Abstract
Implantable sensors for continuous glucose monitoring hold great potential for optimal diabetes management. This is often undermined by a variety of issues associated with: (1) negative tissue response; (2) poor sensor performance; and (3) lack of device miniaturization needed to reduce implantation trauma. Herein, we report our initial results towards constructing an implantable device that simultaneously address all three aforementioned issues. In terms of device miniaturization, a highly miniaturized CMOS (complementary metal-oxide-semiconductor) potentiostat and signal processing unit was employed (with a combined area of 0.665 mm2). The signal processing unit converts the current generated by a transcutaneous, Clark-type amperometric sensor to output frequency in a linear fashion. The Clark-type amperometric sensor employs stratification of five functional layers to attain a well-balanced mass transfer which in turn yields a linear sensor response from 0 to 25 mM of glucose concentration, well beyond the physiologically observed (2 to 22 mM) range. In addition, it is coated with a thick polyvinyl alcohol (PVA) hydrogel with embedded poly(lactic-co-glycolic acid) (PLGA) microspheres intended to provide continuous, localized delivery of dexamethasone to suppress inflammation and fibrosis. In vivo evaluation in rat model has shown that the transcutaneous sensor system reproducibly tracks repeated glycemic events. Clarke’s error grid analysis on the as –obtained glycemic data has indicated that all of the measured glucose readings fell in the desired Zones A & B and none fell in the erroneous Zones C, D and E. Such reproducible operation of the transcutaneous sensor system, together with low power (140 μW) consumption and capability for current-to-frequency conversion renders this a versatile platform for continuous glucose monitoring and other biomedical sensing devices.
Keywords: Implantable sensors, CMOS circuits, amperometric glucose sensors, low-power microelectronics, in vivo monitoring
1. Introduction
A growing amount of research and clinical work has indicated that implantable CGM (continuous glucose monitoring) devices hold great potential for the management of diabetes and related disorders. However, the true prospects of implantable devices are undermined due to three major challenges (Vaddiraju, Burgess et al. 2010; Vaddiraju, Tomazos et al. 2010):
Lack of reliability in the sensing element due to low sensitivity, poor selectivity and inadequate linearity over the entire physiological glucose range (Vaddiraju S 2009; Vaddiraju, Legassey et al. 2011),
Biofouling and other post-implantation effects such as inflammation, fibrosis etc. (Wisniewski and Reichert 2000; Gifford, Kehoe et al. 2006),
lack of miniaturization that is needed to minimize the trauma inflicted during sensor implantation, as well to realize needle based implantation and explantation without the need for complicated surgery (Johnson, Mastrototaro et al. 1992; Errachid, Ivorra et al. 2001; Kvist, Iburg et al. 2006).
To mitigate the aforementioned tribulations, research on implantable glucose sensors is constantly advancing on the following three research frontiers:
Improvement on advanced glucose detection schemes based on electrochemical (Degani and Heller 1987; Dong, Wang et al. 1992; Vaddiraju, Burgess et al. 2009; Vaddiraju S 2009), near-IR (MacKenzie, Ashton et al. 1999; Malchoff, Shoukri et al. 2002; Weiss, Yegorchikov et al. 2007), Raman (Enejder, Scecina et al. 2005; Lyandres, Yuen et al. 2008), fluorescence (Pickup, Hussain et al. 2005; Khan, Saxl et al. 2010), and piezoelectric technologies (Ersöz, Denizli et al. 2005) alongside other transduction mechanisms (Vaddiraju, Burgess et al. 2010), with the common goal of achieving stable and reliable sensor performance,
Development of biocompatible coatings to reduce biofouling as well as other negative tissue responses such as inflammation and fibrosis (Frost and Meyerhoff 2006; Norton, Koschwanez et al. 2007; Bhardwaj, Sura et al. 2010; Morais, Papadimitrakopoulos et al. 2010; Rawat and Burgess 2011),
Engineering of advanced CMOS-based circuits to attain extreme miniaturization and reduce the overall power consumption (McKean and Gough 1988; Shults, Rhodes et al. 1994; Haider, Islam et al. 2007; Serra, Rocchitta et al. 2007; Zhang, Haider et al. 2007; Ahmadi and Jullien 2009; Bolomey, Meurville et al. 2009; Valdastri, Susilo et al. 2009).
While a given research strategy is focused on specific aspects of implantable glucose sensors, further complications may arise from possible interrelationships between the aforementioned strategies. For example, the utilization of biocompatible coatings to minimize negative tissue response and extend sensor lifetime introduces an additional barrier to glucose diffusion that can reduce sensor response time and sensitivity (Vaddiraju S 2009). Similarly, device miniaturization stands as a major impediment for amperometric techniques, since reduction in the area of the working electrode generally leads to weaker signal intensity that rapidly approaches noise level. Along these lines, nanomaterials have been utilized to improve glucose sensor performance, albeit at the cost of heightened immunogenicity. (Vaddiraju, Tomazos et al. 2010) Finally, advanced electronic circuits that are critically needed to detect small changes in sensor response consume more power, necessitating larger power sources which inadvertently increase the overall size of the implanted device (Wilkins, Atanasov et al. 1995).
Over the years, our group has been working on a miniaturized (0.5 X 0.5 X 5 mm) completely implantable glucose sensor with particular emphasis on the aforementioned issues. In order to eliminate foreign body response upon implantation, our group has developed a composite coating of polyvinyl alcohol (PVA) hydrogel and dexamethasone-loaded poly (lactic-co-glycolic acid) microspheres for localized delivery of dexamethasone, a potent anti-inflammatory agent. Such microsphere-based drug delivery has been shown to be highly local and the dexamethasone dosage required for complete suppression of negative tissue response is well below the threshold needed to cause any systemic effects. Similarly, in order to bypass toxicity-issues associated with the use of mediators, the sensing element is designed in such a way that optimal sensor performance (in terms of sensitivity, linearity and response time) is achieved solely through careful mass transfer balance of the various electrochemical participating species namely glucose, O2 and H2O2. (Vaddiraju, Legassey et al. 2011).
In this report, a custom-made CMOS circuitry with a total area of only 0.665mm2 is interfaced with the aforementioned electrochemical sensor. With a total power consumption of only 140 μW, this unique design presents the ability to operate together with highly-miniaturized powering devices, imperative for robust and long-lasting implantable sensor platforms. Embedded in these electronics is a current-to-frequency converter (where the frequency is proportional to glucose levels), which is employed as the data processing scheme where the raw frequency generated can be ultimately transmitted out of the body via electromagnetic radiation. The stability of the CMOS circuitry versatility of the sensor design together with ease of fabrication renders such current-to-frequency transduction mechanism as a powerful tool for continuous glucose monitoring, which is amenable to wireless communication. Herein, we report on the simultaneous performance of these elements when configured in a transcutaneous fashion, en route to the fabrication of a completely implantable unit. For this the PVA/PLGA-coated glucose sensor is implanted subcutaneously while the CMOS-circuitry (containing the potentiostat and signal processing block) resided externally. The amperometric current from the sensor is fed to the signal processing unit, which converts the analog amperometric glucose current into voltage pulses (whose frequency is proportional to the glucose level). The signal processing unit affords a linear correlation between the potentiostat-generated sensor current and the output frequency, hence rendering this a robust methodology for future, wireless-based, transmission protocols. In vitro amperometric experiments revealed that the sensors respond linearly over the entire physiological glucose concentration range (2–22 mM) with sensitivity as high as 560 Hz/mM. Short-term (3-hr) in-vivo results indicate that the sensor reproducibly tracks repeated glycemic events. Clarke’s error grid analysis of the as-obtained glycemic data has shown that all the glucose readings fell in the desired Zones A & B and none in the erroneous Zones C, D and E. This study represents our first study on the operation of the current-to-frequency transduction mechanism which affords advantages in terms of device size and complexity over traditional frequency modulation methodologies (Jain, Grantham et al. 2008) and forms a guide for future CMOS-based designs.
2. Experimental Details
2.1 Materials
Glucose oxidase enzyme (GOx) (E.C. 1.1.3.4, 157 units/mg, Aspergillus Niger), catalase (Cat) (E.C. 1.11.1.6, 5,000 units/mg), glutaraldehyde (25% w/v solution in water), phenol, bovine serum albumin (BSA), glutaraldehyde (50% w/v) and D-glucose (reagent grade) were purchased from Sigma. PVA (99% hydrolyzed, MW 133 kDa) was obtained from Polysciences Inc. (Warrington, PA). Platinum and silver wires were purchased from World Precision Instruments. Selectophore Polyurethane (PU) (catalog number 81367) was purchased from Fluka and used as is.
2.2 Fabrication of amperometric glucose sensors
The working and reference electrodes were made by coiling 125 μm platinum (Pt) wire and Silver (Ag) wires in close proximity to the working electrode (Vaddiraju, Legassey et al. 2011). The surface of the silver wire was subsequently converted to AgCl via galvanometry (at 0.4 V vs. standard calomel electrode for 5 min) in a stirred 0.1 M HCl solution, to render the Ag/AgCl reference electrode (Vaddiraju, Legassey et al. 2011). Overall, the sensor is cylindrical with a radius of about 0.5 mm and length of 10 mm. The area of the working electrode is 3 mm2.
The working electrode was first electrochemically cleaned in a 0.5 M H2SO4 solution via potential cycling between −0.21 to 1.25 V, until a stable background current has been reached. Next, a film of poly phenol (PPh) was electropolymerized on the working electrode from an aqueous phenol solution (Geise, Adams et al. 1991). Subsequently, the GOx enzyme was immobilized by dip coating the Pt/PPh electrode from a solution of 140 mg/ml glucose oxidase, 56 mg/ml BSA and 25% w/v glutaraldehyde, the latter of which enables enzyme cross-linking, followed by a 2-hr soak in phosphate buffer saline (PBS, pH = 7.4) to remove the uncrosslinked proteins (House, Anderson et al. 2007). The next step involves dip-coating the sensor with a polyurethane (PU) layer from a 3% (w/w) PU solution in 98% tetrahydrofuran (THF)/2% dimethylformamide (DMF) (w/w) (Yang, Chung et al. 2002). Subsequently, a thin layer of catalase enzyme was added via coating it from a solution of catalase, BSA and glutaraldehyde, followed by another 2-hr soak in PBS to remove the uncross-linked proteins. Finally, the sensor was encased within a thick poly(vinyl alcohol) (PVA) hydrogel containing dexamethasone-loaded poly (lactic-co-glycolic acid) (PLGA) microspheres (at concentrations of 75 mg microspheres per ml of PVA) and subjected to three freeze-thaw cycles to induce physical cross-linking of PVA (Galeska, Kim et al. 2005; Vaddiraju S 2009; Vaddiraju, Legassey et al. 2011).
The thickness of poly phenol was estimated to be ca. 10 nm based on prior work on similar electropolymerized films (Malitesta, Palmisano et al. 1990; Chu, Duan et al. 2007). Scanning electron microscopy (operating at 10 kV accelerating voltage and a working distance of 15 mm) was performed on the cross-section of the sensor to determine the thickness of GOx (Layer 2), PU (Layer 3), Catalase (Layer 4) and PVA/PLGA composite (Layer 5). The samples were sputter coated with palladium to eliminate charging during imaging.
2.3 Electronic Circuitry and Data Analysis
Miniaturized potentiostat and signal processing circuitry were fabricated using the MOSIS low-cost prototyping and small-volume production service and utilized a 0.35μm CMOS process. This fabricated MOSIS chip consisted of a fully differential potentiostat and signal processing unit. Prior to the fabrication of these circuits, the design was tested in a basic bread-board configuration, followed by simulation and layout using Cadence design software. In order to access the CMOS device terminals for testing, the fabricated electronic chip was mounted and wire-bonded to a dual in-line package, and integrated with the working, reference and counter electrode of the amperometric sensor. This was then connected to a National Instruments Educational Laboratory Virtual Instrumentation Suite (NI ELVIS) prototyping platform which provided both power to the electronic circuitry, as well as a data acquisition port to receive the incoming frequency-based data from the signal processing unit. This sensor platform is controlled via a home-built LabVIEW module that implements a fast-fourier transform (FFT) algorithm on the output frequency signal of the signal processing unit, as a means to record and display the result on a user-friendly graphical interface. In addition, the LabVIEW program monitors the potential difference between the working and reference electrode. This voltage measurement, along with the output frequency, gives a complete dynamic picture of the operation of the transcutaneous sensor system which allows error-free glucose monitoring during both in vitro and in vivo operations.
2.4 In vitro and in vivo testing
In vitro amperometric experiments were performed in a stirred PBS solution (pH 7.4) maintained at 37 °C. The sensor response current vs. various glucose concentrations were obtained by raising the glucose levels in the test-cell by 2 mM every 100 seconds for up to 30 mM, following an initial background stabilization period of ~8 min. The experiment was performed utilizing either the aforementioned MOSIS chip or a commercial electrochemical work station (CH Instruments 1010B).
In vivo amperometric experiments were performed in young anesthetized male Sprague rats (150 – 175 g) and in accordance with the IACUC (Institutional Animal Care and Use Committee) guidelines. The sensor is implanted into the interscapular s.c. tissue using a thin wall 16-guage hypodermic needle. The implanted sensor is connected to the outer MOSIS chip through three thin (125 μm) insulated wires that exit through the skin. The MOSIS chip and sensor then attached onto the back of the animal using a commercially available rat jacket. The sensors were polarized for 1.5 hr to obtain a stable signal (run-in background decay (Choleau, Klein et al. 2002)), following which 0.3 mL of sterile 50% dextrose was administered intraperitoneally. The amperometric response corresponding to the glycemic event of the rat was recorded continuously, while the tail-vein blood glucose readings were obtained periodically at 5 min intervals. Following the first glycemic event (which constitutes a sequential increase and decrease in blood glucose), a second glucose injection (0.6 ml of 50% dextrose) was administered and the procedure was repeated again. The overall time the sensor resided in the body is about 3 hours per rat for a total of three rats.
In vitro sensitivity is determined as the slope of the linear regression between sensor response and glucose concentration for the entire physiological glucose concentration (2–22 mM). In vivo sensor sensitivity was deduced from the average of I1/G1 and I2/G2, wherein I1 and I2 represent the peak amperometric sensor response for the two glycemic events while G1 and G2 represent the peak glucose concentrations of the two glycemic peaks (Choleau, Klein et al. 2002), as determined by a Bayer Contour® Blood Glucose Monitoring System. The resulting sensitivity was then utilized to convert the output frequency into glucose readings. The as-obtained glucose readings were retrospectively time-shifted to account for the generally observed lag-phase between the blood and s.c. glycemic events. (Pasic, Koehler et al. 2006; Vaddiraju, Burgess et al. 2010) These time-shifted glucose values, along with the corresponding tail-vein glucose values were then utilized to construct the Clarke’s error grid analysis (Choleau, Klein et al. 2002).
3. Results and Discussion
Figure 1a illustrates schematically the cross-section of the 5-layer electrochemical glucose sensing element of the transcutaneous sensor system under study, and Figure 1b shows a picture of the actual electrochemical sensor alongside a 16 gauge needle used for implantation. The Pt working electrode was coated with a thin (ca. 10 nm) electropolymerized polyphenol (PPh) layer in order to prevent the diffusion of large molecular weight electrochemical active species (i.e. acetaminophen, ascorbic acid, uric acid, etc.) to the working electrode, where they are likely to get oxidized at the operating potential of 0.7 V vs. Ag/AgCl (Wilson and Gifford 2005; Gifford, Kehoe et al. 2006). The electrode was then decorated with GOx enzyme that was immobilized via cross-linking with glutaraldehyde. Subsequently, the device was dip coated with polyurethane (PU) to yield a conformal film on top of the GOx layer. This PU film was used to offset the large glucose-to-O2 ratio within the s.c. tissue (typically 30 to 300 in normal vs. hyperglycemic conditions) and render the sensor linear within the physiological glucose concentration (2 to 22 mM) (Yang, Chung et al. 2002; Vaddiraju, Legassey et al. 2011). Following this, a thin layer of catalase was coated on top of the PU membrane to convert H2O2 to O2 and enable facile equilibration among various participating species (Tipnis, Vaddiraju et al. 2007; Vaddiraju, Burgess et al. 2008; Vaddiraju, Legassey et al. 2011). This, in turn, affords adequate sensor sensitivity, fast response times and minimal hysteresis by providing n enzymatic (catalase) driving force for outwards diffusion of H2O2 to match the GOx-induced driving force for inwards diffusion of glucose and O2 (Vaddiraju, Legassey et al. 2011). In addition, the catalase-assisted conversion of outerdiffused H2O2 to O2 also prevents possible tissue irritation by H2O2 leaking out from the sensor to the surrounding tissue (Watt, Proudfoot et al. 2004).
Fig. 1.
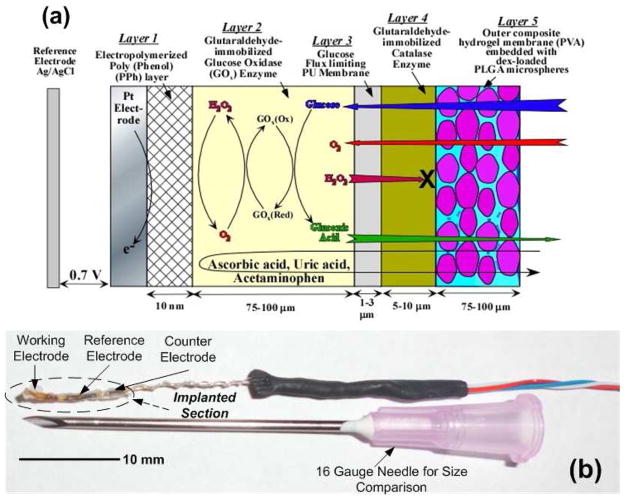
(a) Cross-sectional schematic of the 5-layer electrochemical amperometric glucose sensor employed in the transcutaneous system under investigation (layers not to scale). (b) Picture of the sensor alongside a 16 gauge needle used for implantation.
Following the catalase layer, the device was encased within a poly(vinyl alcohol) (PVA) hydrogel matrix containing dexamethasone-loaded PLGA microspheres, which was cross-linked in place through the application of 3 repetitive freezing and thawing cycles (Galeska, Kim et al. 2005). This is achieved through freeze-induced water micro-crystallization, that causes partial PVA dehydration and the subsequent formation of ordered domains that act as physical cross-links (Galeska, Kim et al. 2005). Upon implantation, the degradation of PLGA microspheres is initiated, which results in the controlled release of dexamethasone (Galeska, Kim et al. 2005; Bhardwaj, Sura et al. 2007; Patil, Papadimitrakopoulos et al. 2007; Bhardwaj, Sura et al. 2010). It has previously been established that the PVA hydrogel in this PVA/PLGA microsphere composite attains a continuous pathway for uninterrupted diffusion of glucose towards the inner layers of the working electrode as well as for outwards diffusion of various enzymatic byproducts and tissue response modifiers (Galeska, Kim et al. 2005; Vaddiraju, Singh et al. 2009).
Figure 2 shows a block diagram of the transcutaneous sensor system (sensing element as well as the associated components) (Figure 2a) as well as a photograph of the CMOS fabricated potentiostat and signal processing unit (Figure 2b). The three electrodes of the transcutaneous glucose sensor interface with the fabricated potentiostat, and the resulting current is sent to a signal processing block which acts as a current-to-frequency (in the form of a square waveform) converter. This square waveform (i) is then sent to the NI ELVIS unit (ii) which is in direct communication with the LabVIEW module that hosts a Fast Fourier Transform (FFT) algorithm (iii). This in turn transforms the square waveform (i) to its frequency Eigen values (iii) and display the strongest frequency (i.e. f1) to the computer graphical interface.
Fig. 2.
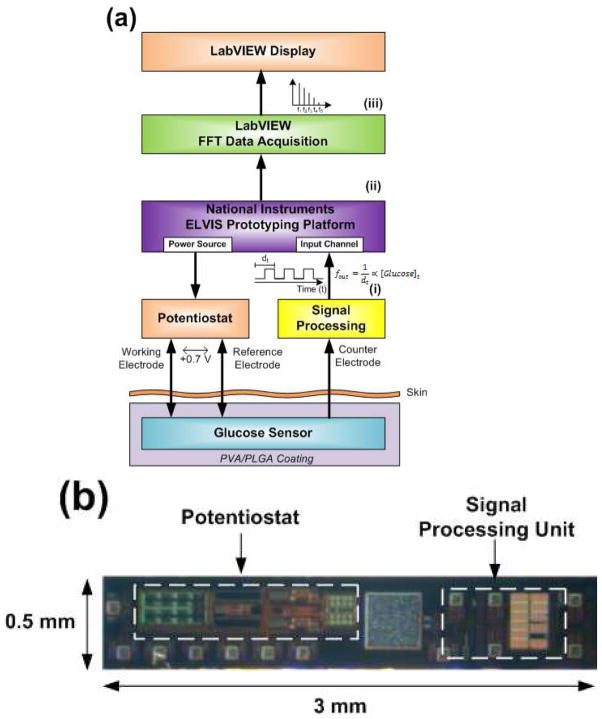
(a) Block diagram of the in vitro and in vivo transcutaneous sensor system under investigation. The CMOS potentiostat and signal processing unit interface with the electrochemical glucose sensor to produce a frequency in the form of square pulses (i). This frequency is then sent to the National Instruments ELVIS Prototyping Platform (ii) where and the resulting data are gathered and processed via the fast-fourier transform (FFT) algorithm (iii) to extract the frequency with the maximum amplitude (i.e. f1) and graphically display it on a computer interface using LabVIEW software. (b) Photograph of the CMOS fabricated potentiostat and signal processing unit.
The potentiostat employed in this design is of the fully differential type, which uses two operational amplifiers to buffer the working and reference electrode, and a control amplifier to maintain +0.7 V between the working and reference electrode (Martin, Gebara et al. 2009). This potentiostat occupies an area of 0.407 mm2 and consumes ca. 108 μW of power. The signal processing unit, employs a current-to-frequency converter (Haider, Islam et al. 2007; Zhang, Haider et al. 2007) that takes the output from the electrochemical sensor (i.e. the current from the counter electrode) as its input. Using a current mirror, the sensor current modulates the charging and discharging of an integrating capacitor, whose output frequency is directly proportional to the sensor current. The signal processing circuitry occupies an area of 0.258 mm2 and consumes ca. 19 μA of current. This affords considerable reduction in overall device dimensions that could be particularly important for future completely implantable systems.
In light of the current-to-frequency transduction protocol introduced above, a series of studies are presented below to establish its functionality and robustness. First, we evaluated the signal processing unit with an ideal current source from 0.1 to 1.5 μA, a range that matches closely to that of the amperometric glucose sensor. Figure 3a shows the variation in output frequency from the signal processing unit as a function of the input current. The output frequency varies linearly (with a linear regression factor (R2) of 0.994) and reproducibly with the input current and only a slight deviation from linearity is observed at high current values, which is within error range. To further establish the efficacy of the signal processing unit when operated along with the sensor and potentiostat, the frequency response of a three layer sensor (Pt/PPh/GOx/PU) was evaluated in vitro as a function of glucose concentration and contrasted with the amperometric response of the same sensor, as obtained from a commercial electrochemical workstation. Here, it is worth mentioning that layers 4 and 5 have been excluded in order to maximize the generated amperometric response which in turn allows characterization of the signal processing block for a wider range of current values. Figure 3b illustrates that the glucose dependant output frequency (left ordinate) and amperometric current (right ordinate) closely match each other, to mirror the linear response of the glucose sensor, as previously reported (Vaddiraju, Legassey et al. 2011). This confirms the operational reliability of the signal processing unit in converting the amperometric current response to output frequency. Here it is worth noting that the sensitivity of the sensor deduced as a slope of linear regression line between the amperometric current and glucose concentration is about 62 nA/mm2-mM, which is comparable to previously reported values (Trzebinski, Moniz et al. 2011).
Fig. 3.
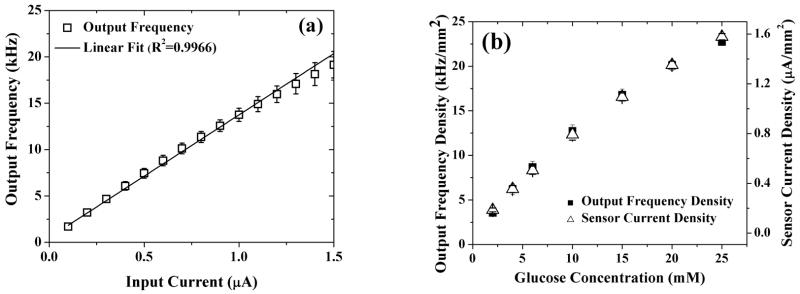
(a) Characterization of the signal processing unit in the current range of the sensors using an ideal current source as the input. (b) Comparison of the output frequency of the fabricated potentiostat and signal processing unit against the saturation amperometric response of a Pt/PPh/GOx/PU sensor as a function of glucose concentration.
In order to attain reliable sensor operation in an in vivo environment, the remaining two layers (i.e. catalase and PVA hydrogel/Dex-loaded PLGA microsphere composite) were introduced to prevent H2O2 outer diffusion (Vaddiraju, Legassey et al. 2011) and suppress foreign body response (Bhardwaj, Sura et al. 2010; Morais, Papadimitrakopoulos et al. 2010), respectively. Figure 4a illustrates the in vitro amperometric response of the five layer transcutaneous sensor under study to sequential additions of glucose, when tested after 1-hr incubation in PBS buffer maintained at 37 °C. Here, the sensor is interrogated with the MOSIS fabricated potentiostat and signal processing unit and the data is recorded continuously as response frequency obtained by the FFT LabVIEW program. As evident, the sensor responded quickly and reproducibly to glucose additions with a response time of 40 seconds for each glucose step, which is comparable to our earlier reports (Vaddiraju, Legassey et al. 2011).
Fig. 4.
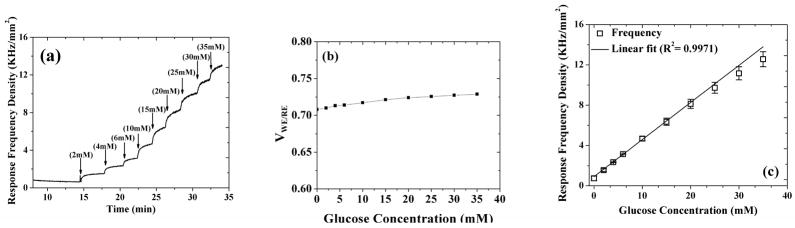
(a) Transient sensor response as a function of glucose concentration (0–35mM); (b) Variation in the applied voltage between the working and reference electrode as a function of glucose concentration measured during the experiment of (a); (c) Saturation response frequency, as obtained from (a).
The stability of the potentiostat in terms of the potential it applied between the working and reference electrode of the electrochemical sensor is critical for a reproducible detection of glucose. This is because of the fact that any significant change in the applied potential will advertently change the resulting amperometric current. Based on that the CMOS potentiostat-applied potential between the working and reference electrode is monitored during the amperometric test of Figure 4a. Figure 4b shows the resulting variation of applied voltage as a function of glucose additions. As seen in Figure 4b, the potential remained quite constant with an overall change of 0.1% per mM of glucose concentration. The observed minor elevation in the applied voltage of the MOSIS potentiostat to increasing glucose concentrations can be attributed to the slight loading of the NI ELVIS data acquisition module on the electrochemical cell. This results in a minute drift in applied bias of the sensor, which for all practical purpose is within error when contrasted from a typical sensor-to-sensor variability.
Figure 4c illustrates the saturation frequency response as a function of glucose concentrations, as obtained from the data of Figure 4a. The addition of glucose resulted in a linear increase of frequency response for glucose concentrations as high as 30 mM, which is well beyond the physiological range of 2 to 22 mM of glucose. This confirms the stability of the potentiostat as well as the efficacy of the LabVIEW program. Here it worth mentioning that the response illustrated in Figure 4c is about 50% lower than that of Figure 3b due to the presence of Layer 4 and mostly Layer 5, which act as additional flux limiting membranes for the inwards diffusion of glucose to the GOx layer (Vaddiraju, Legassey et al. 2011).
Reproducible operation in vivo forms the ultimate test for the functionality of an implantable glucose sensor. With this in mind, the in vivo performance of the transcutaneous sensor has been evaluated in an unconscious (anesthetized) rat. Figure 5a shows the continuous response from the subcutaneously implanted sensor for duration of ca. 3 hours, along with the plasma glucose values determined from tail vein sampling. Following the initial sensor background stabilization, an intraperitoneal injection of 250 μl glucose solution (0.5g/ml) was given to the rat. As shown in Figure 5a, the plasma glucose levels started to increase three minutes after the glucose injection and peaked at around 20 minutes, (following the injection) and then started to come back to normal levels. Correspondingly, the sensor current also increased following the initial glucose injection. Similarly, in response to a second intraperitoneal injection of glucose, both the plasma glucose and sensor response showed a steady increase followed by a decrease. As seen in Figure 5a, a lag phase of about 10±5 min (average of 3 rats totaling to 6 glycemic peaks) was observed between the plasma glucose and the change in the sensor current. This lag phase is due to: (i) the physical time lag between the interstitial fluid (ISF) and plasma which can vary between 4 and 10 min and (ii) time needed for the sensor to reach saturation value following a change in the glucose concentration (i.e. the sensor response time). Considering the fact that in vitro sensor response time (Figure 4a) is only 40 seconds, it can be easily concluded that the lag phase observed in Figure 5a is due to physical lag time for the glucose to arrive in the subcutaneous fluid which is in accordance with previous reports of lag in glucose levels between the blood and the subcutaneous fluid (Kovatchev, Shields et al. 2009). Here it should be noted that a minute increase (of about 1–2 minutes) in lag time was observed from the first glycemic peak to the second which could be a result of changes in the interstitial glucose kinetics due to healing of the trauma over a few hours (Boyne, Silver et al. 2003) as well as possible changes in permeability with sensor membranes due to protein adsorption. Figure 5b shows the variation of applied voltage (between the reference and working electrode) as a function of glucose additions during the amperometric test of Figure 5a. Similar to the in vitro results (Figure 4b), the potential remained fairly constant with a negligible increase of about 0.1% per mM of glucose during the two glycemic events. The observed minor elevation in the applied voltage of the MOSIS potentiostat to increasing glucose concentrations can be attributed to the slight loading of the NI ELVIS data acquisition module on the electrochemical cell. This results in a minute drift in applied bias of the sensor, which for all practical purpose is within error when contrasted from a typical sensor-to-sensor variability.
Fig. 5.
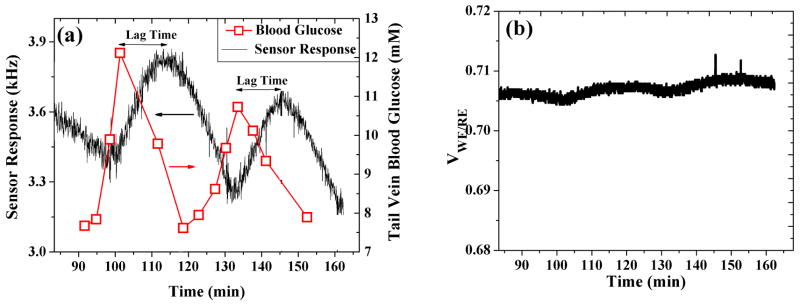
(a) Real-time in vivo sensor response when interfaced with the CMOS-fabricated potentiostat and signal processing unit. This is plotted against the tail vein plasma glucose levels (right ordinate). (b) Recorded potential between the working and reference electrodes for the duration of in vivo testing of (a).
Figure 6 shows the Clarke’s grid analysis of the glucose values obtained after retrospectively time-shifting the sensor frequency response to account for the observed lag phase. This grid is a plot of the sensor-measured glucose levels against the reference glucose values and is divided into five different zones: points falling in Zones A and B are considered acceptable for taking therapeutic decisions whereas those falling in zones C, D and E are considered unreliable and may be risky for clinical decisions (Vaddiraju, Burgess et al. 2010). As shown in Figure 6, all of the predicted glucose values fell in Zones A and B and none in the dangerous Zones C, D and E, demonstrating the efficacy of the sensor system for reliable glucose monitoring.
Fig. 6.
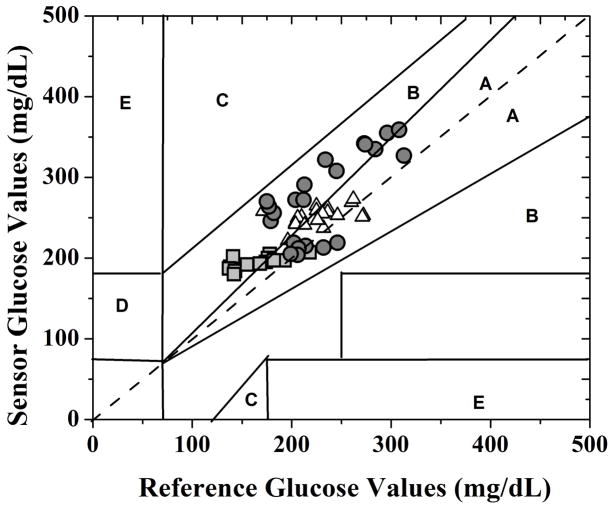
Clarke’s error grid analysis of the blood glucose values as-obtained from the transcutaneous glucose sensor of Figure 1 (after retrospectively accounting for the lag phase between the blood and subcutaneous glycemic events) shown in Figure 5 when interfaced with the MOSIS-fabricated potentiostat and signal processing block versus tail vein reference glucose values. The three different symbols (square, circle and triangle) corresponds to the three rats evaluated in vivo.
The high accuracy of the glucose readings obtained in this short term (3 hour) study is in part due to the inflammation-suppressing PVA/PLGA coating (Figure 1), whose efficacy to control negative tissue response (for up to three months) has already been demonstrated for sizes similar to the one used in this study (Hickey, Kreutzer et al. 2002; Hickey, Kreutzer et al. 2002; Patil, Papadimitrakopoulos et al. 2004; Galeska, Kim et al. 2005; Bhardwaj, Sura et al. 2007; Patil, Papadmitrakopoulos et al. 2007; Bhardwaj, Sura et al. 2010). A more thorough analysis (using a larger sample size) of the accuracy of the as-measured glucose values in terms of the mean absolute relative difference (MARD) as well as the time-variation of the accuracy will shed more light on the reliability of the implantable electrochemical sensor when interfaced with our custom fabricated CMOS circuitry. This along with topics concerning the shape of the implantable electrochemical sensor (Helton, Ratner et al. 2011) when coupled with the PVA/PLGA coating on the extent of foreign body response, is currently under investigation.
4. Conclusions
A transcutaneous amperometric glucose sensing system consisting of miniaturized coil-type wire sensors coupled to a low power CMOS (complementary metal-oxide-semiconductor)-based circuitry has been demonstrated. The use of 0.35 μm CMOS design rules together with an advanced signal processing block that linearly converts the amperometric current to frequency afforded device miniaturization down to an area of 0.665 mm2 with an overall power consumption of only 140 μW. A unique stratification of five functional layers has been employed on the amperometric glucose sensor that extended the sensor dynamic range from 0 to 30 mM, which is well beyond the physiological range of 2 to 22 mM. The sensor has been evaluated in vivo in rat model and has reproducibly tracked repeated glycemic events. Clarke’s error grid analysis on the as-obtained glycemic data has indicated that all of the measured glucose readings fell in the desired Zones A & B and none fell in the erroneous Zones C, D and E. Such reproducible operation of the transcutaneous sensor system, together with smaller footprint and low power consumption along with a unique capability for current-to-frequency conversion renders this a versatile platform for continuous glucose monitoring and other biomedical sensing devices.
Acknowledgments
Financial support for this study was obtained from US Army Medical Research Grants # W81XWH-07–10668 and W81XWH-09–1–0711), NIH grants (#1-R21-HL090458–01, R43EB011886 and R01EB014586–06AL) and NSF/SBIR grant (1046902).
References
- Ahmadi MM, Jullien GA. A Wireless-Implantable Microsystem for Continuous Blood Glucose Monitoring. Biomedical Circuits and Systems, IEEE Transactions on. 2009;3(3):169–180. doi: 10.1109/TBCAS.2009.2016844. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, et al. Controlling acute Inflammation with fast-releasing dexamethasone- PLGA microsphere/PVA hydrogel composites for implantable devices. Journal of Diabetes Science and Technology. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, et al. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. International Journal of Pharmaceutics. 2010;384(1–2):78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, et al. Controlling Acute Inflammation with Fast Releasing Dexamethasone-PLGA Microsphere/PVA Hydrogel Composites for Implantable Devices. Journal of Diabetes Science and Technology. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolomey L, Meurville E, et al. Implantable ultra-low power DSP-based system for a miniature chemico-rheological biosensor. Proceedings of the Eurosensors XXIII conference. 2009;1(1):1235–1238. [Google Scholar]
- Boyne MS, Silver DM, et al. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- Choleau C, Klein JC, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients: Part 2. Superiority of the one-point calibration method. Biosensors and Bioelectronics. 2002;17(8):647–654. doi: 10.1016/s0956-5663(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Chu X, Duan D, et al. Amperometric glucose biosensor based on electrodeposition of platinum nanoparticles onto covalently immobilized carbon nanotube electrode. Talanta. 2007;71(5):2040–2047. doi: 10.1016/j.talanta.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Degani Y, Heller A. Direct electrical communication between chemically modified enzymes and metal electrodes. 1. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. Journal of Physical Chemistry. 1987;91(6):1285–1289. [Google Scholar]
- Dong S, Wang B, et al. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosensors and Bioelectronics. 1992;7(3):215–222. [Google Scholar]
- Enejder AMK, Scecina TG, et al. Raman spectroscopy for noninvasive glucose measurements. Journal of Biomedical Optics. 2005;10(3):1–9. doi: 10.1117/1.1920212. [DOI] [PubMed] [Google Scholar]
- Errachid A, Ivorra A, et al. New technology for multi-sensor silicon needles for biomedical applications. Sensors and Actuators, B: Chemical. 2001;78(1–3):279–284. [Google Scholar]
- Ersöz A, Denizli A, et al. Molecularly imprinted ligand-exchange recognition assay of glucose by quartz crystal microbalance. Biosensors and Bioelectronics. 2005;20(11):2197–2202. doi: 10.1016/j.bios.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Frost M, Meyerhoff ME. In vivo chemical sensors: Tackling biocompatibility. Analytical Chemistry. 2006;78(21):7370–7377. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- Galeska I, Kim TK, et al. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS Journal. 2005;7(1):E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeska I, Kim TK, et al. Controlled Release of Dexamethasone from PLGA Microspheres Embedded Within Polyacid- Containing PVA Hydrogels. AAPS J. 2005;07(01):E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geise RJ, Adams JM, et al. Electropolymerized films to prevent interferences and electrode fouling in biosensors. Biosensors and Bioelectronics. 1991;6(2):151–160. [Google Scholar]
- Gifford R, Kehoe JJ, et al. Protein interactions with subcutaneously implanted biosensors. Biomaterials. 2006;27(12):2587–2598. doi: 10.1016/j.biomaterials.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Haider MR, Islam SK, et al. A Low-Power Signal Processing Unit for in vivo Monitoring and Transmission of Sensor Signals. Sensors and Transducers. 2007;84(10):1625–1632. [Google Scholar]
- Helton KL, Ratner BD, et al. Biomechanics of the Sensor -Tissue Interface-Effects of Motion, Pressure, and Design on Sensor Performance and the Foreign Body Response-Part II: Examples and Applications. Journal of diabetes science and technology. 2011;5(3):647–656. doi: 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey T, Kreutzer D, et al. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- Hickey T, Kreutzer D, et al. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. Journal of Biomedical Materials Research. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- House JL, Anderson EM, et al. Immobilization Techniques to Avoid Enzyme Loss from Oxidase-Based Biosensors: A One-Year Study. J Diabetes Sci Technol. 2007;1(1):18–22. doi: 10.1177/193229680700100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain F, Grantham H, et al. Implantable Biosensor and Methods of Use Thereof, 2008, Patent Pending. Application No. 20080154101 US Patent. 2008
- Johnson KW, Mastrototaro JJ, et al. In vivo evaluation of an electroenzymatic glucose sensor implanted in subcutaneous tissue. Biosensors and Bioelectronics. 1992;7(10):709–714. doi: 10.1016/0956-5663(92)85053-d. [DOI] [PubMed] [Google Scholar]
- Khan F, Saxl TE, et al. Fluorescence intensity- and lifetime-based glucose sensing using an engineered high-Kd mutant of glucose/galactose-binding protein. Analytical Biochemistry. 2010;399(1):39–43. doi: 10.1016/j.ab.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Kovatchev BP, Shields D, et al. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technology and Therapeutics. 2009;11(3):139–143. doi: 10.1089/dia.2008.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist PH, Iburg T, et al. Biocompatibility of an enzyme-based, electrochemical glucose sensor for short-term implantation in the subcutis. Diabetes Technology and Therapeutics. 2006;8(5):546–559. doi: 10.1089/dia.2006.8.546. [DOI] [PubMed] [Google Scholar]
- Lyandres O, Yuen JM, et al. Progress toward an in vivo surface-enhanced Raman spectroscopy glucose sensor. Diabetes Technology and Therapeutics. 2008;10(4):257–265. doi: 10.1089/dia.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie HA, Ashton HS, et al. Advances in photoacoustic noninvasive glucose testing. Clinical Chemistry. 1999;45(9):1587–1595. [PubMed] [Google Scholar]
- Malchoff CD, Shoukri K, et al. A novel noninvasive blood glucose monitor. Diabetes Care. 2002;25(12):2268–2275. doi: 10.2337/diacare.25.12.2268. [DOI] [PubMed] [Google Scholar]
- Malitesta C, Palmisano F, et al. Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly(o-phenylenediamine) film. Analytical Chemistry. 1990;62(24):2735–2740. doi: 10.1021/ac00223a016. [DOI] [PubMed] [Google Scholar]
- Martin SM, Gebara FH, et al. A Fully Differential Potentiostat. Sensors Journal, IEEE. 2009;9(2):135–142. [Google Scholar]
- McKean BD, Gough DA. A Telemetry-Instrumentation System for Chronically Implanted Glucose and Oxygen Sensors. IEEE Transactions on Biomedical Engineering. 1988;35(7):526–532. doi: 10.1109/10.4581. [DOI] [PubMed] [Google Scholar]
- Morais JM, Papadimitrakopoulos F, et al. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS Journal. 2010;12(2):188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton LW, Koschwanez HE, et al. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. Journal of Biomedical Materials Research - Part A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic A, Koehler H, et al. Fiber-optic flow-through sensor for online monitoring of glucose. Analytical and Bioanalytical Chemistry. 2006;386(5):1293–1302. doi: 10.1007/s00216-006-0782-x. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadimitrakopoulos F, et al. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technology and Therapeutics. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadimitrakopoulos F, et al. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadmitrakopoulos F, et al. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Hussain F, et al. Fluorescence-based glucose sensors. Biosensors and Bioelectronics. 2005;20(12):2555–2565. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rawat A, Burgess DJ. Effect of physical ageing on the performance of dexamethasone loaded PLGA microspheres. International Journal of Pharmaceutics. 2011;415(1–2):164–168. doi: 10.1016/j.ijpharm.2011.05.067. [DOI] [PubMed] [Google Scholar]
- Serra PA, Rocchitta G, et al. Design and construction of a low cost single-supply embedded telemetry system for amperometric biosensor applications. Sensors and Actuators B: Chemical. 2007;122(1):118–126. [Google Scholar]
- Shults MC, Rhodes RK, et al. A telemetry-instrumentation system for monitoring multiple subcutaneously implanted glucose sensors. IEEE Transactions on Biomedical Engineering. 1994;41(10):937–942. doi: 10.1109/10.324525. [DOI] [PubMed] [Google Scholar]
- Tipnis R, Vaddiraju S, et al. Layer-by-Layer Assembled Semipermeable Membrane for Amperometric Glucose Sensors. J Diabetes Sci Technol. 2007;1(2):193–200. doi: 10.1177/193229680700100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzebinski J, Moniz ARB, et al. Hydrogel Membrane Improves Batch-to-Batch Reproducibility of an Enzymatic Glucose Biosensor. Electroanalysis. 2011;23(12):2789–2795. [Google Scholar]
- Vaddiraju S, Burgess DJ, et al. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosensors and Bioelectronics. 2008;24:1557–1562. doi: 10.1016/j.bios.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Burgess DJ, et al. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosensors and Bioelectronics. 2009;24(6):1557–1562. doi: 10.1016/j.bios.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Burgess DJ, et al. Technologies for continuous glucose monitoring: current problems and future promises. Journal of diabetes science and technology. 2010;4(6):1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Legassey A, et al. Design and Fabrication of a High-Performance Electrochemical Glucose Sensor. Journal of Diabetes Science and Technology. 2011;5(5):1044–1051. doi: 10.1177/193229681100500504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju SSH, Burgess DJ, Jain FC, Papadimitrakopoulos F. Enhanced glucose sensor linearity using poly(vinyl alcohol) hydrogels. Jouranl of Diabetes Science and Technology. 2009;(4):863–874. doi: 10.1177/193229680900300434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Singh H, et al. Enhanced glucose sensors linearity using PVA hydrogels. Journal of Diabetes Science and Technology. 2009;3(4):863–874. doi: 10.1177/193229680900300434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Tomazos I, et al. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosensors and Bioelectronics. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdastri P, Susilo E, et al. Wireless implantable electronic platform for blood glucose level monitoring. Proceedings of the Eurosensors XXIII conference. 2009;1(1):1255–1258. [Google Scholar]
- Watt BE, Proudfoot AT, et al. Hydrogen peroxide poisoning. Toxicological Reviews. 2004;23(1):51–57. doi: 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- Weiss R, Yegorchikov Y, et al. Noninvasive continuous glucose monitoring using photoacoustic technology - Results from the first 62 subjects. Diabetes Technology and Therapeutics. 2007;9(1):68–74. doi: 10.1089/dia.2006.0059. [DOI] [PubMed] [Google Scholar]
- Wilkins E, Atanasov P, et al. Integrated implantable device for long-term glucose monitoring. Biosensors and Bioelectronics. 1995;10(5):485–494. doi: 10.1016/0956-5663(95)96894-5. [DOI] [PubMed] [Google Scholar]
- Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosensors and Bioelectronics. 2005;20(12):2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids and Surfaces B: Biointerfaces. 2000;18(3–4):197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- Yang H, Chung TD, et al. Glucose sensor using a microfabricated electrode and electropolymerized bilayer films. Biosensors and Bioelectronics. 2002;17(3):251–259. doi: 10.1016/s0956-5663(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Haider MR, et al. A low power sensor signal processing circuit for implantable biosensor applications. Smart Materials and Structures. 2007;16:525–530. (Journal Article) [Google Scholar]


