Abstract
Most studies of the mouse hindlimb locomotor network have used neonatal (P0–5) mice. In this study, we examine the postnatal development of intrinsic properties and serotonergic modulation of intersegmental commissural interneurons (CINs) from the neonatal period (P0–3) to the time the animals bear weight (P8–10) and begin to show adult walking (P14–16). CINs show an increase in excitability with age, associated with a decrease in action potential halfwidth and appearance of a fast component to the afterhyperpolarization at P14–16. Serotonin (5-HT) depolarizes and increases the excitability of most CINs at all ages. The major developmental difference is that serotonin can induce plateau potential capability in P14–16 CINs, but not at younger ages. These plateau potentials are abolished by nifedipine, suggesting that they are mediated by an L-type calcium current, ICa(L). Voltage-clamp analysis demonstrates that 5-HT increases a nifedipine-sensitive voltage-activated calcium current, ICa(V), in P14–16 CINs but does not increase ICa(V) in P8–10 CINs. These results, together with earlier work on 5-HT effects on neonatal CINs, suggest that 5-HT increases the excitability of CINs at all ages studied, but by opposite effects on calcium currents, decreasing N- and P/Q-type calcium currents and, indirectly, calcium-activated potassium current, at P0–3 but increasing ICa(L) at P14–16.
Keywords: modulation, development, central pattern generator, bistability, calcium current
most research on the mouse hindlimb locomotor network has used spinal cords from postnatal day 0–5 (P0–5) mice, which cannot walk. This work is motivated by the assumption that the locomotor central pattern generator (CPG) is reasonably well developed at birth, so neonatal studies can help us understand the adult locomotor network. We address this assumption by studying changes in the properties and neuromodulation of CPG interneurons during the time that mice begin to bear weight (P8–10) and develop mature locomotion (P14–16) (Clarac et al. 1998).
Neonatal spinal cords are more resistant to hypoxia, improving the viability of neurons in slices and enabling fictive locomotion in the isolated cord (Wilson et al. 2003). However, evidence suggests that there are important maturation events occurring postnatally. Ion channel and receptor expression varies during the first postnatal week (Jakowec et al. 1995; Stegenga and Kalb 2001). Expression of two L-type calcium channel transcripts begins at P7–10 (Jiang et al. 1999). Knowing this, how is the locomotor CPG changing as the mouse develops toward mature locomotion?
To understand how locomotion is generated, we must understand the intrinsic properties and modulation of CPG component neurons (Marder and Bucher 2001). The locomotor CPG is located primarily within the lumbar spinal cord, but descending modulatory input, particularly serotonergic (5-HT) input, is critically important for its function (Kjaerulff and Kiehn 1996; Schmidt and Jordan 2000). Serotonergic neurons projecting to the spinal cord increase firing during locomotion (Fornal et al. 1985). Serotonergic antagonists block brain stem stimulation-induced and NMDA-induced fictive locomotion (Liu and Jordan 2005; MacLean et al. 1998). Descending 5-HT inputs reach the lumbar spinal cord by birth in the mouse but continue to mature over several weeks (Ballion et al. 2002). Thus it is important to determine whether the effects of 5-HT on CPG neurons change during postnatal development.
In this article we focus on developmental maturation of the intrinsic properties and serotonin neuromodulation of known components of the hindlimb CPG, the ascending and descending intersegmental commissural interneurons (CINs). CINs are rhythmically active during fictive locomotion and provide excitatory and inhibitory input to motor neurons and interneurons in the opposite hemicord (Birinyi et al. 2003; Butt et al. 2002; Butt and Kiehn 2003; Quinlan and Kiehn 2007; Zhong et al. 2006a, 2006b). Genetic deletion of a subset of CINs results in poor left-right alternation (Lanuza et al. 2004). At P0–3, 5-HT excites intersegmental CINs (Carlin et al. 2006; Zhong et al. 2006a, 2006b), in part by reducing N- and P/Q-type calcium currents and indirectly reducing calcium-activated potassium currents (Abbinanti and Harris-Warrick 2012; Diaz-Rios et al. 2007).
We show how the intrinsic properties and serotonergic modulation of CINs change during postnatal development. We examine three ages, P0–3 (neonates, unable to walk or bear weight; Zhong et al. 2006a, 2006b), P8–10 (beginning to bear weight), and P14–16 (emergence of mature walking). We find that the spike properties and excitability change over postnatal time. Most importantly, although 5-HT increases the excitability of CINs at all ages, it has opposing effects on different calcium currents at different ages and evokes bistability only in P14–16 CINs.
MATERIALS AND METHODS
Slice preparation.
Experiments were performed on P0–3, P8–10, and P14–16 C57BL/6 mice. The animal protocol was approved by the Cornell University Institutional Animal Care and Use Committee and is in accordance with National Institutes of Health guidelines. In accordance with our animal protocol, P1–3 and P8–10 mice were killed by rapid decapitation, whereas P14–16 mice were anesthetized with 100% CO2 before decapitation. The spinal cord was dissected out of the animal by vertebrectomy (also called ventral laminectomy) under ice-cold (4°C), oxygenated (95% O2-5% CO2), sucrose-based low-calcium artificial cerebrospinal fluid (aCSF; in mM: 188 sucrose, 25 d-glucose, 26 NaHCO3, 25 NaCl, 10 MgSO4, 1.2 NaH2PO4, and 1.9 KCl, pH = 7.4). The isolated spinal cord was pinned ventral side up, and CINs in the lumbar 2 (L2) spinal segment were fluorescently retrograde labeled by making small slits in the contralateral hemicord rostral and caudal to the L2 region and placing crystals of fluorescent dextran amines in the slits (3,000 MW Texas red dextran amine rostral to L2 and 3,000 MW fluorescein dextran amine caudal to L2; Invitrogen, Carlsbad, CA) (Zhong et al. 2006a, 2006b). Differentially labeling the neurons sending axons rostrally or caudally by more than one segment from L2 assured that only intersegmental CINs were labeled.
Preparations were incubated in ice-cold sucrose-based aCSF for 20–45 min to allow the dyes to diffuse to the contralateral cell bodies. Transverse spinal cord slices (250 μm) were made with a vibrating microtome (Leica Microsystems, Wetzlar, Germany). Slices were placed in 30°C normal mouse aCSF (in mM: 111 NaCl, 3 KCl, 25 NaHCO3, 1.2 KH2PO4, 1.25 MgSO4, 2.5 CaCl2, and 11 d-glucose, pH = 7.4) and allowed to come to room temperature (∼20–22°C) over 1 h before use.
Drugs.
The AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX), glycine antagonist strychnine, and sodium channel antagonist tetrodotoxin (TTX) were purchased from Tocris Bioscience (Ellisville, MO). The following drugs were purchased from Sigma (St. Louis, MO): NMDA and AMPA/kainate antagonist kynurenic acid, NMDA receptor antagonist d-(-)-2-amino-5-phosphopentanoic acid (APV), GABA antagonist picrotoxin, transient potassium current antagonist 4-aminopyridine (4-AP), potassium channel antagonist tetraethylammonium chloride (TEA-Cl), L-type calcium channel antagonist nifedipine, and serotonin creatinine sulfate (5-HT).
Whole cell current-clamp recordings.
Slices were transferred to the recording dish on the stage of a Zeiss Axioskop 2 FS Plus upright microscope and perfused with oxygenated normal aCSF at 3 ml/min at room temperature (21–22°C). CINs were identified as Texas red- or fluorescein-labeled neurons contralateral to the dye application sites under epifluorescent illumination and visualized using differential interference contrast (DIC) optics. Only single-labeled cells, representing either ascending (aCINs) or descending neurons (dCINs), were recorded. Double-labeled, bifurcating adCINs (ascending and descending) were eliminated from our study. Electrodes were pulled from thick-wall borosilicate glass (WPI, Sarasota, FL) on a Narishige vertical puller (Narishige International USA, East Meadow, NY) and had resistances of 5–8 MΩ. The pipette solution contained (in mM) 128 K-gluconate, 10 HEPES, 5 ATP-Mg, 0.3 GTP-Li, and 0.0001 CaCl2 (pH = 7.4 with KOH). A minimum 1-GΩ seal resistance was obtained before all recordings. The extracellular recording solution designed to block fast synaptic transmission contained, in addition to the normal aCSF described above, (in μM) 20 APV, 9 strychnine, 30 CNQX, and 10 picrotoxin. Kynurenic acid (1 mM) was substituted for APV and CNQX in some experiments. Recordings were made with a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA) and Digidata 1322A digitizer (Axon Instruments) using Clampex (from pClamp 9; Axon Instruments). Data were filtered at 2 kHz and digitized at 5 or 10 kHz.
Whole cell voltage-clamp recordings.
Procedures for voltage-clamp experiments were similar to those described above except for the following. Slices were incubated in at room temperature (21–22°C) in a solution of (in mM) 111 NaCl, 3.1 KCl, 25 NaHCO3, 1.2 KH2PO4, 1 MgCl2, 2.5 CaCl2, and 11 d-glucose (pH = 7.4). To isolate calcium currents, the voltage-clamp experiment intracellular solution contained (in mM) 100 CsCl, 30 TEA-Cl, 1 MgCl2, 10 EGTA, 5 NaCl, 1 CaCl2, 10 HEPES, 3 ATP-Mg, 0.5 GTP-Li, and 10 sucrose (pH = 7.3). For voltage-clamp experiments, a sulfate-free extracellular solution was used which contained (in mM) 111 NaCl, 3.1 KCl, 25 NaHCO3, 1.2 KH2PO4, 1 MgCl2, 2.5 CaCl2, 7 d-glucose, 10 TEA-Cl, 2 4-AP, 2 CsCl, and 0.001 TTX (pH = 7.4). In some experiments, BaCl2 was substituted for CaCl2. To block any spontaneous synaptic events, the same concentrations of fast synaptic transmission blockers described above were included in the recording solutions. Patch electrodes had resistances of 3–5 MΩ. Series resistance and capacitance were compensated ≥70%. Leak currents were corrected either online using P/4 leak subtraction or offline by subtraction of a scaled current determined by the average response to −10-mV hyperpolarizing pulses from −60 mV.
Data analysis.
Ascending and descending CINs were held at −60 mV with bias current to assure uniformity in current-clamp measurements. Only cells with action potentials (APs) overshooting 0 mV were included. We measured the threshold for AP generation as the peak of the second derivative of voltage vs. time during the rising phase of the AP. The width of the AP halfway between the threshold and the peak was measured as the half-width of the AP. The amplitude of the spike afterhyperpolarization (AHP) was measured relative to 0 mV. The time between the peak of the AP and the AHP minimum was defined as the AHP time. In many P14–16 CINs, there were two local minima during the AHP; these are reported as the fast and slow components of the AHP. We measured the input resistance by application of small hyperpolarizing current pulses from −60 mV.
To determine the frequency-current (f-I) relationship, 1- to 2-s depolarizing current steps of increasing amplitude were delivered and the average spike frequency during the first 1,000 ms of the step was determined and plotted against the amplitude of the injected current. The slope of the plot was linearly fitted. We determined the “average f-I value” by calculating the ratio of the firing frequency during the first 1,000 ms of each step divided by the amplitude of the injected current for that step; we then averaged this ratio over all the steps for each f-I run. The average integral of poststep afterpotentials was determined by averaging the area under the membrane potential and above the baseline voltage over 4 s after the termination of the current step. We averaged this value for all the steps of an f-I run.
Peak currents in response to voltage steps in voltage clamp were fitted to the Boltzmann equation in Clampfit (Clampex) using the equation
where Imax is the maximal current, Vmid is the voltage of half-activation, and Vc is the slope of the curve.
Data are means ± SD. One-way analysis of variance and applicable posttests were used to compare data between the three age groups and when more than two conditions were tested. The appropriate Student's t-tests were used to compare two treatments within age groups. Chi-square tests were used to compare the proportions of cells displaying a given intrinsic property where appropriate to analyze statistical significance. An analysis of covariance (ANCOVA) was used to analyze the statistical significance of changes in the f-I relationships between ages and treatments.
RESULTS
Baseline electrophysiological properties of CINs.
Our labeling method allowed us to differentiate aCINs and dCINs from adCINs by their pattern of fluorescent labeling. Because adCINs are not rhythmically active during fictive locomotion and do not respond to 5-HT at P0–3 (Zhong et al. 2006b), we eliminated this class of neurons from our analysis. The remaining aCINs and dCINs showed similar properties and responses to 5-HT at P8–10 and P14–16, and their data were combined for this study. Our first goal was to determine the postnatal development of the electrophysiological and firing properties of CINs. Our hypothesis was that the intrinsic properties of the CINs are immature at P0–3 and that their properties change with time. Using whole cell patch-clamp recordings after blocking fast synaptic inputs, we measured AP and passive electrical properties of CINs from three age groups, P0–3, P8–10, and P14–16 (Table 1, Fig. 1; Zhong et al. 2006a, 2006b). At P0–3, only 20% of synaptically isolated CINs were spontaneously active without the addition of bias current. However, by P8–10 and P14–16, most CINs (67% and 63%, respectively) were spontaneously active, firing on average at 5 and 7 Hz, respectively. A significantly greater proportion of CINs were active at later ages than at P0–3 [C2(2, n = 104) = 21.75, P < 0.001]. The resting potential of silent cells and the input resistance of all cells were not significantly different between age groups. There was a significant decrease in the membrane capacitance of CINs between P0–3 and P8–10 (F2,98 = 5.599, P < 0.01; Table 1), which remained unchanged between P8–10 and P14–16.
Table 1.
Properties of CINs from different postnatal age groups
| P0–3 | P8–10 | P14–16 | |
|---|---|---|---|
| Spontaneous firing at zero current, Hz | 3.0 ± 0.6 (11/56) | 4.5 ± 4.7 (14/21) | 6.7 ± 7.0 (17/27) |
| Rest potential of silent cells, mV | −60.0 ± 4.3 (45/56) | −56.1 ± 6.0 (7/21) | −56.9 ± 6.3 (10/27) |
| Input resistance, MΩ | 618.4 ± 250.9 (31) | 480 ± 282 (25) | 630 ± 333 (19) |
| Mean capacitance, pF | 67.0 ± 13.2 (56) | 51.9 ± 34.8* (22) | 51.7 ± 26.5* (19) |
| AP halfwidth, ms | 3.0 ± 0.5 (57) | 1.6 ± 0.2* (25) | 1.2 ± 0.4*† (19) |
| AP threshold, mV | −39.7 ± 6.6 (57) | −35.6 ± 5.6* (25) | −37.7 ± 5.1 (19) |
| AP amplitude, mV | 62.3 ± 7.8 (57) | 80.4 ± 9.8* (25) | 65.0 ± 10.0† (19) |
| Minimum AHP voltage, mV | −56.6 ± 5.8 (57) | −58.5 ± 6.4 (25) | −56.8 ± 5.7 (19) |
| AHP time (slow), ms | 25.3 ± 7.5 (31) | 34.7 ± 8.4* (25) | 35.3 ± 12.0* (16) |
| AHP time (fast), ms | 3.2 ± 1.4 (8) |
Values are means ± SD in different postnatal (P) age groups; numbers in parentheses indicate no. of commissural interneuons (CINs). AP, action potential; AHP, afterhyperpolarization.
P < 0.05, significantly different from P0–P3.
P < 0.05, significantly different from P8–P10.
Fig. 1.
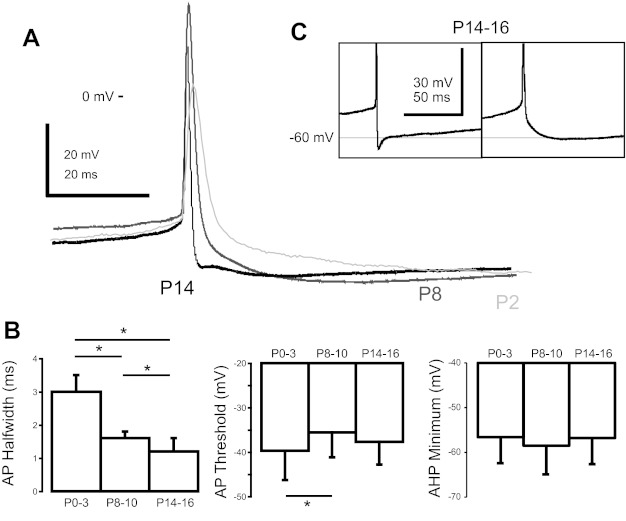
Commisural interneuron (CIN) action potential (AP) properties change during postnatal development. A: representative traces of APs from postnatal day 2 (P2; light gray), P8 (dark gray), and P14 (black) CINs. B: average measurements of AP halfwidth, threshold, and afterhyperpolarization (AHP) minimum for P0–3, P8–10, and P14–16 CINs. *P < 0.05. C: expanded view of the AHP of 2 different P14–16 CINs demonstrating a fast and slow AHP.
The properties of the APs in these neurons changed during postnatal development (Fig. 1, Table 1). The recordings for our measurements of AP properties were taken during tonic injection of bias current to just below threshold, where APs occurred spontaneously (<2 Hz). CIN APs narrowed during the first two postnatal weeks; by P14–16, their halfwidth was only one-half that of neonatal APs (F2,98 = 173.44, P < 0.001; Fig. 1B). Action potential amplitude, defined as the membrane potential difference between the peak of the AP and the trough of the AHP, increased by 33% from P0–3 to P8–10, but decreased by 19% between P8–10 and P14–16 (F2,98 = 37.94, P < 0.001; Fig. 1A). The threshold for AP generation was slightly depolarized at P8–10 compared with P0–3 (F2,98 = 4.027, P < 0.05). The P14–16 AP threshold, although also slightly depolarized, was not significantly different from the other age groups (Fig. 1B).
At all ages, CINs had a significant AHP following the AP. The minimum AHP voltage following a spike was not significantly different between any ages studied (Fig. 1B). We measured the AHP onset time as the time from spike peak to minimum voltage during the AHP. P0–3 and P8–10 CINs had a single-component slow AHP, whereas P14–16 CINs showed heterogeneity in AHP components, with 42% of cells showing both a slow and a fast component of the AHP (Fig. 1C). The slow component of the AHP was significantly slower in P8–10 and P14–16 CINs compared with P0–3 CINs [P0–3: onset time 25.3 ± 7.5 ms (n = 31); P8–10: 34.7 ± 8.4 ms (n = 25); P14–16: 35.3 ± 12.0 ms (n = 16); F2,69 = 10.24, P < 0.001]. In those P14–16 CINs that had a fast component of the AHP, its average time to peak was 3.2 ± 1.4 ms (Fig. 1C). We also examined the AHP halfwidth as a measure of AHP duration and found no difference between age groups (P > 0.5). Thus, by P14–16, a significant number of the CINs displayed APs shaped by the interaction of a fast and slow AHP component, mediated by currents with different activation and deactivation rates; this was not seen at earlier ages.
Baseline excitability of CINs.
We next examined the baseline excitability of CINs by holding the membrane potential at −60 mV and applying increasing current steps, and then plotting the average firing frequency during the step vs. injected current. These f-I plots illustrate how a given neuron responds to current and are useful for comparing the CIN excitability at different ages or in response to a neuromodulator such as serotonin. Zhong et al. (2006a, 2006b) found that the large majority of P0–3 ascending and descending CINs fire tonically in response to current injection. All P8–10 and P14–16 CINs fired tonically in response to a sufficiently large current pulse.
We developed a measure to compare f-I plots between the three CIN ages by averaging the instantaneous slope for each point of the f-I plots. The ratio of the average AP frequency to the injected current was calculated for each step and averaged over the current range to yield an average f-I value. An increase in the average f-I value quantitatively demonstrates an “upward shift” of the f-I plot, even when there is no change in the slope of the f-I relation (Diaz-Rios et al. 2007; Zhong et al. 2006a, 2006b). The average f-I value between P0–3 and P8–10 CINs did not change significantly, but there was a significant increase between P8–10 and P14–16 (P0–3: 0.13 ± 0.03 Hz/pA; P8–10: 0.18 ± 0.11 Hz/pA; P14–16: 0.31 ± 0.14 Hz/pA; F2,50 = 13.27, P < 0.001; Fig. 2C). The slopes of the f-I plots increased from P0–3 to P8–10, as well as from P8–10 to P14–16 (P0–3: 0.13 ± 0.02 Hz/pA; P8–10: 0.22 ± 0.09 Hz/pA; P14–16: 0.34 ± 0.17 Hz/pA; F2,50 =15.81, P < 0.001; Fig. 2F). The minimal measured current to evoke firing from −60 mV was not different between the three ages (P0–3: 38 ± 23 pA; P8–10: 29 ± 27 pA; P14–16: 28 ± 25 pA). These data demonstrate that CINs become more excitable with age and that the increase in firing with larger depolarizations becomes greater with age.
Fig. 2.
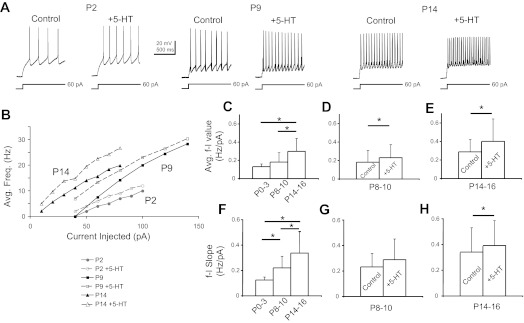
Effects of 5-HT on excitability of P0–3, P8–10, and P14–16 CINs. A: representative responses to 60-pA depolarizing current injections in P2, P9, and P14 CINs before and during application of 9 μM 5-HT. B: representative frequency-current (f-I) plots from individual P2, P9, and P14 CINs before and during 5-HT application. Average f-I values are shown for P0–3, P8–10, and P14–16 CINs (C), P8–10 CINs before and during 5-HT (D), and P14–16 CINs before and during 5-HT (E). f-I slope values are shown for P0–3, P8–10, and P14–16 CINs (F), P8–10 CINs before and during 5-HT (G), and P14–16 CINs before and during 5-HT (H). *P < 0.05.
Postnatal changes in effects of serotonin on CIN firing properties.
Serotonin (5-HT) plays a critical role in enabling rhythmic output from the mouse spinal locomotor network (Liu and Jordan 2005; MacLean et al. 1998; Nishimaru et al. 2,000), so we studied the postnatal maturation of CIN responses to 5-HT. As previously noted by Diaz-Rios et al. (2007), at all ages tested, we found that 5-HT's effects are difficult to wash out after longer application times. Even with application times as short as 5 min, it often took more than 45 min to see an appreciable reversal of its effects.
We found that 9 μM 5-HT depolarized and increased the excitability of neonatal (P0–3) CINs, as we reported earlier (Zhong et al. 2006a, 2006b). CINs were depolarized by an average of 9.2 ± 2.5 mV, and most silent cells began to fire APs. Serotonin also depolarized P8–10 and P14–16 CINs. In all silent P8-P10 CINs and most silent P14–16 CINs, the serotonin-evoked depolarization was strong enough for the neuron to begin to fire (7/7 silent P8–10, 4/5 silent P14–16 CINs). We quantified this depolarization by determining the bias current needed to hold the cell at −60 mV before, during, and after the application of 5-HT. At P8–10, the bias current at −60 mV increased from −33 ± 25 to −60 ± 41 pA in 9 μM 5-HT (P < 0.005, n = 15). The average firing frequency of tonically firing P8–10 CINs with no bias current increased from 6.9 ± 4.8 Hz in control to 11.7 ± 6.3 Hz during 5-HT application (P < 0.005, n = 11). The P8–10 CINs that were silent under control conditions began to fire during 5-HT application, with an average frequency of 6.8 ± 5.7 Hz. Serotonin also increased neuronal excitability of P14–16 CINs. Application of 9 μM 5-HT depolarized the older neurons, as indicated by an increased bias current at −60 mV (−31 ± 22 pA in control to −51 ± 28 pA during 5-HT; P < 0.001, n = 17). The average frequency of tonically firing P14–16 CINs with no bias current increased from 10.0 ± 6.3 to 16.8 ± 7.8 Hz (P < 0.001, n = 14). There was no significant difference in the increase of firing frequency by 5-HT between the P8–10 and P14–16 CINs (F1,32 = 1.891, P = 0.179).
Serotonin increased the excitability of a majority of CINs at all ages, as measured by the f-I relation (Fig. 2, A and B). At P0–3, serotonin shifted the f-I plot of both aCINs and dCINs in the hyperpolarizing direction, without changing the slope (Zhong et al. 2006a, 2006b). Using our method of averaging the f-I plots discussed above, 9 μM 5-HT increased the average f-I value from 0.13 ± 0.03 to 0.17 ± 0.04 Hz/pA (n = 21). In the P8–10 CINs that depolarized in response to 5-HT, we observed a 45% decrease in the minimum current step to evoke spiking from −60 mV (32 ± 34 vs. 18 ± 18 pA in 5-HT, P < 0.05, n = 11). Serotonin caused a hyperpolarizing shift in the f-I relation in 11/15 P8–10 CINs, with no significant change in the average slope (Fig. 2, B and G); this resulted in an increased average f-I value from 0.18 ± 0.13 to 0.23 ± 0.14 Hz/pA (P < 0.03, n = 11; Fig. 2D). Although there was no repeatable change in the average slope of the f-I relationship at this age, ANCOVA revealed that 5 of the 11 cells in which a hyperpolarizing shift was observed had a significant change in the slope of the regressions, precluding further analysis of these cells by ANCOVA. Of the remaining 6 cells that responded to 5-HT, 5 showed a significant (P < 0.05) hyperpolarizing shift in the f-I relationship with an average increase in the y-intercept of 2.6 ± 1.5 Hz during 5-HT. This increase in the y-intercept represents an average increase in firing rate of 2.6 ± 1.5 Hz for a given current step during 5-HT.
Serotonin also excited the P14–16 CINs: 9 μM 5-HT decreased the minimum current step to evoke spiking from −60 mV by 39% in the 15 CINs excited by 5-HT (28 ± 29 pA in control vs. 17 ± 15 pA in 5-HT, P < 0.05; n = 15). In 15/17 P14–16 CINs, 5-HT shifted the f-I plots in the hyperpolarizing direction and increased the average f-I value from 0.29 ± 0.13 Hz/pA in control to 0.40 ± 0.24 Hz/pA (P < 0.02, n = 15; Fig. 2E). In contrast to the younger CINs, in P14–16 CINs excited by 5-HT, the average slopes of the f-I plots also increased slightly but significantly during 5-HT application (0.34 ± 0.18 Hz/pA in control vs. 0.39 ± 0.19 Hz/pA during 5-HT, P < 0.005, n = 15; Fig. 2H). These results were supported by ANCOVA, which demonstrated that 8 of the 15 P14–16 CINs that responded to serotonin had a significant increase in slope; the remaining 7 demonstrated a significant (P < 0.05) increase in the y-intercept of their regressions (4.3 ± 3.3 Hz), again showing the increased excitability of the CINs during 5-HT. In summary, 5-HT increases CIN excitability at all ages studied.
Serotonin affects AP shape at all ages.
Serotonin also affected the AP shape at older ages (Fig. 3, A and B). In P0-P3 CINs (Zhong et al. 2006a, 2006b), 5-HT increased the AP halfwidth by 16% in aCINs (control: 33 ± 10 ms, 5-HT: 36 ± 10 ms) but not in dCINs, reduced the AHP amplitude by 14% and 20% in aCINs and dCINs, respectively (aCIN control: 18.3 ± 5.5 mV; 5-HT: 15.7 ± 4.7 mV; dCIN control: 16.1 ± 3.7 mV; 5-HT: 12.7 ± 3.1 mV), and hyperpolarized the AP threshold by 5% and 8% in aCINs and dCINs, respectively (aCIN control: −30.5 ± 7.5 mV; 5-HT: −32.1 ± 8.3 mV; dCIN control: −38.2 ± 4.3 mV; 5-HT: −41.2 ± 4.0 mV; Zhong et al. 2006a, 2006b). At P8–10 and P14–16, both aCINs and dCINs responded similarly to 5-HT, with no significant differences between the groups; as a result we combined their responses. At P8–10, 9 μM 5-HT reversibly increased the AP by 24% (1.6 ± 0.3 to 2.0 ± 0.4 ms, P < 0.005, n = 12; Fig. 3C). The AHP minimum voltage was depolarized by 3.3 mV (−58.6 ± 4 to −55.3 ± 5 mV, P < 0.01, n = 12; Fig. 3D). Interestingly, in contrast to the younger P0–3 neurons, the AP threshold at P8–10 was not affected by 5-HT application (−35.5 ± 5.0 vs. −35.1 ± 5.6 mV; Fig. 3E). At P8–10, a significant rundown of the AP parameters such as amplitude, halfwidth, threshold, and AHP minimum voltage during prolonged recordings made it difficult to obtain significant washout of 5-HT's effects. To address this problem, we performed a series of experiments on P8–10 CINs in which AP properties were recorded every 30–60 s for the duration of the experiment. By using this method, the time course of 5-HT's effects could be observed, superimposed on the rundown observed before and after 5-HT application. We observed an effect of 5-HT on both AP halfwidth and AHP minimum in most of these experiments (n = 4/5 for both), whereas we observed no effect on other parameters measured (AP amplitude, threshold, and AHP time). These experiments confirmed our earlier results and showed that the 5-HT effect was not simply an artifact of rundown.
Fig. 3.
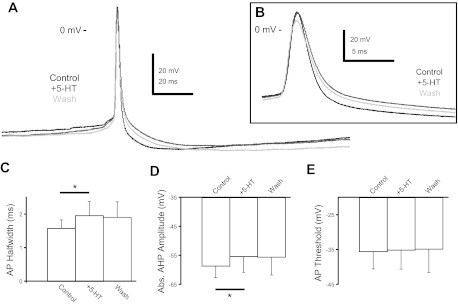
5-HT modulates P8–10 AP shape. A: representative traces of P8–10 AP shape in control (black), 9 μM 5-HT (dark gray), and washout (light gray). B: a slower time course view of the same APs to demonstrate 5-HT's increase of AP halfwidth. C: 5-HT increased the AP halfwidth in P8–10 CINs. D: 5-HT decreased the absolute (Abs.) AHP amplitude in P8–10 CINs. E: 5-HT had no effect on the AP threshold of P8–10 CINs (*P < 0.05).
Serotonin's effects on the AP shape of P14–16 CINs were in general similar to thonse on both younger groups of CINs, although the AP properties showed more rapid rundown at this age. For example, 5-HT reversibly reduced the AHP amplitude from −57.1 ± 4.9 to −52.3 ± 5.4 mV (P < 0.02). These results suggest that 5-HT has similar effects to depolarize CINs, change the AP shape, and enhance the response to depolarizing inputs during the first two postnatal weeks.
Serotonin evokes plateau potentials in P14–16 CINs.
One important developmental difference that arose from our studies was the emergence of bistability during 5-HT application in older CINs. Bistability and plateau potentials allow a neuron to respond to a brief input with a shift from a silent to a stable depolarized and active firing state. We found that 5-HT can induce plateau potential capability in synaptically isolated P14–16 CINs, but not in P0–3 or P8–10 CINs (Fig. 4, A and B). We defined plateau potential capability as prolonged firing or a prolonged afterdepolarization (ADP) following the termination of a 500- to 2,000-ms current step; prolonged ADPs have been observed as a subthreshold poststimulus response in other bistable cells (Bennett et al. 2001; Hounsgaard and Kiehn 1989). This phenomenon was never observed in P8–10 or P0–3 CINs (Fig. 4B; n = 21 P0–3 CINs, n = 15 P8–10 CINs). To quantify plateau capability in P14–16 CINs, we integrated the area under the membrane potential for 4 s beginning at the end of the current step during f-I plots and averaged across all steps given. Fifteen of 27 P14–16 CINs showed prolonged excitability after a current step in the presence of 9 μM 5-HT. For these 15 neurons, the average integrated poststep depolarization increased nearly fivefold in response to 5-HT (2.4 ± 12.2 V·s in control vs. 11.7 ± 13.1 V·s in 5-HT, P < 0.03, n = 15; Fig. 4C). Eight of those 15 CINs demonstrated full bistability, defined as continued spiking after the termination of the injected current step (Fig. 4B). Six of these 8 neurons showed no bistable properties under control conditions and developed bistability only during the application of 5-HT (Fig. 4, A and B). The other two neurons demonstrated a distinct ADP under control conditions, which was further enhanced by 5-HT in one neuron. The remaining 7 of the 15 CINs showed prolonged ADPs after termination of the current step but did not fire APs during the ADP (Fig. 4D). Of these seven, three CINs had an ADP under control conditions which grew larger during 5-HT, whereas the other four developed an ADP only in the presence of 5-HT. These ADPs were never observed in the younger age groups studied (for example, Fig. 4B, left panels).
Fig. 4.
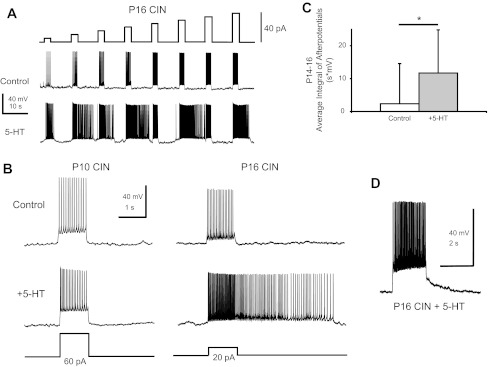
5-HT imparts plateau potential capability in P14–16 CINs. A: increasing steps of current injected into a P16 CIN under control conditions and during 9 μM 5-HT. Note that during 5-HT, firing continued after the termination of the current pulse at a number of steps. B: an example of a P10 (left) and P16 (right) CIN firing in response to an injected current step in control conditions as well as during 9 μM 5-HT. Only P14–16 CINs demonstrated plateau-potential capability. C: average integral of poststep afterpotentials in P14–16 CINs was increased by 5-HT (*P < 0.05). D: a P16 CIN that demonstrated an afterdepolarization (ADP) in 5-HT.
Nifedipine-sensitive current underlies serotonin-evoked plateau potentials in P14–16 CINs.
We next sought to determine which ionic currents mediate 5-HT's ability to enable plateau potential capability in P14–16 CINs. In other preparations, bistability requires the activation of persistent calcium and/or sodium currents (Housgaard and Kiehn 1989; Lee and Heckman 1996; Li and Bennett 2003; Li et al. 2007). In all P14–16 CINs tested (n = 4), 10 μM nifedipine, an L-type calcium channel blocker, eliminated the plateau potential capability (Fig. 5A). Nifedipine reduced the average poststep integrated ADP by 90% (14.9 ± 7.9 V·s during serotonin before nifedipine vs. 1.4 ± 2.1 V·s with added nifedipine, P < 0.03, n = 4; Fig. 5B), which was not significantly different from the pre-5-HT control value. These results suggest that a nifedipine-sensitive L-type calcium current sustains the bistability in P14–16 CINs.
Fig. 5.
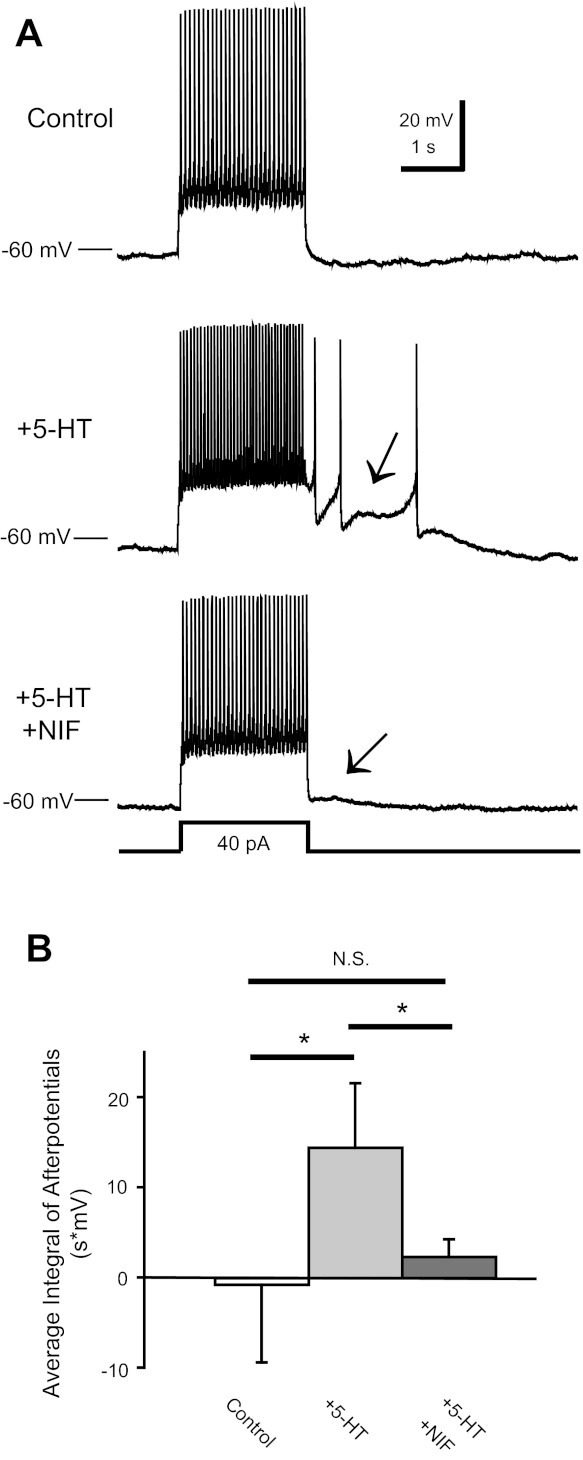
Nifedpine abolishes plateaus in P14–16 CINs. A: a P16 CIN fired in response to an injected current pulse in control, 9 μM 5-HT, and 5-HT + 10 μM nifedipine (NIF). Note that 5-HT induced a plateau potential (middle, arrow) and that addition of NIF abolished the plateau and ADP (bottom, arrow). B: the average integral of afterpotentials in P14–16 CINs was increased by 5-HT and abolished by NIF (*P < 0.05). There was no difference between the control and 5-HT + NIF conditions.
Effect of 5-HT on calcium currents in CINs.
Because 5-HT acts to enhance plateau potential capability in P14–16 CINs, and the 5-HT-enabled plateaus are blocked by nifedipine, we hypothesized that 5-HT enhances an L-type calcium current in P14–16 CINs. In whole cell voltage-clamp, with sodium and potassium currents blocked, a distinct inward current was observed in all P14–16 cells examined (n = 21 in calcium-containing aCSF). The current was also observed with external calcium replaced by barium (n = 7; Fig. 6A). The threshold of activation of calcium currents was around −40 mV, and the voltage at peak calcium current was between −20 and −10 mV (Fig. 6C). First-order Boltzmann analysis of calcium currents showed that the average Imax value was highly variable between neurons (600 ± 376 pA), with an average voltage for half-activation (Vmid) at −26.5 ± 5.2 mV (n = 21) and slope (Vc) of 5.5 ± 1.5 mV/e-fold. Serotonin increased the total current in almost one-half of these neurons (Fig. 6A). Boltzmann analysis showed that Imax was increased by 11 ± 5% in 43% of cells tested (756 ± 574 pA in control vs. 832 ± 615 pA in 5-HT, P < 0.02, 6/14 CINs in Ca2+; Fig. 6B). There was no effect of 5-HT on Vmid or slope Vc (P > 0.60 and 0.40, respectively). In the other 8/14 CINs, 5-HT did not significantly increase Imax, consistent with the inability of 5-HT to evoke bistability in about one-half of P14–16 CINs.
Fig. 6.
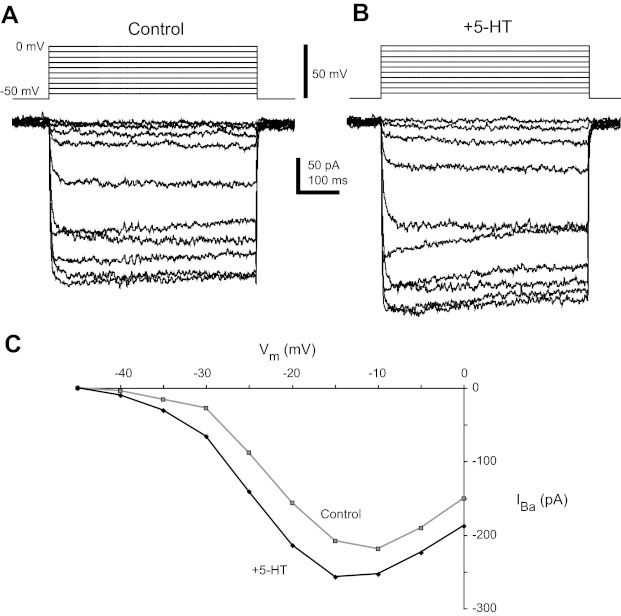
5-HT increases barium current (IBa) in P14–16 CINs. A and B: current steps in response to 5-mV incremental voltage steps from −50 to 0 mV in control conditions (A) and during application of 9 μM 5-HT (B). C: current-voltage (I-V) plot of peak of current in control (gray) and 5-HT (black).
Next, we examined whether nifedipine would block the serotonin-induced increase in calcium current, since it blocks 5-HT-evoked bistability. Because we were unable to hold individual cells long enough to test 5-HT's effects on voltage-activated calcium current, wash it out, and then add nifedipine along with 5-HT, we performed parallel experiments by adding nifedipine before 5-HT to see whether it could occlude 5-HT's effects. Nifedipine (10 μM) reduced the calcium current in all P14–16 cells tested (n = 7). The Boltzmann fit showed that nifedipine caused a 34 ± 18% reduction in Imax (265 ± 119 pA in control, 172 ± 74 pA in nifedipine, n = 5, P < 0.05; Fig. 7, A and B). After nifedipine blockade of L-type calcium current, 5-HT did not increase the remaining nifedipine-insensitive calcium current (Imax : 180 ± 83 pA in nifedipine, 155 ± 72 pA in nifedipine and 5-HT, n = 4; Fig. 7, A and B).
Fig. 7.

5-HT does not increase NIF-insensitive current in P14–16 CINs. A: calcium current (ICa) in response to incremental voltage steps from −50 to 0 mV in a P16 CIN in control conditions, during application of 10 μM NIF, and during application of NIF and 9 μM 5-HT. B: I-V plot from the traces in A showing that 5-HT does not increase the current in the presence of NIF.
Serotonin's effects on calcium currents in P8–10 CINs.
These results present an interesting question. Previous work in our laboratory (Abbinanti and Harris-Warrick 2012; Diaz-Rios et al. 2007) showed that 5-HT increases excitability in P0–3 CINs (but does not induce plateau potential capability) by reducing P/Q and N-type calcium currents and indirectly decreasing calcium-activaetd potassium current; thus the sign of the effect of 5-HT on calcium currents changed during the first two postnatal weeks. We next examined serotonergic modulation of calcium currents at the intermediate age, P8–10. In experiments with barium replacing calcium, a barium current was observed with an average Imax of 376 ± 329 pA (n = 5; Fig. 8A). The threshold for activation of P8–10 currents was near −40 mV, and the voltage of peak current was around −10 mV. Serotonin did not increase the total barium or calcium current in P8–10 CINs (Fig. 8, A and B; 4/5 CINs measured with calcium, 5/5 CINs measured with barium). Serotonin did not produce any significant effects on Boltzmann parameters such as Imax, Vmid, or slope at this age. Typically, the calcium current decreased with 5-HT, but rundown made it difficult to determine whether 5-HT was in fact decreasing the current. To resolve this issue, we monitored barium current with voltage steps at 30-s intervals while washing in 5-HT. In 4/5 P8-P10 CINs, we did not observe any deflection from the progressive barium current rundown when 9 μM 5-HT was added (Fig. 8, C and D), suggesting that any decrease seen during 5-HT at this age was an artifact of the slow rundown of the current with time.
Fig. 8.
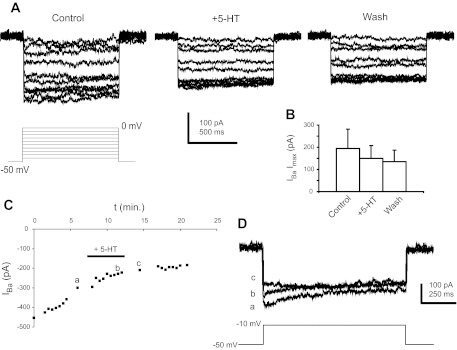
5-HT does not increase IBa in P8–10 CINs. A: IBa in response to incremental voltage steps from −50 to 0 mV in control, 9 μM 5-HT, and washout. B: 5-HT did not affect the maximal current (Imax) from a Boltzmann fit of the currents in control, 5-HT, and washout. C: time course of IBa amplitude in response to steps from −50 to −10 mV taken every 30 s. D: 5-HT did not have any appreciable effects superimposed on the rundown of the current: a, b, and c are traces taken at the times indicated in C.
DISCUSSION
We examined the changes in the intrinsic properties and serotonergic modulation of CINs in the lumbar spinal cord during postnatal development. We sought to examine changes in the properties of intersegmental CINs as an indication of qualitative, as well as quantitative, changes in the overall network with age.
Postnatal changes of intrinsic properties of CINs.
At P8–10 and P14–16, about two-thirds of CINs were spontaneously active in the absence of synaptic input, compared with only 20% of P0–3 CINs. In addition, the AP narrowed, as has been seen during development in many neuronal types. This could arise from changes in potassium and/or sodium currents. Song et al. (2006) showed that expression of the Kv3.1 voltage-gated potassium channel involved in repolarization was strongly increased between P10 and P21 in mouse Renshaw cells. Spatial clustering of Kv2.1 delayed-rectifier channels occurs between P2 and P14 in mouse motor neurons (Wilson et al. 2004). Increasing density of voltage-gated sodium current, INa(V), coupled with increased delayed-rectifier potassium current, IK(V), and calcium-activated potassium current, IK(Ca), between embryonic day 16 and P1–3 in rat motor neurons results in a narrowing of the embryonic AP (Gao and Ziskind-Conhaim 1998). This trend may continue into the postnatal period for CINs.
In addition, we found a decrease in capacitance between the neonatal and the two older CIN groups. Several factors could explain this result. Although no studies have examined potential morphological changes to these cells during postnatal development, a reduction in overall cell size, such as a simplification of the dendritic arborization or a smaller cell body, could account for the capacitance reduction. Additionally, as has been found in motor neurons (Chang et al. 1999), a reduction in gap junctional coupling to other unidentified neurons could be responsible. Zhong et al. (2006a) observed 5-HT-induced membrane oscillations that were blocked by the gap junction blocker carbenoxelone and are truncated APs from electrically coupled neurons. We observed these oscillations in older (P8–10 and P14–16) cells as well (data not shown), but this does not preclude a reduction in overall coupling strength.
The CIN AP threshold depolarized slightly between P3 and P8. We and others have shown that the persistent sodium current (INaP) plays a role in setting the AP threshold in different cell types (Lamas et al. 2009; Zhong et al. 2007). Gao and Ziskind-Conhaim (1998) suggested that hyperpolarizing changes in threshold of INa(V) activation help hyperpolarize the AP threshold in neonatal rat spinal motor neurons. Cooperation between multiple mechanisms may produce the changes in AP threshold.
The shape of the spike AHP changed between P10 and P14, with a rapidly deactivating component appearing only in P14–16 CINs. This is likely due to the appearance of new current(s) contributing to the AHP. An apamin-sensitive small-conductance calcium-activated potassium (SK) channel is responsible for most of the slow AHP in P0–3 CINs (Dias-Rios et al. 2007). In P15–30 rat Purkinje neurons, large-conductance calcium-activated potassium (BK) channels were responsible for the fast-inactivating component of the AHP (Haghdoost-Yazdi et al. 2008). Additionally, enhancement of the delayed-rectifier current, IK(V), could contribute to the fast AHP. Therefore, increases in BK or delayed-rectifier currents during postnatal development may lead to the emergence of the fast AHP.
A number of changes could cause CINs to become more excitable with age, as indicated by the increased average f-I value and slope. Interestingly, we found no significant change in the baseline input resistance at different ages, suggesting that voltage-activated currents are responsible for the increased excitability. Increased INaP, as discussed above, could increase the baseline excitability by amplifying the depolarizing drive of a given current injection step. Decreasing the SK current with apamin caused a similar upward shift in the f-I plot and steeper slope in neonatal CINs, suggesting this current might decrease over time (Diaz-Rios et al. 2007). The emergence of new persistent inward currents, as discussed in more detail below, could also be responsible for this increase in excitability with age (Carlin et al. 2000; Li and Bennett 2003; Li et al. 2004). The increase in excitability would allow older cells to be intrinsically more responsive to excitatory synaptic current, perhaps affecting the necessary balance of excitation and inhibition for proper functioning of the network.
Serotonin induces plateau potential capability in older CINs.
In some ways, serotonergic excitation of CINs at older ages is similar to that at P0–3. At all ages, 5-HT evokes a leftward shift of the f-I plot, a reduction in current required for the cell to spike, and a concurrent increase in average f-I value (Zhong et al. 2006a, 2006b). Interestingly, P0–3 aCINs showed an increase in AP halfwidth during 5-HT application, whereas dCINs did not (Zhong et al. 2006a, 2006b). 5-HT caused an increase in halfwidth in both aCINs and dCINs at P8–10 and P14–16. In contrast to P0–3 and P8–10 CINs, P14–16 CINs showed two qualitative differences in excitability. First, in older CINs, 5-HT induced a small but significant increase in the slope of the f-I plot. The second and major difference in 5-HT modulation with age was the emergence of plateau potential capability in about 50% of P14–16 CINs during 5-HT application. Some of these neurons continued to fire APs after the end of a depolarizing current step, whereas others simply showed a prolonged ADP. Critically, we never observed 5-HT-induced plateau potential capability or ADPs in CINs at P10 or younger. Plateau potentials have been found in motor neurons of the adult turtle, cat, and P8–15 mouse spinal motor neurons (Carlin et al. 2000; Hounsgaard and Kiehn 1989; Lee and Heckman 1998). This result suggests that serotonin, a critical neuromodulator for locomotion, causes a qualitative change in the firing properties of older CINs compared with neonatal neurons.
Plateau potentials in network neurons like CINs could modify network function. First, 5-HT-induced bistability could provide flexibility within the network by altering the manner in which a cell will respond to a given synaptic input. The output of bistable neurons can become binary, depending on whether the cell is in the depolarized or resting state (Loewenstein et al. 2005). Second, bistability could change the functional role of synaptic inputs to the CINs. Bistable neurons respond to a brief “trigger” of synaptic input with prolonged firing, instead of being driven to fire by the temporal pattern of synaptic input. A third possibility is that the persistent inward currents (PICs) that sustain bistability in our experiments may serve a different role in vivo, as a dendritic postsynaptic amplification system. PICs, in the form of persistent sodium or calcium currents, can provide a voltage-dependent boost to synaptic currents in the dendrites (Elbasiouny et al. 2006; Lee and Heckman 2000; Li and Bennett 2003; Li et al. 2004). 5-HT activation of these dendritic currents could change the cell's output through selective amplification of synaptic inputs. These possible actions of 5-HT are not mutually exclusive.
Plateaus are sustained by an L-type calcium current.
The L-type calcium channel blocker nifedipine blocked the 5-HT-induced plateau potentials in P14–16 CINs. L-type calcium current underlies plateau potentials in P9–16 mouse motor neurons (Carlin et al. 2000). New L-type calcium channel subunits are expressed around P8–10 in presumptive mouse motor neurons, reaching their maximal expression at P18 (Jiang et al. 1999). The time course of this expression roughly parallels our results in CINs, suggesting that plateau potential capability appears around P14 due to the upregulation of L-type calcium channels, ICa(L), at this time. We used voltage-clamp analysis to show that 5-HT increases a nifedipine-sensitive calcium current in about one-half of P14–16 CINs; 5-HT evokes bistability in a similar fraction. There are several possible reasons why 5-HT excites a majority of CINs at all ages but induces bistability in only 50% of P14–16 CINs. First, there may be different 5-HT receptors mediating the increase in excitability and the ICa(L)-mediated bistability, which can be differentially expressed among CINs. Second, P14–16 may be an intermediate age for development of CIN bistability; at later ages, perhaps all CINs become bistable with 5-HT.
These results expose an unusual plasticity in the mechanisms of 5-HT modulation of CINs at different ages. Serotonin increases CIN excitability at all ages, but apparently through opposing effects on voltage-activatred calcium currents, ICa(V). In P0–3 CINs, 5-HT acts in part by decreasing IK(Ca) indirectly through a conductance decrease in ICa(V) (Diaz-Rios et al. 2007). We have recently shown that 5-HT decreases N- and P/Q-type calcium currents in P0–5 CINs (Abbinanti and Harris-Warrick 2012). We show here that by P14–16, 5-HT excites neurons at least in part by enhancing ICa(L), an effect that was not seen at earlier ages. Therefore, 5-HT increases the excitability of CINs at different ages through opposite effects on different calcium currents. SK-type calcium-activated potassium channels, whose current appears to be reduced by 5-HT in P0–3 CINs, are activated by different calcium channel subtypes in different preparations, providing a mechanism by which L-type calcium current can be increased while N- and P/Q-type calcium current coupled with IK(Ca) can be concurrently reduced by 5-HT (Abbinanti and Harris-Warrick 2012; Goldberg and Wilson 2005; Kasten et al. 2007; Perez-Rosello et al. 2005; Protti and Uchitel 1997; Sah 1995; Vilchis et al. 2000; Wikstrom and El Manira 1998). At P8–10, we saw no net effect of 5-HT on total calcium current. Given that Jiang et al. (1999) demonstrated that new CaV1.2 and CaV1.3 L-type calcium channel transcripts begin to be expressed in the ventral horn around P8, perhaps some L-type calcium current is present at this time. If 5-HT was having opposing effects on N-, P/Q-, and L-type ICa(V) at P8–10, it may be difficult to resolve these effects due to the small net change.
Conclusion.
Our results show that CINs in the mouse spinal locomotor CPG are not fully mature at birth and that their neonatal firing properties do not fully reflect those of adult animals. Both the baseline firing properties of CINs and their responses to 5-HT are qualitatively different in young adults compared with newborn mice. This suggests that CPG may mature as the animals learn to walk and may not be identical in neonates and adults. Therefore, we must be careful drawing conclusions on the function of the adult locomotor network from neonatal studies. Even though it has not yet been possible to evoke fictive locomotion in an isolated adult mouse spinal cord, it is important to continue to examine the intrinsic properties of network components in slices of the mature adult cord (Husch et al. 2011; Mitra and Brownstone 2012) in conjunction with neonatal studies using the intact spinal cord. Future investigations should focus on developing a functionally mature intact spinal cord preparation to determine the extent of changes in network properties during postnatal development.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants NS17323 and NS057599 (to R. M. Harris-Warrick) and NIH Training Grant T32 GM007469.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.A. and R.M.H.-W. conception and design of research; M.D.A. and G.Z. performed experiments; M.D.A. and G.Z. analyzed data; M.D.A., G.Z., and R.M.H.-W. interpreted results of experiments; M.D.A. prepared figures; M.D.A. and R.M.H.-W. drafted manuscript; M.D.A. and R.M.H.-W. edited and revised manuscript; M.D.A., G.Z., and R.M.H.-W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bruce Johnson, Andreas Husch, and Shelby Dietz for useful discussions and comments on the manuscript.
Present address of G. Zhong: Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138.
REFERENCES
- Abbinanti MD, Harris-Warrick RM. Serotonin modulates multiple calcium current subtypes in commissural interneurons of the neonatal mouse. J Neurophysiol 107: 2212–2219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballion B, Branchereau P, Chapron J, Viala D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res Dev Brain Res 137: 81–88, 2002 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001 [DOI] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Weber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol 461: 429–440, 2003 [DOI] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22: 9961–9971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res Brain Res Rev 40: 107–117, 2002 [DOI] [PubMed] [Google Scholar]
- Carlin KP, Dai Y, Jordan LM. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol 95: 1278–1284, 2006 [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci 12: 1635–1646, 2000 [DOI] [PubMed] [Google Scholar]
- Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap juntional coupling and patterns of connexin expression among neonatal rat lumbar spinal cord neurons. J Neurosci 19: 10813–10828, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Vinay L, Cazalets JR, Fady JC, Jamon M. Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Res Brain Res Rev 28: 35–43, 1998 [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of l-dopa and clonidine in the spinal cat. J Physiol 405: 369–384, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rios M, Dombeck DA, Webb WW, Harris-Warrick RM. Serotonin modulates dendritic calcium influx in commissural interneurons in the mouse spinal locomotor network. J Neurophysiol 98: 2157–2167, 2007 [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570: 355–374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol 88: 590–608, 1985 [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol 80: 3047–3061, 1998 [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci 25: 10230–10238, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghdoost-Yazdi H, Janahmadi M, Behzadi G. Iberiotoxin-sensitive large conductance Ca2+-dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons. Brain Res 1212: 1–8, 2008 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Cramer N, Harris-Warrick RM. Long-duration perforated patch recordings from spinal interneurons of adult mice. J Neurophysiol 106: 2783–2789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience 67: 893–907, 1995 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci 11: 3481–3487, 1999 [DOI] [PubMed] [Google Scholar]
- Kasten MR, Rudy B, Anderson MP. Differential regulation of action potential firing in adult murine thalamocortical neurons by Kv3.2, Kv1, and SK potassium and N-type calcium channels. J Physiol 584: 565–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas JA, Romero M, Reboreda A, Sanchez E, Ribeiro SJ. A riluzole- and valproate-sensitive persistent sodium current contributes to the resting membrane potential and increases the excitability of sympathetic neurones. Pflügers Arch 458: 589–599, 2009 [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998 [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005 [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Hausser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci 8: 202–211, 2005 [DOI] [PubMed] [Google Scholar]
- MacLean JN, Cowley KC, Schmidt BJ. NMDA receptor-mediated oscillatory activity in the neonatal rat spinal cord is serotonin dependent. J Neurophysiol 79: 2804–2808, 1998 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001 [DOI] [PubMed] [Google Scholar]
- Mitra P, Brownstone RM. An in vitro spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse. J Neurophysiol 107: 728–741, 2012 [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett 280: 187–190, 2000 [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol 93: 2507–2519, 2005 [DOI] [PubMed] [Google Scholar]
- Protti DA, Uchitel OD. P/Q-type calcium channels activate neighboring calcium-dependent potassium channels in mouse motor nerve terminals. Pflügers Arch 434: 406–412, 1997 [DOI] [PubMed] [Google Scholar]
- Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci 27: 6521–6530, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Different calcium channels are coupled to potassium channels with distinct physiological roles in vagal neurons. Proc Biol Sci 260: 105–111, 1995 [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000 [DOI] [PubMed] [Google Scholar]
- Song ZM, Hu J, Rudy B, Redman SJ. Developmental changes in the expression of calbindin and potassium-channel subunits Kv3.1b and Kv3.2 in mouse Renshaw cells. Neuroscience 139: 531–538, 2006 [DOI] [PubMed] [Google Scholar]
- Stegenga SL, Kalb RG. Developmental regulation of N-methyl-d-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience 105: 499–507, 2001 [DOI] [PubMed] [Google Scholar]
- Vilchis C, Bargas J, Ayala GX, Galvan E, Galarraga E. Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience 95: 745–752, 2000 [DOI] [PubMed] [Google Scholar]
- Wikstrom MA, El Manira A. Calcium influx through N- and P/Q-type channels activate apamin-sensitive calcium-dependent potassium channels generating the late afterhyperpolarization in lamprey spinal neurons. Eur J Neurosci 10: 1528–1532, 1998 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol 474: 13–23, 2004 [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Chersa T, Whelan PJ. Tissue Po2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience 117: 183–196, 2003 [DOI] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol 95: 1545–1555, 2006 [DOI] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci 26: 6509–6517, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 27: 4507–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


