Abstract
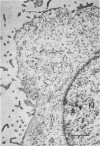
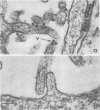
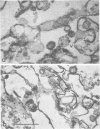
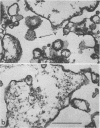
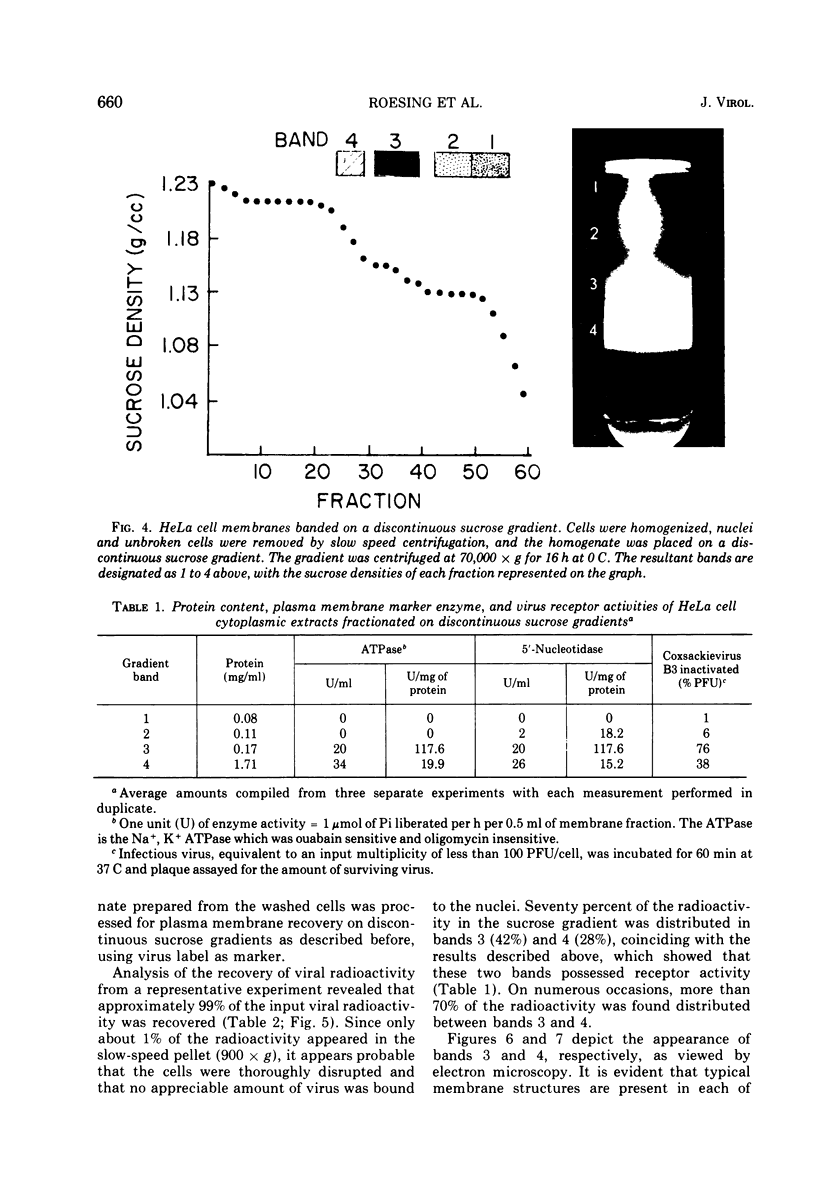
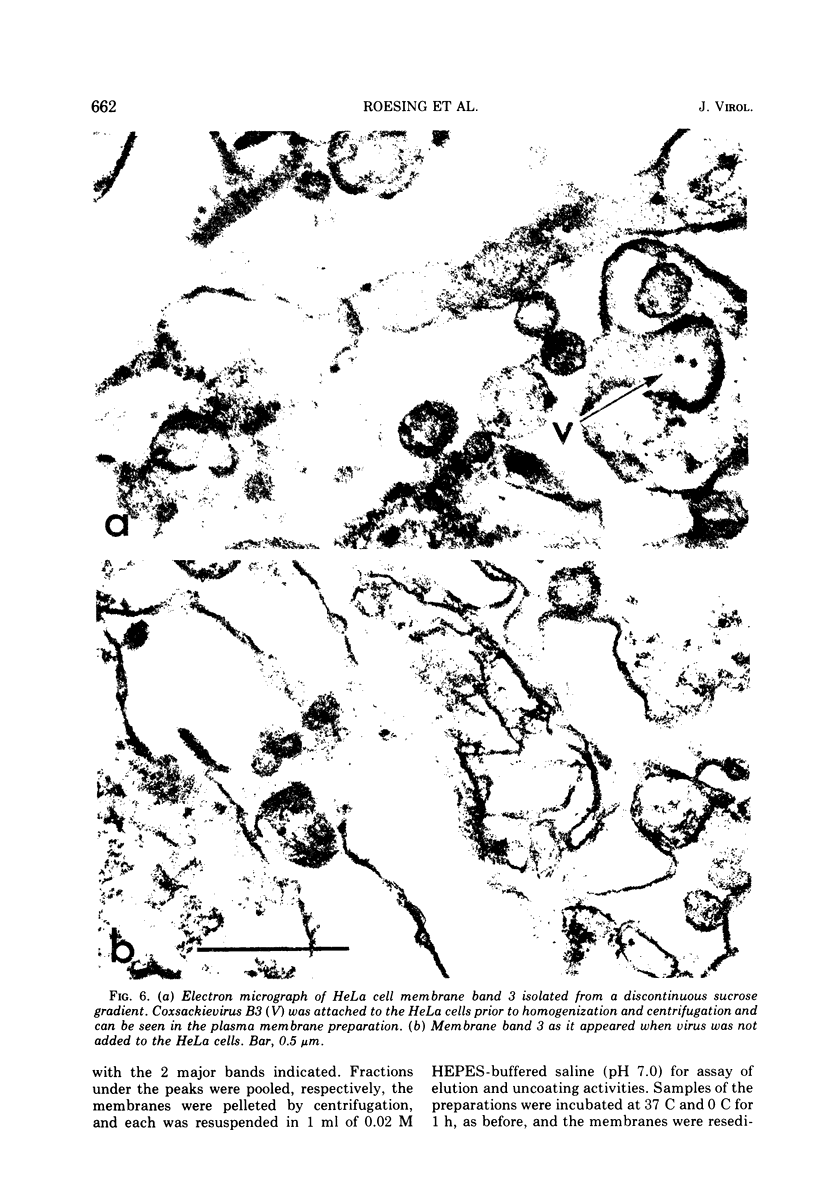
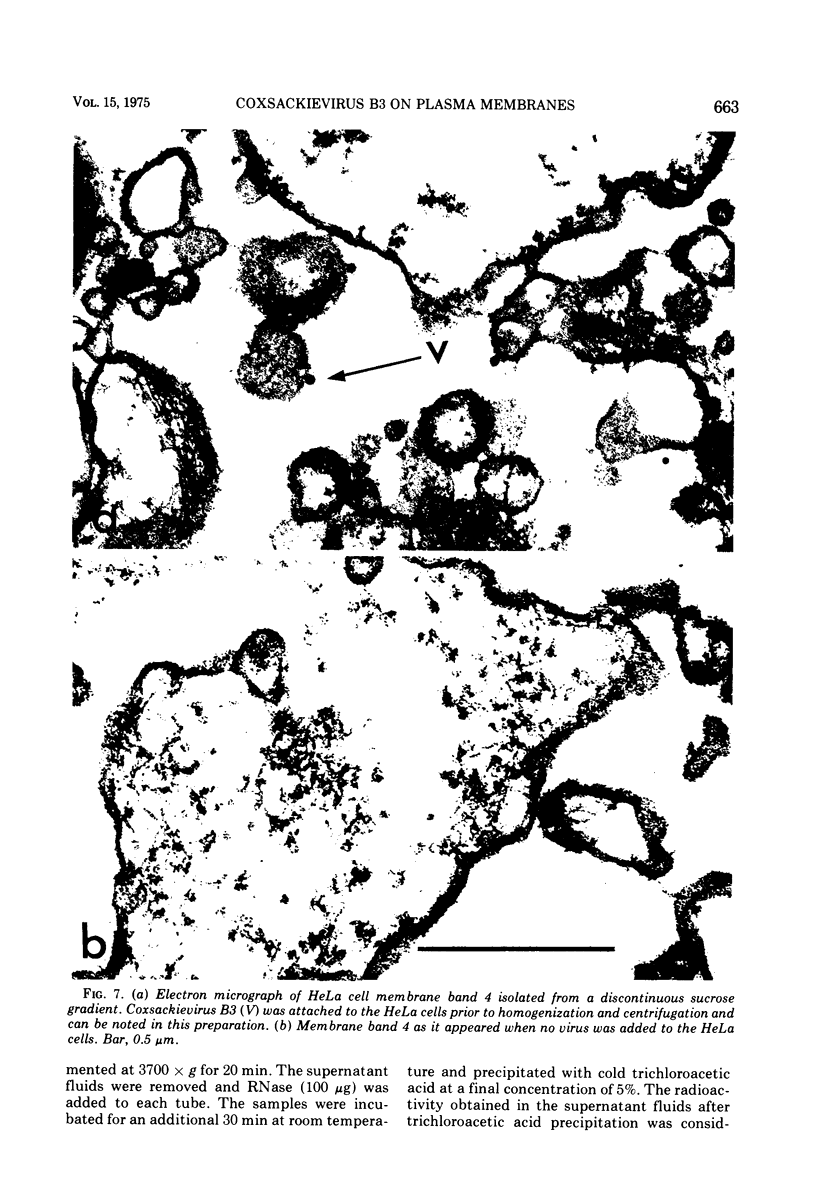
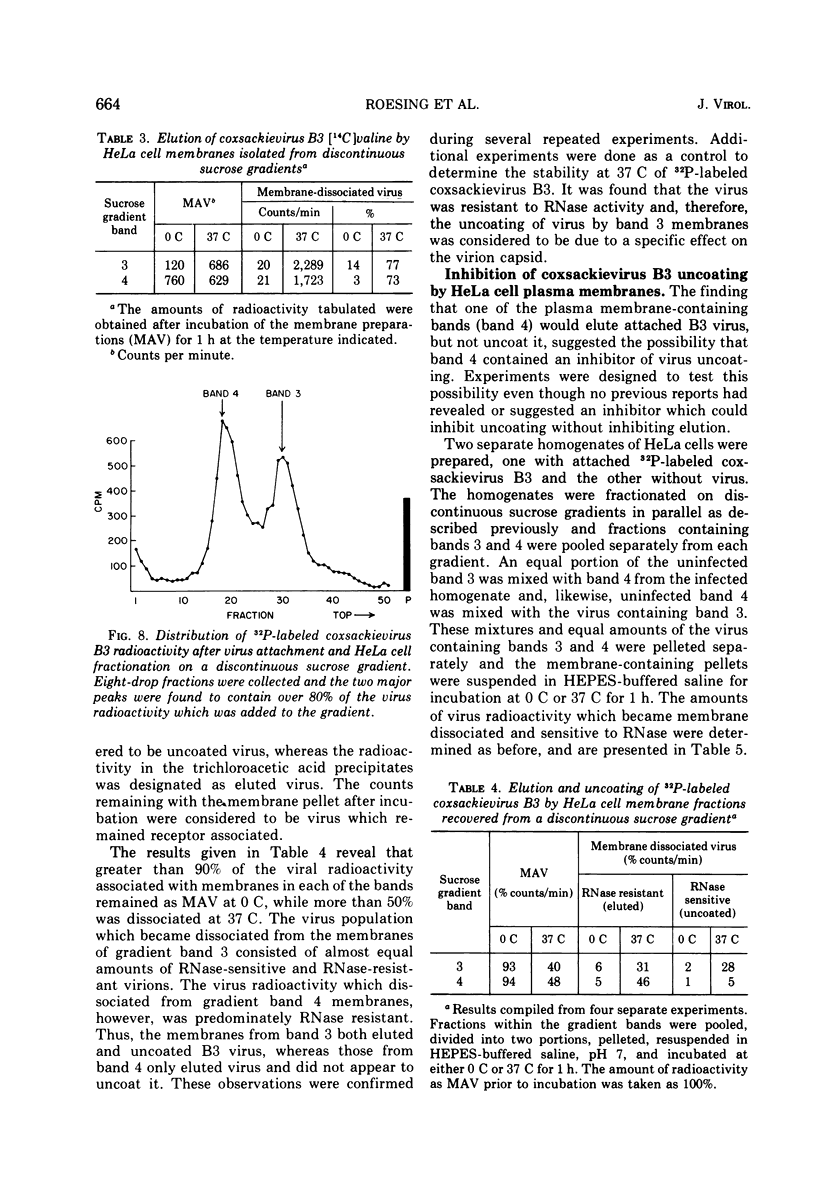
Plasma membranes isolated from HeLa cells on discontinuous sucrose gradients were assayed for their capacity to elute and uncoat coxsackievirus B3 at 37 C. Because the viral receptors are limited to the surface of HeLa cells, the addition of radioactively labeled virus to the cells prior to cell homogenization provided a useful marker for locating the plasma membranes during the fractionation procedure. Four bands were formed on the discontinuous sucrose gradients with approximately 70% or more of the membrane-associated viral label being recovered in the most dense bands, designated as bands 3 and 4. Bands 3 and 4 also possessed the plasma membrane marker enzymes, Na+, K+ adenosine triphosphatase and 5'-nucleotidase and revealed typical structures characteristic of plasma membranes as revealed by electron microscopy. Pelleted and washed membranes from band 3 both eluted and uncoated B3 32P-labeled virus, whereas membranes from band 4 eluted virus but failed to uncoat it. The membranes from band 4 were shown to inhibit the viral uncoating activity when mixed with membranes of band 3. Characteristically, unfractionated homogenates of cell membranes eluted but did not uncoat virus. The finding of a naturally occurring inhibitor of virus uncoating provides for the first time a way to distinguish between the membrane activities of virus elution and virus uncoating. The inhibitor remains to be characterized.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H. HeLa cell plasma membranes. Methods Cell Biol. 1973;7:157–188. doi: 10.1016/s0091-679x(08)61776-8. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. F., Black F. L. Uncoating of poliovirus by isolated plasma membranes. J Virol. 1970 Mar;5(3):309–312. doi: 10.1128/jvi.5.3.309-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L., Landau B. J., Philipson L. The early interaction of coxsackievirus B3 with HeLa cells. Proc Soc Exp Biol Med. 1971 Jul;137(3):1082–1088. doi: 10.3181/00379727-137-35732. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- FINCK H. Epoxy resins in electron microscopy. J Biophys Biochem Cytol. 1960 Feb;7:27–30. doi: 10.1083/jcb.7.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitt N. H., Crowell R. L. Comparative studies of the regeneration of HeLa cell receptors for poliovirus T1 and coxsackievirus B3. J Virol. 1967 Aug;1(4):693–700. doi: 10.1128/jvi.1.4.693-700.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL B. THE FATE OF THE INOCULUM IN HELA CELLS INFECTED WITH POLIOVIRUS. Virology. 1965 Jan;25:152–154. doi: 10.1016/0042-6822(65)90264-3. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L., BENGTSSON S., BRISHAMMAR S., SVENNERHOLM L., ZETTERQVIST O. PURIFICATION AND CHEMICAL ANALYSIS OF THE ERYTHROCYTE RECEPTOR FOR HEMAGGLUTINATING ENTEROVIRUSES. Virology. 1964 Apr;22:580–590. doi: 10.1016/0042-6822(64)90080-7. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L., LIND M. ENTEROVIRUS ECLIPSE IN A CELL-FREE SYSTEM. Virology. 1964 Jul;23:322–332. doi: 10.1016/0042-6822(64)90254-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Lin P. S. A critical evaluation of plasma membrane fractionation. Biochim Biophys Acta. 1973 Nov;300(3):211–254. doi: 10.1016/0304-4157(73)90005-1. [DOI] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. LOCATION AND REGENERATION OF ENTERIOVIRUS RECEPTORS OF HELA CELLS. J Bacteriol. 1965 Apr;89:1097–1100. doi: 10.1128/jb.89.4.1097-1100.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac I., Crowell R. L. Differential inhibition of attachment and eclipse activities of HeLa cells for enteroviruses. J Virol. 1969 Apr;3(4):422–428. doi: 10.1128/jvi.3.4.422-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]