Abstract
The nucleus tractus solitarii (nTS) is the primary termination and integration point for visceral afferents in the brain stem. Afferent glutamate release and its efficacy on postsynaptic activity within this nucleus are modulated by additional neuromodulators and transmitters, including serotonin (5-HT) acting through its receptors. The 5-HT2 receptors in the medulla modulate the cardiorespiratory system and autonomic reflexes, but the distribution of the 5-HT2C receptor and the role of these receptors during synaptic transmission in the nTS remain largely unknown. In the present study, we examined the distribution of 5-HT2C receptors in the nTS and their role in modulating excitatory postsynaptic currents (EPSCs) in monosynaptic nTS neurons in the horizontal brain stem slice. Real-time RT-PCR and immunohistochemistry identified 5-HT2C receptor message and protein in the nTS and suggested postsynaptic localization. In nTS neurons innervated by general visceral afferents, 5-HT2C receptor activation increased solitary tract (TS)-EPSC amplitude and input resistance and depolarized membrane potential. Conversely, 5-HT2C receptor blockade reduced TS-EPSC and miniature EPSC amplitude, as well as input resistance, and hyperpolarized membrane potential. Synaptic parameters in nTS neurons that receive sensory input from carotid body chemoafferents were also attenuated by 5-HT2C receptor blockade. Taken together, these data suggest that 5-HT2C receptors in the nTS are located postsynaptically and augment excitatory neurotransmission.
Keywords: serotonin, electrophysiology, immunohistochemistry, autonomic nervous system, respiratory system, carotid body
located in the dorsomedial medulla, the nucleus tractus solitarii (nTS) is vital to the autonomic and respiratory systems. The nTS serves as the central site for sensory afferent input for visceral sensory reflexes, including cardiovascular, respiratory, cardiopulmonary, and gastroesophagointestinal (Andresen and Kunze 1994; Dean 2011). Glutamate is the primary neurotransmitter at the sensory afferent-nTS cell synapse and is required for the initiation of these reflexes (Andresen and Kunze 1994; Kline 2008). However, other neurochemicals play a vital role in the modulation of information transfer at this synapse, augmenting or reducing synaptic transmission or postsynaptic neuronal activity (Andresen and Mendelowitz 1996; Gordon and Sved 2002; Lawrence and Jarrott 1996). Such additional neurochemicals may derive from the sensory afferents, interneurons, or the numerous reciprocal connections to other central regions related to autonomic and respiratory function (Andresen and Kunze 1994). Overall, the properties of the nTS as a whole, its synapses in particular, and their modulation by these neurochemicals will strongly influence the initiation, maintenance, or efficiency of the visceral sensory reflexes.
Serotonin (5-hydroxytryptamine, 5-HT) modulates the central control of stress and thermoregulation, as well as cardiovascular, respiratory, and gastrointestinal systems (Browning and Travagli 1999; Jordan 2005; Morrison and Nakamura 2011; Nalivaiko and Sgoifo 2009; Raul 2003; Tache et al. 1995; Villalon and Centurion 2007). The raphé nuclei serve as the primary origin of 5-HT neurons in the brain, and projections from the raphé serve as a major source of 5-HT to the nTS (Schaffar et al. 1988). However, the nTS may also receive serotonergic input from cells within the nTS itself as well as the nodose ganglion, the location of visceral afferent cell bodies (Nosjean et al. 1990; Raul 2003). Microinjection of 5-HT into the nTS and activation of its receptors elicit robust changes in blood pressure (Raul 2003) and breathing (Sessle and Henry 1985). While numerous 5-HT receptors have been identified in the nTS, with several playing a functional role in cardiorespiratory and autonomic regulation, the 5-HT2 receptor subtype has been a major focus of study (Comet et al. 2007; Raul 2003). Depending on the receptor subtype, activation of the 5-HT2 receptor family in nTS alters cardiorespiratory function and increases or decreases in vivo neuronal discharge (Comet et al. 2007; Sevoz-Couche et al. 2000a, 2000b, 2000c) or in vitro signaling (Feldman 1995; Takenaka et al. 2011). A potential excitatory role for 5-HT2C receptors (5-HT2CRs) in the nTS has been suggested by studies illustrating that systemic administration of 5HT2A/2C receptor agonists increases Fos expression, a neuronal activation marker, in the nTS (Cayetanot et al. 2001). These studies support a potential role for 5-HT2CRs in the nTS, but as yet their impact at the synaptic level as well as their influence on sensory modalities are not fully understood. This work was undertaken to examine the localization of the 5-HT2CR in the nTS and the role these receptors play in synaptic and membrane properties in monosynaptically driven nTS neurons. Previous portions of this work have been presented in abstract form (Austgen et al. 2011a).
METHODS
Animals.
Experiments were conducted according to the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the University of Missouri Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Indianapolis, IN; n = 30) aged 3–5 wk were used. Animals were housed within an in-house animal facility on a 12:12-h day-night cycle with food and water available ad libitum. Room temperature and humidity were maintained at 22°C and 40%, respectively.
Real-time reverse transcriptase-polymerase chain reaction.
The presence of 5-HT2CR mRNA in the nTS was examined through reverse transcriptase-polymerase chain reaction (RT-PCR), similar to our previous studies (Austgen et al. 2011b). Briefly, rats were anesthetized with isoflurane (Vet-One, Meridian, IN) and decapitated, the brain stem was removed, and horizontal nTS sections (∼350 μm) were generated with a vibratome (VT 1000s; Leica, Wetzler, Germany). Nodose-petrosal ganglia (NPG) were also removed. Tissue (nTS and NPG) was placed into RNAlater (Qiagen) until RNA isolation. RNA was isolated (RNAqueous-Micro; Ambion), concentration was determined, and 100 ng of RNA was used to generate cDNA (SuperScript III; Invitrogen). PCR amplification with 2 μl of cDNA used primers for 5-HT2CR (forward: TAT CGC TGG ACC GGT ATG TA, reverse: GAG AAC GAA GTT GGG GTC AT, 10 μM; Real Time Primers, Elkins Park, PA) and β-actin (forward: CAC ACT GTG CCC ATC TAT GA, reverse: CCG ATA GTG ATG ACC TGA CC, 10 μM; Real Time Primers). Control PCR was run on samples that had no template, no primers, or no reverse transcriptase. PCR products were separated and visualized on 1.5% agarose gel.
Immunoblot.
5-HT2CR antibody specificity was examined by immunoblot. Four-week-old rats were deeply anesthetized with isoflurane and decapitated. Brain stem tissue was quickly removed and lysed by sonication in two volumes of RIPA buffer [final concentrations in mM: 1% NP-40, 150 NaCl, 50 Tris, 1 ethylenediaminetatraacetic acid (EDTA), 10 NaF, 10 sodium orthovanadate, 1 phenylmethylsulfonyl fluoride (PMSF), and 0.25% sodium deoxycholate] containing protease inhibitor cocktail (Roche, Indianapolis, IN). The insoluble protein was removed after centrifugation at 14,000 g and 4°C, and supernatant was collected. Protein concentrations were determined by the Micro BCA method (Pierce, Thermo Scientific, Rockford, IL). Forty micrograms of protein was separated on 4–20% Tris·HCl gels (Bio-Rad Laboratories, Hercules, CA) and subsequently transferred to PVDF membranes. Membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS)-0.1% Tween 20 (2 h, 23°C). Membranes were subsequently incubated (48 h, 4°C) with primary antibodies against 5-HT2CR (rabbit, 1:1,000; Abcam, Cambridge MA) or tubulin (mouse, 1:1,000; Sigma, St. Louis, MO), washed, and incubated with anti-rabbit or anti-mouse HRP-linked secondary antibody (1:10,000, 1 h, 23°C). Blots were developed with the Immuno-Star WesternC kit (Bio-Rad) and visualized with the ChemiDoc Imaging system (Bio-Rad).
Immunohistochemistry.
Presence and distribution of 5-HT2CR in the nTS were examined through immunohistochemistry, as described previously (Austgen et al. 2008, 2011b; Kline et al. 2010). In short, animals were deeply anesthetized with isoflurane and transcardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brain stems were removed and sectioned coronally at 30 μm on a vibratome (Leica VT 1000s). Tissue sections were then subjected to heat-induced epitope retrieval in a decloaking chamber (Biocare Medical, Concord, CA) followed by a PBS rinse. Tissue was subsequently blocked in 10% normal donkey serum (NDS; Millipore, Billerica, MA) in PBS with 0.3% Triton X-100 (PBS-Tx), rinsed, and incubated for 40 h in PBS with a primary antibody raised against 5-HT2CR (rabbit, 1:750; Abcam) as well as one or more of the following primary antibodies: microtubule-associated protein (MAP)-2, a dendrite marker (mouse, HM-2 clone, 1:500; Sigma), postsynaptic density-95 (PSD-95) MAGUK, a postsynaptic protein responsible for the tethering of glutamate receptors to the synaptic membrane (mouse, clone K28/43, 5 μg/ml; NeuroMab, Davis, CA), synaptophysin, a presynaptic terminal marker (guinea pig, 1:500; SySy, Göttingen, Germany), and tryptophan hydroxylase (TPH, mouse, WH-3 clone, 1:1,000; Sigma), the rate-limiting enzyme for 5-HT production. Antibody specificity for 5-HT2CR was confirmed by two methods, immunoblot studies (see above and results) and the reduction of immunostaining by preabsorption of the immunizing peptide. For the latter, anti-5-HT2CR (1:750) and a 5-HT2CR peptide (1:750; Abcam) were coincubated on a laboratory shaker for 2 h at room temperature. This was followed by tissue incubation of the antibody-peptide cocktail for 48 h as above. Tissue sections not containing preabsorption peptide were also run in parallel. Specificity of other antibodies has been previously established: Sakakibara et al. 2008 (MAP-2), NeuroMab (PSD-95), Hodges et al. 2011 (TPH), the vendor indicated, and The Journal of Comparative Neurology Antibody Database (version 7). As an additional control, primary antibodies were also withheld from individual sections. The next day, sections were rinsed and either incubated for 2 h in PBS-Tx with a fluorescent Cy-conjugated secondary antibody or, for 5-HT2CR, subjected to tyramide signal amplification according to the manufacturers' specifications (PerkinElmer). Sections were then rinsed a final time, mounted onto gelatin-coated sides, dried, and coverslipped with ProLong Gold mounting medium (Invitrogen, Carlsbad, CA). Slides were sealed with nail polish.
Sections were examined with an Olympus epifluorescent spinning disk confocal system or a FluoView FV1000 confocal system. Each image set with multiple fluorophores was acquired in the same focal plane. Images were imported into Photoshop (v. 11.0.2, Adobe, San Jose, CA) or ImageJ, with image brightness and contrast adjusted for clarity.
Carotid body labeling.
In a subset of animals (n = 13), sensory afferent terminals originating from the carotid body chemoreceptors were identified in live brain slices through fluorescent labeling in horizontal nTS slices, as previously shown by us (Kline et al. 2002, 2010) and others (Accorsi-Mendonca et al. 2011; Zhang et al. 2008). Rats were anesthetized with isoflurane (5% induction, 2–3% maintenance), their ventral neck region was cleared of hair, and the left carotid artery was exposed. After the carotid bifurcation was located, a small crystal of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Invitrogen) was placed on the carotid body and fixed in place with Kwik-Sil (World Precision Instruments, Sarasota, FL). Care was taken to ensure that the DiI remained in place prior to incision closure. After closure of the neck, rats were treated postoperatively with Baytril (0.03 ml im; Bayer, Shawnee Mission, KS) and Buprenex (0.6 mg/ml sc; Reckitt Benckiser Pharmaceuticals, Richmond, VA). Anterograde transport of dye took place over the next 5 days. One potential limitation of this approach is that baroreceptor-like afferents may course near or through the carotid body to innervate blood vessels in the carotid body (Gonzalez et al. 1994); thus a small portion of the labeled chemoafferent-rich population may also contain such fibers. Brain slice electrophysiology studies were subsequently performed on this chemoafferent-rich population of cells.
In vitro brain slice preparation and electrophysiology.
Rats were anesthetized with isoflurane and decapitated, and their brain stems were rapidly removed and placed into ice-cold low-Ca2+, high-Mg2+ artificial cerebrospinal fluid (aCSF, in mM: 124 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, 1 CaCl2, and 2 MgCl2, saturated with 95% O2-5% CO2, pH 7.4, 315–325 mosM). To generate horizontal slices that contain the nTS and the afferent-containing tractus solitarii (TS) in a single plane, a wedge of the ventral surface of the brain stem was removed and the resulting medulla was fixed ventral side down to the chuck of a vibrating microtome (Leica) with cyanoacrylate. Tissue slices (∼280 μm) were cut from the dorsal surface of the brain stem, placed in the recording chamber, and secured with a nylon mesh harp. Slices were superfused at 3–4 ml/min with recording aCSF (in mM: 124 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, and 2 CaCl2, saturated with 95% O2-5% CO2, pH 7.4, 315–325 mosM) at 33°C.
Neurons were identified with an Olympus BX51WIF microscope equipped with differential interface contrast (DIC), fluorescence and an infrared-sensitive camera (Retiga; Q-Imaging, Burnaby, BC, Canada). In sections with carotid body afferent labeling, fluorescent DiI-labeled boutons were visualized with a xenon lamp and a TRITC filter set (Semrock, Rochester, NY). All recordings were made from caudal nTS neurons. To record excitatory postsynaptic currents (EPSCs), recording electrodes (8250 glass, 3.5–4.5 MΩ; King Precision Glass, Claremont, CA) were filled with (mM) 10 NaCl, 130 K+ gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 MgATP, and 0.2 NaGTP, pH 7.3, 295–300 mosM. In experiments in which we recorded miniature (m)EPSCs, tetrodotoxin (1 μM) and gabazine (SR 95531 HBr, 25 μM) were added to the bath. The recording pipette was guided with a piezoelectric micromanipulator (PCS-6000; Burleigh, Victor, NY). Neurons were voltage clamped (−60 mV) in the whole cell configuration. Evoked synaptic currents were generated by placing a concentric bipolar stimulating electrode (F. Haer, Bowdoinham, ME) on the TS and stimulating at a duration of 0.1 ms with an isolated programmable stimulator (AMPI, Jerusalem, Israel). Stimulation intensity was increased until an EPSC was evoked. Final TS intensity was set at 1.5 × threshold and was stimulated at a frequency of 0.5 and 20 Hz (20 events, with intersweep interval of 10 s). A 5-mV step (−60 to −65 mV) following 0.5 Hz-evoked EPSCs was used to determine changes in membrane conductance (i.e., input resistance) of the cell. The resting membrane potential (RMP) of the cell was measured under current clamp with no holding currents (I = 0). Data were recorded with a Multiclamp700B amplifier, filtered at 2 kHz, and sampled at 10 kHz with pCLAMP10 software (Molecular Devices, Palo Alto, CA).
Drugs.
CP809101 (a 5-HT2CR agonist; Tocris) and RS102221 (a 5-HT2CR antagonist; Tocris) were used to examine the role of 5-HT2CR in electrophysiological studies. CP809101 has >500-fold specificity for 5-HT2CRs over other 5-HT2 subtypes (Sharman et al. 2011; Siuciak et al. 2007). RS102221 exhibits >100-fold selectivity for 5-HT2CR over the 5-HT2A and 5-HT2B receptors (Bonhaus et al. 1997; Sharman et al. 2011). In addition, tetrodotoxin citrate (TTX; Tocris), and SR 95531 HBr (gabazine, GABAA antagonist; Tocris) were used in mEPSC protocols. Bath solutions were delivered by gravity feed from 60-ml reservoirs bubbled with 95% O2-5% CO2. Switching among solution reservoirs occurred by the use of pinch valve controllers (Warner Instruments, Hamden, CT). In a subset of cells, AMPA was applied locally to the cell with a picospritzer (Parker, Cleveland, OH). A glass recording pipette was filled with aCSF containing AMPA (1 mM; Tocris) and placed ∼150–200 μm upstream from the recorded cell. AMPA was briefly applied (50 ms, 5–10 psi) to the cell. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data analysis.
Relative differences in 5-HT2C mRNA expression between nTS and nodose ganglia were evaluated with the ΔΔCT method (where CT is cycle threshold) (2−ΔΔCT; Livak and Schmittgen 2001). This method allows the examination of the relative differences in mRNA expression between different conditions or tissues (Livak and Schmittgen 2001). CT values were obtained via Cepheid Smartcycler software. β-Actin served as our internal housekeeping gene. Electrophysiological data were analyzed with Clampfit software (Molecular Devices, Sunnyvale, CA). Cells with holding currents less than −50 pA and a RMP depolarized more than −45 mV upon initial membrane rupture were not considered for further analysis. Second-order neurons were identified by jitter analysis, defined as the standard deviation of the TS-EPSC latency from the shock artifact (Doyle and Andresen 2001). Neurons with jitter values below 300 μs were considered monosynaptic and directly connected to sensory TS fibers (Accorsi-Mendonca et al. 2011; Hisadome et al. 2010; Jin and Andresen 2011; Kline et al. 2010; Laaris and Weinreich 2007; Miles 1986). The paired-pulse ratio (PPR) of two consecutive TS-EPSCs was determined from the ratio of the amplitude of the second TS-EPSC to the first TS-EPSC (TS-EPSC2/TS-EPSC1; Kline et al. 2002). The failure rate was the percentage of TS stimulations that failed to evoke an EPSC response determined from 30 current sweeps. mEPSCs were discriminated from noise by thresholding events 2.5 times above the root mean square value of noise. The magnitude of change in EPSC amplitude to a given pharmacological agent is also reported as percentage of baseline control, given as 100%. Statistical analyses were performed with Microsoft Excel or SigmaPlot 11 (Systat). EPSC peak amplitude (pA), instantaneous frequency (Hz), percent failure, holding currents, PPR, TS-EPSC decay time (tau, τ90–10%, ms), membrane potential, and input resistance in control and drug periods were all compared by a paired t-test. EPSC amplitude and its alteration from the first event during 20-Hz stimulation between control and drug periods were tested with two-way repeated-measures ANOVA. Data are presented as means ± SE. P values < 0.05 were considered significant.
RESULTS
5-HT2C receptors are located in the medial and commissural nTS.
We examined the presence of mRNA for the 5-HT2CR subtype in the caudal (medial and commissural) nTS and NPG, location of visceral afferent cell bodies that project to the caudal nTS. Across the animals tested, real-time RT-PCR demonstrated 5-HT2CR mRNA (206 bp) in the caudal nTS, with reduced message in the NPG (Fig. 1A). When normalized to the housekeeping gene β-actin, relative 5-HT2CR mRNA was 25-fold greater in the caudal nTS compared with the NPG (Fig. 1B; P < 0.05, n = 6). These results suggest a potential prominent role for 5-HT2CR produced in the nTS rather than NPG.
Fig. 1.
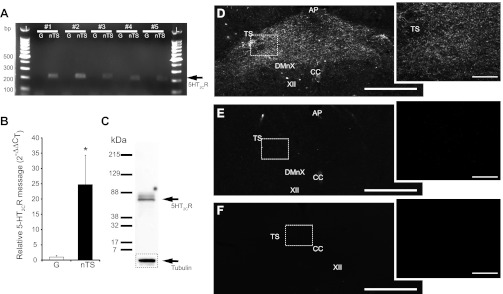
5-HT2C receptor (5-HT2CR) message and protein is expressed in nucleus tractus solitarii (nTS). A: agarose gel image from 5 of 6 individual rats illustrating 5-HT2CR cDNA (arrow, 206 bp) in nTS and nodose-petrosal ganglia (NPG, denoted as G) tissue. Note the greater expression of 5-HT2CR in nTS compared with NPG. L, 100-bp ladder. B: relative expression of 5-HT2CR cDNA in nTS compared with NPG, as determined by the 2−ΔΔCT method (where CT is threshold cycle). Note the increase in relative nTS expression compared with NPG (*P < 0.05). n = 6, 5 of which are shown in A. C: immunoblot analysis of whole brain stem tissue (40 μg) using the 5-HT2CR antibody. The presence of a single band at ∼52 kDa suggests specificity of the antibody used in immunohistochemistry. The loading control marker, tubulin, is shown below. D: light photomicrograph of the caudal (medial and commissural) nTS. Note the robust 5-HT2CR staining in the caudal nTS. E: preincubation of anti-5-HT2CR with 5-HT2CR recombinant protein results in diminished positive immunoreactivity compared with nonpeptide (D)-incubated tissue. F: nonprimary antibody control tissue incubated with secondary antibody alone. Note that staining is similar between E and F. D–F, right: zoomed photomicrograph (×20 objective, confocal) of boxed area on ×4 objective photomicrograph, left. Scale bars, 250 μm (left) and 100 μm (right). TS, solitary tract; DMnX, dorsal motor nucleus of the vagus; AP, area postrema; CC, central canal; XII, hypoglossal nucleus.
The presence and distribution of 5-HT2CR protein within caudal (medial and commissural) nTS tissue were explored. Baroreceptor and chemoreceptor afferents terminate in these nTS subnuclei and were the focus of our electrophysiological studies. Immunoblot analysis of 5-HT2CR from whole brain stem tissue demonstrated a single band at the appropriate size (∼52 kDa), confirming primary antibody specificity (Fig. 1C). Immunohistochemistry demonstrated the presence of 5-HT2CR immunoreactivity (IR) throughout the medial and commissural nTS (Fig. 1D). Furthermore, preabsorption of the antibody with the 5-HT2CR peptide reduced or eliminated immunoreactivity (Fig. 1E), which was similar to that of negative controls (no primary antibody; Fig. 1F). These data demonstrate antibody specificity and 5-HT2CR localization to the nTS.
Cellular localization of 5-HT2CR-IR was examined, in part, through coexamination of its IR with several additional neuronal markers. Dendrites were labeled with MAP-2 and presynaptic terminals with synaptophysin. 5-HT2CR-IR was often observed as long fiberlike processes and in complexes with MAP-2 and synaptophysin. As shown in Fig. 2A, 5-HT2CR-IR was associated with MAP-2 and apposed by presynaptic terminals identified by synaptophysin. 5-HT2CR-IR was observed occasionally along the soma membrane (not shown). PSD-95, a postsynaptic protein responsible for anchoring of glutamate receptors to the synaptic membrane, also exhibited colabeling with 5-HT2CR-IR in the nTS, especially along fibers (Fig. 2B). 5-HT2CR-IR tended to be apposed to TPH, the rate-limiting enzyme for serotonin production (Fig. 2C). Together, these data demonstrate that 5-HT2CRs are localized in the nTS and suggest that at the confocal level they are found postsynaptically. Electrophysiology was performed to confirm the synaptic location.
Fig. 2.
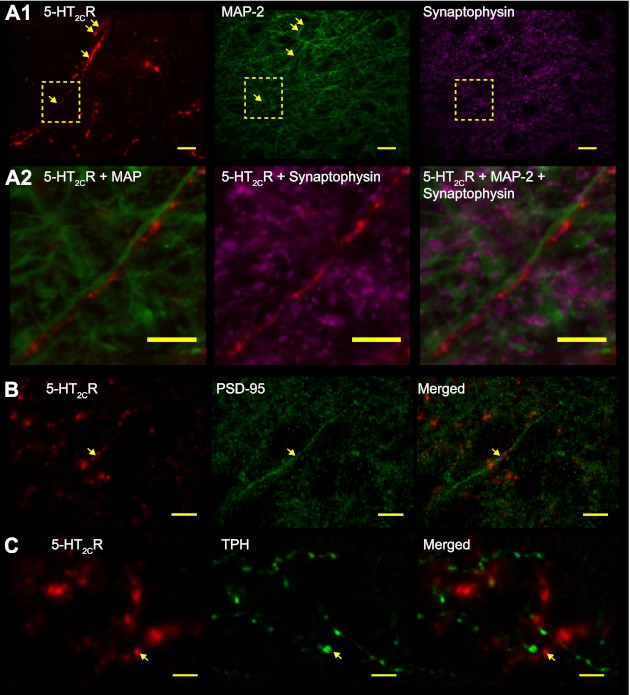
5-HT2CR localization in the nTS. A1: confocal images of immunoreactivity (IR) for 5-HT2CR (pseudocolored red, left), microtubule-associated protein-2 (MAP-2; pseudocolored green, center), and synaptophysin (pseudocolored magenta, right). Dashed box in A1 is enlarged in A2. Arrows depict 5-HT2CR-IR in close apposition to MAP-2. Note the 5-HT2CR fibers in nTS that run in parallel with MAP-2 dendrites. Shown is a z-projection image of 5 images separated by 0.2 μm. A2: merged photomicrographs of IR in A1 boxed area for 5-HT2CR with MAP-2 (left) and synaptophysin (center) and merge of all markers (right). Shown is an z-projection image of 3 images separated by 0.2 μm. Note that 5-HT2CR coincides with MAP-2 in long fibers, with synaptophysin apposed to this complex. B: 5-HT2CR (red) colabeling with postsynaptic density-95 (PSD-95; green, arrow). C: 5-HT2CR (red, arrow) did not colabel with tryptophan hydroxylase (TPH; green). In B and C, merged images are shown on right for each marker. Scale bars, 10 μm
Role of 5-HT2C receptors in monosynaptic nTS neurons.
Electrophysiology in horizontal brain stem slices was performed to confirm 5-HT2CR synaptic localization and examine its potential function. Cells were recorded from the medial and commissural nTS. Stimulation of the TS evoked invariant EPSCs with a latency of 4.8 ± 0.2 ms and a standard deviation of latency (jitter) of 176 ± 9.9 μs (n = 48). These results are consistent with the recording of monosynaptic nTS neurons, i.e., those that form a direct synapse with afferent fibers in the TS. Overall, across the cells studied, TS-EPSC amplitude averaged 134.2 ± 13.7 pA (n = 48). We subsequently examined how activation and blockade of 5-HT2CRs affect EPSCs in these cells.
Activation of 5-HT2C receptors augments TS-EPSCs.
The role of 5-HT2CRs in TS-evoked synaptic transmission was tested by application of the specific 5-HT2CR agonist CP809101. As demonstrated in the representative traces in Fig. 3A, 5-HT2CR activation by CP809101 (50 μM) increased the amplitude of TS-EPSCs evoked at 0.5 Hz (30 sweeps, averaged) from baseline aCSF control. TS-EPSC amplitude increased from 104.2 ± 18.7 pA (baseline control) to 137.1 ± 21.5 pA with 50 μM CP809101 (P < 0.05, n = 7; Fig. 3B), an augmentation to 132.2 ± 13.6% of baseline control. With washout of CP809101, TS-EPSC amplitude was 146.0 ± 51.2 pA. On the other hand, 1 and 10 μM CP809101 did not significantly alter TS-EPSC amplitude [1 μM (n = 5) control 101.4 ± 24.9 pA vs. CP809101 97.1 ± 23.5 pA and 10 μM (n = 5) control 109.8 ± 31.8 pA vs. CP809101 127.9 ± 19.8 pA]. Therefore, 50 μM was used for the remainder of the protocols.
Fig. 3.
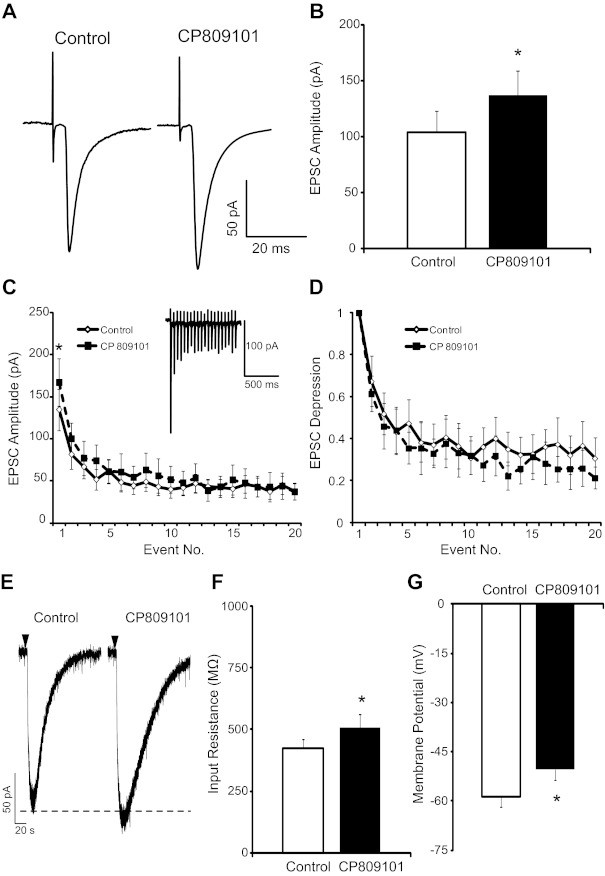
5-HT2CR activation augments TS excitatory postsynaptic currents (EPSCs). A: representative traces of TS-EPSCs (30 sweeps, averaged) evoked at 0.5 Hz from a single cell during control and 5-HT2CR activation by CP809101. Note the augmented amplitude after the 5-HT2CR agonist CP809101 (50 μM, 5 min). B: group raw data of TS-EPSC amplitude during control and CP809101 (50 μM, 5 min, n = 7). C: TS stimulation at 20 Hz produced use-dependent depression (◇), which was maintained after CP809101 (■, n = 7). An example of the observed use-dependent depression is shown in inset. There was an increase in event 1 amplitude with CP809101 (P < 0.05) but no change in amplitude of subsequent events. D: relative magnitude of EPSC depression from the 1st event (plotted as 1). E: brief application of AMPA (1 mM, 50 ms) evoked inward currents that were increased by CP809101. Downward arrowhead depicts application of AMPA. F and G: 5-HT2CR activation with CP809101 (n = 7) increased input resistance (F) and depolarized membrane potential (G). *P < 0.05.
To further define the role of 5-HT2CR activation on synaptic currents and to mimic sustained increases in in vivo sensory afferent activity to the nTS, stimulation frequency of the TS was increased to 20 Hz (20 events). As is typical of the first synapse of the nTS, during baseline control (aCSF) repeated TS stimulation progressively reduced synaptic current amplitude following the first event (use-dependent depression; Fig. 3C, example shown in inset). Bath application of 50 μM CP809101 increased the amplitude of the first TS-EPSC (Fig. 3C; n = 7, P < 0.05) but not subsequent events. The relative magnitude of use-dependent depression following the first TS-EPSC, a measure sensitive to changes in presynaptic neurotransmitter release properties, was not altered by CP809101 compared with control (Fig. 3D; n = 7). In addition, the ratio of TS-EPSC2 to TS-EPSC1 (PPR), another indicator of alterations in presynaptic release or postsynaptic receptor properties, was not altered by CP809101 (control 0.67 ± 0.12 vs. CP809101 0.61 ± 0.08, n = 7).
To further define the synaptic locus of 5-HT2CR actions, we examined the effects of CP809101 (50 μM) on exogenous AMPA-induced currents. A representative example of the effect of CP809101 on AMPA-induced currents is shown in Fig. 3E. Brief application of AMPA (1 mM, 50 ms) induced inward current averaging 149 ± 48 pA (n = 4). CP809101 significantly increased the relative amplitude of AMPA currents to 108.9 ± 2.4% of control (P = 0.035, n = 4).
Compared with control, CP809101 did not alter TS-EPSC decay time constant (control 3.9 ± 0.5 ms vs. CP809101 6.5 ± 3.7 ms), holding currents (control −5.1 ± 12.4 pA vs. CP809101 −21.3 ± 11.1 pA), or failure rate (control 0.5 ± 0.5% vs. CP809101 1.4 ± 1.0%). However, CP809101 increased input resistance (Fig. 3F; n = 7, P < 0.05) and, under current clamp conditions (I = 0), depolarized the membrane (Fig. 3G; n = 7, P < 0.05).
5-HT2C receptors augment TS-EPSCs independent of GABAA receptor signaling.
To examine the possibility that activation of 5-HT2CRs alters synaptic transmission, membrane conductance, or holding currents in nTS by altering GABAergic signaling, we examined these parameters in the presence of the GABAA receptor antagonist gabazine (25 μM) and CP809101 (50 μM). Figure 4A is an example of the effect of CP809101 on TS-EPSCs in the presence of gabazine. In the company of gabazine, CP809101 increased TS-EPSC amplitude from 134.9 ± 26.5 pA (baseline gabazine control) to 159.6 ± 28.2 pA (P = 0.024, n = 6; Fig. 4B), an increase to 123.3 ± 7.9% of gabazine alone. A representative time course for the effect of CP809101 is shown in Fig. 4C. In addition, input resistance increased from 714.7 ± 135.6 MΩ (gabazine control) to 854.4 ± 178.4 MΩ (gabazine + CP809101; P = 0.033, n = 7). Holding currents were not altered by CP809101 in the presence of gabazine (gabazine control −37.5 ± 17.6 mV vs. gabazine + CP809101 −36.3 ± 13.7 mV). These data indicate that the alteration in TS-EPSC amplitude or input resistance observed in the presence of 5-HT2CR activation cannot be explained solely by shifts in GABAergic transmission.
Fig. 4.
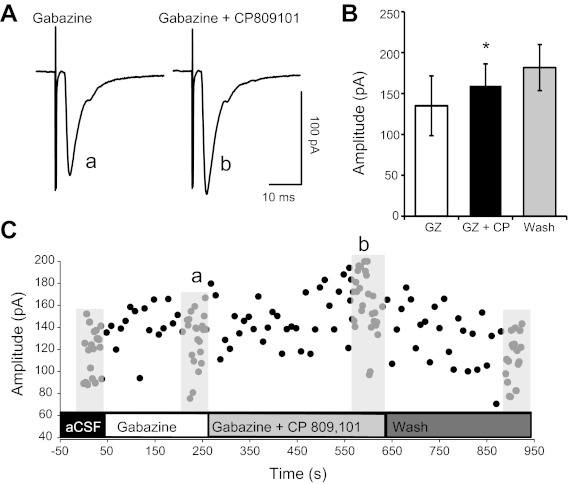
Enhanced TS-EPSCs with 5-HT2CR activation occur in presence of GABAA receptor blockade. A: representative example of TS-EPSCs evoked in the presence of gabazine and the 5-HT2CR agonist CP809101 (50 μM). Application of gabazine did not prevent the increase in TS-EPSC amplitude by CP809101. B: average amplitude for each TS-EPSC stimulated at 0.5 Hz for gabazine (GZ), gabazine + CP809101 (GZ+CP), and wash. CP809101 significantly increased TS-EPSC amplitude from control. *P < 0.05. C: time course of the example shown in A. Lower case letters in A and C depict the time of representative examples, evoked at 0.5 Hz. Other time points were recorded at 0.1 Hz.
5-HT2C receptor activation and miniature EPSCs.
To identify a possible pre- or postsynaptic locus of 5-HT2CR's role in neurotransmission, mEPSCs were examined. Tetrodotoxin (1 μM) and gabazine (25 μM) were added to the aCSF to block action potentials and GABAA receptor-mediated inhibitory currents, respectively. After bath application of CP809101 (Fig. 5A), there was a small but nonsignificant increase in mEPSC amplitude across the neurons studied (control 18.3 ± 2.2 pA vs. CP809101 20.8 ± 2.3, n = 12, P = 0.14; Fig. 5B). During washout, mEPSC amplitude was 18.6 ± 1.8 pA. In the presence of CP809101, the frequency of mEPSC was not altered (control 14.9 ± 2.1 Hz vs. CP809101 15.0 ± 2.2 Hz, n = 12, P = 0.95; Fig. 5C). During washout, mEPSC frequency was 13.9 ± 2.3 Hz.
Fig. 5.
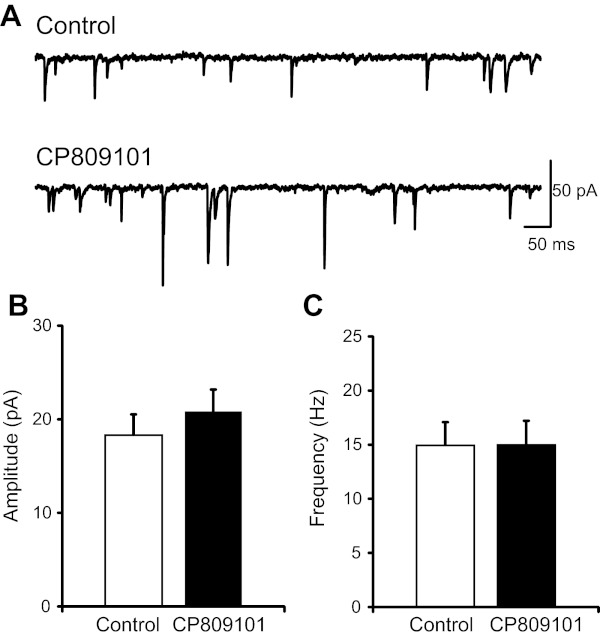
5-HT2CR activation does not alter miniature (m)EPSCs. A: representative traces of mEPSCs during aCSF control (top) and after bath application of CP809101 (50 μM, 5 min, bottom). B and C: quantitative analysis of mEPSC events demonstrated that CP809101 (n = 12) does not alter the amplitude (B) or frequency (C) of mEPSCs.
Activation of 5-HT2CR reduces K+ currents.
Activation of 5-HT2CR may alter TS-EPSCs, depolarize membrane potential, and increase input resistance by modulation of one or more ion channels. One possibility includes reduction in G protein-coupled potassium currents, in particular G protein inward-rectifying potassium (GIRK) channels that have been suggested to be modulated by 5-HT2CR (Qui et al. 2007). We explored this possibility by monitoring holding current during voltage ramps from −110 to −40 mV over 200 ms during control and 5-HT2CR activation by CP809101 (50 μM). An example of one cell is shown in Fig. 6A. Slope conductance during baseline control was 1.10 ± 0.29 pA/mV, which was significantly reduced by CP809101 to 0.88 ± 0.21 pA/mV (P = 0.031, n = 7, a significant reduction to 83.0 ± 5.3% of control). The reversal potential of this CP809101-sensitive current was −91.70 ± 6.7 mV (n = 7; an example is shown in Fig. 6B), close to the calculated K+ equilibrium potential in our slice preparation (−99 mV).
Fig. 6.
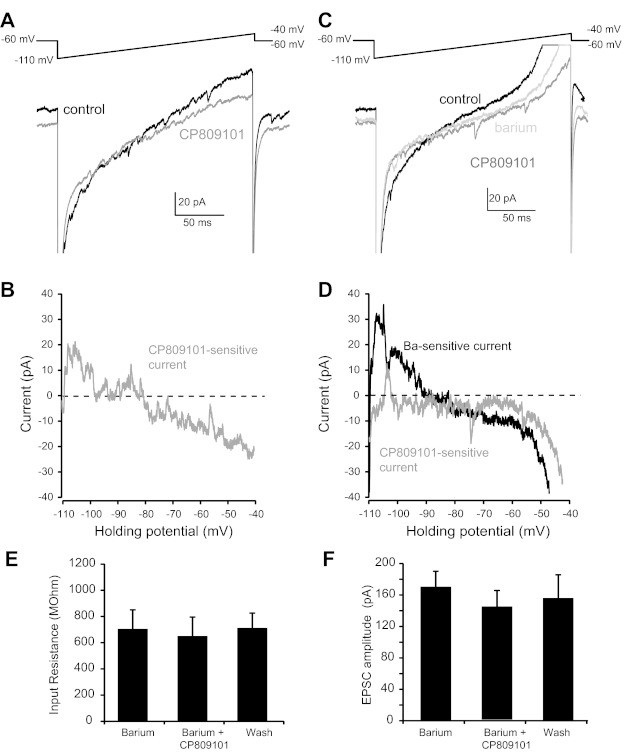
Activation of 5-HT2CR inhibits potassium currents. To better characterize the ionic basis of CP809101 on membrane properties, a depolarizing ramp protocol (−110 to −40 mV, 200 ms; A and C, top) was performed and membrane currents were recorded. A: compared with control (black trace), CP809101 (dark gray trace) decreased slope conductance which reversed at ∼90 mV. B: the subtracted CP809101-sensitive curve reverses near the K+ equilibrium potential. C: barium (light gray trace) alone decreased membrane conductance from control (black traces). Barium also prevented the change in slope conductance by CP809101 (dark gray trace). D: the subtracted barium-sensitive current reversed ∼90 mV, while the CP-sensitive current was eliminated in barium. E and F: barium prevented the CP809101-induced alterations in input resistance (E; from −60 mV to −65 mV) and TS-EPSCs (F; 0.5 Hz).
Inward-rectifying potassium currents, including those sensitive to 5-HT, are blocked by low concentrations of barium (Bayliss et al. 1997). In a separate group of neurons, we tested whether the CP809101-sensitive current could be blocked by pretreatment with barium (100 μM, n = 4). An example of the reduction in slope conductance by barium is shown in Fig. 6C. This barium-sensitive current reversed at −90.29 ± 1.8 mV, close to our calculated K+ equilibrium potential and not significantly different from the reversal potential for the CP-sensitive current (P = 0.87, barium-sensitive vs. CP809101-sensitive current). Bath application of CP809101 in the presence of barium did not further alter slope conductance compared with barium alone (barium 0.46 ± 0.07 pA/mV vs. CP809101 0.44 ± 0.07 pA/mV, P = 0.11). A representative example of the lack of effect of CP809101 on conductance in the presence of barium is shown in Fig. 6C, with subtracted currents shown in Fig. 6D. In the presence of barium, CP809101 did not increase input resistance measured between −60 and −65 mV (Fig. 6E) or TS-EPSC amplitude (Fig. 6F). Taken together, these data suggest that the CP809101-sensitive current is predominantly carried by a barium-sensitive K+ current.
Blockade of 5-HT2C receptors attenuates agonist-induced increases in TS-EPSCs.
We examined whether the synaptic and membrane actions of the 5-HT2CR agonist CP809101 could be attenuated or eliminated by preblockade of 5-HT2CR with the antagonist RS102221. Prior application of RS102221 (100 μM, 60 s) eliminated the increase in synaptic currents and membrane actions of CP809101 [50 μM, 5 min, in the presence of RS102221 (100 μM), n = 6]. Compared with baseline control, CP809101 in the presence of RS102221 (CP + RS) did not alter TS-EPSCs evoked at 0.5 Hz (control 235.3 ± 77.8 pA vs. CP + RS 213.6 ± 76.4 pA), holding currents (control 5.7 ± 23.5 pA vs. CP + RS −5.8 ± 25.6 pA), and input resistance (control 641.8 ± 127.7 MΩ vs. CP + RS 606.9 ± 126.0 MΩ). Likewise, there was no significant change with CP + RS from control in TS-EPSCs during a 20-Hz stimulus train, including the overall EPSC amplitude and the magnitude of synaptic depression (data not shown). Taken together, these results demonstrating that CP809101 actions are blocked by RS102221 suggest that its actions occur through the 5-HT2CR.
Blockade of 5-HT2C receptors reduces TS-EPSCs.
5-HT2 receptors may exhibit tonic activity (Aloyo et al. 2009; Roth et al. 1998). To determine whether 5-HT2CRs have a basal tonic level of activation in the nTS slice, we bath applied the 5-HT2CR antagonist RS102221 alone and recorded synaptic currents. As shown in the representative traces in Fig. 7A, 5-HT2CR blockade with RS102221 reduced TS-EPSC amplitude evoked at 0.5 Hz (30 sweeps, averaged) from aCSF control. Quantitatively, bath application of RS102221 significantly reduced TS-EPSC current amplitude (Fig. 7B; n = 9, P < 0.05) to 82.7 ± 6.9% of baseline control. When stimulation frequency was increased to 20 Hz, use-dependent depression was observed during control aCSF (Fig. 7C). 5-HT2CR blockade with RS102221 reduced the overall TS-EPSC amplitude (Fig. 7C; P < 0.05, n = 9). There was, however, no alteration in the relative magnitude of synaptic depression from the first event (Fig. 7D; n = 9) or PPR (control 0.52 ± 0.07 vs. RS102221 0.47 ± 0.08, n = 9).
Fig. 7.
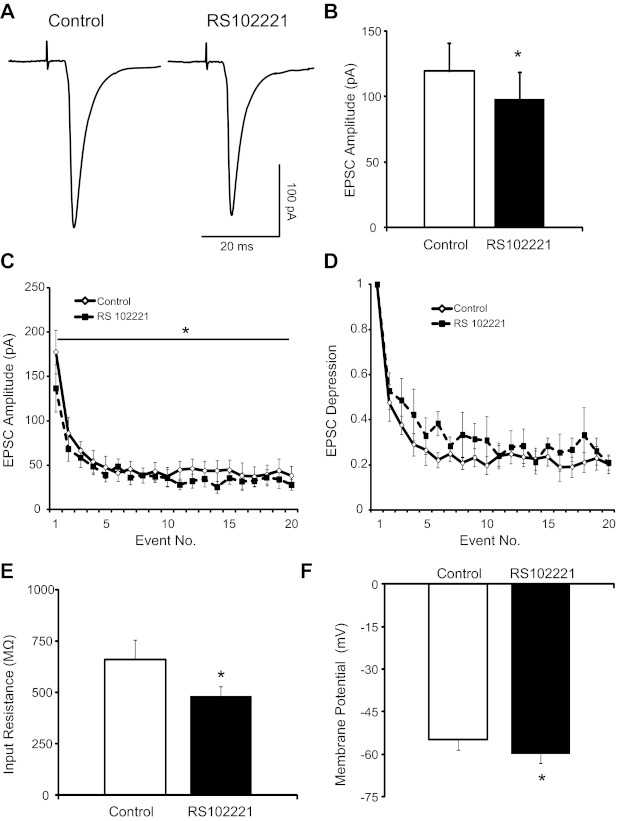
5-HT2CR blockade reduce TS-EPSCs. A: representative traces of TS-EPSCs (30 sweeps, averaged) evoked at 0.5 Hz from a single cell during control and 5-HT2CR blockade by RS102221. Note the reduced amplitude after RS102221 (100 μM, 5 min). B: group raw data (n = 9) of TS-EPSC amplitude during control and RS102221. C: TS stimulation at 20 Hz produced use-dependent depression (◇), which was maintained after RS102221 (■). There was a decrease in the overall amplitude of TS-ESPC compared with control (P < 0.05, 2-way repeated-measures ANOVA). D: relative magnitude of EPSC depression from the 1st event. E and F: 5-HT2CR blockade with RS102221 (n = 9) decreased input resistance (E) and hyperpolarized membrane potential (F). *P < 0.05.
Compared with control, RS102221 did not alter TS-EPSC decay time constant (control 5.1 ± 1.1 ms vs. RS102221 5.8 ± 2.2 ms), holding currents (control −21.4 ± 6.1 pA vs. RS102221 −23.7 ± 10.0 pA), or failure rate (control 2.5 ± 1.5% vs. RS102221 5.0 ± 3.1%). However, RS102221 decreased input resistance (Fig. 7E; n = 9, P < 0.05) and, under current-clamp conditions (I = 0), hyperpolarized the membrane (Fig. 7F; n = 9, P < 0.05).
Blockade of 5-HT2C receptors reduces mEPSC amplitude.
mEPSCs were recorded to define the site of action of the decrease in EPSC amplitude by 5-HT2CR blockade. As demonstrated in the representative traces in Fig. 8A, RS102221 reduced the amplitude of miniature synaptic currents but had little effect on their frequency compared with aCSF control. Quantitatively, RS102221 reduced the amplitude of mEPSCs (control 19.8 ± 2.9 pA vs. RS102221 15.2 ± 2.4 pA; Fig. 8B; n = 6, P < 0.05). During washout, mEPSC amplitude was 13.6 ± 2.6 pA. By contrast, RS102221 did not alter mEPSC frequency (control 15.7 ± 3.1 Hz vs. RS102221 15.6 ± 2.9 Hz; Fig. 8C; n = 6). During washout, mEPSC frequency was 16.9 ± 2.5 Hz.
Fig. 8.
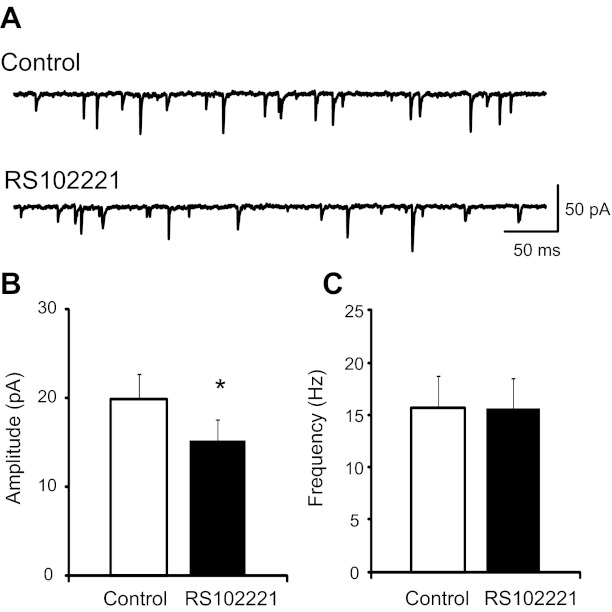
5-HT2CR blockade reduces mEPSCs. A: representative traces of mEPSCs during control (top) and after bath application of RS102221 (100 μM, 5 min; bottom). B and C: quantitative analysis of mEPSC events demonstrated that RS102221 (n = 6) significantly reduced the amplitude (B) but not frequency (C) of events. *P < 0.05.
5-HT2C receptors modulate synaptic transmission in the chemoafferent-nTS pathway.
In a subset of animals, we examined whether 5-HT2CRs modulate synaptic transmission in nTS cells that receive afferents from the carotid body chemoreceptors. Labeling of the carotid body with DiI prior to slice generation produced fluorescent terminals throughout the medial and commissural nTS, as previously described (Kline et al. 2002, 2010) and consistent with carotid body terminals in the nTS (Finley and Katz 1992). These chemosensory-rich, DiI-labeled afferents were in close apposition to nTS cells and identified neurons that received monosynaptic projections from afferent fibers (Fig. 9A). As in the general population, stimulation of the TS evoked invariant EPSCs in nTS cells directly apposed to DiI-labeled chemoafferents. Overall, DiI-labeled cells (n = 13) had a latency of 6.0 ± 0.9 ms and jitter of 181 ± 20.8 μs, suggesting they are monosynaptic (Fig. 9B, inset). Comparing DiI-labeled to unlabeled nTS neurons, synaptic latency and jitter were comparable (P > 0.05 for both). However, TS-EPSC amplitude from DiI-labeled neurons averaged 80.2 ± 13.3 pA (n = 13), which was significantly smaller than that from unlabeled neurons (DiI-labeled vs. unlabeled 134.2 ± 13.7 pA, n = 48, P < 0.05).
Fig. 9.
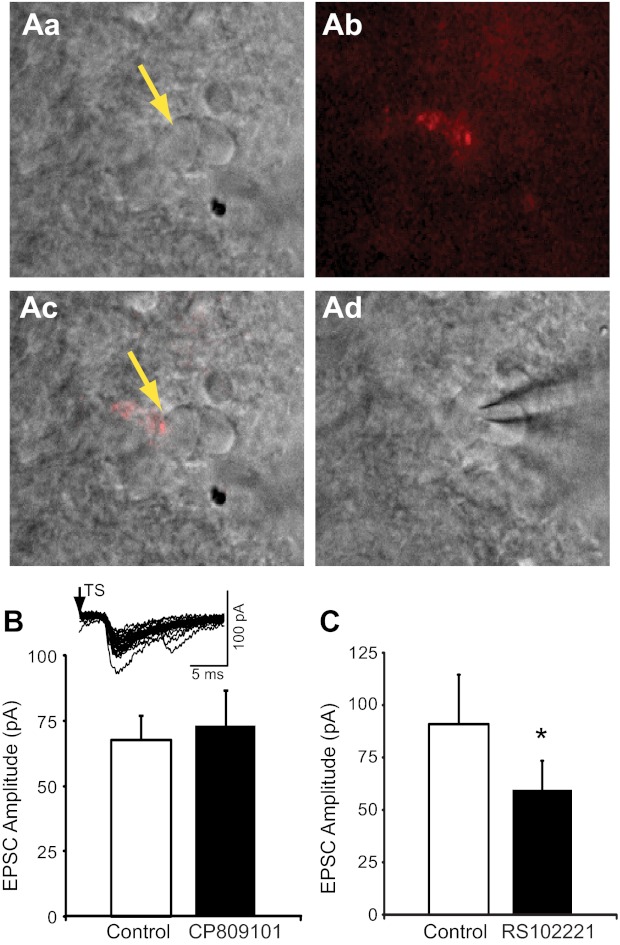
5-HT2CRs modulate synaptic transmission from chemosensory afferents in the nTS. A: example of a DiI fluorescent synaptic bouton labeled from the carotid body on a caudal nTS cell. Aa: infrared-differential interface contrast (IR-DIC) image of nTS cells medial to the TS. Yellow arrow denotes labeled cell. Ab: DiI labeling (pseudocolored red) of synaptic boutons from chemoafferent fiber visualized under fluorescence. Ac: overlay of images from Aa and Ab illustrates that the fluorescent boutons were on the soma of the nTS neuron. Ad: after identification of a bouton-labeled nTS cell, a patch electrode was guided to the cell under IR-DIC for recording. B: group raw data (n = 6) of TS-EPSC amplitude during control and CP809101. Inset: representative example of TS-EPSCs evoked from a DiI-labeled nTS cell. Note the low variability of TS-evoked EPSCs, indicative of a monosynaptic cell. C: group raw data (n = 7) of TS-EPSC amplitude that was reduced by RS102221 compared with control. *P < 0.05.
Activation of 5-HT2CR with 50 μM CP809101, which produced significant and robust changes in TS-EPSCs in unlabeled neurons (see Fig. 3), did not increase TS-EPSC amplitude evoked at 0.5 Hz in DiI-labeled cells (control 67.8 ± 9.4 pA vs. CP809101 73.2 ± 13.5 pA; Fig. 9B; n = 6, 105.3 ± 0.2% of baseline control; wash 46.6 ± 18.6 pA). Moreover, in these DiI-labeled cells CP809101 did not alter EPSC amplitude at 20 Hz (20 events), the relative magnitude of synaptic depression (data not shown), or the PPR (control 0.39 ± 0.1 vs. CP809101 0.55 ± 0.05, n = 6). Compared with aCSF, no alteration to TS-EPSC decay time constant (3.9 ± 0.9 vs. 3.1 ± 0.5 ms), holding currents (18.8 ± 23.1 vs. 3.2 ± 20.4 pA), or percent failure rate (4.2 ± 2.5% vs. 1.7 ± 1.7%) was observed with CP809101, respectively. Exogenous 5-HT2CR activation also did not alter input resistance (control 404.1 ± 52.4 MΩ vs. CP809101 503.1 ± 86.8 MΩ, n = 6). There was, however, a significant depolarization of the membrane (I = 0; control −63.2 ± 5.3 mV vs. CP809101 −53.5 ± 5.2 mV, n = 6, P < 0.05).
We also examined the potential tonic activation of endogenous 5-HT2CR in DiI carotid body-labeled nTS cells (n = 7). This again was tested through application of the 5-HT2CR antagonist RS102221 (100 μM), which produced robust decreases in synaptic activity in unlabeled cells (see Fig. 7). RS102221 significantly reduced TS-EPSC amplitude evoked at 0.5 Hz from baseline control (control 90.9 ± 23.5 pA vs. RS102221 59.6 ± 13.8 pA; Fig. 9C; n = 7, P < 0.05; wash 41.9 ± 12.3 pA). Overall, RS decreased TS-EPSC amplitude to 72.9 ± 9.1% of control. When stimulation frequency was increased to 20 Hz, 5-HT2CR blockade with RS10221 reduced the amplitude of the first three TS-EPSCs but not subsequent events, yet did not alter the relative magnitude of synaptic depression (data not shown) or the PPR (control 0.32 ± 0.06 vs. RS102221 0.64 ± 0.15, n = 7). Compared with control, no changes to TS-EPSC decay time constant (3.4 ± 0.8 vs. 4.1 ± 1.6 ms), holding currents (−7.4 ± 2.9 vs. −16.3 ± 7.1 pA), or percent failure rate (11.4 ± 5.3% vs. 11.4 ± 4.5%) were observed in DiI-labeled cells with RS102221. RS102221 also did not alter input resistance (control 811.9 ± 257.3 MΩ vs. RS102221 571.1 ± 107.2 MΩ, n = 7) or membrane potential (I = 0; control −57.1 ± 3.6 mV vs. RS102221 −58.0 ± 3.0 mV, n = 7).
DISCUSSION
In the present study, we demonstrate that 5-HT2CRs are present in the nTS. Activation of 5-HT2CRs augments excitatory synaptic transmission, whereas blockade of 5-HT2CRs decreases synaptic currents. Blockade of 5-HT2CRs in the carotid body afferent-nTS pathway also reduced synaptic amplitude, suggesting that 5-HT2CRs also function in the chemoafferent-nTS circuit. These data are consistent with a tonic role of 5-HT2CRs in the nTS. Modulation of synaptic currents by 5-HT2CRs occurs primarily through postsynaptic mechanisms.
The nTS contains a dense network of 5-HT fibers and a multitude of 5-HT receptors. The demonstration that 5-HT2CRs are present and functional in the nTS is consistent with reports detailing 5-HT2CR message (Fonseca et al. 2001) and physiological relevance (Jordan 2005) in this nucleus. We extended these studies to show that 5-HT2CR-IR was found in fiberlike processes associated with MAP-2-labeled dendrites and PSD-95 in the nTS. This is consistent with previous reports demonstrating that 5-HT2 receptors colabel with MAP-1A in the cortex (Cornea-Herbert et al. 2002) and PSD-95 in other central nuclei (Abbas et al. 2009; Anastasio et al. 2010). By contrast, 5-HT2CR-IR did not colabel with synaptophysin- or TPH-labeled synaptic terminals, suggesting that 5-HT2CRs are not located in presynaptic terminals, including the terminals of raphé neurons that project to the nTS. The greater relative expression of 5-HT2CR message in the nTS compared with that of NPG would further suggest a propensity of 5-HT2CRs to modulate postsynaptic nTS activity through its production in this nucleus rather than afferent neurotransmitter release. Future electron microscopy studies are required to fully identify 5-HT2CR synaptic location in nTS. Overall, we interpret these finding that 5-HT2CRs are localized, although perhaps not exclusively, to the postsynaptic membrane of nTS cells.
Activation of 5-HT2CRs in the nTS augmented the amplitude of TS-evoked EPSCs and AMPA-induced inward currents, increased input resistance, and depolarized membrane potential indicative of postsynaptic alterations. Conversely, there was no change in the PPR, use-dependent depression, or failure rate, presynaptic neurotransmitter release properties that were not altered by 5-HT2CR activation. Together, these data are indicative of a postsynaptic mechanism of action that occurs through non-NMDA receptors, results that were supported by our immunohistochemical observations. Our results also support studies from other central nuclei that 5-HT2CRs augment excitatory synaptic transmission via postsynaptic mechanisms (Bonsi et al. 2007; Di Giovanni et al. 2001; Rick et al. 1995). An excitatory role for 5-HT2CRs was also observed in 5-HT2CR knockout mice, which exhibit impaired hippocampal long-term potentiation (Tecott et al. 1998). Overall, 5-HT2CRs in the nTS enhance synaptic transmission through postsynaptic mechanisms.
In contrast to their activation, blockade of 5-HT2CRs decreased TS-EPSC and mEPSC amplitude, reduced input resistance, and hyperpolarized the membrane. The changes in the above parameters to 5-HT2CR blockade suggest that its effects occur primarily by postsynaptic mechanisms, similar to those of receptor activation. This is further supported by the lack of alteration in failure rate, PPR, and use-dependent depression. The alterations in synaptic parameters to 5-HT2CR blockade further indicate that these receptors may be tonically active in the nTS. Tonic, constitutive activity has been observed in several G protein-coupled receptors, including the 5-HT2A and 5-HT2C receptors (Aloyo et al. 2009; Roth et al. 1998). This tonic activation of 5-HT2CRs may also underlie the modest effect of 5-HT2CR activation on mEPSC amplitude. Whether 5-HT2CRs are near saturation or the downstream pathways responsible for their effect are constitutively activated requires further study. Nevertheless, 5-HT2CR activation enhanced TS-EPSC and AMPA-induced inward current amplitude to indicate that not all receptors are fully saturated or activated. Tonic activity of 5-HT2 receptors has been demonstrated in behavioral studies, associative learning and many central nuclei (see Aloyo et al. 2009), including the nTS (Raul 2003).
Several mechanisms may play a role in 5-HT2CR modulation of glutamatergic synaptic transmission in nTS. Activation of 5-HT2CR may inhibit GABAergic transmission, resulting in synaptic disinhibition and increased glutamatergic signaling. However, blockade of GABAA receptors with gabazine did not alter agonist-induced augmentation in TS-EPSC amplitude or input resistance. Furthermore, blockade of 5-HT2CRs modulated mEPSC amplitude in the presence of gabazine. Cells were also held near the reversal potential for chloride, minimizing GABA's influence on EPSCs. Thus 5-HT2CR may modulate glutamatergic neurotransmission independent of GABA in the nTS. It is likely that 5-HT2CRs modulate glutamatergic signaling via its downstream pathways. 5-HT2CRs activate, through G protein-coupled proteins, phospholipase C (PLC), and protein kinase C, to subsequently alter downstream pathways and synaptic signaling (Raymond et al. 2006), including glutamatergic signaling (Bonsi et al. 2007; Di Giovanni et al. 2001; Rick et al. 1995). 5-HT2CRs have also been shown to interact with PSD-95, an essential regulator of ionotropic glutamatergic signaling (Anastasio et al. 2010), and knockout of PSD-95 in mice severely reduces 5-HT2CR targeting, expression, and signaling (Abbas et al. 2009). Our results illustrating that 5-HT2CR colabeled with PSD-95 and altered EPSCs support a role for 5-HT2CRs in modulating glutamatergic signaling through their action at the postsynaptic cell.
Additional postsynaptic events may be superimposed on alterations in glutamatergic transmission, as evidenced by changes in input resistance and membrane potential by 5-HT2CR activation and blockade. 5-HT2CRs may modulate one or more ion channels in the postsynaptic membrane, including GIRK channels. Interestingly, these channels are inhibited by 5-HT2CRs via a PLC mechanism in hypothalamic proopiomelanocortin neurons (Qui et al. 2007). Our data demonstrating that 5-HT2CR activation decreases slope conductance of membrane currents between −110 and −40 mV, with a CP809101-sensitive current that reverses near the reversal potential of potassium, are consistent with the closing of a potassium channel. Moreover, the effects of CP809101 on slope conductance and agonist-sensitive current are blocked by barium, which at the present concentration inhibits inward-rectifying potassium channels (Bayliss et al. 1997). Together, these data are consistent with the studies of Qui et al. (2007) showing that GIRK channels are inhibited by 5-HT2CR. Barium also blocked the CP809101-induced changes in input resistance and TS-EPSCs, suggesting a potential common mechanism. These results are consistent with inward-rectifying channels in other systems modulating membrane properties and synaptic transmission through postsynaptic mechanisms (John and Manchanda 2011; Luscher et al. 1997; Podda et al. 2010; Takigawa and Alzheimer 2002). Thus 5-HT2CRs likely modulate postsynaptic properties, in part though modulation of inward-rectifying potassium channels.
Our data are in contrast to others that suggest that 5-HT2 receptors inhibit nTS synaptic activity (Feldman 1995; Sevoz-Couche et al. 2000b; Takenaka et al. 2011). Takenaka et al. (2011) demonstrated a reduction in TS-EPSC amplitude by α-methyl 5-HT, a purported 5-HT2 receptor agonist, that was abolished by the general 5-HT2 receptor antagonist ketanserin. These studies suggested a presynaptic mechanism of action, while our data demonstrate that location and activity of 5-HT2CR in the nTS are postsynaptic. The difference in the studies may result from the pharmacological agents used. For instance, in the rat, α-methyl 5-HT binds to 5-HT2B receptors with the highest affinity, but also 5-HT2A, 5-HT4, and 5-HT6 receptors (Sharman et al. 2011). Likewise, ketanserin blocks 5-HT2A with high affinity, but also 5-HT7 and 5-HT2B receptors (Sharman et al. 2011). Given that 5-HT2A, 5-HT4, and 5-HT7 receptors are functionally present in the nTS (Edwards and Paton 1999; Oskutyte et al. 2009; Sevoz-Couche et al. 2000c), they may also play a role in previously observed synaptic responses. The difference between our study and the study of Sevoz-Couche et al. (2000b) that demonstrated an inhibitory role of 5-HT2CR in vivo may be due to not only the agonists used but also the preparation. This may include not only influences from an intact GABAergic system, among others, but also influences from other nTS cells or central nuclei. However, more studies are required to fully discern that information. In the present study, we utilized an agonist and antagonist that to date are only known to affect 5-HT2 receptors, with the most potent response directed to the 5-HT2CRs. Our results demonstrating the effects of the 5-HT2CR agonist are blocked by the antagonist further suggest that they both work through the 5-HT2CR.
We also examined the role of 5-HT2CRs in modulating synaptic transmission between carotid body chemoafferents and second-order nTS cells. We reasoned that 5-HT2CRs may play a role in the chemoafferent-nTS pathway based on 5-HT's role in mediating carotid body sensory activity, breathing, and central respiratory activity (Cayetanot et al. 2001; Hodges and Richerson 2008; Prabhakar 2011; Sessle and Henry 1985). Results demonstrated that TS-EPSC amplitude in chemosensory-rich (DiI) nTS neurons was reduced compared with general unlabeled visceral neurons. The intrinsic properties of the chemoafferent fibers likely contribute to their reduced EPSC amplitude. In particular, EPSC amplitude in the nTS is lower in synapses innervated by C-type fibers compared with A-type fibers (Andresen and Peters 2008). Given that carotid body chemosensory afferents are made up of 80–85% unmyelinated C-fibers (McDonald 1983), it is not surprising that EPSC amplitude derived from activation of this chemosensory-rich population may be reduced compared with other afferents including A-type and other lightly myelinated afferents. Interestingly, 5-HT2CRs also modulate synaptic activity in the chemosensory-nTS circuit. While activation and blockade of 5-HT2CRs in general visceral unlabeled afferents modulated TS-EPSCs, in chemoafferent-rich nTS neurons only blockade of 5-HT2CR modified TS-EPSC amplitude. This may indicate a tonic activation of 5-HT2CRs in the chemoafferent-nTS synapse, as in the general population. Furthermore, these chemoafferent-nTS synapses may be near saturation, as TS-EPSCs were not further increased upon administration of the receptor agonist. Thus one likely site of 5-HT2CR modulation of the cardiorespiratory system is the initial chemoafferent-nTS synapse.
5-HT is an important neuromodulator in many physiological processes, including in the autonomic control of heart rate and blood pressure, respiration, and gastrointestinal systems (Crowell and Wessinger 2007; Hodges and Richerson 2008; Ramage 2001), as well as responses to their perturbations, such as hemorrhage (Kung and Scrogin 2011). The nTS is the central site for the processing and modulation of the cardiovascular, respiratory, and gastroesophagointestinal systems and recently has been postulated to be involved in coordinated, integrated control of these vital systems (Dean 2011). In the nTS, 5-HT2 receptors modulate blood pressure and heart rate, with activation of 5-HT2CRs inducing bradycardia (Raul 2003). These 5-HT2-mediated cardiovascular effects may be tonic at rest, as lesion of 5-HT neurons in the nodose ganglia raises blood pressure and heart rate. In vivo work has suggested that 5-HT2 receptors are located postsynaptic to vagal afferents (Merahi et al. 1992), and our study is consistent with this conclusion. Systemic injection of 5HT2A/2C agonists decreases respiration and induces Fos, a neuronal activation marker, in the nTS (Cayetanot et al. 2001). Furthermore, application of 5-HT2CR agonists to the 4th ventricle decreases respiratory-like burst frequency in newborn pups (Khater-Boidin et al. 1999). Projections from nTS to medullary respiratory centers, including expiratory nuclei, have been identified in the rat (Alheid et al. 2011; Nunez-Abades et al. 1993). In the nearby dorsal motor nucleus of the vagus, 5-HT2 receptors modulate synaptic transmission in neurons that project to the stomach and gut (Browning and Travagli 1999), with part of their actions likely occurring in the nTS. While the projection site(s) of the presently recorded nTS neurons is not known, our results would suggest that 5-HT2CR plays an important role in the integrated cardiorespiratory and/or gastroesophagointestinal system via, in part, its actions in the nTS. The general excitatory role of 5-HT2CR in the nTS may serve to augment the gain of a particular glutamatergic synapse, such as those involved in the baroreflex to induce bradycardia. Moreover, its tonic activation may also allow greater control of synaptic signaling, enabling the fine-tuning of incoming reflex information. Overall, the synaptic and intrinsic properties of 5-HT2CRs in the nTS likely influence reflex regulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-085108 (D. D. Kline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.A. and D.D.K. conception and design of research; J.R.A., H.A.D., B.K.B., and D.D.K. performed experiments; J.R.A., H.A.D., B.K.B., and D.D.K. analyzed data; J.R.A. and D.D.K. interpreted results of experiments; J.R.A. and D.D.K. prepared figures; J.R.A. and D.D.K. drafted manuscript; J.R.A., H.A.D., B.K.B., and D.D.K. edited and revised manuscript; J.R.A., H.A.D., B.K.B., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Diana L. Kunze and Dr. Tim D. Ostrowski for helpful comments on the manuscript.
REFERENCES
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci 29: 7124–7136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi-Mendonca D, Castania JA, Bonagamba LG, Machado BH, Leao RM. Synaptic profile of nucleus tractus solitarius neurons involved with the peripheral chemoreflex pathways. Neuroscience 197: 107–120, 2011 [DOI] [PubMed] [Google Scholar]
- Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience 190: 207–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121: 160–173, 2009 [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem 113: 1504–1515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius—common denominators. Chem Senses 21: 387–395, 1996 [DOI] [PubMed] [Google Scholar]
- Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol 295: H2032–H2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Dantzler HA, Kline DD. 5-Hydroxytryptamine receptor 2C (5-HT2C) augments synaptic currents in the nucleus tractus solitarii (nTS) (Abstract). FASEB J 25: 844.9, 2011a [Google Scholar]
- Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of Group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience 159: 701–716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Hermann G, Dantzler HA, Rogers RC, Kline DD. Hydrogen sulfide (H2S) augments synaptic neurotransmission in the nucleus of the solitary (NTS). J Neurophysiol 106: 1822–1832, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol 77: 1349–1361, 1997 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36: 621–629, 1997 [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier D, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32: 1840–1854, 2007 [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurons of the rat dorsal motor nucleus of the vagus (DMV). Br J Pharmacol 128: 1307–1315, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. 5-HT2A/2C receptor-mediated hypopnea in the newborn rat: relationship to fos immunoreactivity. Pediatr Res 50: 596–603, 2001 [DOI] [PubMed] [Google Scholar]
- Comet MA, Vernard JF, Hamon M, Laguzzi R, Sevoz-Couche C. Activation of nucleus tractus solitarius 5-HT2A but not other 5-HT2 receptor subtypes inhibits the sympathetic activity in rats. Eur J Neurosci 26: 345–354, 2007 [DOI] [PubMed] [Google Scholar]
- Cornea-Herbert B, Watkins K, Roth B, Kroeze W, Gaudreau P, Leclerc N, Descarries L. Similar ultrastructural distribution of the 5-HT2A serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience 113: 23–35, 2002 [DOI] [PubMed] [Google Scholar]
- Crowell MD, Wessinger SB. 5-HT and the brain-gut axis: opportunities for pharmacologic intervention. Expert Opin Investig Drugs 16: 761–765, 2007 [DOI] [PubMed] [Google Scholar]
- Dean JB. Theory of gastric CO2 ventilation and its control during respiratory acidosis: implications for central chemosensitivity, pH regulation, and diseases causing chronic CO2 retention. Respir Physiol Neurobiol 175: 189–209, 2011 [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience 103: 111–116, 2001 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vivo. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Edwards E, Paton JF. 5-HT4 receptors in nucleus tractus solitarii attenuate cardiopulmonary reflex in anesthetized rats. Am J Physiol Heart Circ Physiol 277: H1914–H1923, 1999 [DOI] [PubMed] [Google Scholar]
- Feldman PD. Effects of serotonin-1 and serotonin-2 receptor agonists on neuronal activity in the nucleus tractus solitarius. J Auton Nerv Syst 56: 119–124, 1995 [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992 [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Ni YG, Dunning DD, Miledi R. Distribution of serotonin 2A, 2C, and 3 receptor mRNA in spinal cord and medulla oblongata. Mol Brain Res 89: 11–19, 2001 [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol 29: 522–524, 2002 [DOI] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes 59: 1890–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol 177: 133–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol 164: 222–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Andresen MC. GABAB restrains release from singly-evoked GABA terminals. Neuroscience 193: 54–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Manchanda R. Modulation of synaptic potentials and cell excitability by dendritic KIR and KAs channels in nucleus accumbens medium spiny neurons: a computational study. J Biosci 36: 309–328, 2011 [DOI] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 90: 175–181, 2005 [DOI] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Glerant JC, Duron B. Central effects of 5-HT on respiratory rhythm in newborn rats in vivo. Eur J Neurosci 11: 3433–3440, 1999 [DOI] [PubMed] [Google Scholar]
- Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- Kung LH, Scrogin KE. Serotonin nerve terminals in the dorsomedial medulla facilitate sympathetic and ventilatory responses to hemorrhage and peripheral chemoreflex activation. Am J Physiol Regul Integr Comp Physiol 301: R1367–R1379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Weinreich D. Prostaglandin E2 depresses solitary tract-mediated synaptic transmission in the nucleus tractus solitarius. Neuroscience 146: 792–801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol 48: 21–53, 1996 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DeltaDeltaC(T) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997 [DOI] [PubMed] [Google Scholar]
- McDonald DM. Morphology of the rat carotid sinus nerve. I. Course, connections, dimensions, and ultrastructure. J Neurocytol 12: 345–372, 1983 [DOI] [PubMed] [Google Scholar]
- Merahi N, Orer HS, Laguzzi R. 5-HT2 receptors in the nucleus tractus solitarius: characterisation and role in cardiovascular regulation in the rat. Brain Res 575: 74–78, 1992 [DOI] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol 55: 1076–1090, 1986 [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 16: 74–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. Central 5-HT receptors in cardiovascular control during stress. Neurosci Biobehav Rev 33: 95–106, 2009 [DOI] [PubMed] [Google Scholar]
- Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ, Laguzzi R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neurosci Lett 114: 22–26, 1990 [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Morillo AM, Pasaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst 42: 99–118, 1993 [DOI] [PubMed] [Google Scholar]
- Oskutyte D, Jordan D, Ramage AG. Evidence that 5-hydroxytryptamine7 receptors play a role in the mediation of afferent transmission within the nucleus tractus solitarius in anaesthetized rats. Br J Pharmacol 158: 1387–1394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda MV, Riccardi E, D'Ascenzo M, Azzena GB, Grassi C. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 167: 678–690, 2010 [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir Physiol Neurobiol 178: 375–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui J, Xue C, Bosch MA, Murphy JG, Fan W, Ronnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharmacol 72: 885–896, 2007 [DOI] [PubMed] [Google Scholar]
- Ramage A. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 56: 425–439, 2001 [DOI] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol 23: 709–726, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Turner J, Gelasco AK, Ayiku HB, Coaxum S, Arthur JM, Garnovskaya MN. 5-HT receptor signal transduction pathways. In: The Serotonin Receptors: from Molecular Pharmacology to Human Therapeutics, edited by Roth B. Totowa, NJ: Humana, 2006, p. 143–206 [Google Scholar]
- Rick CE, Stanford IM, Lacey MG. Excitation of rat substantia nigra pars reticulata neurons by 5-hydroxytryptamine in vitro: evidence for a direct action mediated by 5-hydroxytryptamine2C receptors. Neuroscience 69: 903–913, 1995 [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther 79: 231–257, 1998 [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Nakadate K, Tanaka-Nakadate S, Yoshida K, Nogami S, Shirataki H, Ueda S. Developmental and spatial expression pattern of alpha-taxilin in the rat central nervous system. J Comp Neurol 511: 65–80, 2008 [DOI] [PubMed] [Google Scholar]
- Schaffar N, Kessler JP, Bosler O, Jean A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labeling study in the rat. Neuroscience 26: 951–958, 1988 [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Henry JL. Effects of enkephalin and 5-hydroxytryptamine on solitary tract neurones involved in respiration and respiratory reflexes. Brain Res 327: 221–230, 1985 [DOI] [PubMed] [Google Scholar]
- Sevoz-Couche C, Spyer KM, Jordan D. In vivo modulation of vagal-identified dorsal medullary neurones by activation of different 5-hydroxytryptamine2 receptors in rats. Br J Pharmacol 131: 1445–1453, 2000a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevoz-Couche C, Spyer KM, Jordan D. Inhibition of rat nucleus tractus solitarius neurones by activation of 5-HT2C receptors. Neuroreport 11: 1785–1790, 2000b [DOI] [PubMed] [Google Scholar]
- Sevoz-Couche C, Wang Y, Ramage AG, Spyer KM, Jordan D. In vivo modulation of nucleus tractus solitarius (NTS) neurones by activation of 5-hydroxytryptamine2 receptors in rats. Neuropharmacology 39: 2006–2016, 2000c [DOI] [PubMed] [Google Scholar]
- Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, Laudet V, Harmar AJ, NC-IUPHAR. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res 39: D534–D538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52: 279–290, 2007 [DOI] [PubMed] [Google Scholar]
- Tache Y, Yang H, Kaneko H. Caudal raphe-dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides 16: 431–435, 1995 [DOI] [PubMed] [Google Scholar]
- Takenaka R, Ohi Y, Haji A. Distinct modulatory effects of 5-HT on excitatory synaptic transmissions in the nucleus tractus solitarius of the rat. Eur J Pharmacol 22: 222–223, 2011 [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol 539: 67–75, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Logue SF, Wehner JM, Kauer JA. Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice. Proc Natl Acad Sci USA 95: 15026–15031, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon CM, Centurion D. Cardiovascular responses produced by 5-hydroxytryptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol 376: 45–63, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


