Abstract
A major tissue engineering challenge is the creation of multilaminate scaffolds with layer-specific mechanical properties representative of native tissues, such as heart valve leaflets, blood vessels, and cartilage. For this purpose, poly(ethylene glycol) diacrylate (PEGDA) hydrogels are attractive materials due to their tunable mechanical and biological properties. This study explored the fabrication of trilayer hydrogel quasilaminates. A novel sandwich method was devised to create quasilaminates with layers of varying stiffnesses. The trilayer structure was comprised of two “stiff” outer layers and one “soft” inner layer. Tensile testing of bilayer quasilaminates demonstrated that these scaffolds do not fail at the interface. Flexural testing showed that the bending modulus of acellular quasilaminates fell between the bending moduli of the “stiff” and “soft” hydrogel layers. The bending modulus and swelling of trilayer scaffolds with the same formulations were not significantly different than single layer gels of the same formulation. The encapsulation of cells and the addition of phenol red within the hydrogel layers decreased bending modulus of the trilayer scaffolds. The data presented demonstrates that this fabrication method can make quasilaminates with robust interfaces, integrating layers of different mechanical properties and biofunctionalization, and thus forming the foundation for a multilaminate scaffold that more accurately represents native tissue.
Keywords: Tissue Engineering, Biomaterials, Hydrogels, Laminate, Composites, Flexure
INTRODUCTION
There is growing interest in the development of natural and/or synthetic hydrogel materials for use in tissue engineering. Synthetic hydrogels, like poly(ethylene glycol) (PEG), are particularly interesting because they are intrinsically biocompatible, permit the rapid encapsulation of cells in cytocompatible conditions,28 and allow for extensive modification of their biochemical and biomechanical characteristics.1,31 In order to broaden the application of hydrogels in tissue engineering, this study looks at the challenge of constructing laminate hydrogel scaffolds with layer-specific mechanical properties for the purpose of tailoring layer-specific properties. Many native tissues are layered in structure, which offers several advantages. For example, heart valve leaflets possess a trilayered structure of collagen, elastin, and hyaluronan,24 which confers flexibility to the valve leaflet while maintaining its bulk tensile strength to prevent backflow of blood into the left ventricle.37,38 Other layered tissues like blood vessels,34 cartilage,32 and intervertebral discs,13 have varying extracellular matrix compositions, stiffnesses, cellularities, and cell types across their layers, whether these layers are organized in a planar arrangement or concentrically. Given the biocompatible and tunable properties of PEG hydrogels, this study explores the creation of multilaminate hydrogel scaffolds with the potential of varying mechanical properties and cellularity across their layers.
While many layering methods exist in the literature, including layer-by-layer approaches involving dip or spray coating,29,33 and spatially-controllable thin-film polymer assemblies,30 these methods involve either harsh processing or result in layers that are thinner than the tissues of interest. As a result, they do not translate well to hydrogels or cell-encapsulated scaffolds. The bulk of the literature regarding hydrogel lamination has focused on crosslinking for the creation of “quasilaminates.” Specifically, lamination has been achieved either by taking partially or undercrosslinked hydrogel layers,7,10,18–20,27 assembling them in their final form, then crosslinking the whole laminate scaffold, or by patterning bulk hydrogels to have regionally-specific properties, such as stiffness16,17,21,25 or bioactivity.12 A few studies have used this method to create laminate scaffolds, but did not vary the material properties, nor did they investigate the final material behavior.7,19,20 Some studies have varied the cell type across the layers while keeping the hydrogel formulation constant, and while demonstrating a laminate biological structure, these studies also did not test for the final material properties.10,18 Three studies in literature have investigated layered hydrogels with varying mechanical properties. In one study developing approaches for endochondral tissue engineering, layered PEG dimethacrylate hydrogels were fabricated with varied glycosaminoglycan (GAG) types and content in the different layers. Although the material properties of the layers were initially comparable, the varied hydrogel formulations promoted differential behavior and matrix deposition of the cells encapsulated within each layer, yielding distinct layers and a gradient of compressive strength.27 A second study introduced a hydrogel lamination technique using similar hydrogel layers with different densities.15 This technique created continuous interfaces between the hydrogels compared to sequentially layered hydrogels, although using hydrogels with comparable mechanical properties. Another study looked at laminating chemically crosslinked oligo(poly ethylene glycol fumarate) hydrogels of varying polymer length. Lamination of hydrogels with different mechanical properties was demonstrated, although the mismatch in swelling between the hydrogel layers often resulted in gel tearing.36 The swelling between hydrogel layers would seem to be a major limitation, as large differences in swelling could lead to gross deformity in the overall gel.36 Therefore, this study takes into consideration the mass swelling ratio in the design of quasilaminate hydrogels.
The objective of this study was to generate a quasilaminate hydrogel scaffold that allowed direct control of layer specific cellularity and material properties in an effort to mimic the layered microstructure of the valve leaflet. While previous layering strategies guided the work performed in this research, the data presented herein represents the first successful efforts to: (1) fabricate photopolymerized trilayer composite hydrogels; (2) rigorously test multilayer photopolymerized hydrogel interface strength; and (3) characterize the mechanical behavior of multilayer poly(ethylene glycol) diacrylate (PEGDA) scaffolds with layer-specific composition. This study forms the basis for fabrication of state-of-the-art layered scaffolds that could address the current limitations of basic hydrogels. The results presented have implications for biomaterials and tissue engineering of laminate tissues.
METHODS
Cell Culture
While any generic cell type could have been encapsulated within the hydrogels, aortic valve interstitial cells (VICs) were used due to their availability. Aortic valves were dissected from fresh porcine hearts obtained from a commercial abattoir (Fisher Ham and Meats, Spring, TX). Primary cultures of VICs were isolated from the aortic valve leaflets by a multistep collagenase digestion9,35 and were cultured in DMEM/F12 supplemented with 10% bovine growth serum (Hyclone, Logan, UT) and 1% antibiotic/antimycotic solution (Mediatech, Manassas, VA) in an incubator (37 °C, 5% CO2, 95% humidity). Medium was changed every 2 days and cells were passaged at 80–100% confluence. Cells between passages 4 and 8 were used for encapsulation.
Synthesis of PEGDA
PEGDA was prepared using previously described methods.9 Briefly, PEG (3.4, 6, and 8 kDa, Sigma-Aldrich, St. Louis, MO) was reacted with acryloyl chloride (4:1 acryloyl chloride:PEG molar ratio, Sigma-Aldrich) and triethylamine (TEA, 2:1 TEA:PEG molar ratio, Sigma-Aldrich) overnight. The reaction was then rinsed with 2 M potassium carbonate and phase separated overnight. The PEGDA-containing organic layer was collected and subsequently dried with magnesium sulfate. After filtering out the magnesium sulfate, the solution was precipitated in cold diethyl ether. The PEGDA product was collected by filtration, lyophilized, and finally stored at −20 °C.
Synthesis of Fluorescent PEG-Peptides
Fluorescently tagged PEG-peptides were synthesized as previously described.8,12 Peptides (RGDS and IKVAV) were reacted in DMSO with PEG-SCM (Laysan Bio, Arab, AL) and DIPEA overnight at a 1:1.2 and 1:2 ratio, respectively, then dialyzed against ultrapure H2O at room temperature for 2 days and lyophilized overnight. The subsequent PEG-peptides were then fluorescently tagged by reacting overnight with AlexaFluor succinimidyl ester (488 for PEG-RGDS, 633 for PEG-IKVAV, Invitrogen, Carlsbad, CA) at a 10:1 molar ratio. The resulting product was dialyzed against ultrapure H2O at room temperature for 2 days, lyophilized overnight, and stored at −20 °C.
Creation of Trilayer Quasilaminates
Trilayer quasilaminate gels were fabricated in permutations of two PEGDA formulations: 12.5% (w/v) 3.4 kDa PEGDA in ultrapure water, or formulation A; and 10% (w/v) 6 kDa PEGDA in ultrapure water, or formulation B. Formulation A is stiffer than B due to its lower molecular weight and higher weight percentage.9 The mass swelling ratios of formulations A and B were found to be 9.19 ± 0.49 and 12.86 ± 0.43 (n = 7).6 The following permutations were tested (gels are bracketed, acellular conditions are stated in lowercase): [A-B-A]; [a-b-a]; [a-a-a]; [b-b-b]; and single-layer controls [a], [b], [A], and [B] (n = 4–8). Prepolymer solutions were crosslinked using the addition of the photoinitiator Irgacure 2959 (100 mg/mL in ethanol, Sigma Aldrich, St. Louis, MO) at a concentration of 3% (v/v) in prepolymer solution, and the subsequent exposure of longwave UV light (365 nm, 10 mW/cm2)
Molds were first prepared by coating 3″ ×9 2″ glass slides with Sigmacote (Sigma-Aldrich), a silicone surface-coating lubricant, yielding glass slides with a hydrophobic coating. PTFE spacers with the desired strip geometry and thickness (5 mm wide, 0.5 mm thick) were sandwiched between one untreated slide and one coated slide (Fig. 1). After the prepolymer solution A was pipetted into two molds, they were simultaneously exposed to UV light for 4.5 min, with the untreated slide closest to the illumination source. Following exposure, the coated slides were removed, resulting in two partially crosslinked, gelatinous slabs that preferentially adhered to the untreated slides. Next, a third PTFE spacer was sandwiched between the two slides with partially crosslinked gels were positioned on the outside, yielding an A-empty-A assembly (Fig. 1) with a total thickness of 1.5 mm. Prepolymer B was added to the void between the slab gels and the assembly was again exposed to UV light for 10 min, with a 180° rotation about the length and width of the slide after 5 min. Single-layer controls were similarly crosslinked in a strip mold (5 mm wide, 1.5 mm thick) between two Sigmacote treated slides, with a UV exposure time of 14.5 min to match the exposure experienced in the quasilaminates.
FIGURE 1.
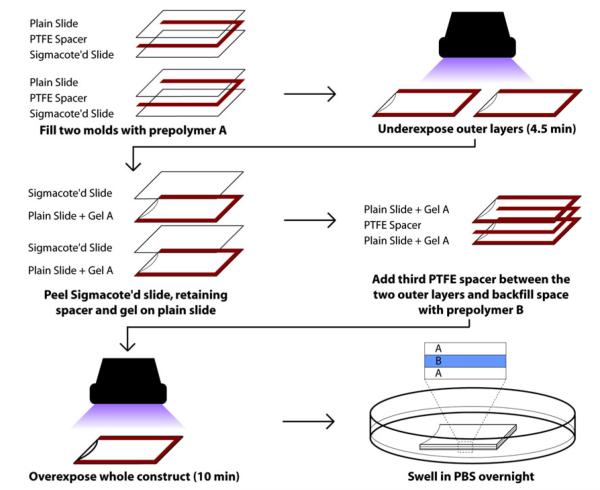
Schematic depicting the fabrication of trilayer quasilaminates with an A-B-A composition. Gel A is 12.5% 3.4 kDa PEGDA, gel B is 10% 6 kDa PEGDA. This fabrication technique can be used to generate scaffolds with different stiffnesses and cellularity in each layer.
For cellular quasilaminates, the prepolymer solutions were made in media with phenol red and sterilefiltered within a laminar flow hood. Cells were added at a concentration of 2.2 × 107 cells/mL, and hydrogels were formed under sterile conditions similarly to acellular scaffolds. The scaffolds were then swelled for 48 h in either PBS at room temperature for acellular scaffolds, or in media inside an incubator (37 °C, 5% CO2) for cellular scaffolds. In order to visually distinguish the mechanically distinct layers, acellular gels were soaked in cresyl violet acetate solution (0.2 mg/mL in PBS, Sigma-Aldrich) overnight. Images were captured under a stereo microscope (MZ6, Leica, Wetzlar, Germany).
In order to demonstrate layer specific bioactivity, fluorescently labeled PEG-RGDS was added to prepolymer solution (1 mM) A and fluorescently labeled PEG-IKVAV was added to prepolymer solution (1 mM) B. The gels were fabricated in the same manner as described above. Furthermore, layer specific cellularity was achieved by generating quasilaminate hydrogels as described above, but with different cell densities between formulation A (2.0 × 107 cells/mL) and formulation B (2.0 × 106 cells/mL). After 2 days of swelling in media, 4′,6-diamidino-2-phenylindole (1:1000, DAPI, KPL, Gaithersburg, MD) was used to stain the cell nuclei. Fluorescent images of layered bioactive hydrogels and hydrogels with different cell densities were imaged on a confocal microscope (LSM 510 META NLO, Zeiss, Oberkochen, Germany) and processed using ImageJ (NIH, Bethesda, MD).
Flexure Testing of Hydrogels
As layering can affect flexural behavior, the quasilaminate hydrogels and the single layer controls were subjected to flexure tests, performed as previously described.9,11,23 Briefly, samples were first soaked in a fluorescein solution to turn the gels bright green for the purpose of providing contrast. The samples were then loaded onto a custom 3-point bending tester and deformed 6 mm at a rate of 1.5 mm/min in the center against a bending bar, which moves relative to a fixed reference bar (Fig. 2). Sample deformation was tracked optically with high-definition video (1280 × 720) at 30 fps (EOS Rebel T1i, Canon, Tokyo, Japan). The resulting video was demultiplexed into 8-bit grayscale images using ImageJ, and analyzed using parallel MATLAB (Mathworks, Natick, MA) code that performed digital image correlation in a method described previously.9 Change in curvature was calculated from fitting the gel to a second-degree polynomial.9,11,23 The moment applied was calculated by measuring the displacement of the bending bar relative to the reference bar, and from that, assuming cantilever beam theory, calculating moment applied to the gel. By graphing the moment against the change in curvature graph, the effective bending modulus (Eeff) could be calculated.9,11,23 Gel thickness was measured using the same images. One-way ANOVA testing was performed on this data, along with post hoc Tukey’s testing to observe pairwise comparisons (JMP, SAS, Cary, NC). Significance was defined as p < 0.05.
FIGURE 2.
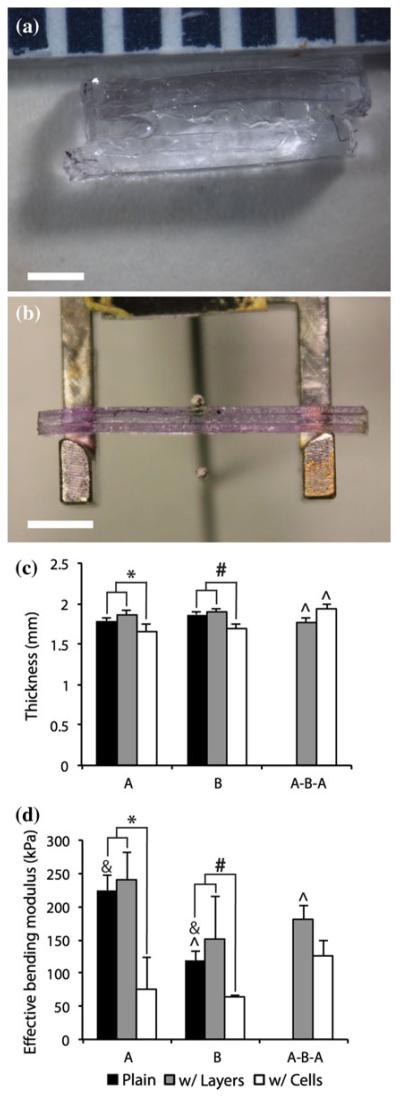
(a) Close-up view of the layers of the quasilaminate hydrogel, with two outer A (12.5% 3.4 kDa PEGDA) layers and an inner B (10% 6 kDa PEGDA) layer. Scale bar = 1 mm. (b) Trilayer quasilaminate prepared for three point flexure. Scale bar = 5 mm. (c) Thickness of quasilaminate gels, either 1.5 mm plain single-layer gels in black columns ([a], [b]), layered gels in gray columns ([a-a-a], [b-b-b], [a-b-a]), or cellularized gels with phenol red in white columns ([A], [B], [A-B-A]). (d) Effective bending modulus of quasilaminate gels. Layering had no significant effect on effective bending modulus or thickness. The addition of cells and phenol red reduced thickness and bending modulus for [A] and [B], but did not affect [A-B-A] bending modulus, and increased thickness. n = 4–8. * and # indicate significant differences (p < 0.05) between cellularized gels with phenol red and either bulk gels or gels with layers; ^ and & indicate significant differences (p < 0.05) between groups marked by pairs of symbols.
Creation of Bilayer Quasilaminates for Interface Strength Testing
Bilaminar A-B PEGDA quasilaminates were prepared to measure interfacial strength. Formulation A was 12.5% 3.4 kDa, whereas formulation B was either 6 kDa, 10% (w/v) or 8 kDa, 5% (w/v) (n = 6). Layered gels were prepared by filling a 1″ × 3″ mold halfway with prepolymer A, exposing for 4.5 min, and then filling completely with prepolymer B and re-exposing for 10 min. The resulting gels were swollen for 48 h in cresyl violet acetate solution to visualize the two distinct layers (Fig. 3). Lower molecular weight gels were stained a darker purple than the high molecular weight gels.25 The gels were punched into a 5 mm wide, 30 mm long rectangular strip for uniaxial tensile testing where the interface was placed at the center of the strip. Hydrogel samples were then pulled to failure at 6 mm/min (ELF 3200, Bose ElectroForce, Eden Prairie, MN), and the tensile elastic modulus (Et) and failure stress (σf) were measured in MATLAB. High-speed video (EOS Rebel T1i) was used to ensure that the samples did not fail at the layer interface. One-way ANOVA testing and post hoc Tukey’s testing were used for statistical analysis (JMP, SAS).
FIGURE 3.
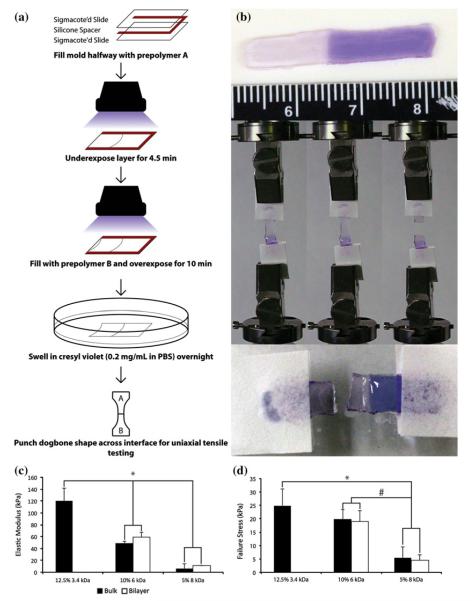
(a) Schematic depicting the fabrication of bilayer quasilaminates between the stiff gel A (12.5% 3.4 kDa PEGDA) and the soft gel B (either 10% 6 kDa or 5% 8 kDa PEGDA) for interface strength testing. (b) Images of a bilayer quasilaminate (soft gel B is 5% 8 kDa PEGDA) before, during and after interface strength testing. Cresyl violet was used to stain the gel, with lower MW gels staining darker. The failure in the quasilaminate gel is located in the higher MW gel layer, and not the interface, demonstrating a robust interface between the two formulations. (c) Elastic modulus and (d) failure stress of bilayer quasilaminates with varying B formulation. n = 6. * and # indicate significant differences (p < 0.05) between formulations.
RESULTS
Generation of Quasilaminate Hydrogels
Quasilaminate gels were successfully fabricated using a novel “sandwich” layering method (Fig. 1). This layering method was also used to create quasilaminate gels with different bioactivities and cellularities (Fig. 4). [A-B-A] quasilaminates swelled the most, to a mean thickness of 1.95 ± 0.04 mm (130% of 1.5 mm original thickness), which is thicker than the predicted thickness of 1.66 mm based on the single-layered cellular controls [A] (1.65 ± 0.11 mm) and [B] (1.69 ± 0.06 mm) (Fig. 2). There was no change in swelling between acellular single-layer and trilayered controls ([a], 1.78 ± 0.04 mm and [a-a-a], 1.87 ± 0.04 mm; [b], 1.90 ± 0.04 mm and [b-b-b], 1.90 ± 0.02 mm). The addition of cells significantly reduced the thickness of the gels compared to single-layer controls, except for the [A-B-A] quasilaminate, which was significantly thicker than the comparable acellular [a-b-a] hydrogel (1.78 ± 0.05 mm).
FIGURE 4.
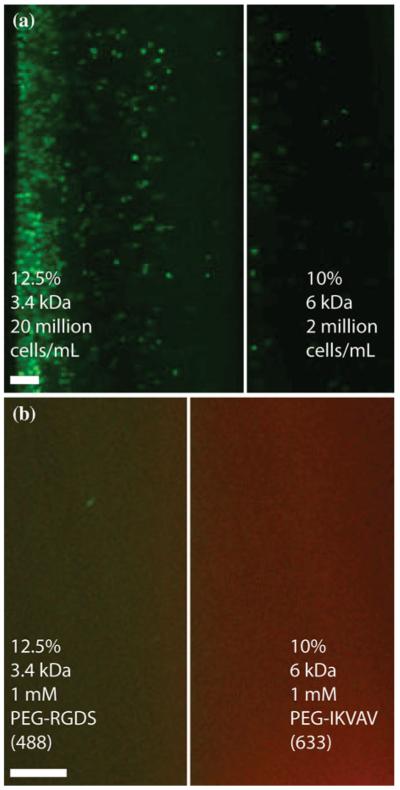
Fluorescent images of trilayer quasilaminates made either (a) varying cellularity (green) or (b) bioactivity. Quasilaminates with varying cellularity had 20 million cells/mL in the A layer, 2 million cells/mL in the B layer. Quasilamiantes with varying bioactivity were doped with 1 mM fluorescently tagged PEG-peptides in each layer [RGDS in (a), green, IKVAV in (b), red]. Scale bar = 200 μm.
Flexure Testing
To assess their flexural behavior, quasilaminates and single layer control hydrogels were subjected to three point flexure testing (Fig. 2). As expected based on their hydrogel formulation, [a] gels (223.40 ± 23.69 kPa) had a significantly higher Eeff than [b] gels (118.25 ± 14.05 kPa). The Eeff of [a-b-a] gels (180.36 ± 22.21 kPa) was lower but not significantly different from [a] gels, but was significantly higher than [b] gels. It was also found that the effects of layering alone did not significantly change the Eeff of the scaffold ([a-a-a] 241.24 ± 41.44 kPa; [b-b-b] 150.23 ± 63.98 kPa). The addition of cells reduced Eeff significantly for both [A] (75.41 ± 47.70 kPa) and [B] (63.00 ± 3.75 kPa), but not for [A-B-A] (125.51 ± 34.53 kPa, p = 0.21).
Interfacial Bonding
As the different layers were covalently crosslinked together, there was the potential for the generation of swelling-mediated stress at the interface. To determine the robustness of the layer interface, tensile testing of bilayer quasilaminate hydrogels was performed. Bilayer quasilaminates were fabricated with 12.5% 3.4 kDa PEGDA and two different B formulations, 10% 6 kDa or 5% 8 kDa PEGDA (Fig. 3). The bilayer quasilaminates were statistically similar to the higher MW bulk gel with regards to Et (bilayer v. bulk, 10% 6 kDa: 58.73 ± 8.45 kPa v. 48.85 ± 3.45 kPa; 5% 8 kDa: 11.1 ± 0.62 kPa v. 6.19 ± 0.97 kPa) as well as σf (bilayer v. bulk, 10% 6 kDa: 19.03 ± 3.91 kPa v. 19.90 ± 3.61 kPa; 5% 8 kDa: 4.55 ± 1.89 kPa v. 5.49 ± 0.75 kPa). Images taken from high-speed video capture demonstrated that the bilayer quasilaminate failed in the less stiff layer and not at the interface of A and B.
DISCUSSION
This study presented a novel method for making quasilaminate hydrogels with layer-specific and mechanically distinct formulations. Rigorous mechanical testing was performed to demonstrate the formation of strong laminate interfaces using the methods presented. These methods for fabricating quasilaminate gels could have broad application as the basis for generating heterogeneous scaffolds for tissue engineering layered structures, such as heart valves, blood vessels, cartilage, and intervertebral discs. A novel “sandwich” layering method was used to fabricate the quasilaminate gels with an A-B-A layer composition. First, the stiff A layers were created by adding prepolymer A to glass slide molds and partially crosslinking using ultraviolet light. The mold was then reconfigured to create a space between the A layers where prepolymer B could be deposited. The entire assembly was subsequently exposed to UV light to polymerize the B layer and crosslink all three layers together. The result was the successful fabrication of quasilaminate hydrogels composed of individual lamina less than 1.0 mm thick. Tensile testing of bilayer quasilaminates showed that the interface between layers created in this method did not fail before the constituent layers themselves. Flexure testing demonstrated that A-B-A quasilaminates had an Eeff between the control moduli of its two constituent formulations. In all groups, the addition of cells reduced the Eeff. In addition, the gels can be modified with either different cell-ularities or bioactivities to tailor quasilaminates for specific applications.
The Eeff of [a-b-a] gels was in between that of [a] and [b] single layer controls, although it was only significantly higher than [b]. This matches our expectation of a an Eeff in between the two controls; based on composition (2/3 A, 1/3 B) and the Eeff of the two controls, [a-b-a] gels should have an Eeff of 188 kPa, close to the 180 kPa ± 22.21 kPa that was recorded. This data suggests that the fabrication method presented created a strong interface with which trilaminate gels flexed as one continuous material across their thickness. This result was confirmed by the tensile testing of bilayer hydrogels, which did not fail at the interface and only in the higher MW gel. The evidence of a strong interface was further supported by the fact that layering in [a-a-a] and [b-b-b] gels did not significantly alter Eeff or the gel thickness. Overall, this study demonstrated the robustness of this method in creating strong laminate interfaces.
A major factor in this work was the matching of swelling between layers, which limited the range of prepolymer formulations and associated variations in mechanical properties that could be used to form layers.36 The largest difference in mass swelling ratio used was 19.03, which was found in the bilayer quasilaminates with 5% 8 kDa as the soft gel B (12.5% 3.4 kDa: 9.19; 5% 8 kDa: 28.22). A potential solution to this limitation is to decouple the material properties of the gel from swelling, which has been demonstrated in recent literature, either with the addition of methacrylated alginate4 or multi-arm PEG.3 Decoupling the two parameters could lead to the use of a wider array of hydrogel formulations, including substantially softer gels with high-swelling capabilities similar to hyaluronan.22
The encapsulation of cells with media containing phenol red did significantly reduce Eeff in [A] and [B] gels, while only nominally having this effect on [A-B-A] gels. This result is consistent with previous studies demonstrating that high densities of encapsulated cells (~107 cells/mL and higher) affected the Eeff of the gels.5,9 Interestingly, when cells were encapsulated only within the soft middle layer (in [a-B-a] hydrogels), there was no significant difference between these partially cellular and the fully acellular hydrogels (data not shown). In addition, the authors found that the removal of phenol red increased the Eeff of cellularized 3.4 kDa gels, but not to the level of bulk gels (data not shown). It should be noted that in this study, the timeframe of maintaining cells within the hydrogels was only 2 days and that these cellularized gels were not functionalized with biological factors to direct cell behavior. Based on previous literature, it is speculated that with a longer culture time, the material properties would change significantly over time with deposition of extracellular matrix components.27 As a result of the limited time frame of the culture in this study, significant extracellular matrix deposition was not expected, and thus, significant differences in material properties of the quasilaminates cannot be attributed to the cells’ production of extracellular matrix. The reduction in Eeff in cellular gels could have resulted either because the cells served as a particular exclusion in the gel that weakened its flexural strength, or the addition of phenol red reduced crosslinking, or a combination of both. Unexpectedly, while the addition of cells to single layer controls [A] and [B] reduced swelling (compared with [a] and [b]), [A-B-A] swelling increased relative to [a-b-a] and was highest of all the gels. A possible explanation could be the differential exposure times used between single-layer controls and quasilaminates, where the inner layer was exposed for 10 out of the total 14.5 min. Additional studies will be necessary to understand the effect of cell encapsulation on hydrogel swelling.
The layered fabrication utilized in this work is appealing due to its relative simplicity. Each layer is created independently, thus allowing for the manipulation of the mechanical, biochemical, and cellular components on a layer specific level. Previous work has shown the ability to use multiple crosslinking steps to pattern areas within hydrogel materials that are stiffer,21,25 nondegradable,16,17 or contain specific biomolecules.12 The integration of patterning techniques with the methods introduced here could lead to the development of complex, layered hydrogel systems that are highly tunable.
The methods developed and implemented in the studies described herein are the first to demonstrate strongly bonded photocrosslinked multiphase hydrogels that retain their layered structure following swelling. These methods allowed the development of a biphasic, trilayered composite hydrogel with distinct stiffness and cellularity between layers. The micro-scale mechanical environment can be engineered to have a desired stiffness that is independent from the overall laminated hydrogel properties. This approach provides a valuable tool to control cellular microenvironment and behavior via layer-specific substrate stiffness while maintaining macro-mechanical properties that mimic the structure and function of the target native tissue. This approach should also seamlessly integrate with layer-specific biofunctionalization strategies. One suggested application of this technology is for control of behavior of cells, such as VICs, that respond to high substrate stiffness by accelerating calcific nodule formation.2 Controlling the bulk and pericellular stiffnesses of quasilaminate hydrogels with encapsulated VICs could result in engineered tissues less prone to calcification.2 Furthermore, composite theory dictates that a larger proportion of stress is imparted to the stiffer layers in a laminate.14 From this it can be inferred that when these scaffolds are flexed, cells in the middle layer are potentially shielded from the high stresses resulting from large flexural deformations, which have been correlated to sites of valve calcification.2 Another suggested application for this methodology, when applied in a concentric manner, would be efforts to tissue engineer the intervertebral disc, in which the inner nucleus pulposus layer is more compliant, and richer in GAGs, than the outer annulus fibrosis layer.26
In conclusion, the described methods allow the fabrication of increasingly complex architectures in hydrogel scaffolds. These studies were the first to demonstrate a photopolymerized quasilaminate scaffold with strong layer–layer adhesion where each layer had distinct composition, bioactivity, and cellularity. Additionally, these studies tested the flexural stiffness of “stiff-soft-stiff” quasilaminates. These methods will likely find their first utility in tissue engineering, where layered structures are diffiult to create with existing synthetic hydrogel fabrication techniques. This straightforward technique presented in this study could allow for the control of mechanical properties, bioactivity, and cell type between distinct layers, and could be expanded and improved upon with the decoupling of mechanical properties and swelling.3,4 The methods described herein serve as a basis for the creation of cell-type specific microenvironments that leverage the cell’s synthetic ability to induce tissue regeneration. Finally, the use of a photopolymerizable hydrogel system provides the ability to fabricate various architectures outside of the rectangular structures presented, further increasing the ability to mimic tissue microstructures. These methods permit the recapitulation of tissue-specific anatomical features in hydrogels, which are expected to yield superior mechanical performance of the resulting scaffolds and allow the creation of diverse functional engineered tissues.
ACKNOWLEDGMENTS
This work was supported by March of Dimes Research Grant 1-FY08-409; an American Heart Association Predoctoral Fellowship (to H.T.); and NIH R01HL107765. Flexural data analysis was performed in part using the Shared University Grid at Rice funded by NSF (EIA-0216467), and a partnership between Rice University, Sun Microsystems, and Sigma Solutions, Inc. The authors thank Roger Moye, Rice University, for assistance with supercomputing, as well as Joseph Hoffmann, Rice University, for the use of his fluorescent PEG peptides.
REFERENCES
- 1.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J. Heart Valve Dis. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 3.Browning MB, Wilems T, Hahn MS, Cosgriff-Hernandez E. Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size. J. Biomed. Mater. Res. A. 2011;98:268–273. doi: 10.1002/jbm.a.33109. [DOI] [PubMed] [Google Scholar]
- 4.Cha C, Kim SY, Cao L, Kong H. Decoupled control of stiffness and permeability with a cell-encapsulating poly(ethylene glycol) dimethacrylate hydrogel. Biomaterials. 2010;31:4864–4871. doi: 10.1016/j.biomaterials.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Chou AI, Akintoye SO, Nicoll SB. Photocrosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthritis Cartilage. 2009;17:1377–1384. doi: 10.1016/j.joca.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of inter-facially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 7.Cuchiara MP, Allen ACB, Chen TM, Miller JS, West JL. Multilayer microfluidic PEGDA hydrogels. Biomaterials. 2010;31:5491–5497. doi: 10.1016/j.biomaterials.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Culver JC, Hoffmann JC, Slater RAJH, West JL, Dickinson ME. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv. Mater. 2012;24:2344–2348. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011;7:2467–2476. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elisseeff JH, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod. Craniofac. Res. 2005;8:150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 11.Gloeckner DC, Billiar KL, Sacks MS. Effects of mechanical fatigue on the bending properties of the porcine bioprosthetic heart valve. ASAIO J. 1999;45:59–63. doi: 10.1097/00002480-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann JC, West JL. Three-dimensional photolithographic patterning of multiple bioactive ligands in poly(ethylene glycol) hydrogels. Soft Matter. 2010;6:5056–5063. [Google Scholar]
- 13.Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat. Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 14.Jones RM. Mechanics of Composite Materials. CRC Press; Boca Raton, FL: 1975. [Google Scholar]
- 15.Karpiak JV, Ner Y, Almutairi A. Density gradient multilayer polymerization for creating complex tissue. Adv. Mater. 2012;24:1466–1470. doi: 10.1002/adma.201103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31:8228–8234. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Khetan S, Katz JS, Burdick JA. Sequential crosslinking to control cellular spreading in 3-dimensional hydrogels. Soft Matter. 2009;5:1601–1606. [Google Scholar]
- 18.Kim T-K, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, Elisseeff JH. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–664. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 19.Lu S, Anseth KS. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J. Control Release. 1999;57:291–300. doi: 10.1016/s0168-3659(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Ramirez WF, Anseth KS. Photopolymerized, multilaminated matrix devices with optimized nonuniform initial concentration profiles to control drug release. J. Pharm. Sci. 2000;89:45–51. doi: 10.1002/(SICI)1520-6017(200001)89:1<45::AID-JPS5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Marklein RA, Burdick JA. Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter. 2010;6:136–143. [Google Scholar]
- 22.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Mirnajafi A, Raymer JM, Scott MJ, Sacks MS. The effects of collagen fiber orientation on the flexural properties of pericardial heterograft biomaterials. Biomaterials. 2005;26:795–804. doi: 10.1016/j.biomaterials.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Misfeld M, Sievers H–H. Heart valve macro- and microstructure. Philos. Trans. R. Soc. Lond. B. 2007;362:1421–1436. doi: 10.1098/rstb.2007.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemir S, Hayenga HN, West JL. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 2010;105:636–644. doi: 10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- 26.Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010;43:1017–1030. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 29.Niinomi M. Metallic biomaterials. J. Artif. Organs. 2008;11:105–110. doi: 10.1007/s10047-008-0422-7. [DOI] [PubMed] [Google Scholar]
- 30.Pavlukhina S, Sukhishvili S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011;63:822–836. doi: 10.1016/j.addr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin. Orthop. Relat. Res. 2001;391:S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 33.Shepperd JAN, Apthorp H. A contemporary snapshot of the use of hydroxyapatite coating in orthopaedic surgery. J. Bone Joint Surg. Br. 2005;87:1046–1049. doi: 10.1302/0301-620X.87B8.16692. [DOI] [PubMed] [Google Scholar]
- 34.Silver FH, Christiansen DL, Buntin CM. Mechanical properties of the aorta: a review. Crit. Rev. Biomed. Eng. 1989;17:323–358. [PubMed] [Google Scholar]
- 35.Stephens EH, Carroll JL, Grande-Allen KJ. The use of collagenase III for the isolation of porcine aortic valvular interstitial cells: rationale and optimization. J. Heart Valve Dis. 2007;16:175–183. [PubMed] [Google Scholar]
- 36.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J. Biomed. Mater. Res. 2002;59:429–437. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 37.Tseng H, Grande-Allen KJ. Elastic fibers in the aortic valve spongiosa: a fresh perspective on its structure and role in overall tissue function. Acta Biomater. 2011;7:2101–2108. doi: 10.1016/j.actbio.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesely I, Boughner DR, Song T. Tissue buckling as a mechanism of bioprosthetic valve failure. Ann. Thorac. Surg. 1988;46:302–308. doi: 10.1016/s0003-4975(10)65930-9. [DOI] [PubMed] [Google Scholar]


