Abstract
Two experiments examined the relations among adult aging, mind wandering, and executive-task performance, following from surprising laboratory findings that older adults report fewer task-unrelated thoughts (TUTs) than do younger adults (e.g., Giambra, 1989; Jackson & Balota, 2011). Because older adults may experience more ability- and performance-related worry during cognitive tasks in the laboratory, and because these evaluative thoughts (known as task-related interference, “TRI”) might be sometimes misclassified by subjects as task-related, we asked subjects to distinguish task-related thoughts from TRI and TUTs when probed during ongoing tasks. In Experiment 1, younger and older adults completed either a go/no-go or a vigilance version of a sustained attention to response task (SART). Older adults reported more TRI and fewer TUTs than did younger adults while also performing more accurately. In Experiment 2, subjects completed either a 1- or 2-back version of the n-back task. Older adults again reported more TRI and fewer TUTs than younger adults in both versions, while performing better than younger adults in the 1-back and worse in the 2-back. Across experiments, older adults’ reduced TUT rates were independent of performance relative to younger adults. And, although older adults consistently reported more TRI and less mind wandering than did younger adults, overall they reported more on-task thoughts. TRI cannot, therefore, account completely for prior reports of decreasing TUTs with aging. We discuss the implications of these results for various theoretical approaches to mind-wandering.
Keywords: aging, mind wandering, executive control, consciousness, working memory
1. Introduction
Young adults spend, on average, a third to half of their daily lives thinking about something other than their current activity (Kane et al., 2007; Klinger & Cox, 1987-1988; Killingsworth & Gilbert, 2010; McVay, Kane, & Kwapil, 2009). Unfortunately, these task-unrelated thoughts (TUTs) can sometimes result in “absentminded” mistakes (e.g., McVay et al., 2009; Reason, 1990; Schooler, Reichle, & Halpern, 2004; Smallwood et al., 2004). Mind wandering is thus a frequent, yet occasionally costly, experience. It also occurs more frequently for some people than others: College students who have poorer cognitive-control abilities, such as those with lower working memory capacity (WMC; Kane et al., 2007; McVay & Kane, 2009, 2012a, 2012b) and Attention Deficit/Hyperactivity Disorder (AD/HD; Shaw & Giambra, 1993; McVay et al., 2008), report more TUTs during challenging tasks than do people with better control abilities.
1.1. Mind Wandering and Adult Aging
As we age, then, does the propensity to mind-wander increase? Do TUTs account, in part, for older adults’ performance deficits in many tasks involving cognitive control? Based on age-related decline in many domains, including WMC (see Craik & Salthouse, 2008), and on theoretical accounts that propose deficits in goal maintenance or attentional inhibition to explain age differences in executive control (e.g., Braver & West, 2008; Hasher & Zacks, 1988), we might expect that older adults are often mind wandering. For example, the Braver-West view claims that older adults have difficulty maintaining task-related goals to intentionally guide actions; an age-related inability to keep task-irrelevant information from becoming conscious (as TUTs) should thus disrupt active maintenance or accessibility of task goals, thereby leading to errors. Indeed, Hasher and Zacks originally theorized that such impaired inhibition is the root of much age-related variance in cognition (see Hasher, Zacks, & May, 1999; Hasher, Lustig, & Zacks, 2007).
Counter to this prediction, however, and in contrast to most WMC-related findings with younger adults (e.g., Kane et al., 2007; McVay & Kane, 2009; but see Levinson, Smallwood, & Davidson, 2012), older adults actually report less frequent TUTs than do younger adults (Giambra, 1989; Grodsky & Giambra, 1990-1991; Jackson & Balota, 2011; Krawietz, Tamplin, & Radvansky, in press). The negative correlation between age and mind-wandering rate was first established via retrospective questionnaires (e.g., Giambra, 1977-78; Singer & McCraven, 1961), and may have reflected age-related memory or metacognitive deficits, or a reporting bias. To demonstrate aging’s effect on mind wandering in a controlled setting, Giambra (1989) measured TUTs during a laboratory vigilance task with instructions aimed to encourage reporting and to limit self-censure. Across five experiments, older adults reported fewer TUTs than did younger adults.
Giambra’s (1989) findings are surprising from the perspective that aging impairs executive control and that executive-control failures predict TUTs. Indeed, Giambra discussed these results as contradicting the Hasher and Zacks (1988) view that older adults have decreased inhibitory ability. Giambra argued, instead, that TUTs represent trains of thoughts, or “unfinished business,” which come to a conclusion during unconscious processing and then require attentional capacity to enter awareness. In other words, when performing a task that does not require full attention, excess attentional capacity can be devoted to mind wandering. Accordingly, younger adults should experience more TUTs than should older adults because they have more attentional capacity and more often an excess to allow TUTs. Giambra further explained, however, that his tasks were designed to allow plenty of attentional capacity to spare (supported by ceiling-level performance), and proposed that older adults have less “unfinished business” than do younger adults, leading to fewer and less urgent unconscious thoughts.
1.2. Executive Processes in Mind Wandering
Giambra (1989) thus foreshadowed a current debate about the role of executive resources in TUTs. The Smallwood and Schooler (2006) theory of mind wandering characterizes TUTs as requiring the resources typically used for executive control (see also Teasdale et al., 1995), with evidence drawn from studies showing that: (1) tasks imposing greater cognitive loads reduce TUT rates (e.g., Antrobus, 1968; Teasdale et al., 1993, 1995) and, conversely, practice-driven automaticity increases TUT rates (e.g., Mason et al., 2007; Teasdale et al., 1995); (2) executive-control brain networks, along with “default mode” networks, are active during mind wandering (e.g., Christoff, Gordon, Smallwood, Smith, & Schooler, 2009); (3) in-the-moment TUT reports predict performance errors, suggesting competition for a unitary processing capacity (e.g., Smallwood et al., 2004); (4) individual differences in control capabilities may be positively associated with TUT rates during very simple tasks, such as breath monitoring, indicating that people with more available resources use them to mind-wander (Levinson et al., but see McVay & Kane, 2012).
McVay and Kane (2010a), in contrast, propose a “control failures × current concerns” view that takes the opposite stance on the role of executive capacity: The contents of TUTs are automatically and continuously generated unconsciously in response to environmental cues to subjects’ current concerns and goals (following from Klinger, 1971, 1999, 2009), similar to Giambra’s concept of “unfinished business.” Cued TUTs then enter awareness as a result of an executive-control failure, as opposed to the availability of excess capacity. Their main sources of evidence were: (1) TUTs predict performance deficits on attention-demanding tasks (e.g., McVay & Kane, 2009; McVay et al., 2009; Smallwood et al., 2004), which may indicate that TUTs enter awareness when control falters, rather than when there is capacity to spare; (2) contexts that impair control abilities, such as fatigue (e.g., Antrobus et al., 1966; McVay & Kane, 2009; Smallwood, et al., 2004; 2005; Teasdale, 1995) and inebriation (Finnigan, Schulze, & Smallwood, 2007; Sayette, Reichle, & Schooler, 2009), increase TUTs; (3) individual differences in control are negatively associated with TUT rates during demanding tasks (e.g., McVay & Kane, 2009, 2012a, 2012b; Shaw & Giambra, 1993); (4) subjects who have greater attention control suffer as much a performance cost as those with poorer control on occasions when they experience TUTs, in conflict with a resource-availability view that predicts spare resources to minimize dual-tasking costs.1
Giambra’s (1989) findings of TUTs decreasing with age seem to fit more comfortably with the executive-resource view of mind wandering than the control failures × concerns view. Older adults, who have reduced WMC (Bopp & Verhaeghen, 2005) and poorer attention control (e.g., Cohn, Dustman, & Bradford, 1984; Hartley, 1993; Spieler, Balota, & Faust, 1996; Hamm & Hasher, 1992; West & Baylis, 1998), should experience more control failures than should younger adults. If control failures drive mind-wandering, then older adults should mind-wander more frequently. Thus, older adults’ reduced rate of mind-wandering seems to suggest, instead, that they have insufficient resources to maintain TUTs in the face of simultaneous tasks. Given the potential importance of aging findings to general theories of mind-wandering, we thought it necessary to confirm and expand on Giambra’s results. In the current study, we improve upon Giambra’s methods and address an alternative explanation for the age-related differences in TUTs he found.
1.3. Age Differences in Mind-Wandering: Methodological and Theoretical Considerations
Although Giambra’s (1989) laboratory studies improved upon retrospective surveys of mind-wandering tendencies, he asked subjects whether they had experienced any TUTs during 25-30 s task periods, a long enough delay to allow forgetting or confabulation. In our studies, as is now common (Smallwood & Schooler, 2006), we further reduced retrospective biases by probing subjects randomly throughout the task and having them report on their immediately preceding thoughts. In addition, Giambra’s vigilance tasks yielded ceiling performance, which prevented assessment of TUT-performance associations (which bears on whether TUTs draw on executive resources). The current studies kept performance below ceiling to allow tests of whether in-the-moment costs of TUTs were similar across age groups.
While we were conducting the current studies, both Jackson and Balota (2011) and Krawietz, Tamplin, and Radvansky (in press) similarly reported age-related decreases in TUTs. Moreover, they did so using random, in-the-moment, thought probes (like ours) during either variations of a go/no-go task (the “sustained attention to response task” [SART]; Jackson & Balota, 2011) or reading comprehension tasks (Jackson & Balota, 2011; Krawietz et al., in press). Although Jackson and Balota did not assess age differences in the consequences of TUTs for performance, Krawietz et al. reported that both older and younger adults were similarly inaccurate in answering reading comprehension questions following TUT reports versus on-task-thought reports; moreover, Krawietz et al. found age differences in TUT rates within tasks that yielded either no age differences in accuracy (Experiment 1) or significant age differences in accuracy favoring younger adults (Experiment 2). A growing body of data thus points consistently to reduced TUT rates in older versus younger adults, regardless of age differences in corresponding task performance.
The primary purpose of our study was to test an alternative explanation age differences in TUT rates by probing for a particular thought category. In our previous young-adult work (McVay & Kane, 2009, 2011), subjects reported not only on-task thoughts versus TUTs, but also evaluative thoughts about their performance (e.g., “I’m good at this!” “I’m screwing up.”), or so-called “task-related interference” (TRI; e.g., Smallwood et al., 2006; see also Mikulincer & Nizan, 1988; Sarason, 1988). TRI differs conceptually from TUTs because it is, in a sense, task-related; however, TRI experiences are also not quite “on-task,” or directly about the task stimuli or demands, either. TRI is also empirically distinguishable from both TUTs and on-task thoughts. On one hand, TRI and on-task thoughts both decline with time on go/no-go tasks, while TUTs increase (McVay & Kane, 2009, 2011a). On the other hand, TRI and TUTs are similarly associated with higher in-the-moment go/no-go errors than are on-task reports (McVay & Kane, 2012a). Note that the control failures × concerns view argues that mind-wandering propensity reflects an interaction of control abilities and the cuing of personal goal-related concerns. Although typical laboratory contexts, with computer equipment and young-adult researchers in campus buildings, are less likely to cue many of the personal goals for older adults (i.e., their present-oriented, relationship-related goals; Carstensen 1993; 1995), they may trigger other, non-goal-related, concerns about their cognitive abilities and potential intellectual decline (e.g., Hertzog & Hultsch, 2000). Thus, unless subjects are asked about TRI (and they have not been in prior aging studies; Giambra, 1989; Jackson & Balota, 2011; Krawietz et al., in press), we cannot know whether older adults actually experience less off-task thought than do younger adults.
Here, we tested whether apparent age differences in TUTs stem from subjects misclassifying TRI. That is, older adults may experience increased TRI (see Parks, Klinger, & Perlmutter, 1988-89), but may also misclassify TRI as “on-task” thoughts because of forced-choice, on- versus off-task reporting. At least occasionally, subjects may classify performance-evaluative thoughts as “on-task” because they are more task-related than are TUTs about romantic getaways or dinner plans. If older adults experience elevated TRI, due to stereotype threat (Hess, Auman, Colcombe, & Rahal, 2003; Rahhal, Hasher, & Colcombe, 2001) or concerns about cognitive decline (Hertzog & Hultsch, 2000), and if TRI has similar behavioral consequences to TUTs (McVay & Kane, 2009, 2011), then misclassifications of TRI as on-task thoughts should result in older adults appearing more task-focused and less distracted than are younger adults. Moreover, if older adults actually experience fewer TUTs, and yet increased TRI, relative to younger adults, it strains any resource-based explanation of age differences in off-task thought: If older adults’ reduced TUT rate is due to deficient resources, then they ought to engage in little TRI-type thinking, as well. Finally, if age differences in TUT reports were largely caused by older adults’ inability to assess their subjective experiences (a concern not yet directly tested in the literature), then older adults should show similarly low rates of TUTs and TRI.
Both Jackson and Balota (2011) and Krawietz et al. (in press) reported several findings that underscore the need to distinguish TUT from TRI when assessing age differences. First, in the Jackson-Balota study, older adults showed increased post-error slowing — where response times were longer following incorrect than correct responses — which may reflect TRI (see also Cheyne et al., 2009). Second, the older subjects tested by Jackson and Balota had higher conscientiousness scores than the younger subjects, indicating a general propensity to care about performing well; they also indicated higher levels of interest in the SART (see also Germain and Hess, 2007), consistent with the older subjects tested by Krawietz et al., who reported greater interest in the reading task than did the younger adults. Such age differences in interest and conscientiousness could lead to more TRI experiences for older than for younger adults in the laboratory. Again, if people frequently misclassify TRI experiences as on-task thoughts, then age-related difference in thought reports might misrepresent the extent to which older adults engage in TUTs.
2. Experiment 1
In both Experiments 1 and 2 we assessed TRI, a form of off-task thought that may be especially relevant to older adults and may be sometimes misclassified. We also analyzed age differences in the effects of TUTs on performance in several executive-control tasks, as a potential means to distinguish resource-consuming views of mind wandering (Giambra, 1989; Smallwood & Schooler, 2006) from the control failures × concerns view (McVay & Kane, 2010a, 2010b). Experiment 1 presents a manipulation of the SART, where some subjects performed a standard, go/no-go version requiring cognitive control (Robertson et al., 1997), in the form of inhibiting habitual responses (withhold response to rare targets), while other subjects performed a less executive, non-inhibitory “vigilance” version (respond to rare targets). Our “vigilance SART” conceptually replicates Giambra’s (1989) task, whereas the standard SART: (a) seems to require executive control over habitual responding; (b) is more commonly used in recent mind-wandering research (e.g., Jackson & Balota, 2011; McVay & Kane, 2009; Smallwood et al., 2004), and; (3) shows that TUTs predict errors at the level of occasions (errors occur when TUTs occur) and individuals (people who TUT often err often). Because McVay and Kane (2012a) found that the executively demanding go/no-go version of the SART elicited WMC-related variation in task performance and TUT rates, whereas the vigilance version yielded null WMC effects in both, we tested here whether similar, selective patterns would emerge for age differences in performance and thoughts.
2.1. Method
2.1.1. Subjects
One hundred and eight adults between ages 18 and 28 (M = 19.3, 69% female) and ninety-nine adults between ages 60 and 75 (M = 66.9, 74% female) participated. All were prescreened for basic health issues and all had good corrected visual acuity (20/50 or better). We recruited younger adults from the UNCG Psychology research pool, who received credit towards a course requirement, and older adults from the Greensboro, NC area, who earned $30. Table 1 presents demographic and basic cognitive measures.
Table 1. Means (and Standard Errors) of Subject Characteristics.
| Experiment 1 | ||||
|---|---|---|---|---|
| Measure | YoungStandard | YoungVigilance | OldStandard | OldVigilance |
| Education | 12.85 (0.18) | 12.98 (0.20) | 15.22 (0.39) | 15.62 (0.36) |
| Medicationa | 0.66 (0.18) | 0.77 (0.16) | 2.39 (0.27) | 2.64 (0.33) |
| Vocabularya | 28.05 (0.44) | 28.26 (0.48) | 33.63 (0.47) | 34.20 (0.44) |
| Digit symba | 60.62 (1.32) | 63.65 (1.82) | 51.14 (1.89) | 50.40 (1.73) |
| DS memorya | 7.93 (0.19) | 7.67 (0.23) | 5.80 (0.31) | 5.92 (0.33) |
| Experiment 2 | ||||
| Measure | Young1-back | Young2-back | Old1-back | Old2-back |
|
| ||||
| Education | 12.41 (0.11) | 12.70 (0.15) | 15.58 (0.38) | 15.00 (0.42) |
| Medicationa | 0.70 (0.14) | 0.82 (0.16) | 2.35 (0.25) | 3.12 (0.34) |
| Vocabularya | 27.96 (0.43) | 27.84 (0.47) | 33.86 (0.43) | 33.05 (0.56) |
| Digit symba | 61.76 (1.49) | 62.21 (1.25) | 49.00 (1.81) | 46.14 (2.04) |
| DS memorya | 7.65 (0.25) | 7.59 (0.23) | 5.23 (0.38) | 4.93 (0.37) |
Note. Education = number of years education completed; medication = self-reported number of medications taken daily; vocabulary = number correct out of 40 on the Shipley Vocabulary Test (Zachary, 1986); digit symb = WAIS Digit-Symbol subtest (Wechsler, 1981); DS memory = symbol recall following the WAIS Digit-Symbol subtest (Wechsler, 1981); Standard = subjects completing the standard, go/no-go, version of the SART; Vigilance = subjects completing the vigilance version of the SART; 1-back = subjects completing the 1-back task; 2-back = subjects completing the 2-back task.
Age comparison p < .05. No comparisons of, or interactions with, the task-condition variables were significant. No statistical comparison of education was made because the young adults were currently enrolled at a university.
2.1.2. Design and Materials
The design was a 2 × 2 factorial model, with Age Group (Younger, Older) and SART Typee (Standard, Vigilance) as between-subjects factors. We modified the standard and vigilance SART from previous mind-wandering studies of younger adults (McVay & Kane, 2009, 2012a).
Both SARTs serially presented 900 words: Each was centered for 350 ms and followed by a 900 ms mask. We instructed subjects to respond with a key-press to either frequent non-target words (animal names) or to infrequent target words (foods; 11% of trials). In the standard (go/no-go) SART, subjects responded to frequent non-targets by pressing the spacebar and to infrequent targets by withholding response. The frequency of ‘go’ stimuli thus built up a habitual response that had to be suppressed on target no-go trials. In contrast, the vigilance SART, where subjects responded only to infrequent targets, did not generate a “go” prepotency to be controlled. The first 10 (unanalyzed) trials presented non-targets; remaining trials comprised four seamless blocks, each presenting 225 trials consisting of 45 words repeated five times in a different random order. Five target words appeared randomly within each set of 45 trials; different targets appeared in each block. Thought probes followed 60% of the targets, yielding 60 probes over a 20 min task.
Thought probes presented a screen asking, “What were you just thinking about?” with seven response options (McVay & Kane, 2009, 2012a). Subjects were to report what they were thinking just before the probe appeared, and were instructed at length about these response choices: (1) the task: thinking about the stimulus words or appropriate response; (2) task experience/performance: evaluating one’s performance; (3) everyday things: thinking about recent or impending events; (4) current state of being: thinking about conditions such as hunger or sleepiness; (5) personal worries: thinking about ongoing concerns or troubles; (6) daydreams: fantasies disconnected from reality; or (7) other. During the task, probes presented only the numbers and italicized category names; subjects pressed the corresponding number key. For analysis, responses of (1) were coded as on-task thoughts, (2) as TRI, and (3) – (7) as TUTs. Instructions attempted to minimize bias against TUT reporting by stressing the normalcy of off-task thoughts during such tasks:
“During the task, you may find yourself thinking about something other than the task. We are interested in what types of things people think about during a task like this (and during other kinds of tasks). In order to examine this, the computer will periodically ask you what you were *just* thinking about. It is perfectly normal to think about things that are not related to the task, and to have different kinds of thoughts during different kinds of tasks. We will give you several categories of things that people might think about during a task like this. Please try your best to honestly assess your thoughts and choose a category that best describes your thoughts at the time when we ask.”
We conducted this experiment using Dell desktop computers with 17-inch LCD monitors and E-Prime 1.2 software. Stimuli were black against a white background, in 18-point Courier-New bold font.
2.1.3. Procedure
Subjects were tested in groups of 1 – 6, with cubicle partitions between them. Before completing the SART, all subjects provided informed consent and demographic information, had their visual acuity tested, completed a brief cognitive battery (see Table 1 for tests and results), and then completed a source memory task (part of an unrelated project). These activities took approximately 1 hour to complete. We offered all subjects a break before the SART. Experimenters read all on-screen SART instructions aloud to subjects. Subjects completed 10 practice SART trials before seeing thought-probe instructions.
2.2. Results
We report non-directional null-hypothesis significance tests with alpha = .05 and partial eta squared (ηp2) as an effect-size estimate.
2.2.1. Thought Content
Here we addressed our primary question: Do younger and older adults differ in either TUTs or TRI? We also assessed whether our two SART versions elicited different rates of TUTs and TRI experiences, and whether off-task thoughts were predicted by local changes in performance (i.e., reaction time; RT).
As illustrated in Figure 1, subjects reported a higher proportion of TUTs on the vigilance SART than on the standard SART, F(1, 203) = 11.94, p < .001, ηp2 = .06, and, consistent with previous findings, older adults reported fewer TUTs than younger adults on both SART versions, F(1, 203) = 125.32, p < .001, ηp2 = .38. Age group did not interact with SART Type (F < 1). In contrast, and as predicted, older adults reported more TRI than did younger adults, F(1, 203) = 10.21, p < .01, ηp2 = .05 (see Figure 2), and both groups reported more TRI in the standard than the vigilance SART, F(1, 203) = 29.39, p < .001, ηp2 = .13; again, Age group and SART version did not interact (F < 1).
Figure 1.
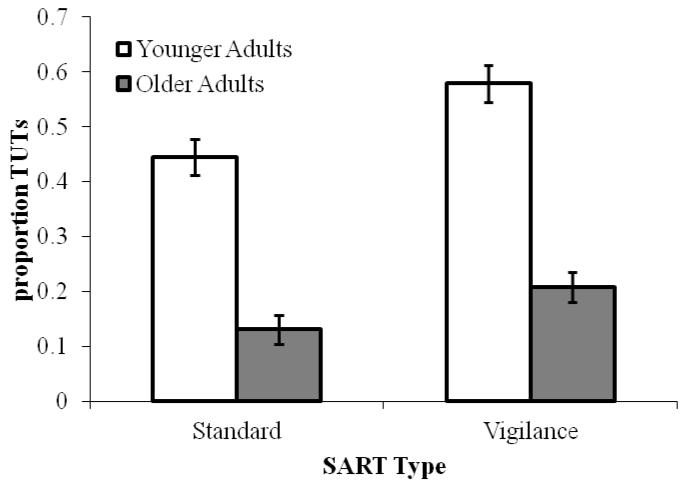
Mean proportion of TUT reports, by SART Type (Standard, Vigilance) and Age (Younger and Older adults) in Experiment 1. Error bars represent standard errors. Note: TUT = task-unrelated thought.
Figure 2.
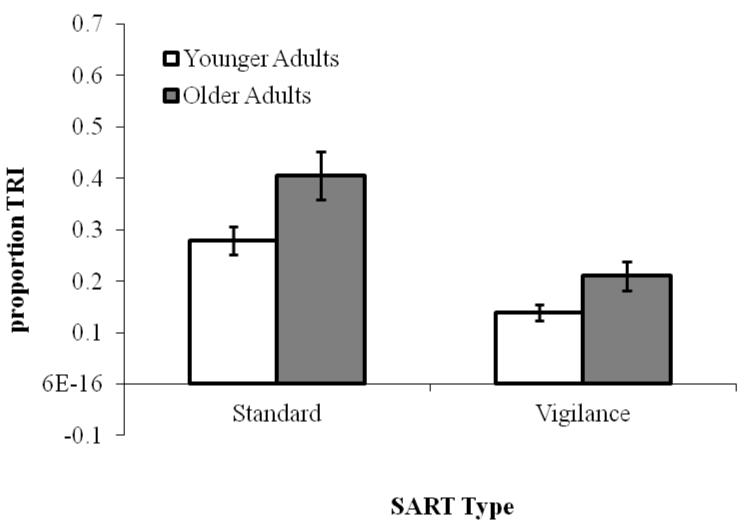
Mean proportion of TRI reports, by SART Type (Standard, Vigilance) and Age (Younger and Older adults) in Experiment 1. Error bars represent standard errors. Note: TRI = task-related interference.
Previous SART studies have demonstrated an RT speed-up prior to TUT versus on-task reports (McVay & Kane, 2009; Smallwood et al., 2004) by comparing the average RT from the four non-target trials directly preceding TUT reports to those preceding on-task reports; these pre-TUT speed-ups are sometimes interpreted as reflecting attentional lapses and “mindless” responding (but see McVay & Kane, 2009, 2012a). Using standardized RTs from the Standard SART to account for general slowing in older adults (Jackson & Balota, 2011), we conducted a Thought Type × Age Mixed ANOVA, which indicated a main effect of thought type, F(2, 170) = 4.91, p =.008, ηp2 = .06. As illustrated in Figure 3A, subjects responded more quickly leading up to a TUT report than an on-task thought report, F(1, 85) = 11.84 p =.001, ηp2 = .12, and more quickly leading up to a TRI report than an on-task thought report, F(1, 85) = 4.12 p =.045, ηp2 = .05, but with no difference between TUTs and TRI (F < 1). We found no main effect of Age (F < 1) and no interaction of Thought Type and Age (F < 1).
Figure 3.
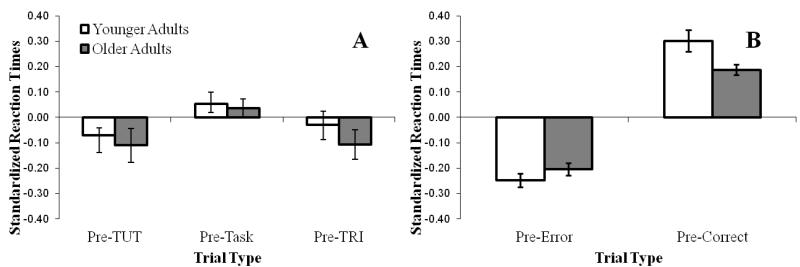
Mean “go” RTs (to non-target trials in the Standard SART) leading up to a thought report (TUT, TRI, or TASK; Panel A) or to a target trial (error or correct; Panel B), standardized to account for general slowing due to age, in Experiment 1. Error bars represent standard errors. Note: TUT = task-unrelated thought; TRI = task-related interference; TASK = on-task thoughts.
2.2.2. SART Performance
Here we tested for performance differences in accuracy and RT across two SART versions, while determining whether any age- or task-related differences would parallel those we found in subjects’ thought reports. We also tested whether standard-SART errors were either predicted or followed by RT changes (i.e., speed-ups before errors; slow-downs after errors), because both have been argued to reflect off-task thinking.
As in previous SART research (McVay & Kane, 2009, 2012a), we calculated a signal-detection sensitivity score for each subject using the formula for logistic distributions (Snodgrass & Corwin, 1988): dL = ln{[H(1 − FA)]/[(1 − H)FA]}, and a CL score, representing bias, using: CL = 0.5[ln{[(1 − FA)(1 − H)]/[(H)(FA)]}], where ln = natural log, H = hit proportion, and FA = false-alarm proportion. We adjusted individual hit or false-alarm rates of 0 and 1 by .01. Overall, subjects’ accuracy (dL) was higher in the vigilance than in the standard SART, F(1, 203) = 132.24, p < .001, ηp2 = .39 (see Figure 4). Also as expected, a “go” bias (i.e., CL < 0) was elicited by the standard SART (M = −2.14) and a “no-go” bias (CL > 0) by the vigilance SART (M = 0.62), F(1, 203) = 1044.57, p < .001, ηp2 = .84. Contrary to our intentions of using executive tasks that would elicit age-related deficits in performance, older adults showed higher accuracy (dL) than did younger adults on both SART versions, F(1, 203) = 8.02, p < .01, ηp2 = .04, and age did not interact with SART type, F(1, 203) = 1.63, p = .20, ηp2 = .008. The Age Groups did not differ in bias (CL), F(1, 203) = 1.36, p = .25, ηp2 = .01, and, again, age did not interact with SART type, F(1, 203) = 2.49, p = .12, ηp2 = .012.
Figure 4.
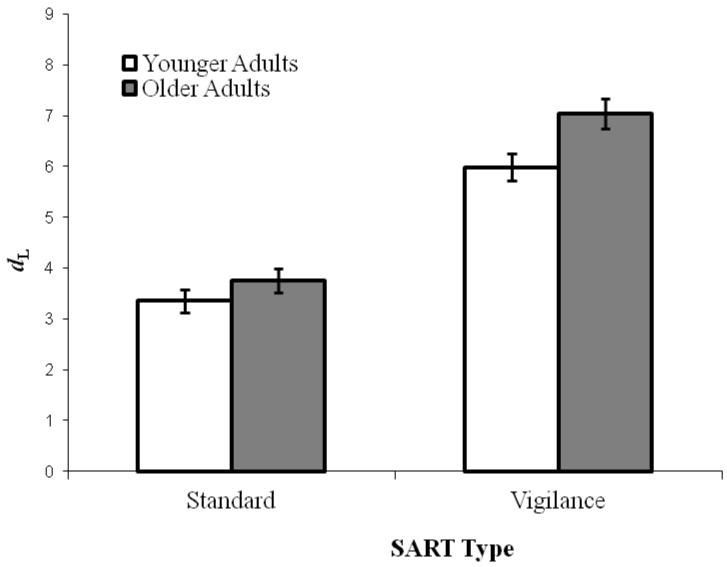
Mean signal-detection sensitivity (dL) by SART Type (Standard, Vigilance) and Age (Younger and Older adults) in Experiment 1. Error bars represent standard errors.
Because RTs were assessed on different trial types across SART versions (frequent no-go trials in standard SART; rare go trials in vigilance SART), we analyzed age differences separately by SART type. Older adults were slower than younger adults to respond correctly in Standard SART (Ms = 469 and 425 ms); t(102) = 2.44, p < .05, but not in vigilance SART (Ms = 628 and 615 ms); t(100) = −.95, p = .34. Because prior studies have found that individual RT variability (i.e., subjects’ RT standard deviations) covaries with TUT rate, we calculated it for each subject on non-target “go” trials in the standard SART (McVay & Kane, 2009, 2012a, 2012b); RT variability did not differ between age groups, t(102) < 1.
As in previous SART studies, we examined pre-error speeding in the standard go/no-go SART (e.g., Jackson & Balota, 2011; McVay & Kane, 2009, 2012a; Robertson et al., 1997; Smallwood et al., 2004) by comparing the average RT from the four non-target trials directly preceding a target error (i.e., responding to a no-go target) to those directly preceding a correct withholding of response. Some researchers have argued that such pre-error speeding is reflective of “mindless” habitual responding, and thus may provide a behavioral marker of mind-wandering (e.g. Robertson, Manly, Andrade, Baddeley, & Yiend, 1997; Smallwood, McSpadden, Luus et al., 2008; but see McVay & Kane, 2009; 2012a). Using standardized RTs (see Figure 3B), we found a main effect of accuracy, whereby subjects responded more quickly leading up to an error than to a correct target trial, F(1, 100) = 11.23 p < .001, ηp2 = .61. There was no main effect of Age (F < 1), but, in contrast to our prior analysis of pre-TUT-report RTs, age interacted with accuracy, F(1, 100) = 4.41, p = .04, ηp2 = .04. Although both older and younger adults were faster on the non-target trials leading up to an error than a correct response [t(52) = 8.60, p < .001; t(49) = 10.95, p < .001], younger adults had a greater disparity between their RTs prior to an error versus a correct response (M difference = .55) than did older adults (M difference = .39).
To examine post-error slowing in standard SART (see Figure 5), which may be a behavioral manifestation of self- or performance-referential thoughts (i.e., TRI; Jackson & Balota, 2011), we analyzed standardized RTs on non-target trials after correct and incorrect targets that did not precede thought probes (which affect RTs). We found that main effects of accuracy and age were not significant, but age group interacted with accuracy, F(1, 100) = 10.31, p = .002, ηp2 = .09. Consistent with our prediction that older adults would experience more TRI than would younger adults, older adults slowed down after committing errors, t(48) = −1.97, p = .055, but younger adults actually sped up, t(52) = 2.58, p = .013.
Figure 5.
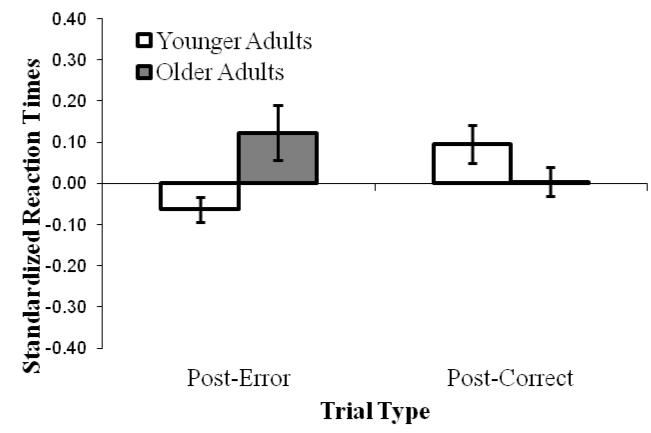
Mean “go” RTs (to non-target trials in the Standard SART) following a non-probe target trial (error or correct), standardized to account for general slowing due to age, in Experiment 1. Error bars represent standard errors.
2.2.3. Mind Wandering and Performance
Here we tested, first, whether individual differences in overall TUT rate predicted aspects of SART performance in either or both versions of the task (as they typically do within samples of young-adult subjects; e.g., McVay & Kane 2009, 2012a). We then assessed whether TUT or TRI experiences, in the moment, were associated with committing an error on either or both SARTs (again, as they typically are within samples of young-adult subjects).
SART performance was associated with TUTs. Regarding individual differences, overall TUT rates correlated negatively with standard SART dL (r = −.25) and positively with intra-subject RT variability (r = .31), even after partialing Age (rs = −.23 and .38, respectively). In the vigilance SART, as well, TUT rate correlated with dL (r = −.28) and with intra-subject RT variability on target trials (r = .24), but only intra-subject RT variability was significantly correlated with TUT rate after partialing Age (r = .27). When TUT was included as a covariate in the Age Group × SART Type ANOVA on performance (dL), the main effect of Age on performance was no longer significant (F < 1), indicating that older adults outperformed younger adults in the SART due, at least in part, to their reduced TUT rate. When TRI was included as a covariate, in contrast, the main effect of Age was still significant, F(1, 202) = 6.67, p = .01, ηp2 = .03.
Within subjects, we calculated the in-the-moment accuracy associated with TUTs and TRI experiences. An Age Group × SART Type × Thought Type (TUT, TRI, and on-task) mixed ANOVA on target accuracy yielded no main effect or interactions involving Age (all Fs < 1). However, a main effect of Thought Type, F(2, 336) = 40.16, p < .001, ηp2 = .19, indicated that subjects were more likely to report TUT or TRI than on-task thoughts on error trials. Thought Type also interacted with SART Type, F(2, 336) = 8.85, p < .001, ηp2 = .05. Follow-up contrasts indicated that in the standard SART, TUTs and TRI were both associated with worse performance on targets than were on-task thoughts, F(1, 83) = 50.57, p < .001, ηp2 = .38; F(1,83) = 38.15, p < .001, ηp2 = .32, but they were not different from each other (F < 1). Subjects were accurate on 57% (SD = 29%) of target trials when they reported on-task thoughts, but only 32% accurate (SD = 30%) when reporting a TUT and 35% accurate (SD = 26%) when reporting TRI. In the vigilance SART, TUTs and TRIs were, again, both associated with worse performance on targets than were on-task thoughts, F(1,87) = 23.87, p < .001, ηp2 = .22, and F(1, 87) = 3.89, p = .05, ηp2 = .04, respectively. But, unlike in the standard SART, TUTs were associated with worse performance than was TRI, F(1, 87) = 6.17, p = .02, ηp2 = .06. Subjects were accurate 89% (SD = 20%) of the time when they reported on-task thoughts but only 78% accurate (SD = 24%) when reporting TUTs and 84% accurate (SD = 26%) when reporting TRI.
2.3. Discussion
As expected, TRI explained some age-related differences in reporting off-task thoughts. Older adults experienced more TRI than did younger adults, but TRI did not account entirely for the difference between younger and older adults’ off-task thinking. Younger adults reported 21% TRI and 51% TUT, for a total of 72% off-task thoughts, while older adults reported 31% TRI and 17% TUT, for a total of 48% off-task thoughts. In previous studies, the absence of TRI as a response choice may have inflated age differences in TUT rate by categorizing older adults’ TRI reports as on-task thoughts. In the current study, though, older adults still reported more on-task thoughts and fewer TUTs than did younger adults.
Mind wandering was equally detrimental to older and younger adults, conflicting with Giambra’s (1989) prediction that TUTs occur when there is attentional capacity to spare, as well as with one reasonable interpretation of the executive-resource view of Smallwood and Schooler (2006; but see Smallwood, 2010). Both views propose that resources are divided between task and TUTs, and so, by resource-theory logic (e.g. Norman & Bobrow, 1975), performance of individuals with fewer resources to divide (e.g., older adults) should be impacted more when TUTs occur (but see footnote 1). This was not the case.
Following Jackson and Balota (2011), we also analyzed age differences in post-error slowing on the Standard SART. Here, again, older adults slowed more following errors than did younger adults. Although subjects’ degree of slowing did not correlate with their probed TRI rates (r = −.10, p = .92), post-error slowing may nonetheless represent another indication of increased attention to performance evaluation by older adults (Jackson & Balota, 2011). We recommend that future studies assess thought content when post-error slowing occurs to test explicitly whether such slowing is connected to TRI experiences.
There is a notable limitation to Experiment 1: Older adults outperformed younger adults on both versions of the SART. Jackson and Balota (2011) and others (e.g., Carriere, Cheyne, Solman, & Smilek, 2010) have also recently reported better accuracy for older than for younger adults on the standard SART. Older adults’ superior accuracy was likely due to a speed-accuracy tradeoff in all of these studies, as they responded much more slowly than did younger adults. The standard SART, in particular, benefits from slow responding, which allows time to catch errant habitual responses by overcoming the prepotent action (McVay & Kane, 2012a). So, although our finding is not an outlier, the lack of performance differences between ages prevents us from assessing TUTs’ contributions to cognitive-control declines in aging, and from evaluating the paradox of older adults’ control deficits in the context of reduced TUTs. Indeed, if anything, our ANCOVA results from Experiment 1 indicate that older adults’ propensity for on-task thoughts helps to reduce their performance deficits relative to younger adults (i.e., younger adults’ deficit in SART accuracy was not significant after accounting for their increased TUT rate). For Experiment 2, then, we chose a task known to elicit age differences in performance, the n-back task (e.g., Dobbs & Rule, 1989; Vaughan, Basak, Hartman, & Verhaeghen, 2008).
3. Experiment 2
Experiment 2 attempted to replicate the age differences we found in TUTs and TRI during go/no-go and vigilance SARTs, but in an n-back task of working memory (e.g., Gevins et al., 1990; Kirchner, 1958; Mackworth, 1959) that should yield significant age-related deficits in performance. The n-back requires subjects to decide whether each stimulus in a sequence matches the one presented n items ago. N-back tasks generally show age differences for ns of 2 or higher (e.g., Missonnier et al., 2004; Vaughan et al., 2008). Here we compared TUT and TRI rates in older and younger adults, and tested for thought-performance associations, during 1-back versus 2-back tasks. As in Experiment 1, then, we contrasted two similar tasks that nonetheless differed in cognitive-control demands, to test whether age-related differences in TUTs might depend on age-related differences in performance (but see Krawietz et al., in press).
3.1. Method
3.1.1. Subjects
One hundred and twelve younger adults between ages 18 and 29 (M = 19.0, 71% female) and 85 older adults between ages 60 and 75 (M = 67.4, 64% female), who had not participated in Experiment 1, participated. We screened, recruited, and compensated subjects the same way as in Experiment 1 (for characteristics, see Table 1).
3.1.2. Design and Materials
The design was a 2 × 2 factorial model, with Age Group (Younger, Older) and Memory Load (1-back, 2-back) as between-subjects factors. The n-back task required subjects to respond overtly only when the current stimulus matched the stimulus presented “n” trials ago. Stimuli were twelve 1-syllable, 4- or 5-letter, semantically unrelated words (corn, fence, green, guard, jump, large, month, name, push, star, tape, waive); subjects pressed the space bar only when they identified targets. In the 1-back task, targets matched the immediately preceding word and, in 2-back, targets matched the word presented prior to the immediately preceding word (e.g., in corn-green-corn, the second corn is the target). Targets appeared on 25% of the trials. Each n-back task also presented lures — 3-back matches in both tasks (e.g., corn-green-jump-corn), 2-back matches in the 1-back task (e.g., corn-green-corn), and 1-back matches in the 2-back task (e.g., green-corn-corn) — on 21% of trials. A word never appeared simultaneously as a target and lure. To minimize perceptual recognition, we varied the location of consecutive words in one of three vertical positions in a repeating sequential order (Oberauer, 2005).
Both n-back tasks presented ten blocks of 48 words but this block structure was not apparent to subjects. Each block presented 12 targets and 10 lures. Each word was presented for 500 ms and followed by a fixation cross for 2500 ms, allowing 3000 ms for response. Every block contained thought probes following two target trials and one lure (alternating between 3-back and 1- or 2-back lures) for a total of 30 probes over a 25-minute task. Stimuli appeared in black against a white background, in 36-point Courier-New bold font. We used the same computer hardware and software as in Experiment 1.
3.1.3. Procedure
Subjects performed the same pre-n-back activities as in Experiment 1, and were again tested in groups of one to six. Experimenters read all on-screen n-back instructions aloud. Subjects completed 40 practice n-back trials (10 targets) before seeing the thought-probe instructions from Experiment 1.
3.2. Results
3.2.1. Thought Content
As in Experiment 1, our key question was whether younger and older adults differed in either TUTs or TRI. We also assessed whether our two n-back tasks elicited different TUT and TRI rates (i.e., whether memory load moderated any of our thought-content findings).
Figure 6 illustrates that older adults again exhibited lower TUT rates than did younger adults, F(1, 192) = 175.89, p < .001, ηp2 = .48, and that subjects reported more TUTs in the 1-back than 2-back task, F(1, 192) = 11.69, p < .001, ηp2 = .06; although older adults reported fewer TUTs than did younger adults in both tasks, the Age Group × Memory Load interaction approached conventional significance, F(1, 192) = 3.33, p = .07, ηp2 = .02. Younger adults reported more TUTs in the 1-back than in the 2-back, t(109) = 3.77, p < .001, but older adults did not differ between tasks, t(83) = 1.12, p = .27. As indicated in Figure 7, TRI reports showed a different pattern. Older adults, as in Experiment 1, reported more TRI than did younger adults, F(1, 192) = 14.99, p < .001, ηp2 = .07, and subjects reported more TRI during the 2-back than 1-back, F(1, 192) = 12.13, p = .001, ηp2 = .06. Furthermore, Age Group interacted with Memory Load, F(1, 192) = 6.86, p < .01, ηp2 = .03, such that older adults reported more TRI than younger adults in 2-back, t(97) = −4.04, p < .001, but not in 1-back, t(95) = −1.06, p = .29.
Figure 6.
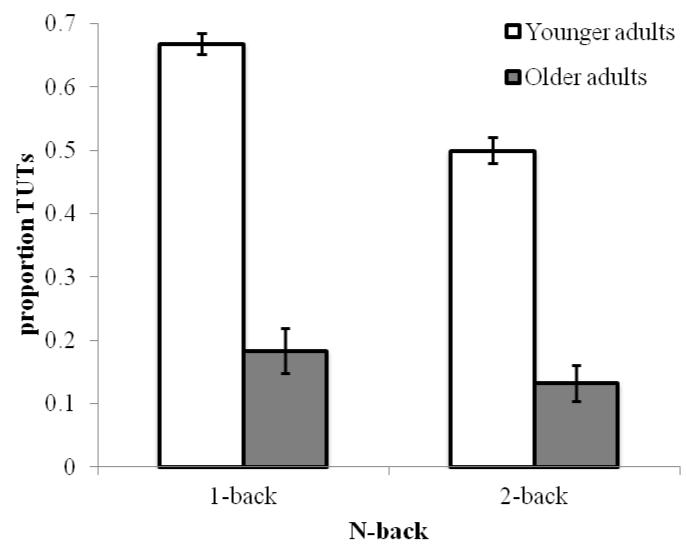
Mean proportion of TUT reports, by n-back memory load (1-back, 2-back) and Age (Younger and Older adults) in Experiment 2. Error bars represent standard errors. Note: TUT = task-unrelated thought.
Figure 7.
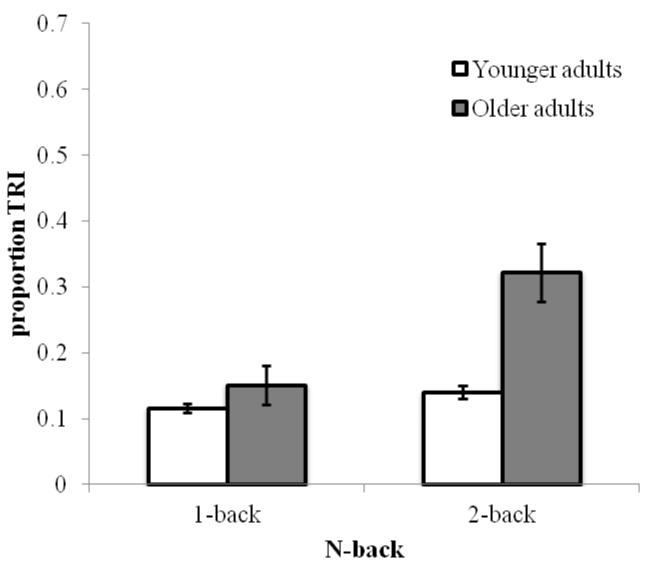
Mean proportion of TRI reports, by n-back memory load (1-back, 2-back) and Age (Younger and Older adults) in Experiment 2. Error bars represent standard errors. Note: TRI = task-related interference.
3.2.2. N-back Performance
In order to test whether our Experiment 2 manipulation of executive-control requirements was more successful in producing age differences than was Experiment 1, we examined accuracy and RT differences between our two n-back versions, while also determining whether any age or memory-load differences in performance would parallel those we found in subjects’ thought reports.
Target accuracy was better on the 1-back than 2-back task, F(1, 193) = 139.73, p < .001, ηp2 = .42, and although accuracy did not differ between older and younger adults (F < 1), Age Group interacted with Memory Load, F(1, 193) = 9.08, p < .001, ηp2 = .05. In 1-back, older adults identified more targets than did younger adults (M proportions = .98 and .95, respectively), t(95) = −2.66, p < .01, but in 2-back, younger adults identified more targets and did older adults (Ms = .88 vs. .85), t(97) = 1.99, p = .05. Similarly, for lure accuracy, Age Group and Memory Load produced main effects and an interaction. Younger adults committed fewer false alarms on lures overall, F(1, 192) = 8.66, p = .004, ηp2 = .04, and subjects committed fewer false alarms on the 1-back than 2-back, F(1, 192) = 183.36, p < .001, ηp2 = .49. There was also a significant interaction of Age Group and Memory Load on lure accuracy, F(1, 192) = 13.91, p < .001, ηp2 = .07. In the 1-back task, older and younger adults correctly rejected lures equally well (Ms = .97 and .96), t(95) = −1.27, p = .21, but in 2-back, younger adults correctly rejected more lures (M = .83) than did older adults (M = .74), t(97) = 3.53, p = .001. On non-lure trials, subjects had more false alarms in the 2-back than 1-back, F(1, 192) = 27.97, p < .001, ηp2 = .13, but older and younger adults did not differ, F(1, 192) = 1.53, p = .22, ηp2 = .01, and Age Group did not interact with Memory Load (F < 1).
We calculated signal-detection sensitivity (dL) and bias (CL) scores for each subject as we did for the SART. As indicated in Figure 8, accuracy was higher in the 1-back than in the 2-back, F(1, 192) = 274.96, p < .001, ηp2 = .59. Older adults and younger adults performed equally well, overall, F(1, 192) = 1.80, p = .18, ηp2 = .01, but these main effects were modified by a significant interaction, F(1, 192) = 23.99, p < .001, ηp2 = .11. In 1-back, older adults performed better than younger adults (Ms = 7.40 and 5.92), t(95) = −3.87, p < .001, but in 2-back, younger adults (M = 3.15) outperformed older adults (M = 2.30), t(97) = 2.98, p = .004. A “non-response” bias (CL > 0) was elicited by both n-back tasks (M = 0.28); bias did not differ by Memory Load, F(1, 192) = 3.47, p = .06, ηp2 = .02, or Age Group, F(1, 192) = 2.95, p = .09, ηp2 = .02, and Memory Load and Age Group did not interact, F(1, 192) = 2.73, p = .10, ηp2 = .01. Overall then, our 2-back task elicited the expected age difference in performance favoring young adults, while still demonstrating older adults to mind-wander less frequently than younger adults. (Analyses of RTs indicated overall slowing for older adults, but did not suggest any speed-accuracy trade-offs that might complicate interpretation of the age differences we found in n-back accuracy.2)
Figure 8.
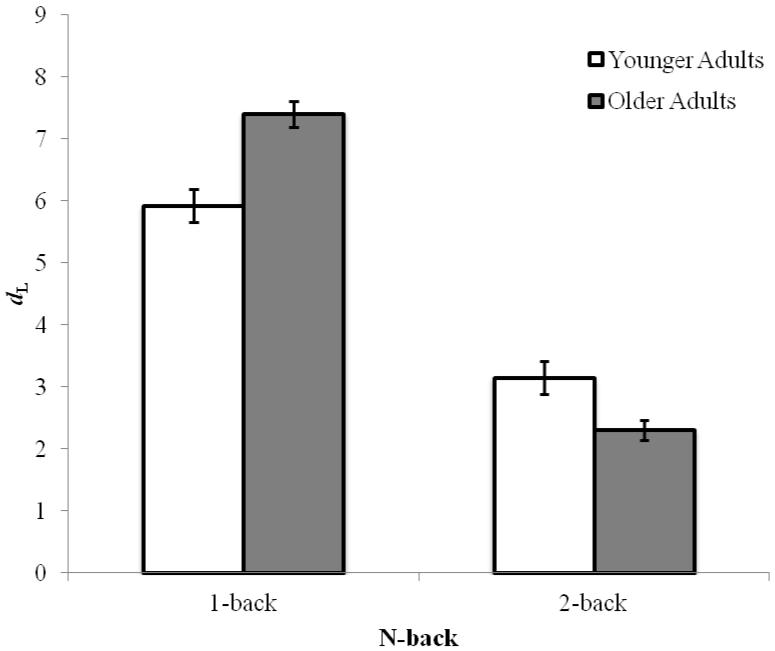
Mean signal-detection sensitivity (dL) by n-back memory load (1-back, 2-back) and Age (Younger and Older adults) in Experiment 2. Error bars represent standard errors.
3.2.3. Mind Wandering and Performance
Here we tested for both individual-differences and in-the-moment associations between TUTs and n-back performance. Regarding individual differences, TUT rates correlated negatively with 1-back accuracy (r = − .37) and dL (r = − .50), and positively with intra-subject RT variability to 1-back targets (r = .22). With Age partialed out, TUTs correlated negatively with 1-back accuracy (r = − .27) and dL (r = − .37), but not RT variability (r = .16; p = .13). In 2-back, as well, TUT rate predicted accuracy (r = − .19), but not dL (r = − .15; p = .13) or RT variability (r = .07). With Age partialed out, TUTs correlated negatively with 2-back accuracy (r = − .43), dL (r = − .46), and positively with RT variability (r = .23).
When TUT was included as a covariate in the Age Group × Memory Load ANOVA on performance (dL), the interaction of Age and Memory Load was still significant, F(1, 191) = 20.34, p < .001, ηp2 = .10. In the 1-back task, as in the SART in Experiment 1, the main effect of Age group (favoring older adults) was no longer significant when TUT was included as a covariate (F < 1), suggesting that older adults performed more accurately because they were more task-focused than younger adults. In 2-back, the main effect of Age group was still significant when TUT was included as a covariate, F(1, 96) = 33.53, p < .001, ηp2 = .26, indicating an effect of Age above and beyond the relationship of TUTs with performance – this makes sense because, unlike Experiment 1, older adults performed worse here than did younger adults while having more on-task thoughts. When TRI was included as a covariate, the interaction of Age and Memory Load was still significant, F(1, 191) = 24.29, p < .001, ηp2 = .11, as was the main effect of Age in both the 1-back and 2-back tasks, F(1, 94) = 13.81, p < .001, ηp2 = .13, and F(1, 96) = 6.73, p = .01, ηp2 = .07, respectively.
Regarding in-the-moment associations of TUTs and TRI with target accuracy, an Age Group × Memory Load × Thought Type (TUT, TRI, on-task) mixed ANOVA indicated a main effect of Thought Type, F(2, 228) = 15.80, p < .001, ηp2 = .12: Accuracy was worse when subjects reported TUTs or TRI versus on-task thoughts. Thought type also interacted with Memory Load, F(2, 228) = 3.37, p = .04, ηp2 = .03. Follow-up contrasts revealed that in 1-back, only TUTs were associated with worse performance on targets than were on-task thoughts, F(1, 60) = 16.39, p < .001, ηp2 = .22. TRI did not differ significantly from on-task thoughts, F(1, 60) = 2.40, p = .13, ηp2 = .04, although, numerically, TRI and TUTS were very similar: Compared to 95% (SD = 10%) target accuracy for on-task reports, subjects were accurate on 90% (SD = 23%) of trials when reporting TRI and 89% (SD = 13%) when reporting a TUT. In 2-back, both TUTs and TRI were associated with worse performance on targets than were on-task thoughts, F(1, 70) = 19.59, p < .001, ηp2 = .22; F(1,70) = 8.75, p = .004, ηp2 = .11, but they were not different from each other, F(1, 70) = 1.66, p =.20, ηp2 = .02. Subjects were accurate 79% (SD = 19%) of the time when reporting on-task thoughts but only 65% (SD = 24%) when reporting a TUT and 69% (SD = 28%) when reporting TRI. TUTs and TRIs seemed equally detrimental to subjects’ performance on target trials in the 2-back task. Age did not interact with thought type, F(2, 228) = 2.14, p = .12, ηp2 = .02.
The n-back task also allowed us to attach thought probes to designated lure trials, as well as to targets. Given that off-task thinking is associated with relatively shallow processing of external stimuli (e.g., Smallwood et al., 2011), we questioned whether mind-wandering episodes might make subjects especially vulnerable to responding on the basis of familiarity, leading to commission errors to lure stimuli. An Age Group × Memory Load × Thought Type (TUT vs. on-task) mixed ANOVA on lure accuracy, however, indicated no main effect of Thought Type, F(2, 148) = 1.91, p =.15, ηp2 = .03, nor any interactions with Age group or n-back (Fs < 1). TUT experiences were associated only with misses, and not with false alarms to lures.
3.3. Discussion
Older adults reported fewer TUTs than did younger adults during both n-back tasks, while demonstrating the predicted performance deficit in 2-back. Thus, older adults may exhibit executive-control difficulties despite relatively infrequent mind wandering. Also consistent with Experiment 1, older adults reported greater TRI than did younger adults, but here only during the more difficult 2-back task. Younger adults reported 58% TUTs and 13% TRI, for a total of 71% off-task thoughts, while older adults reported 16% TUTs and 24% TRI, for a total of 40% off-task thoughts. The findings confirm, again, that older adults mind-wander less than do younger adults, even when TRI is taken into account.
Younger adults outperformed older adults on both target (omission errors) and lure trials (commission errors) in the 2-back task. Older adults were also slower to respond, but unlike the SART, where slowing helps subjects stop more effectively on no-go trials, slowing here did not aid accuracy. As in Experiment 1, TUTs negatively affected performance, in the moment, equally for older and younger adults. The equivalent impact of TUTs on performance between younger and older adults’ does not seem to support the view that those with less attention to spare – older adults in this case – are especially hurt by TUTs.
4. General Discussion
The current experiments expanded upon previous aging and mind-wandering studies. First, we used randomly occurring probes to assess immediately preceding thoughts, rather than predictable probes to assess thought content over 20-30 s periods (cf. Giambra, 1989), as a way to reduce contributions of retrospective-memory deficits in older adults. Second, we probed for an additional category of thought, TRI (cf. Giambra, 1989; Jackson & Balota, 2011; Krawietz et al., in press). TRI reflects self- and performance-evaluative thoughts and thus might be important when testing older adults in the laboratory. Both experiments indicated that older adults report fewer TUTs than do younger adults, even after accounting for their elevated TRI.
4.1. Task-Related Interference
As we predicted, older adults reported more TRI than did younger adults. TRI does not map directly onto on-task thoughts or TUTs, but stands alone as a separate type of thought. TRI is task-related, like on-task thoughts, but was associated with performance deficits, similar to TUTs. Unlike TUTs, however, TRI was only associated with errors in the more difficult tasks – Standard SART and 2-back – but not Vigilance SART or 1-back. Finally, age differences in TRI were in the opposite direction to those in TUTs, with older adults reporting more TRI experiences but fewer TUTs. Self-evaluative thoughts may thus be similar to the compensatory strategies older adults use in response to awareness of age-related deficits (e.g., Dixon, deFrias, & Backman, 2001), although here we found no evidence that TRI experiences aid performance.
Although we suggest that age differences in TRI are important to laboratory-based studies of executive control and thought content, we do not know whether they also reflect age-related thought differences in everyday contexts. When older subjects choose to spend time participating in the laboratory and younger subjects participate as a course requirement, variables like task novelty, environmental novelty, task interest, and task-elicited effort, are likely to differ between age groups. Indeed, Jackson and Balota (2011) and Krawietz et al. (in press) found that older subjects expressed more interest in their lab tasks than did younger subjects. Moreover, anxiety about being compared directly to younger adults, a form of stereotype threat that will be specific to laboratory contexts (Hess et al., 2003; Rahhal et al., 2001), may trigger intrusive thoughts about task performance for older adults. Thus, laboratory tasks should provoke age differences in TRI that may be more subtle, or largely nonexistent, in daily life.
4.2. Aging, Mind-Wandering Reports, and Performance
Multiple and diverse findings of reduced TUTs with age may seem puzzling in light of age-related declines in executive control (see Braver & West, 2008; Hasher et al., 2007). However, some of the previous aging studies of mind wandering did not systematically examine the association between thought and performance because their tasks elicited age equivalence in performance (Giambra, 1989; Jackson & Balota, 2011; but see Krawietz et al., in press). Here, the seemingly paradoxical result, that older adults mind wander less often than do younger adults, persisted even in the 2-back task where younger adults outperformed older adults. This finding suggests that the entry of TUTs into awareness is not a primary cause of age-related declines in performance on executive tasks like 2-back. Furthermore, we assessed age differences in the relationship of TUTs with task performance. As in previous studies, analyses of both the overall TUT rate and in-the-moment analysis of TUTs and errors revealed a negative association between TUTs and accuracy. Importantly, the current studies showed that this relationship is the same for younger and older adults, and so TUTs were not differentially costly for older adults, in contrast to what executive-resource accounts would seem to predict (Giambra, 1989; Smallwood & Schooler, 2006; Teasdale et al. 1995).
One potential criticism of our (and others’) methods is that subjects may have reported TUTs as a reaction to a recent error, rather than as a valid report of their thought content (note this this explanation cannot handle well the replicable associations between TUT rate and RT variability; McVay & Kane 2009, 2012a, 2012b). However, in the n-back task, we examined in-the-moment thought reports attached to both target and lure trials and found that TUTs were associated with only target misses rather than lure false alarms. This distinction adds validity to the thought probe method in that, if subjects were simply reacting to all their errors by reporting TUTs, rather than genuinely reporting their thought content, TUTs reports should have followed both types of error. Furthermore, in the standard SART, TUTs accompanied errors on “false alarm” trials and so the particular response or error type does not distinguish the impact of TUTs on performance.
Finally, regarding thought-report validity, we must consider whether older adults consistently report few TUTs because meta-consciousness (e.g., Schooler, 2002) is altered with aging, an issue that has not been sufficiently addressed in previous studies of aging and mind-wandering. That is, older adults may be less able to monitor, assess, or remember their own subjective experiences, and so they may fail to perceive and report the TUTs they actually had throughout the task. Although this hypothesis warrants direct investigation in future mind-wandering work, the metacognition literature indicates that older adults are just as able to monitor their memory-encoding experiences as are younger adults (e.g., Hertzog & Dunlosky, 2011; Hertzog, Kidder, Powell-Moman, & Dunlosky, 2002; Robinson, Hertzog,& Dunlosky, 2006). Moreover, we see several direct indications that our older adults’ thought reports were as valid as our younger adults’ and thus were not compromised by meta-awareness deficits. First, older adults reported frequent TRI experiences, and so they were not reluctant to report some forms of off-task thought. Second, younger and older adults’ accuracy rates were similarly associated with thought reports in both experiments, with more errors preceding TUT and TRI reports than preceding on-task thought reports. Third, older and younger adults both sped up similarly on the trials preceding TUT reports in the Standard SART. Fourth, in both experiments, the pattern of response latencies to thought probes were similar for older and younger adults: Subjects in both age groups reported on-task thoughts more quickly than TRI experiences, which in turn were reported more quickly than TUTs (standarized RTs, collapsed across tasks: Experiment 1 younger adults’ Ms = − .106 ± .743, .056 ± .536, and .370 ± .431, respectively; Experiment 1 older adults’ Ms = .027 ± 1.011, .422 ± 1.231, and .878 ± .868, respectively; Experiment 2 younger adults’ Ms = − .172 ± .755, .182 ± .709, and .282 ± .472, respectively; Experiment 2 older adults’ Ms = − .327 ± .249, .401 ± .793, and 1.015 ± .887, respectively). If older adults were unable to report on their own subjective experiences, we would not expect them to be so similar to younger adults in their associations between thought reports and accuracy rates, thought reports and pre-target RTs, and latencies to report different categories of thought.
4.3. Implications for Mind Wandering Theory
Although Giambra’s resource-sharing view of mind wandering, where subjects divide their attentional capacity between task performance and TUTs (see also Smallwood & Schooler, 2006; Teasdale et al., 1995), explained reduced TUTs in older adults, it cannot explain the associations among age, TUTs, and performance reported here. If, as capacity views suggest (e.g., Norman & Bobrow, 1975; Posner & Boies, 1971), subjects divide available resources between tasks (here, between on- and off-task thoughts), and if younger adults have more resources available to divide, then it follows that younger adults should be less affected by TUTs than should older adults (see footnote 1). In contrast, we found that TUTs had the same detrimental effect on younger and older adults’ performance (and this same pattern holds for higher vs. lower WMC younger adults; McVay & Kane, 2010a).
Otherwise, an age-related decline in TUTs appears consistent with a resource view of mind wandering, at least at first glance, as aging has been hypothesized to reduce cognitive resources (e.g., Craik & Byrd, 1982). By this view, older adults report fewer TUTs because they do not have the available resources to entertain task-irrelevant thoughts. This explanation only works, however, until one considers age-related increases in TRI. Unless TRI-type thoughts can be demonstrated to require fewer cognitive resources than do TUTs, and we see no reason to think that they should (indeed, they seem to be equivalently associated with performance deficits in the moment), we probably should not appeal to reduced resources to explain the age-related decline in mind-wandering experiences.3
Given these limits to resource views (see also Navon, 1994), a new framework for understanding age differences in TUTs seems necessary. Indeed, Smallwood and colleagues recently proposed an alternative to resource-based theories of mind wandering (Smallwood, 2010, in press; Smallwood et al., 2011). Their “global availability/perceptual decoupling” view grows out of global workspace conceptions of consciousness (e.g., Baars, 1988; Dehaene, Kerszberg, & Changeux, 1998; Navon, 1989a, 1989b). According to these views, the broadcasting of goal-relevant information to the cognitive system can bring its specialized modules under widespread executive control of that globally available (and verbally reportable) information. The global availability/perceptual decoupling theory argues that mind-wandering experiences result when the broadcast workspace is occupied – in an all-or-none fashion – by off-task, internally generated representations. The attentional system thus becomes decoupled from its externally oriented, perceptual analysis of the outside world, resulting in minimal sensory analysis. Although Smallwood and colleagues continue to couch this theoretical approach in resource language, it lacks the defining features of a resource theory. That is, the theory argues clearly that the global workspace projects either on-task or off-task representations to the cognitive system, rather than acting as a divisible capacity that can be apportioned flexibly between externally and internally focused mentation.
Whatever the merits are of this novel theoretical approach, we know of no research suggesting age-related changes in the extent or quality of verbally reportable, or globally available, experiences, and so the theory does not make as clear predictions about age-related differences in mind wandering as did the original Smallwood-Schooler (2006) resource view. Indeed, to the extent that maintaining global availability to goal-relevant representations reflects a form of executive control, Smallwood’s view might seem to predict that populations with executive-control deficiencies, such as older adults, would also be especially likely to mind-wander [see, e.g., Smallwood (2010) regarding WMC-related individual differences in TUT rate]. With that said, Smallwood and colleagues argue that people with superior executive abilities should mind-wander more than should those with inferior executive abilities, at least during tasks that are easy enough to allow excess executive resources to be devoted to TUTs (e.g., Levinson et al., 2012).
The fact that TUTs decrease with adult age also appears problematic for our control failures × concerns theory (McVay & Kane, 2010a; 2010b): Older adults often exhibit impaired cognitive control (Braver & West, 2008) and so they should be particularly susceptible to the TUT intrusions. However, our view considers not only the influences of executive control on mind wandering, or we would simply refer to it as a “control failures” theory. Instead, we posit interacting influences of control processes and interference, in the form of cue-driven, off-task thoughts about personal concerns.4 The “control” factor reflects the ability of executive-control processes to maintain ready access to task goals and suppress TUTs (and other forms of distraction) before they enter awareness and disrupt goal maintenance. The “concerns” factor reflects the extent to which the environment cues a current concern and thereby interferes with ongoing-task goals (Singer, 1975; Klinger, 1971, 1999, 2009). We consider the automatic cuing of off-task thoughts as analogous to an experimental manipulation of interference within a task, such as during Stroop tasks where incongruent color-words disrupt responding relative to neutral words: Increased control processing is required to maintain task goals when there is more competing information provided by off-task thoughts cued by the environment. In fact, when the environment is manipulated to elicit more (or more urgent) current concerns (Antrobus, et al., 1966; Horowitz & Becker, 1971; Horowitz, Becker, & Moskowitz, 1971), subjects report more TUTs. Age differences in TUTs, therefore, should be viewed as an interaction of reduced control processes and the generation and cuing of concern-related, off-task thoughts.
By this view, older adults may have fewer TUTs than younger adults because they are less likely to produce off-task thoughts in response to the contextual cues available in a laboratory setting. For example, socioemotional selectivity theory (Carstensen 1993; 1995) explains age differences in the types of goals and current concerns people hold. Older adults’ goals tend to involve close relationships and emotional well-being, whereas younger adults profess more novelty-seeking and accomplishment-oriented goals. Few of older adults’ social or emotional goals are likely to be cued while sitting with an unfamiliar undergraduate in front of a flat panel LCD screen. That is, the college campus setting, with bustling hallways, high-tech computer workstations, and young student experimenters, is more likely related to the goals and current concerns of the undergraduate population, and so the typical context for aging studies is less likely to trigger current-concern-related TUTs in older subjects (McVay & Kane, 2010). With that said, note that older adults’ increased TRI reports are consistent with a concerns view: Although TRI experiences may not reflect older adults general life concerns outside the laboratory, they do seem to reflect contextually primed concerns about intellectual aging (e.g., Sindi et al., 2012). They thus provide mixed support for the control × concerns view: Older adults do report more off-task thought related to performance concerns than do younger adults, but older adults’ TRI still does not rise to the frequency level of younger adults’ TUT reports. Future research will be needed to determine whether cuing additional, personally relevant concerns for older adults will bring their total off-task thinking rate to the level of younger adults. For example, if familiar objects from older adults’ homes or lives (e.g., family portraits, keepsakes, print newspapers) were incorporated into the experimental setting, particularly those that might activate socioemotional goals, it may help overcome the concern-related cue disparity in the laboratory between older and younger adults. Alternatively, an exploration of TUTs within the daily life and routines of older adults, but without the dependence on retrospective reports, such as with an experience-sampling method (e.g., Kane et al., 2007; Killingsworth & Gilbert, 2010; Klinger & Cox, 1987-1988; McVay et al., 2009) may reveal a different pattern of age differences than do laboratory studies.
5. Conclusions
Two experiments addressed the possibility that age-related differences in TUTs were due to misclassifications of TRI experiences, and confirmed the finding that older adults mind wander less than do younger adults during ongoing laboratory tasks (Giambra, 1989; Jackson & Balota, 2011; Krawietz et al., in press). We suspect that the age difference in TUTs derive from differential cuing of concern-related, interfering thoughts, and our TRI findings support this, but further research is needed to more fully address this possibility. If the interference from off-task thoughts is somehow equated between younger and older adults, by analogy to aging studies where task demands are manipulated to equate performance across ages, we suspect that older adults would be less able than younger adults to exert cognitive control to keep those thoughts out of awareness.
Highlights.
Two experiments test for adult age differences in mind-wandering during cognitive tasks
Older adults show reduced rates of task-unrelated thought
Older adults show increased rates of performance-related thought
Findings are not easily accommodated by executive-resource theories of mind-wandering
Footnotes
According to a view that mind-wandering episodes draw on general executive resources and that tasks that require more resources allow for less mind wandering, McVay & Kane (2012a, 2012b) argued that it should also follow that the performance of people with more resources available should be less affected by mind-wandering than should that of people with fewer resources available. Someone with more resources should be able to dedicate more of them to two simultaneous activities (task performance and maintaining TUTs) than should someone with fewer resources (e.g., Norman & Bobrow, 1975; Posner & Boies, 1971). However, we acknowledge that resource theories are generally flexible enough – indeed, often to the point of unfalsifiability – to allow for multiple, if not contradictory, predictions (see Navon, 1984). For example, one might claim that ongoing task performance should be hurt equally for people with more and with fewer resources because those with more resources will engage in more complex, resource-intensive TUTs than might those with fewer resources.
Age Group and Memory Load exerted main effects on target RTs. Older adults were significantly slower (M = 771 ms) than were younger adults (M = 680 ms), F(1, 193) = 12.15, p = .001, ηp2 = .06), and RTs to 2-back targets were slower (M = 814 ms) than to 1-back targets (M = 622 ms), F(1, 193) = 53.47, p < .001, ηp2 = .22; the Age Group × Memory Load interaction was marginally significant, F(1, 193) = 3.34, p = .07, ηp2 = .02, whereby older adults slowed to a greater degree of slowing in the 2-back task. In the analysis of standardized RTs to account for age-related slowing, however, older adults’ marginally greater slowdown to 2-back versus 1-back trials was not significant, F < 1. For raw RTs to lure trials, only Memory Load exerted a main effect: Erroneous responses to 2-back lures (M = 966 ms) were slower than to 1-back lures (M = 841 ms), F(1, 187) = 8.84, p = .003, ηp2 = .05. Older adults and younger adults responded to lures with similar RTs, F < 1, and Age Group did not interact with Memory Load, F(1, 187) = 2.52, p = .11, ηp2 = .01; standardized RT analyses yielded a similar non-significant Age interaction with Memory Load, F < 1.
The same logic applies to TUT rates varying with task demands in the present study (i.e., during the less demanding tasks in Experiments 1 and 2 – vigilance SART and 1-back – versus the more demanding tasks – standard SART and 2-back). On one hand, the easier tasks, which should consume fewer resources than the harder tasks, also elicited higher TUT rates, seemingly consistent with the claim that TUTs depend on resource availability. But, again, on the other hand, this framework is unhelpful in explaining the TRI data, because the higher-demand tasks also produced the higher TRI rates. Without positive evidence that TRI and TUT experiences vary in resource demands, we cannot explain task-related variability in both by appeal to resource theory.
Smallwood’s global availability/perceptual decoupling view (Smallwood 2010; Smallwood et al., 2011) similarly emphasizes the influence of “default system” goal-related activity on thought content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Perceptual and Motor Skills. 1966;23:399–417. [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. Journal of Gerontology: Psychological Sciences. 2005;60(5):223–233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, West RL. Working memory, executive processes, and aging. In: Craik FIM, Salthouse TL, editors. Handbook of Aging and Cognition. 3rd Edition Psychology Press; New York: 2008. pp. 311–372. [Google Scholar]
- Carriere JSA, Cheyne JA, Solman JF, Smilek D. Age trends for failures of sustained attention. Psychology and Aging. 2010;25:569–574. doi: 10.1037/a0019363. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Motivation for social contact across the life span: A theory of socio-emotional selectivity. In: Jacobs J, editor. Nebraska Symposium on Motivation: Vol. 40. Developmental perspectives on motivation. University of Nebraska Press; Lincoln, NE: 1993. pp. 209–254. [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4:151–156. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne J, Solman G, Carriere J, Smilek D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111:98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop Color Test performance. Journal of Clinical Psychology. 1984;40(5):1244–1250. doi: 10.1002/1097-4679(198409)40:5<1244::aid-jclp2270400521>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub SE, editors. Aging and cognitive processes. Plenum Press; New York: 1982. [Google Scholar]
- Craik FIM, Salthouse TA. Handbook of aging and cognition. 3rd ed. Psychology Press; New York: 2008. [Google Scholar]
- Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proceedings of the National Academy of Sciences, USA. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM, Backman L. Characteristics of self-reported memory compensation in older adults. Journal of Clinical and Experimental Neuropsychology. 2001;23(5):650–661. doi: 10.1076/jcen.23.5.650.1242. doi: 10.1076/jcen.23.5.650.1242. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychologyand Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Finnigan F, Schulze D, Smallwood J. Alcohol and the wandering mind: A new direction in the study of alcohol on attentional lapses. International Journal on Disability and Human Development. 2007;6:189–199. [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Germain CM, Hess TM. Motivational influences on controlled processing: Moderating distractibility in older adults. Aging, Neuropsychology, and Cognition. 2007;14(5):462–486. doi: 10.1080/13825580600611302. doi: 10.1080/13825580600611302. [DOI] [PubMed] [Google Scholar]
- Gevins A, Bressler SL, Cutillo BA, Illes J, Miller JC, Stern J, Jex HR. Effects of prolonged mental work on functional brain topography. Electroencephalography and Clinical Neurophysiology. 1990;76:339–350. doi: 10.1016/0013-4694(90)90035-i. [DOI] [PubMed] [Google Scholar]
- Giambra LM. Task-unrelated thought frequency as a function of age: A laboratory study. Psychology and Aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Giambra LM. Adult male daydreaming across the life span: A replication, further analyses, and tentative norms based upon retrospective reports. International Journal of Aging and Human Development. 1977-78;8:197–228. doi: 10.2190/2bej-t9m9-mnja-l64l. [DOI] [PubMed] [Google Scholar]
- Grodsky A, Giambra LM. The consistency across vigilance and reading tasks of individual differences in the occurrence of task-unrelated and task-related images and thoughts. Imagination, Cognition and Personality. 1990-91;10:39–52. [Google Scholar]
- Hamm VP, Hasher L. Age and the availability of inferences. Psychology and Aging. 1992;7:56–64. doi: 10.1037//0882-7974.7.1.56. [DOI] [PubMed] [Google Scholar]
- Hartley AA. Evidence for the selective preservation of spatial selective attention in old age. Psychological Aging. 1993;8(3):371–379. doi: 10.1037//0882-7974.8.3.371. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Speer NK, Jonides J, Reuter-Lorenz PA, Smith EE. Is the dissociability of working memory systems for name identity, visual-object identity, and spatial location maintained in old age? Neuropsychology. 2001;15:3–17. doi: 10.1037//0894-4105.15.1.3. [DOI] [PubMed] [Google Scholar]
- Hartman M, Hasher L. Aging and suppression: Memory for previously relevant information. Psychology and Aging. 1991;6:587–594. doi: 10.1037//0882-7974.6.4.587. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford University Press; Oxford: 2007. pp. 227–249. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 22. Academic Press; San Diego, CA: 1988. pp. 193–225. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance, XVII. Cognitive regulation of performance: Interaction of theory and application. MIT Press; Cambridge, MA: 1999. pp. 653–675. [Google Scholar]
- Hertzog C, Dunlosky J. Metacognition in later adulthood: Spared monitoring can benefit older adults’ self-regulation. Current Directions in Psychological Science. 2011;30:167–173. doi: 10.1177/0963721411409026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Hultsch DF. Metacognition in adulthood and old age. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Lawrence Erlbaum Associates Publishers; Mahwah, NJ US: 2000. pp. 417–466. [Google Scholar]
- Hertzog C, Kidder DP, Powell-Moman A, Dunlosky J. Aging and monitoring associative learning: Is monitoring accuracy spared or impaired? Psychology and Aging. 2002;17:209–225. [PubMed] [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. Journal of Gerontology: Psychological Sciences. 2003;58B:P3–P11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ, Becker SS. Cognitive response to stress and experimental demand. Journal of Abnormal Psychology. 1971;78:86–92. doi: 10.1037/h0031386. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ, Becker SS, Moskowitz ML. Intrusive and repetitive thought after stress: A replication study. Psychological Reports. 1971;29:763–767. doi: 10.2466/pr0.1971.29.3.763. [DOI] [PubMed] [Google Scholar]
- Jackson JD, Balota DA. Mind-wandering in younger and older adults: Converging evidence from the Sustained Attention to Response Task and reading for comprehension. Psychology and Aging. 2011 doi: 10.1037/a0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Kirchner WK. Age-differences in short-term retention of rapidly changing information. Journal of Experimental Psychology. 1958;55(4):352–358. doi: 10.1037/h0043688. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagination, Cognition and Personality. 1987-1998;7:105–128. [Google Scholar]
- Klinger E. Structure and functions of fantasy. Wiley; New York: 1971. [Google Scholar]
- Klinger E. Thought flow: Properties and mechanisms underlying shifts in content. In: Singer JA, Salovey P, editors. At play in the fields of consciousness: Essays in honor of Jerome L. Singer. Erlbaum; Mahwah, NJ: 1999. pp. 29–50. [Google Scholar]
- Klinger E. Daydreaming and fantasizing: Thought flow and motivation. In: Markman KD, Klein WMP, Suhr JA, editors. Handbook of imagination and mental simulation. Psychology Press; NewYork, NY: 2009. pp. 225–239. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mackworth JF. Paced memorizing in a continuous task. Journal of Experimental Psychology. 1959;58(3):206–211. doi: 10.1037/h0049090. doi: 10.1037/h0049090. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2005) and Watkins (2008) Psychological Bulletin. 2010a;136:188–197. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Adrift in the stream of thought: The effects of mind wandering on executive control and working memory capacity. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of individual differences in cognition: Attention, memory and executive control. Springer; New York: 2010b. [Google Scholar]
- McVay JC, Kane MJ. Drifting from slow to “D’oh!”: Working memory =apacity and mind wandering predict extreme reaction times and executive-control Errors. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012a doi: 10.1037/a0025896. Advance online publication. doi: 10.1037/a0025896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General. 2012b doi: 10.1037/a0025250. Advance online publication. doi:10.1037/a0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind-wandering across controlled and ecological contexts. Psychonomic Bulletin & Review. 2009;16:857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Knouse L, Mitchell J, Brown L, Kane MJ, Kwapil TR. Impaired conductors in the train of thought? Mind wandering in Attention-Deficit/Hyperactivity Disorder; Poster presented at the annual meeting of Association for Psychological Science; Chicago, IL. 2008; May, 2008. [Google Scholar]
- Mikulincer M, Nizan B. Causal attribution, cognitive interference, and the generalization of learned helplessness. Journal of Personality and Social Psychology. 1988;55:470–478. doi: 10.1037//0022-3514.55.3.470. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Gold G, Leonards U, Costa-Fazio L, Michel JP, Ibanez V. Aging and working memory: Early deficits in EEG activation of posterior cortical areas. Journal of Neural Transmission. 2004;111:1141–1154. doi: 10.1007/s00702-004-0159-2. [DOI] [PubMed] [Google Scholar]
- Navon D. Resources – A theoretical soup stone? Psychological Review. 1984;91:216–234. [Google Scholar]
- Norman DA, Bobrow DB. On data-limited and resource-limited processes. Cognitive Psychology. 1975;7:44–64. [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, Verguts T. Post-error slowing: An orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Binding and inhibition in working memory: Individual and age differences in short-term recognition. Journal of Experimental Psychology: General. 2005;134:368–387. doi: 10.1037/0096-3445.134.3.368. [DOI] [PubMed] [Google Scholar]
- Parks CW, Klinger E, Perlmutter M. Dimensions of thought as a function of age, gender and task difficulty. Imagination, Cognition and Personality. 1988-89;8:49–62. [Google Scholar]
- Posner MI, Boies SJ. Components of attention. Psychological Review. 1971;78:391–408. [Google Scholar]
- Rahhal TA, Hasher L, Colcombe SJ. Instructional manipulations and age differences in memory: Now you see them, now you don’t. Psychology and Aging. 2001;16:697–706. doi: 10.1037//0882-7974.16.4.697. [DOI] [PubMed] [Google Scholar]
- Reason J. Human error. Cambridge University Press; New York, NY US: 1990. [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neurospsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Robinson AE, Hertzog C, Dunlosky J. Aging, encoding fluency, and metacognitive monitoring. Aging, Neuropsychology, and Cognition. 2006;13:458–478. doi: 10.1080/13825580600572983. [DOI] [PubMed] [Google Scholar]
- Sarason IG. Anxiety, self-preoccupation and attention. Anxiety Research. 1988;1:3–7. [Google Scholar]
- Sayette MA, Reichle ED, Schooler JW. Lost in the sauce: The effects of alcohol on mind wandering. Psychological Science. 2009;20:747–752. doi: 10.1111/j.1467-9280.2009.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler JW, Reichle ED, Halpern DV. Zoning out while reading: Evidence for dissociations between experience and metaconsciousness. In: Levin D, editor. Thinking and seeing: Visual metacognition in adults and children. MIT Press; Cambridge, MA: 2004. pp. 203–226. [Google Scholar]
- Shaw GA, Giambra LM. Task unrelated thoughts of college students diagnosed as hyperactive in childhood. Developmental Neuropsychology. 1993;9:17–30. [Google Scholar]
- Sindi S, Juster R-P, Wan N, Nair NPV, Kin NY, Lupien SJ. Depressive symptoms, cortisol, and cognition during human aging: The role of negative aging perceptions. Stress: The International Journal on the Biology of Stress. 2012;15:130–137. doi: 10.3109/10253890.2011.599047. [DOI] [PubMed] [Google Scholar]
- Singer J. The inner world of daydreaming. Harper Colophon Books; New York: 1975. [Google Scholar]
- Singer J, McCraven V. Some characteristics of adult daydreaming. Journal of Psychology. 1961;51:151–164. [Google Scholar]
- Smallwood J. Why the global availability of mind wandering necessitates resource competition: Reply to McVay and Kane (2010) Psychological Bulletin. 2010;136:202–207. [Google Scholar]
- Smallwood J, Brown KS, Tipper C, Giesbrecht B, Franklin MS, Mrazek MD, Carlson JM, Schooler JS. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS ONE. 2011;6(3):e18298. doi: 10.1371/journal.pone.0018298. doi 10.1371/journal.pone.0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry MV, O Connor RC, Obonsawain MC. Subjective experience and the attentional lapse. Task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;4:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Heim D, Riby L, Davies JD. Encoding during the attentional lapse: accuracy of encoding during the semantic SART. Consciousness and Cognition. 2005;15:218–231. doi: 10.1016/j.concog.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, Schooler JW. The restless mind. Psychological Bulletin. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Memory and Cognition. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- Van Gerven PWM, Meijer WA, Prickaerts JHM, Van der Veen FM. Aging and focus switching in working memory: Excluding the potential role of memory load. Experimental Aging Research. 2008 doi: 10.1080/03610730802274165. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Basak C, Hartman M, Verhaeghen P. Aging and working memory inside and outside the focus of attention: Dissociations of availability and accessibility. Aging, Neuropsychology, and Cognition. 2008;15:703–724. doi: 10.1080/13825580802061645. [DOI] [PubMed] [Google Scholar]
- West R, Baylis GC. Effects of increased response dominance and contextual disintegration on the Stroop interference effect in older adults. Psychology & Aging. 1998;13(2):206–217. doi: 10.1037//0882-7974.13.2.206. [DOI] [PubMed] [Google Scholar]


