Abstract
We previously generated a knock-in mouse line with a cocaine-insensitive dopamine transporter (DAT-CI mice). These mice lost several behavioral responses to cocaine, but retained their response to amphetamine. DAT-CI mice are hyperdopaminergic due to reduced DAT function, and may thus be a good model for studying attention deficit hyperactivity disorder (ADHD). These mice had been behaviorally characterized while they were on a mixed genetic background. – However as the colony was propagated over time, the mixed genetics were shifted toward a pure C57Bl/6J background – via a common breeding scheme known as “backcrossing.” Several phenotypes appeared to have changed during this time frame. In this study, we investigated whether backcrossing altered the hyperlocomotive phenotype and behavioral responses to amphetamine, a drug used to treat ADHD.
C57-congenic DAT-CI mice had high spontaneous locomotor activity that could be suppressed by low doses of amphetamine. Furthermore, their locomotion was not stimulated by very high doses of amphetamine (20 mg/kg). After the reversion to a mixed genetic background by breeding with the 129 strain, the C57:129 hybrid DAT-CI mice displayed reduced basal locomotor activity compared to the C57-congenic mutant mice, and regained locomotor stimulation by high-dose amphetamine. The calming effect of amphetamine at low doses was retained in both strains.
In summary, reduced DAT function in DAT-CI mice leads to a hyperdopaminergic state, and an ADHD-like phenotype in both strains. The data show that the genetic background of DAT-CI mice affects their locomotor phenotypes and their responses to amphetamine. Since the differences in genetic background between the strains of mice have a significant impact on the ADHD-like phenotype and the response to amphetamine, further study with these strains could identify the genetic underpinnings affecting the severity of ADHD-related symptoms and the treatment response.
Keywords: ADHD, Amphetamine, Dopamine, Habituation, Knock-in
1. Introduction
In the recent past, knock-out/in mouse generation was limited by the lack of hardiness of stem cells derived from inbred embryos other than those from the 129 strain. Viable stem cells are derived from the 129Sv/J strain of mice relatively easily, and are still almost exclusively used in the generation of knock-out/in mice. In contrast, the large majority of behavioral experiments are performed on inbred C57Bl/6J mice, because of the unique robustness of this strain’s behavioral repertoire (Owen et al., 1997). It is therefore conventional to backcross the chimeric founders with C57 mice. However, this backcrossing process produces >99% C57-congenic mice only after 10 generations of breeding – and it is commonplace for initial behavioral experiments to be conducted before this time-consuming process has been completed. This may not be ideal for building upon seemingly related data from inbred lines.
Nonetheless, the initial characterization of many genetically engineered knock-out/in mice is performed and published on a mixed C57:129 background, whereas subsequent studies are performed on a C57-congenic background. This results in a gradual shift of the strain genetic background. If such a shift leads to changes in phenotype, future studies may reveal genetic variants responsible for the phenotypic changes.
In the present study, we test whether the strain background is critically involved in the locomotor behavior of our mouse line. Previously, we generated a knock-in mouse line with a cocaine-insensitive dopamine transporter (DAT-CI mice) (Chen et al., 2006). As expected, cocaine responses were altered in these mice while amphetamine responses were not. Specifically, 10 mg/kg amphetamine stimulated locomotion in DAT-CI mice while the line was on a mixed C57:129 background. However, after the mice were backcrossed to a C57-congenic background, we no longer observed locomotor stimulation to 10 mg/kg amphetamine. This prompted us to test the hypothesis that the backcrossing procedure caused a shift in phenotypes related to amphetamine-induced locomotion.
2. Materials and Methods
2.1 Animal Subjects
In these studies, a knock-in mouse line containing three point mutations (L104V/F105C/A109V) in the dopamine transporter gene was used. These mice (DAT-CI mice) were generated as described previously (Chen et al., 2006). In brief, a targeting vector containing the point mutations was incorporated into the genome of ES-cells derived from 129/SvJ mice. Positive ES cell clones were used to generate the chimeric founder mice in a series of embryonic procedures (Yale University Core Facility). These chimeras were then backcrossed to C57Bl/6J as is conventional for behavioral analysis. Heterozygous F1 offspring indicated successful germline transmission of the ES-cell DNA. This F1 generation would be an approximately 1:1 mixture of the C57 and 129 strains. Until backcrossing to the C57 line was completed, these mice were varying mixtures of the C57 and 129 parent strains. Backcrossing heterozygous DAT-CI mice to C57Bl/6J mice (Jackson Laboratories) for at least 10 generations would produce greater than 99% genetic homology with the C57 strain. Heterozygous male and female F14 mice were bred to produce wild-type and mutant C57-congenic mice used as a behavioral cohort in this study.
In order to generate the other experimental cohorts, the 129 strain background was reintroduced by crossing male homozygous F14 DAT-CI mice with female 129/SvImJ mice (Jackson Laboratories). The heterozygous siblings were crossed, generating wild-type, heterozygous, and homozygous DAT-CI mutants on an approximately 1:1 mixture of C57Bl/6J and 129/SvImJ backgrounds (C57:129 hybrid mice).
All mice were kept in standard housing conditions, including ad libitum access to food/water and 12 hours each of dark/light. Only male mice were used, and all mice were between 6–10 weeks of age at the time of behavioral testing. All animal procedures were approved by The Ohio State University Internal Laboratory Animal Care and Use Committee (ILACUC).
2.2 Drugs Administered
Mice were administered either a single dose of amphetamine sulphate (2.5, 5, 10, and 20 mg/kg) dissolved in 0.9% saline, or the vehicle alone, intraperitoneally (i.p.). The solutions were prepared so that 10μL was injected for every gram of body weight.
2.3 Locomotor Activity Measurements
Amphetamine dose-response experiments
Mice were habituated to handling for three days prior to behavioral testing. On the test day, mice were brought into the experiment room and acclimated for one hour. They were then placed individually into 25 × 25-cm acrylic boxes where their locomotor activity was recorded by the AnyMaze (Stoelting) software system for 45 minutes. The mice were then immediately injected with either saline, or amphetamine, and their locomotor activity was recorded for another 45 minutes. The mice were then removed, and their genotypes confirmed.
It should be noted that nearly 75 mice were needed for the double biphasic DAT-CI locomotor dose-response curves for amphetamine, depicted in figure 3B. For wild-type controls, only three of the doses were performed (n = 45) because their dose-response curve is not thought to have a suppressive phase at low doses, and therefore requires less definition. Furthermore, a 25 cm2 apparatus was used – rather than a more conventional 40 cm2 apparatus – for higher throughput because the total number of mice to be tested was very large.
Figure 3. Amphetamine-induced locomotor dose-response curve for both strains of DAT-CI mice.
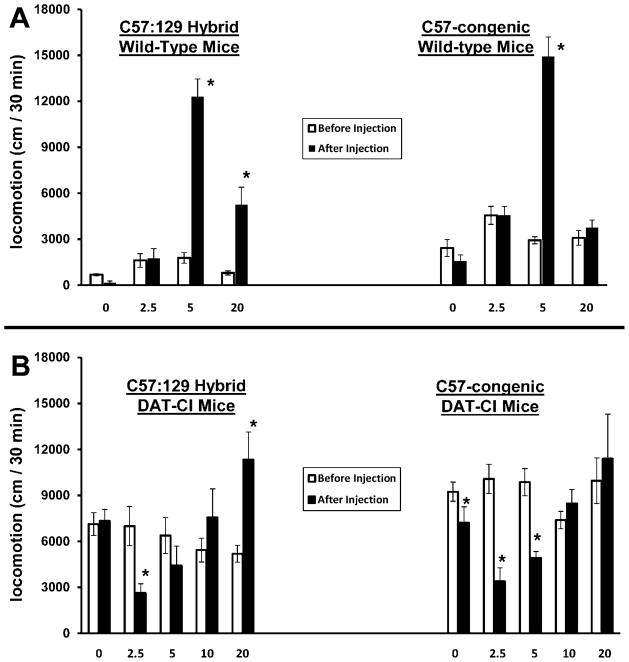
Mice were placed into a 25 × 25 cm open-field apparatus and allowed to habituate for 45 min. (A) Wild-type mice from both strains were injected with amphetamine at the doses indicated (0, 2.5, 5, and 20; in mg/kg), and returned to the apparatus for another 45 min. The locomotion over the 30 minutes before injection (open bars) and after injection (filled bars) is plotted. Both strains of wild-type mice have locomotor stimulation, but never suppression, from amphetamine (*, p < 0.05; relative to pre-injection locomotion). (B) DAT-CI mice from both strains were also injected with amphetamine at the same doses, plus 10 mg/kg, and measured in the same way. Both strains of DAT-CI mice have suppression of locomotion from 2.5 mg/kg amphetamine, but only the C57:129 hybrid DAT-CI mice have locomotor stimulation from 20 mg/kg. All statistics are comparisons of post-injection locomotion relative to the corresponding pre-injection score. All data are means ± SEM.
Locomotor habituation experiments
Mice were habituated to handling for three days prior to behavioral testing and acclimated to the room on test days. They were placed into 40 × 40-cm acrylic and their locomotor activity was measured for 30 minutes, once each day. A total of six such sessions were carried out on the subsequent days. The 40 cm2 apparatus was used in this case, because direct comparison with similar experiments (Zhuang et al., 2001) is desirable, and the larger apparatus should be better for detecting subtle differences in spontaneous locomotion and habituation.
2.4 Data Analysis
For the spontaneous locomotion results, a repeated measures ANOVA was used to detect within-session habituation for the two strains of DAT-CI mice (Fig 1A). A repeated measures ANOVA was also used to detect habituation/hyperactivity induced by repetitive, daily testing within the groups (Fig 2). The Bonferroni-Dunn procedure was used to determine pairwise differences between various groups (Fig 1A/B & Fig 2). \
Figure 1. C57:129 hybrid DAT-CI mice are less hyperactive and habituate more efficiently than C57-congenic DAT-CI mice.
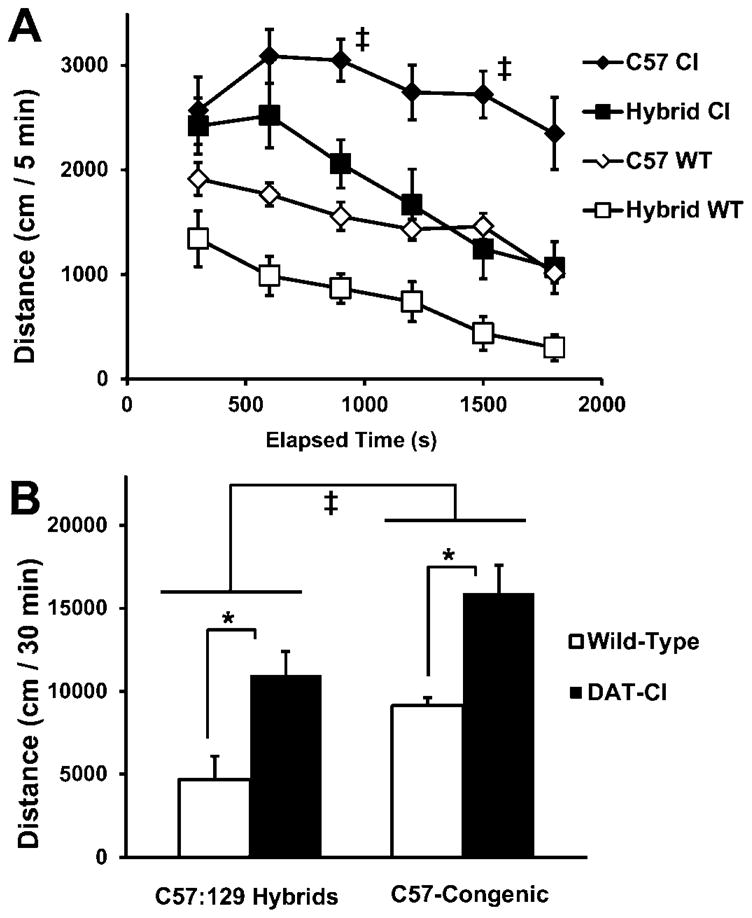
Mice were placed in 40 × 40 cm boxes for 30 min, and their horizontal movement was recorded in six, daily sessions. (A) The time-course for the locomotion of two strains of DAT-CI mice in the first session – the C57-congenic DAT-CI mice (n = 8) habituate less efficiently than the C57:129 hybrids (n = 8). (B) The total locomotion during the first session for both the mutant (filled bars) and wild-type mice (open bars) for each of the two strains (n = 8, in all four groups). DAT-CI mice are more hyperactive than wild-type mice in both cases (*, p < 0.05 within strains; ‡, p < 0.05 between strains of DAT-CI mice). All data are means ± SEM.
Figure 2. C57-congenic DAT-CI mice grow more hyperactive over days of repeated testing.
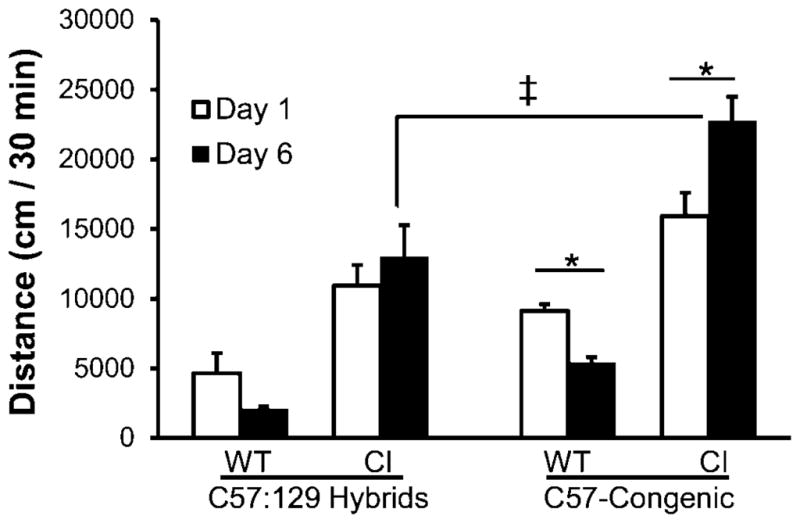
Mice were placed in 40 × 40 cm boxes for 30 min, and their horizontal movement was recorded in six daily sessions. Presented is the day 1 (open bars) vs. day 6 (filled bars) comparison of locomotor results for both the wild-type and DAT-CI mice in both strains (n = 8 C57:129 hybrid; and n = 8 C57-congenic mice). Wild-type mice (n = 8 in each strain) display habituation by the sixth session, whereas DAT-CI mice do not (main effects). Notably, the C57-congenic DAT-CI mice undergo significant “reverse habituation”, causing differences between the strains on day six (double dagger). (*, p < 0.05 within strains; ‡, p < 0.05 between strains). All data are means ± SEM.
For the amphetamine dose-response locomotor results (Fig 3), a two-way ANOVA (testing phase and dose as fixed factors) was performed on each of the four genotypes (wild-type and mutant; at both strains). The Bonferroni-Dunn procedure was then used to detect amphetamine effects at specific doses.
The data were analyzed with SPSS (ver. 19). The Bonferroni correction was used for all main effect models, and the Welch ANOVA was used when there were unequal variances (Fig 3A). In the figures, asterisks represent differences within strains, or between DAT-CI mutants and their wild-type littermates. The double daggers represent differences between the strains of DAT-CI mutants.
3. Results
Two mouse lines were used in this study: 1) DAT-CI mice on a C57-congenic background and 2) DAT-CI mice on a C57:129 hybrid background. The corresponding wild-type littermates were used as controls.
A side-by-side characterization of the locomotor behavior of the C57-congenic and the C57:129 hybrid mice was performed in two separate testing paradigms. First, spontaneous locomotion and locomotor habituation were observed; then, the amphetamine dose-response curves were determined for locomotion.
3.1 C57:129 hybrid DAT-CI mice are less hyperactive and habituate more efficiently than C57-congenic DAT-CI mice
In addition to hyperactivity, another characteristic feature of ADHD is a deficit in habituation of locomotion over time (ie. within-session) – especially within familiar environments (ie. across sessions). This feature has been recapitulated in mice – as the dopamine transporter knock-down mouse displays a less efficient locomotor habituation over a 1 hour session and sustained hyperactivity throughout the 6 days of testing (Zhuang et al., 2001). A similar paradigm was used in our study in order to measure the relative activity and locomotor habituation characteristics of the strains of mice (Fig. 1 & 2).
We placed untreated mice in open field apparatuses and their locomotor activities were monitored for 30 min. The same experiments were performed for 6 consecutive days. We found that on day 1 DAT-CI mice in both strains are hyperactive relative to wild-type mice (Fig 1B), as was reported previously (Chen et al., 2006). Importantly, C57-congenic DAT-CI mice show a deficit in within-session habituation, and are more hyperactive than the C57:129 hybrid DAT-CI mice (Fig. 1A). In the subsequent five days, we found that the C57-congenic strain of DAT-CI mice grew more hyperactive each day and their locomotor activities were significantly higher on day 6 than those on day 1 (Fig 2).
Specifically, a two-way ANOVA showed that there was a main effect of genetics (F3, 52 = 41.03; p < 0.001) on spontaneous activity. Figure 1B shows that both strains of DAT-CI mice are hyperactive relative to their wild-type counterparts (Day 1: p < 0.05; Day 6: p < 0.001). The results also show that there is a lack of habituation over days, in both strains of DAT-CI mice relative to their wild-type controls (Fig 2). In a repeated-measures ANOVA for locomotion over days, there was a day × genotype interaction (F3, 24 = 8.080; p < 0.01). Wild-type mice exhibit habituated locomotion on day 6 relative to day 1 (main effect; p < 0.01) whereas DAT-CI mice have either unchanged (hybrids) or higher locomotion (C57-congenic: p < 0.01) after repeated exposure (Fig 2). The C57-congenic DAT-CI mice are significantly more hyperactive than their C57:129 hybrid counterparts after repeated exposure (p < 0.001).
In addition to altered between-session habituation, there are differences in within-session habituation between the strains of DAT-CI mice. In a repeated-measures ANOVA for locomotion over 5-minute bins of the day 1 data (Fig. 1A), there was a main effect of time (F5, 70 = 11.754; p < 0.001), of genotype (F1, 14 = 7.899; p < 0.05), and a time × genotype interaction(F5,70 = 4.287; p < 0.01). The time-course results also show that the C57-congenic DAT-CI mice have less efficient habituation than the C57:129 hybrid mice. There is a time × strain interaction (F1, 14 = 5.961; p < 0.5) for locomotion, and a one-way ANOVA identifies differences between specific time points across the two strains of DAT-CI mice (p < 0.01 for latter 15 minute time-bin). The daggers in Fig 1A (subdivided by 5-minute bins) denote repeated measures ANOVA pairwise comparisons (p < 0.05) between DAT-CI mice.
3.2 Amphetamine has a locomotor suppressive phase of the dose-response curve for both strains of DAT-CI mice
We also aimed to determine whether the change in genetic background, due to backcrossing, was responsible for differences in the amphetamine response of DAT-CI mice which we saw over time. Therefore, a thorough dose-response curve for amphetamine-induced locomotion was characterized (0, 2.5, 5, 10, and 20 mg/kg doses) for DAT-CI mice. Wild-type mice from both strains were also tested with the same doses, with the omission of 10 mg/kg.
For wild-type mice, a two-way ANOVA shows that there was a main effect of the drug dose (F3, 41 = 33.619; p < 0.001) and of the testing phase (F1, 41 = 68.207; p < 0.001), and there is a phase x dose interaction (F3, 41 = 47.624; p < 0.001). The Bonferroni post hoc results show that C57:129 hybrid wild-type mice have significant locomotor stimulation at most doses (2.5A: p = .903; 5A: p < 0.001; 20A: p < 0.001) when comparing post-injection locomotion to their respective pre-injection scores. The C57-congenic wild-type mice have significant locomotor stimulation only at 5 mg/kg amphetamine (p < 0.001). The number of mice used, for each strain and at each dose, is as follows: C57:129 hybird (0A: n = 3, 2.5A: n = 5, 5A: n = 4, and 20A: n = 9) C57 congenic – (0A: n = 3, 2.5A: n = 6. 5A: n = 7, 20A: n = 8).
For DAT-CI mice, a two-way ANOVA shows that there was a main effect of the drug dose (F4,68 = 3.073; p < 0.05) but not of the testing phase (F1,68 = 3.734; p = 0.057). There was also a significant phase × dose interaction (F4,68 = 20.203; p < 0.001). The Bonferroni post hoc results show that the C57-congenic DAT-CI mice do not have locomotor stimulation even at the very high-dose (20 mg/kg) of amphetamine (p = 0.146). On the other hand, the C57:129 hybrid DAT-CI mice have significant locomotor stimulation at this dose (p < 0.001). Furthermore, both strains have the paradoxical calming effect at lower doses (2.5 mg/kg) of amphetamine (Hybrid: p < 0.01 Congenic: p < 0.001). The C57-congenic DAT-CI mice also have suppression to saline (p < 0.05) and 5 mg/kg amphetamine (p < 0.001), which is in contrast to hybrid DAT-CI mice. The apparently reduced activity after saline injection (relative to before injection) is expected if mice have no significant response to saline, but continue to habituate.
The number of mice used, for each strain and at each dose, is as follows: C57:129 hybrid – (0A: n = 10, 2.5A: n = 6, 5A: n = 10, 10A: n = 7, and 20A: n = 8) C57 congenic – (0A: n = 8, 2.5A: n = 8. 5A: n = 6, 10A: n = 4, 20A: n = 6).
4. Discussion
In this study, we generate a second line of knock-in mice with a cocaine-insensitive dopamine transporter (DAT-CI mice) that are a C57:129 hybrid, in addition to the C57-congenic mice already available. We used both strains of DAT-CI mice in a side-by-side comparison of spontaneous and amphetamine-induced locomotion. One aim was to determine whether changes in the genetic background could explain the locomotor responses to amphetamine observed recently that are discrepant to those observed in earlier studies. DAT-CI mice have been shown to exhibit phenotypes considered representative of human ADHD symptoms (Castelli et al., 2011; Napolitano et al., 2010). Thus the second aim was to characterize any ADHD-related phenotypes that are also affected by the genetic background. A recent bioinformatics study shows that both genetic and environmental factors contribute to ADHD in humans (Hudziak et al., 2005). Further studies using these two mouse lines may reveal possible genetic factors contributing to ADHD.
We found that DAT-CI mice in both strain backgrounds had elevated locomotion relative to their respective wild-type littermates (Fig. 1B), indicating that reduced DAT function leads to hyperlocomotion. We also found that the C57:129 hybrid DAT-CI mice had lower spontaneous locomotion relative to their C57-congenic counterparts. Since wild-type C57:129 hybrids also have lower spontaneous locomotion than wild-type C57 mice, the difference in locomotion between the two strains of DAT-CI mice appears to be due to a main effect of strain.
However, we also found that the C57-congenic DAT-CI mice had a less efficient within-session locomotor habituation compared to the hybrid DAT-CI mice (Fig. 1A) and a significant “reverse habituation” over repeated testing (Fig. 2). The persistence of hyperactivity within a familiar environment is an important characteristic of ADHD present in purported rodent models (Williams et al., 2009), that is not present in normal mice (Voikar et al., 2004). The lack of within-session habituation observed in the C57-congenic DAT-CI mice, but not in the C57:129 hybrid DAT-CI mice (Fig. 1A), therefore suggests that certain genetic variants that are different between the two strains play an important role in the hyperactive aspect of ADHD. The further potentiation of this effect across sessions, again present in the C57 congenic but not C57:129 hybrid DAT-CI mice (Fig. 2), strengthens this conclusion.
Another finding is the difference in the amphetamine response between the two strains of DAT-CI mice. Both strains exhibit a therapeutic calming effect of amphetamine at low doses. In contrast, the C57:129 hybrid DAT-CI mice have a stimulatory phase at high doses while the C57-congenic DAT-CI mice are not stimulated by amphetamine, even at 20 mg/kg (Fig. 3B). This may be viewed as a main effect of strain, since C57-congenic wild-type mice also do not have locomotor stimulation in response to 20 mg/kg amphetamine (Fig. 3A).
Amphetamine, at low doses, has a calming effect in ADHD patients and is one of the frequently prescribed drugs for the disorder. In contrast, a high dose of the drug is a powerful psychostimulant. The double biphasic dose-response curve for amphetamine in 129:C57 hybrid DAT-CI mice is similar to the amphetamine effect in human ADHD patients (Stein et al., 2011). The behavior of other models – such as the complete lack of stimulation found in the C57-congenic DAT-CI mice – is less similar to ADHD patients. A heavily studied rat model of ADHD notably lacks amphetamine’s calming effect (Langen and Dost, 2011). In this regard, the 129:C57 hybrid DAT-CI mice have good face and predictive validity as an ADHD animal model.
Although this study focuses on hyperactivity, ADHD has two main components: attention deficit and hyperactivity. Additional studies on the attention of these mice are needed to further validate the mouse models. It may also be interesting and important to characterize impulse control and reward deficits in DAT-CI mice as these related dysfunctions have been seen in ADHD and comorbid disorders, but are less studied (Sagvolden, 2011; Volkow et al., 2009). In this context, having even more than two background strains characterized with the locomotor phenotype would be beneficial, because each may have varying degrees of attentional deficits. Studies containing both genetic and behavioral analysis of multiple DAT-CI strains – each containing partially overlapping phenotypes and genetic backgrounds – would be best suited for identifying common causes of hyperactivity and inattention.
While the specific genetic underpinnings of the ADHD phenotypes and therapeutic responses are not well understood, there is an increasingly clear involvement of a network of related genetic factors (Poelmans et al., 2011). Many of these genes encode products related to the dopamine system, but the involvement of DAT specifically, is controversial (Contini et al., 2010; Kooij et al., 2008) (for review, see (Thapar et al., 2005)). Some studies have indicated that increases in dopamine function are associated with ADHD, while others have found the opposite effect, or bidirectionality (Granon et al., 2000; Russell, 2002; Viggiano et al., 2003). Still others have found that NET/norepinephrine function is more critically associated with the symptoms (Viggiano et al., 2004). Importantly, imbalances in single neurotransmitters between interacting regions have been observed (Ventura et al., 2004), and likely make many of these distinctions overly simplified. Our results support the dopamine hypothesis, without excluding a potential role for norepinephrine in these phenotypes. Our results also show a clear involvement of “background” genetic elements that do not co-segregate with the DAT mutations selected during genotyping.
5. Conclusion
These results show that differences in the genetic background of DAT-CI mice affect several ADHD-related phenotypes: the spontaneous locomotion, the habituation to an environment, and the responses to amphetamine, a drug used to treat ADHD. ADHD is a highly heritable and polygenic disorder in humans and the contributing genetic factors are not known. Additional comparison studies using the congenic C57-congenic and C57:129 hybrid DAT-CI mice may reveal genetic polymorphisms and underlying mechanisms that contribute to the variations in the severity of ADHD symptoms and the variations in responses to drug treatment.
Highlights.
A triple mutant knock-in of the dopamine transporter results in hyperlocomotion in mice
The hyperlocomotive behavior is suppressed by a range of doses of amphetamine
The basal and amphetamine-induced locomotion is modulated by the mouse strain used
The C57 strain of knock-in mice has higher symptom severity, and does not habituate
Acknowledgments
The authors would like to acknowledge Dr. Bradley Martin for his valuable assistance in the data interpretation and writing processes. This study was supported by grants from the NIH (DA014610 and DA020124).
Abbreviations
- ADHD
Attention Deficit Hyperactive Disorder
- ANOVA
Analysis of Variance
- DA
Dopamine
- DAT
Dopamine Transporter
- DAT-CI mice
Cocaine-insensitive dopamine transporter knock-in mice
- NET
Norepinephrine Transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian O’Neill, Email: oneill.131@osu.edu.
Howard H. Gu, Email: gu.37@osu.edu.
References
- Castelli M, Federici M, Rossi S, De Chiara V, Napolitano F, Studer V, et al. Loss of striatal cannabinoid CB1 receptor function in attention-deficit / hyperactivity disorder mice with point-mutation of the dopamine transporter. Eur J Neurosci. 2011;34:1369–77. doi: 10.1111/j.1460-9568.2011.07876.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–8. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, Gnegy ME. C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacol Biochem Behav. 2007;87:158–63. doi: 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini V, Victor MM, Marques FZ, Bertuzzi GP, Salgado CA, Silva KL, et al. Response to methylphenidate is not influenced by DAT1 polymorphisms in a sample of Brazilian adult patients with ADHD. J Neural Transm. 2010;117:269–76. doi: 10.1007/s00702-009-0362-2. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak JJ, Derks EM, Althoff RR, Rettew DC, Boomsma DI. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ Rating Scales--Revised. Am J Psychiatry. 2005;162:1614–20. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- Kooij JS, Boonstra AM, Vermeulen SH, Heister AG, Burger H, Buitelaar JK, et al. Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1) Am J Med Genet B Neuropsychiatr Genet. 2008;147B:201–8. doi: 10.1002/ajmg.b.30586. [DOI] [PubMed] [Google Scholar]
- Langen B, Dost R. Comparison of SHR, WKY and Wistar rats in different behavioural animal models: effect of dopamine D1 and alpha2 agonists. Atten Defic Hyperact Disord. 2011;3:1–12. doi: 10.1007/s12402-010-0034-y. [DOI] [PubMed] [Google Scholar]
- Napolitano F, Bonito-Oliva A, Federici M, Carta M, Errico F, Magara S, et al. Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine. J Neurosci. 2010;30:11043–56. doi: 10.1523/JNEUROSCI.1682-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–99. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:365–77. doi: 10.1176/appi.ajp.2010.10070948. [DOI] [PubMed] [Google Scholar]
- Russell V. A Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2002;130:191–6. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Impulsiveness, overactivity, and poorer sustained attention improve by chronic treatment with low doses of l-amphetamine in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behav Brain Funct. 2011;7:6. doi: 10.1186/1744-9081-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Waldman ID, Charney E, Aryal S, Sable C, Gruber R, et al. Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol. 2011;21:581–8. doi: 10.1089/cap.2011.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14(Spec No 2):R275–82. doi: 10.1093/hmg/ddi263. [DOI] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Arcieri S, Sadile AG. Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast. 2004;11:133–49. doi: 10.1155/NP.2004.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Ruocco LA, Sadile AG. Behavioural, pharmacological, morpho-functional molecular studies reveal a hyperfunctioning mesocortical dopamine system in an animal model of attention deficit and hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:683–9. doi: 10.1016/j.neubiorev.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. Jama. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Sagvolden G, Taylor E, Sagvolden T. Dynamic behavioural changes in the Spontaneously Hyperactive Rat: 2. Control by novelty. Behav Brain Res. 2009;198:283–90. doi: 10.1016/j.bbr.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


