg movements across the sleep period. No statistically significant differences were noted between the non-fragmented and non-fragmented nights.
Table 3.
Leg movement activity parameters obtained at baseline (non-fragmented sleep, night 1) and during the 2 nights of sleep fragmentation (nights 2 and 3).
| Baseline night 1 | Fragmentation night 2 | Fragmentation night 3 | Friedman ANOVA p-value | ||||
|---|---|---|---|---|---|---|---|
| median | interquartile range | median | interquartile range | median | interquartile range | ||
| NREM sleep | |||||||
| Total index, n/hour | 3.4 | 2.2–6.3 | 5.2 | 3.9–10.5 | 4.0 | 2.5–6.1 | NS |
| PLMS index, n/hour | 0.0 | 0.0–0.8 | 0.4 | 0.0–1.5 | 0.0 | 0.0–1.0 | NS |
| Isolated LMs index, n/hour | 3.0 | 2.2–3.8 | 5.2 | 3.8–8.0 | 4.0 | 2.5–5.3 | NS |
| REM sleep | |||||||
| Total index, n/hour | 4.6 | 2.8–7.6 | 5.8 | 3.3–9.6 | 4.4 | 2.9–8.4 | NS |
| PLMS index, n/hour | 0.0 | 0.0–0.6 | 0.0 | 0.0–3.5 | 0.0 | 0.0–0.0 | NS |
| Isolated LMs index, n/hour | 4.3 | 2.8–7.6 | 5.8 | 3.3–7.6 | 4.4 | 3.1–9.1 | NS |
| Total sleep | |||||||
| Total index, n/hour | 3.5 | 3.2–5.6 | 6.2 | 4.3–9.4 | 4.9 | 3.6–6.0 | NS |
| PLMS index, n/hour | 0.1 | 0.0–0.7 | 1.2 | 0.0–1.3 | 0.0 | 0.0–1.0 | NS |
| Isolated LMs index, n/hour | 3.2 | 2.7–4.3 | 5.7 | 4.3–8.0 | 4.0 | 3.3–5.3 | NS |
| PLMS sequence number | 0.0 | 0.0–1.0 | 1.0 | 0.0–2.0 | 0.0 | 0.0–2.0 | NS |
| Periodicity index | 0.000 | 0.000–0.118 | 0.000 | 0.000–0.092 | 0.053 | 0.000–0.077 | NS |
| PLMS/Arousalindex | 0.0 | 0.0–0.4 | 0.4 | 0.0–1.1 | 0.0 | 0.0–0.4 | NS |
Fig. 2.
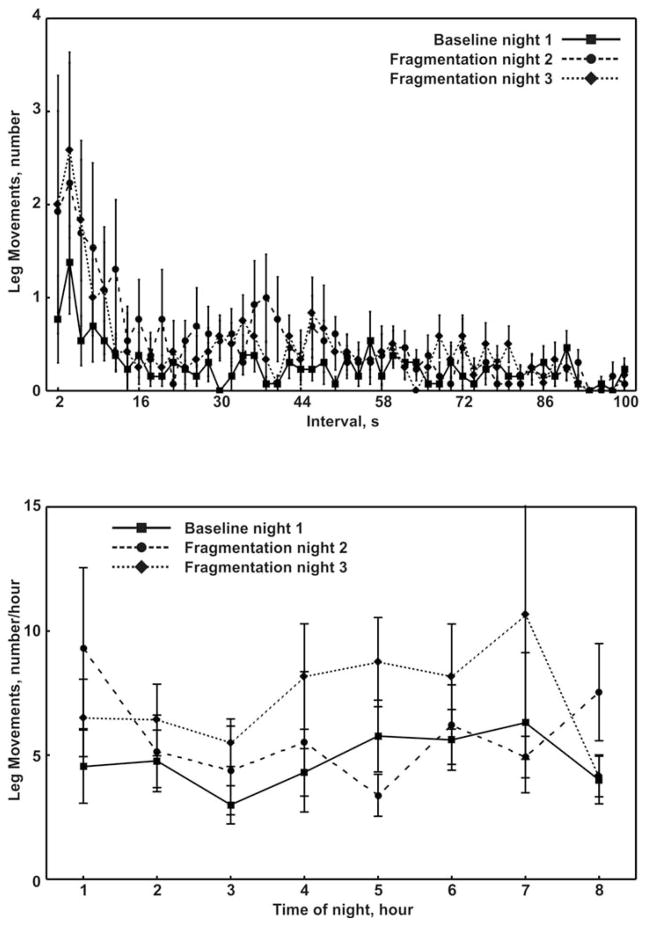
Distribution of leg inter-movement intervals (top panel) and total leg movements (bottom panel) during sleep at baseline (non-fragmented sleep, night 1) and during the 2 nights of sleep fragmentation (nights 2 and 3). Values are means and standard error of the means.
In the one subject with PLMS at baseline, the arousal index was 9.7 events/hr, the PLMS index was 33.7 events/hr and the periodicity index was 0.75. On the fragmented nights, this subject had an arousal index of 11.1 and 14.0 events/hr, PLMS index of 26.7 and 40.7 events/hr, and a periodicity index of 0.68 and 0.66. Figure 3 compares the distributions of leg inter-movement intervals and the total leg movements during sleep on the non-fragmented and fragmented nights. The stability in leg motor activity is evident across nights given the peak of the inter-movement interval distribution in the 10–40s range and a decreasing number of leg movements across the sleep period on all nights.
Fig. 3.
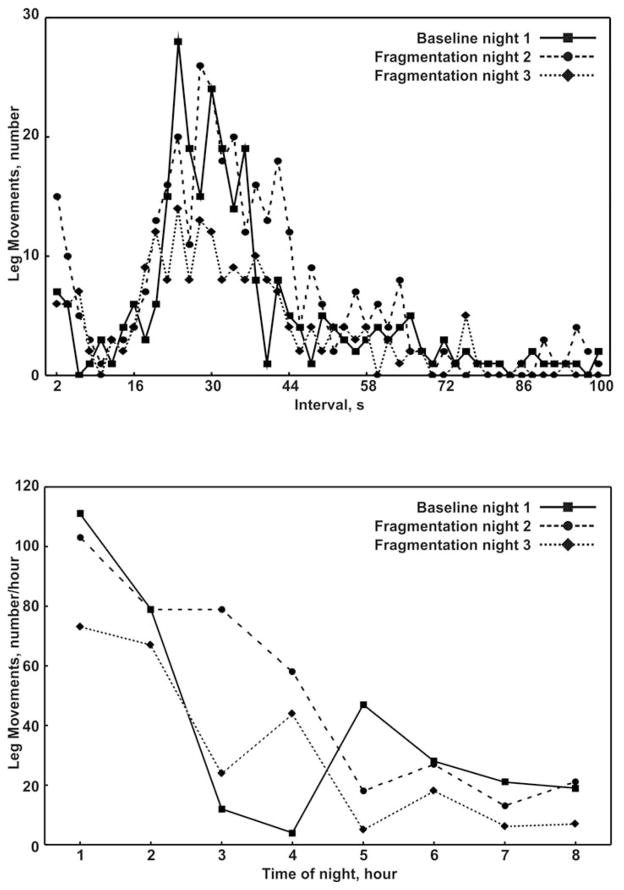
Distribution of leg inter-movement intervals (top panel) and total leg movements (bottom panel), at baseline (non-fragmented sleep, night 1) and during the 2 nights of sleep fragmentation (nights 2 and 3), in the single patient with PLMS at baseline.
4. Discussion
The overarching aim of the current study was to evaluate whether increasing the frequency of arousals and the accompanying autonomic activity would be associated with an increase in PLMS in normal subjects. The results reported herein suggest that an increase in arousals induced by auditory or mechanical stimuli is not associated with an increase in PLMS in normal subjects. In the one subject with evidence of PLMS at baseline, sleep fragmentation and an increase in arousals did not significantly alter the frequency of observed PLMS. Collectively, these data suggest that, at least in normal subjects, cortical arousals and an increase in autonomic events do not alter the occurrence of PLMS.
Although there is often a clustering of cortical arousals, autonomic events, and PLMS, previous work by our group [5,14,15,22] and others [6–8,23] suggests that the inter-relationships between these events are not likely to follow the simple paradigm of any one event leading to another [24]. The lack of a simple one cause event sequence between these events does not exclude the possibility that, when they do occur concurrently, one modifies the biological effects of the other [25–27]. The resulting effect modification may form the basis of adverse consequences of various clinical conditions in which PLMS are abundant such as RLS [28–31] or periodic limb movement disorder [32,33]. Whether such an effect modification occurs will require further research on the independent and interactive effects of cortical arousals, autonomic events, and PLMS.
PLMS in normal individuals are generally observed after the age of 40 years and usually with a PLMS index that is considerably lower than that observed in our subject with PLMS at baseline [2]. It is now increasingly recognized that PLMS are possibly a genetically-determined phenomenon, as recently demonstrated by a large genome-wide associated study in Iceland [34]. PLMS are considered to be an endophenotype of RLS [35] and gene variants associated with PLMS (i.e., BTBD9 and Meis1) confer an increased risk for RLS [34,36]. In fact, asymptomatic PLMS are often a precursor to RLS and more common in relatives of RLS patients [37] and various in ethnic groups with the highest frequencies of RLS/PLMS risk alleles (e.g., North Americans of European vs. African descent) [38]. It is thus possible that the one subject in the current study with PLMS at baseline with a time structure (intervals and night distribution) identical to that of typical PLMS of RLS [21] may be at risk for developing RLS.
5. Limitations
There are several limitations in the current study that merit discussion. First, only one EMG channel which combined two tibialis anterior muscles was used to record and assess the presence of leg movement activity. While such a montage is not standard, it did not hinder our ability to detect and characterize the presence and severity of leg movements. Second, fragmentation of sleep was only undertaken for two nights and thus inferences are limited regarding the impact of other chronic conditions that are associated with recurrent arousals (i.e., sleep apnea) on PLMS. Third, given the small sample size, the results are not generalizable and are prone to a type 2 error. Finally, we have only analyzed the effects of experimentally-induced arousals; thus, we cannot rule out that an increase in natural arousals in an individual (perhaps with aging) could actually lead to an increase in PLMS. These limitations notwithstanding, there are several strengths in the current study. Given that the effects of sleep fragmentation on PLMS were characterized in normal subjects, the effects of arousals on PLMS were assessed in the absence of numerous confounding conditions (e.g., sleep apnea, habitual sleep deprivation, and smoking). Moreover, the relatively high frequency of experimentally evoked arousals allowed for an assessment of whether frequent arousals can increase the propensity for PLMS.
6. Conclusion
In conclusion, the current study suggests that cortical arousals, autonomic activation, and PLMS, which often occur concurrently, are not related in a simple cause-and-effect sequence. Specifically, increasing the number of arousals does not increase the frequency of PLMS in normal young adults indicating that the pathophysiology of PLMS in various conditions is likely to be more complex than just a simple representation of an arousal response.
Acknowledgments
This study was supported by the National Institutes of Health Grant HL075078.
The work was performed at the Johns Hopkins University, School of Medicine, Baltimore, MD, USA, and at the Sleep Research Centre, Department of Neurology I.C., Oasi Institute (IRCCS), Troina, Italy.
Footnotes
Financial disclosures
Raffaele Ferri has consulted for Merck & Co., Sapio Life, and EB Neuro; for the remaining authors there are no financial interests that represent potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Pennestrì M-H, Whittom S, Benoit A, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: Prevalence and interval distribution. Sleep. 2006;29:1183–1187. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 3.Ferri R. The time structure of leg movement activity during sleep: The theory behind the practice. Sleep Med. 2012;13:433–441. doi: 10.1016/j.sleep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–448. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12:47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–766. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan BJ, Dempsey JA, Pegelow DF, Jacques A, Finn L, Palta M, et al. Blood pressure perturbations caused by subclinical sleep-disordered breathing. Sleep. 1998;21:737–746. doi: 10.1093/sleep/21.7.737. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 11.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000;111:1611–1619. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 12.Ferini-Strambi L, Bianchi A, Zucconi M, Oldani A, Castronovo V, Smirne S. The impact of cyclic alternating pattern on heart rate variability during sleep in healthy young adults. Clin Neurophysiol. 2000;111:99–101. doi: 10.1016/s1388-2457(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 13.Ferri R, Parrino L, Smerieri A, Terzano MG, Elia M, Musumeci SA, et al. Cyclic alternating pattern and spectral analysis of heart rate variability during normal sleep. J Sleep Res. 2000;9:13–18. doi: 10.1046/j.1365-2869.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Manconi M, Arico D, Sagrada C, Zucconi M, Bruni O, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33:793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manconi M, Ferri R, Zucconi M, Bassetti C, Fulda S, Arico D, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–844. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 16.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis K, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2009;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferri R, Drago V, Arico D, Bruni O, Remington RD, Stamatakis K, et al. The effects of experimental sleep fragmentation on cognitive processing. Sleep Med. 2010;11:378–385. doi: 10.1016/j.sleep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington Public Health service; US Government Printing Office; Washington: 1968. [Google Scholar]
- 20.Ferri R, Zucconi M, Manconi M, Bruni O, Miano S, Plazzi G, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 21.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–769. [PubMed] [Google Scholar]
- 22.Ferri R, Zucconi M. Heart rate and spectral EEG changes accompanying periodic and isolated leg movements during sleep. Sleep. 2008;31:16–17. doi: 10.1093/sleep/31.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie S, de Bilbao F, Haba-Rubio J, Ibanez V, Sforza E. Influence of sleep stage and wakefulness on spectral EEG activity and heart rate variations around periodic leg movements. Clin Neurophysiol. 2004;115:2236–2246. doi: 10.1016/j.clinph.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Ferri R. Two legs, one heart, one sleeping brain. Sleep Med. 2006;7:299–300. doi: 10.1016/j.sleep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–580. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 26.Yang CK, Jordan AS, White DP, Winkelman JW. Heart rate response to respiratory events with or without leg movements. Sleep. 2006;29:553–556. doi: 10.1093/sleep/29.4.553. [DOI] [PubMed] [Google Scholar]
- 27.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–791. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 28.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters AS, Rye DB. Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep. 2010;33:287. doi: 10.1093/sleep/33.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 31.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2. American Academy of Sleep Medicine; Westchester, Illinois: 2005. International Classification of Sleep Disorders. [Google Scholar]
- 33.Saletu M, Anderer P, Saletu B, Hauer C, Mandl M, Semler B, et al. Sleep laboratory studies in periodic limb movement disorder (PLMD) patients as compared with normals and acute effects of ropinirole. Hum Psychopharmacol. 2001;16:177–187. doi: 10.1002/hup.239. [DOI] [PubMed] [Google Scholar]
- 34.Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 35.Winkelman JW. Periodic limb movements in sleep--endophenotype for restless legs syndrome? N Engl J Med. 2007;357:703–705. doi: 10.1056/NEJMe078129. [DOI] [PubMed] [Google Scholar]
- 36.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 37.Birinyi PV, Allen RP, Hening W, Washburn T, Lesage S, Earley CJ. Undiagnosed individuals with first-degree relatives with restless legs syndrome have increased periodic limb movements. Sleep Med. 2006;7:480–485. doi: 10.1016/j.sleep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Trotti LM, Bhadriraju S, Rye DB. An update on the pathophysiology and genetics of restless legs syndrome. Curr Neurol Neurosci Rep. 2008;8:281–287. doi: 10.1007/s11910-008-0044-8. [DOI] [PubMed] [Google Scholar]


