Abstract
Tumor islands - large collections of tumor cells isolated within alveolar spaces - can be seen in lung adenocarcinomas. Recently we observed by 3D reconstruction that these structures were connected with each other and with the main tumor in different tissue planes, raising the possibility of tumor islands being a means of invasion. However, the clinical and prognostic significance of tumor islands remain unknown. In this study, we compared clinicopathological and molecular characteristics and prognosis of Stage I–II lung adenocarcinomas with tumor islands (n=58) and those without (n=203). Lung adenocarcinomas with tumor islands were more likely to occur in smokers, exhibit higher nuclear grade and a solid or micropapillary pattern of growth, and harbor KRAS mutations. In contrast, lung adenocarcinomas without tumor islands were more likely to present as minimally invasive adenocarcinoma, show a lepidic pattern of growth, and harbor EGFR mutations. Although there was no difference in stage, the prognosis of lung adenocarcinomas with tumor islands was significantly worse than those without. The five-year recurrence-free survival for patients with tumor islands and those without was 44.6% and 74.4%, respectively (log-rank p = 0.010). The survival difference remained significant (p < 0.020) by multivariate analysis, and the presence of tumor islands was associated with almost two-fold increase in the risk of recurrence. Even in the Stage IA cohort, more than half of the patients with tumor islands experienced recurrence within 5 years. Thus, aggressive surveillance and/or further intervention may be indicated for patients whose tumors exhibit tumor islands.
Keywords: lung adenocarcinoma, 3D, intra-alveolar, tumor islands, prognosis
Introduction
Lung cancer remains the most frequent cause of cancer incidence and mortality worldwide despite efforts toward its early detection and treatment.1–3 The prognosis of patients with lung cancer is generally poor, and the overall 5-year survival rate is 15%.4 Of the four main histologic types of lung cancer, adenocarcinoma is increasing in frequency and accounts for almost half of all lung cancers.3,5 Pathological and radiological studies have revealed significant prognostic subsets of lung adenocarcinoma.5–7 However, a wide morphologic spectrum exists in lung adenocarcinoma, and this heterogeneity has resulted in confusion and difficulty in comparing the results of clinicopathological studies.1,5,6 Despite remarkable advances in understanding the molecular biology of this tumor in the past decade, there remains a need for universally accepted criteria for adenocarcinoma classification according to prognosis.
In 2008 an international multidisciplinary expert panel supported by the International Association for the Study of Lung Cancer (IALC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS) reevaluated the histologic classification of lung adenocarcinoma and formulated a new classification. It recognizes 5 major patterns (lepidic [formerly nonmucinous bronchioloalveolar], papillary, acinar, micropapillary and solid) and 4 variants (mucinous, fetal, colloid and enteric) and correlates these histologic features with clinical features, imaging characteristics, molecular signature, and prognostic and predictive markers.7 This new classification is expected to facilitate diagnostic standardization and improve risk stratification and management of patients with lung adenocarcinoma. In fact, Yoshizawa et al7 and Russell et al 8 recently validated the new classification with cohorts of 514 and 210 lung adenocarcinomas, respectively, and demonstrated a correlation between the predominant pattern(s) of adenocarcinoma and clinical outcome. The results confirm a prognostic role of the new lung adenocarcinoma classification.7–9
Several challenges are associated with this proposed classification, however. Interobserver concordance in assigning patterns remains suboptimal, particularly when multiple patterns are present in one tumor and the proportion of each pattern must be determined.10 Additionally, certain unique morphologic structures cannot easily be classified into one pattern or variant. One example of this is a tumor island, a detached collection of tumor cells within an alveolar space that is separated from the main tumor mass by a distance of at least a few alveoli and cannot be classified as a micropapillary pattern, which has been clearly defined by Amin et al11 and later by Tsutsumida et al,12 based on the shape and large size of these collections. We have recently developed an automated algorithm for 3-dimensional (3D) reconstruction of paraffin embedded tissues and found that tumor islands were interconnected with each other and with the main tumor at different tissue planes in 2 of 4 examined lung adenocarcinoma cases (Figure 1).13 These features could not be clearly recognized by routine (2D) observation of histology slides. Based on these results, we hypothesize that the tumor island is a means of tumor extension in lung adenocarcinoma and may be an important factor for prognosis. In the present study, we sought to examine 1) the prevalence, and clinicopathological and molecular characteristics of subjects with tumor islands and 2) the prognostic significance of tumor islands in resected early stage lung adenocarcinomas.
Figure 1.
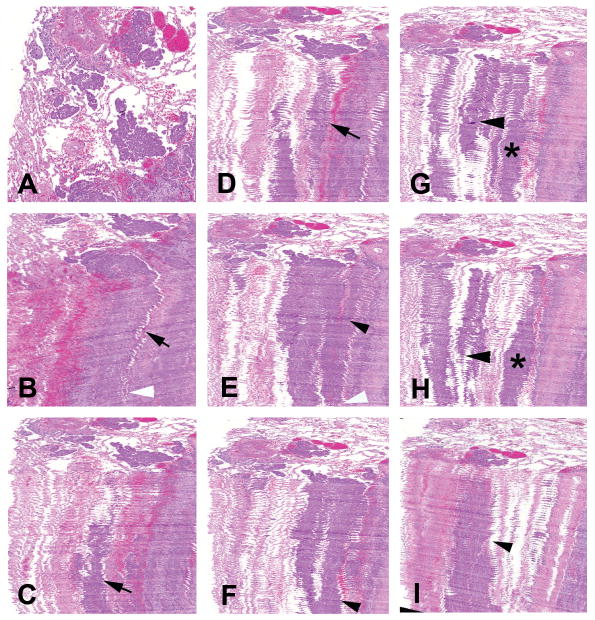
(A) Top view of 3D-reconstructed image of lung adenocarcinoma showing tumor islands. (B–J) Fifty whole-slide images of a serial-sectioned paraffin embedded specimen were combined and a 3D image was obtained to study the structure of the tumor islands and its relation with surrounding structures. (B–D) Different planes of view are shown depicting the islands running deep into the tissue (arrows). (B, E–J) Neighboring islands tend to connect (arrow heads and asterisks) and at certain points merge with the main solid tumor (white arrow heads).
Methods
Subjects
The Massachusetts General Hospital institutional review board approved this study. The study group consisted of 261 patients who had undergone lung resection with curative intent for Stage I or II lung adenocarcinoma without neoadjuvant therapy between January 1998 and December 2010. In 92 of the 261 cases, resected in 2009 and 2010, mutational analysis had been performed for clinical purposes using a recently developed tumor-genotyping assay based on SNaPshot technology (Applied Biosystems) for point mutations and small insertions and deletions in 13 cancer genes, including KRAS and EGFR.14 Clinical and demographic information was obtained from the medical record.
Histological evaluation
Two of the authors (MMK, and AEK or VEK) jointly reviewed all the 5μm hematoxylin and eosin (HE)-stained slides processed for clinical diagnosis of all 261 lung adenocarcinomas, including 3–8 tumor slides per case, and performed comprehensive histologic subtyping, based on recommendations by the classification strategy proposed by IASCLC/ATS/ERS.3 In brief, the proportion of any particular histologic pattern (lepidic, acinar, papillary, micropapillary, solid, mucinous adenocarcinoma or the other variants) was semiquantitatively determined in 5% increments and the predominant histologic pattern was recorded. For each case, a three-tier scoring system for nuclear grade was applied (low-, intermediate-, and high-grade), and the highest grade for the analysis recorded.15 Also recorded was specimen type (wedge resection versus anatomic resection), tumor size based on gross and/or conventional 2D microscopic examination and the presence of lymphovascular invasion, pleural invasion and nodal status. One of the authors (MLO), who was blinded to clinicopathological data, independently reviewed each case and recorded the presence or absence of a tumor island or islands. A tumor island was defined as an isolated, large collection of tumor cells present within alveolar spaces that lacked well-demarcated micropapillary configuration.11,12 The island was located at the periphery of the lesion and was separated from the main tumor by at least a few alveoli. The tumor cells in the island were cytologically similar to those of the main tumor and could easily be differentiated from alveolar macrophages by a high N/C ratio and cytologic atypia.
Statistical Analysis
Fisher’s exact test and Wilcoxon rank-sum test were used to evaluate the association of tumor islands with various clinicopathological characteristics and molecular alterations. Recurrence-free survival was estimated using the Kaplan-Meier method, with patients followed from time of surgery until recurrence. Patients without disease recurrence at the last available follow-up, no later than June 30, 2011, were censored. The log-rank test was applied to assess for differences. To determine the independent effect of tumor islands on recurrence-free survival, multivariate analysis was performed using a Cox regression model. All reported p-values are based on two-sided hypothesis tests, and the statistical analysis was computed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Clinicopathological Characteristics
The clinicopathological and histology findings are summarized in Table 1. Of the 261 subjects, 59.8% were women (n=156), and the median age was 69 years (range 37 to 92 years). Wedge resection was performed in 82 cases (31.4%), and median tumor size was 2.2 cm (range 0.4 to 15.0 cm). The majority of subjects presented with Stage I disease: 151 patients with Stage IA (57.9%) and 66 with stage IB (25.3%). Of the remaining 44 subjects, 23 (8.8%) presented with Stage IIA disease and 21 (8.0%) with Stage IIB disease. In accordance with the IASLC/ATS/ERS proposed classification, 31 cases (11.9%) were reclassified as minimally invasive adenocarcinoma, and the acinar pattern was the most common predominant pattern (115 cases, or 44.1%).
Table 1.
Clinical and pathological characteristics of lung adenocarcinomas with and without tumor islands
| Variable | Island (−) n=203 |
Islands (+) n=58 |
P value |
|---|---|---|---|
| Age (y); median (range) | 69 (37–92) | 68.5 (47–83) | 0.253 |
| Gender (F; %) | 126 (62.1) | 30 (51.7) | 0.173 |
| Smoking History (Pack-year); median (range) | 30 (0–730) | 40 (0–140) | 0.060 |
| Tumor size (cm); median (range) | 2.3 (0.6–15.0) | 2.05 (0.4–6.5) | 0.283 |
| Specimen type (wedge resection; %) | 66 (32.5) | 16 (27.6) | 0.524 |
| pT (%) | 0.949 | ||
| 1a | 82 (40.4) | 22 (37.9) | |
| 1b | 44 (21.7) | 15 (25.9) | |
| 2a | 57 (28.1) | 16 (27.6) | |
| 2b | 7 (3.4) | 1 (1.7) | |
| 3 | 13 (6.4) | 4 (6.9) | |
| pN (%) | 0.609 | ||
| N0 | 173 (85.2) | 49 (84.5) | |
| N1 | 17 (8.4) | 6 (10.3) | |
| Nx | 13 (6.4) | 3 (5.2) | |
| AJCC Stage | 0.960 | ||
| IA | 116 (57.1) | 35 (60.4) | |
| IB | 53 (26.1) | 13 (22.4) | |
| IIA | 18 (8.9) | 5 (8.6) | |
| IIB | 16 (7.9) | 5 (8.6) | |
| Predominant Histological Pattern (%) | 0.003 | ||
| Minimally invasive adenocarcinoma | 29 (14.3) | 2 (3.5) | |
| Lepidic | 34 (16.8) | 4 (6.9) | |
| Acinar | 90 (44.3) | 25 (43.1) | |
| Papillary | 21 (10.3) | 7 (12.1) | |
| Micropapillary | 8 (3.9) | 6 (10.3) | |
| Solid | 16 (7.9) | 13 (22.4) | |
| Mucinous adenocarcinoma | 5 (2.5) | 1 (1.7) | |
| Nuclear Grade (%) | 0.011 | ||
| 1 | 15 (7.4) | 0 (0) | |
| 2 | 104 (51.2) | 24 (41.4) | |
| 3 | 84 (41.4) | 34 (58.6) | |
| Lymphatic invasion (%) | 45 (22.17) | 19 (32.76) | 0.119 |
| Vascular invasion (%) | 23 (11.3) | 8 (13.8) | 0.646 |
| Pleural invasion (%) | 34 (16.8) | 13 (22.4) | 0.336 |
Tumor islands were seen in 58 (22.2%) of these 261 early stage lung adenocarcinomas, with a mean greatest dimension of 155μm (range: 35μm – 366μm). Each case showed 2 – 29 tumor islands in the examined sections. In 18 of the 58 cases, small tufts (micropapillary structures) were also seen in adjacent alveolar spaces, but tumor islands were identified as isolated structures in all cases.
The median size of cases with tumor islands was similar to those without (2.05 cm versus 2.3 cm, p=0.283). Histologically, cases with tumor islands were significantly more likely to have a predominant solid pattern (22.4% versus 7.9%, p = 0.004) and less likely to be minimally invasive (3.5% versus 14.3%, p = 0.022). There were also trends towards a predominant micropapillary pattern seen more frequently in cases with tumor islands (10.3% versus 3.9%, p = 0.090) and a predominant lepidic pattern less frequently seen in cases with tumor islands (6.9% versus 16.8%, p = 0.089). Lung adenocarcinomas with tumor islands tended to have higher nuclear grade than those without and did not include any tumors with low nuclear grade (p = 0.011). Smoking history was significantly associated with tumor islands in lung adenocarcinomas (91.1% vs. 77.9%, p = 0.031). There were no differences in the age, gender distribution, pathologic T (pT) stage, pathologic N (pN) stage, AJCC stage or presence of pleural, lymphatic or vascular invasion between the group presenting with tumor islands and the group without tumor islands (Table 1).
Molecular Alterations
Of the 92 cases that had been analyzed by the SNaPshot assay, 20 (22%) were found to harbor EGFR mutations and 37 (40%) KRAS mutations. Of the remaining 35 cases, one demonstrated a BRAF mutation, two ERBB2 mutations, one a PIK3CA mutation and one a TP53 mutation, with 30 cases showing none of the mutations assayed in the panel. Tumor islands were not observed in any of the cases with EGFR mutations in this cohort. By contrast, tumor islands were identified in 46% (17 of 37) of lung adenocarcinomas with KRAS mutations and in 23% (8 of 35) of the tumors with neither KRAS nor EGFR mutations. The presence of tumor islands was strongly associated with KRAS mutation (p = 0.002). In the 5 lung adenocarcinomas with other mutations, tumor islands were present in one BRAF mutant, another member of the mitogen-activated protein kinase (MAPK) pathway, but not in the other cases including the two cases with ERBB2 mutations.
Survival in context of tumor islands
Subjects with lung adenocarcinomas exhibiting tumor islands had a significantly worse outcome than those without tumor islands. Median survival was 55 months after resection among patients with tumor islands and undefined among those without tumor islands. The five-year recurrence-free survival for patients with tumor islands and those without was 44.6% and 74.4%, respectively (log-rank p = 0.010) (Figure 3A).
Figure 3.
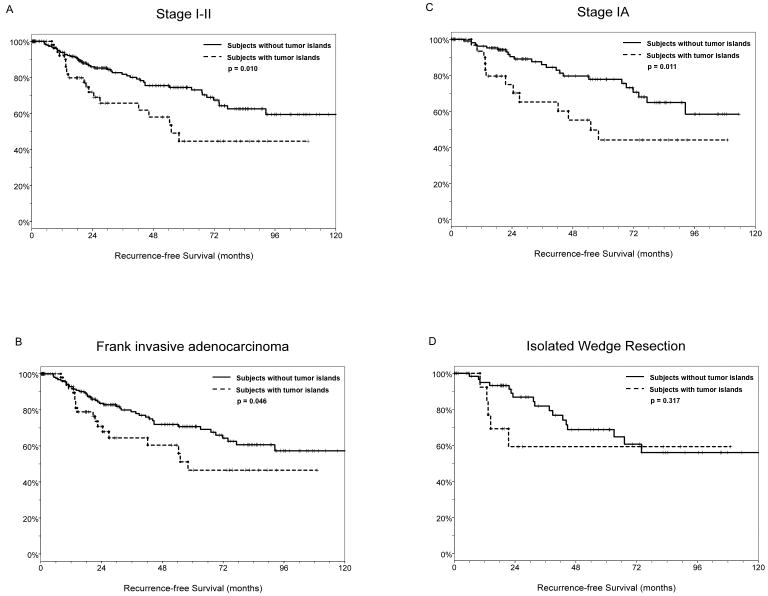
(A) For all stages, median recurrence-free survival was 55 months after resection among subjects with tumor islands (n=56) and undefined among those without tumor islands (n=174). The five-year recurrence-free survival for subjects with tumor islands and those without was 44.6% and 74.4%, respectively (log-rank p = 0.010). (B) For cases with frank invasive carcinomas, median recurrence-free survival was 58 months after resection among subjects with tumor islands (n=56) and undefined among those without tumor islands (n=174). The five-year recurrence-free survival for patients with tumor islands and those without was 46.5% and 70.7%, respectively (log-rank p = 0.046). (C) For Stage IA cases, median survival was 55 months after resection among patients with Stage IA lung adenocarcinomas exhibiting tumor islands (n=35) and undefined among those without tumor islands (n=116). Five-year recurrence-free survival for patients with tumor islands and those without was 44.1% and 77.8%, respectively (log-rank p = 0.011). (D) For subjects who underwent isolated wedge resections, the median survival was undefined among those with tumor islands (n=16) as well as among those without tumor islands (n=66). There was no difference in the five-year recurrence-free survival between subjects with tumor islands and those without (59.3% vs. 68.8%, log-rank p = 0.317); however, the presence of tumor islands was associated with early recurrence (< 2 years) after resection.
When comparing only the subjects with frank invasive carcinoma, the survival difference remained significant. Median recurrence-free survival was 58 months after resection among patients with tumor islands (n=56) and undefined among those without tumor islands (n=174). The five-year recurrence-free survival for patients with tumor islands and those without was 46.5% and 70.7%, respectively (log-rank p = 0.046) (Figure 3B).
Similarly, the presence of tumor islands was significantly associated with poor outcomes in the Stage IA cohort. Median survival was 55 months after resection among patients with Stage IA lung adenocarcinomas exhibiting tumor islands (n=35) and undefined among those without tumor islands (n=116). Five-year recurrence-free survival for patients with tumor islands and those without was 44.1% and 77.8%, respectively (log-rank p = 0.011) (Figure 3C).
Of patients who underwent isolated wedge resection, the median survival was undefined among those with tumor islands (n=16) as well as among those without tumor islands (n=66). There was no difference in the five-year recurrence-free survival between patients with tumor islands and those without (59.3% vs. 68.8%, log-rank p = 0.317); however, the presence of tumor islands was associated with early recurrence (< 2 years) after resection (5 of 5 vs. 7 of 17, p = 0.040) (Figure 3D).
The survival difference remained significant (p<0.020) after controlling for the independent effects of stage and invasiveness by multivariate analysis. The adjusted hazard ratio of 1.9 suggests the presence of tumor islands was associated with an almost two-fold increase in the risk of recurrence.
Discussion
Early stage lung adenocarcinomas with tumor islands compared to those without demonstrated significant differences in pathologic features, molecular alterations and survival. Lung adenocarcinomas with tumor islands were more likely to occur in smokers, exhibit higher nuclear grade, a solid or micropapillary pattern of growth and harbor KRAS mutations. In contrast, lung adenocarcinomas without tumor islands were more likely to present as minimally invasive adenocarcinoma, show a lepidic pattern of growth and harbor EGFR mutations. Although there was no difference in stage or other parameters commonly associated with poor outcomes, the prognosis of lung adenocarcinomas with tumor islands was significantly worse than those without. The same was true when only frank invasive carcinomas were compared. These findings indicate that tumor islands likely dictate the aggressiveness of early stage lung adenocarcinomas.
In a previous study,13 we analyzed early stage lung adenocarcinomas with an automated 3D model that could visualize morphologic features not clearly seen by 2D views of routine histology slides. We found that tumor islands were connected with each other and with the main tumor, thus increasing the total volume (and frequently the maximum dimension) of tumor. In the current practice, pT stage is defined by size of tumor based on gross examination and conventional microscopic assessment. The tumor islands, which are not easily appreciated on gross examination and are seen isolated from the main tumor on microscopy, could be considered as artifact associated with processing by some pathologists. In addition, they are not specified either in the 2004 WHO classification or in the new ISALC/ATS/ERS International Multidisciplinary Classification.3 Thus, it is reasonable to think that lung adenocarcinomas with tumor islands might have been underestimated in size, although in our cohort, the median size of lung adenocarcinomas with tumor islands was similar to those without tumor islands on conventional size assessment. However, whether tumor islands should be considered as part of tumor upon measuring tumor size as well as whether they should be considered as part of tumor tissue for histologic classification need to be evaluated in larger cohort studies.
Kamiya et al studied histopathological features of lung adenocarcinomas with the micropapillary pattern in depth and found that most micropapillary tufts had continuity with other tufts and main tumor in 3 cases studied using serial sections.16 Given the ambiguous definition in size of a micropapillary pattern in the reported studies,11,12,16–25 and some association of tumor islands with the micropapillary predominant pattern, there is the possibility of tumor islands and micropapillary tufts being at the opposite ends of the same spectrum. However, in our cohort, the majority of the tumor islands were seen as isolated structures without micropapillary tufts in the background. In addition, the presence of micropapillary pattern in lung adenocarcinomas was associated with lymphatic and/or vascular invasion and lymph node metastasis in the reported studies,11,12,16,17,21–25 but these associations were not identified in lung adenocarcinomas with tumor islands in our cohort. Therefore, we believe that tumor islands represent a unique morphology of lung adenocarcinoma.
It has been shown that the presence of a micropapillary pattern in > 5% of tumor is associated is a strong predictor of recurrence in patients undergoing limited resection for small lung adenocarcinomas.26 Based on their results, the authors hypothesized that micropapillary structures might spread through airspaces and extend beyond the resection margin (Travis WD, personal communication). Islands of tumor cells that appear isolated and away from the main mass on conventional microscopic examination, but are found in continuum with it on 3D assessment, likely represent another manner of extension. In our cohort, the presence of tumor islands was associated with early recurrence (< 2 years) after resection in subjects who underwent wedge resection, even though the parenchymal margin was free of tumor on microscopic examination in these cases (data not shown). The results indicate the possibility of residual, grossly invisible tumor cells in the remaining lung parenchyma after wedge resection.
We found an association of tumor islands with MAPK pathway alterations, mainly KRAS mutations, and the inverse correlation with minimal invasion. Interestingly, Sakamoto et al evaluated lung cancer resections from a Japanese cohort with foci of atypical adenomatous hyperplasia (AAH) and found KRAS gene mutations in one third of AAH lesions but rarely in minimally invasive adenocarcinomas or well-differentiated invasive adenocarcinomas, although KRAS mutations were present in 17–18% of higher-grade adenocarcinomas.27 Conversely, EGFR mutations were evenly distributed from AAH to poorly differentiated adenocarcinomas. Based on the results, the authors concluded that AAH lesions harboring KRAS mutations might not progress further to invasive carcinomas through a conventional “adenoma–carcinoma sequence” but rather that high-grade adenocarcinomas with KRAS mutations might develop through de novo or alternative unknown pathways.27 Another possible interpretation of their results is that lung adenocarcinomas driven by KRAS mutations may progress rapidly from preinvasive lesions to high-grade invasive carcinoma and replace preinvasive lesions when they are small. Irrespective of the presence of de novo or alternative unknown pathways, based the findings of the current study, we hypothesize that at least a subset of lung adenocarcinomas harboring KRAS mutations become invasive when they are small and expand the tumor mass via tumor islands and/or some other means of extension.
In conclusion, tumor islands associated with a solid pattern of growth and KRAS mutations likely dictate adverse prognosis after resection in early stage lung adenocarcinomas. Even in the Stage IA cohort, more than half of the patients experienced recurrence within 5 years. Thus, aggressive surveillance and/or further intervention may be indicated for those with tumor islands.
Figure 2.
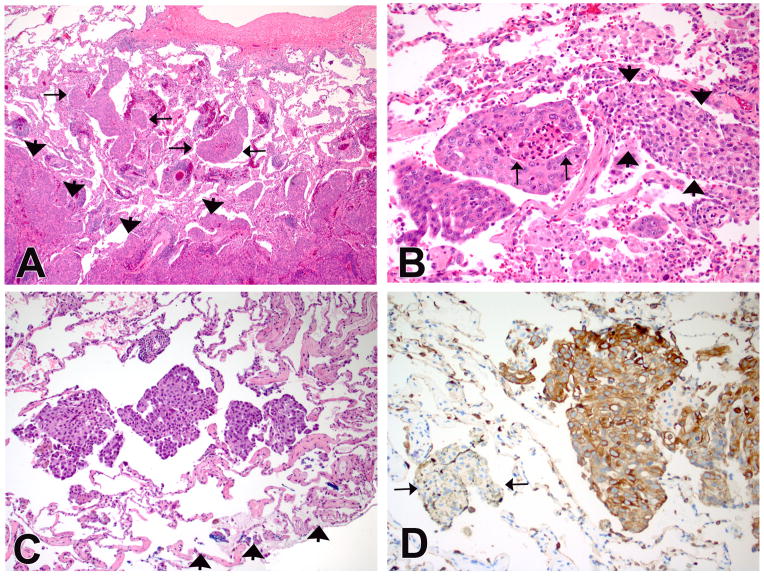
(A) An example of lung adenocarcinoma with tumor islands. The islands are isolated within airspaces (arrows) and are several alveoli away from the main tumor (arrow heads). (B) A high-power view shows clusters of atypical cells with necrosis (arrows). The arrowheads indicate a collection of benign alveolar macrophages in the adjacent air space with significant difference in cytology between the two groups. (C) Another example of lung adenocarcinoma with tumor islands. The islands are present in the alveoli adjacent to the blue-inked wedge resection margin (arrowheads). (D) Keratin stain highlights the tumor islands confirming an epithelial origin. Conversely, a cluster of alveolar macrophages (arrows) are negative for keratin. (Immuno stain for pan-keratin on a deeper section of C).
Acknowledgments
We also thank Dr. Jie (Jenny) Zhao and Peggy Sherwood of the Wellman Center for Photomedicine, Histopathology Core Laboratory (Boston, MA) and Gregory Mannheim for their technical assistance. We gratefully acknowledge Kurabo Industries and 3D Histech for their technical support.
Source of Funding:
B.Y. was supported by grants from the National Cancer Institute Lung SPORE P50-CA090578.
Footnotes
Conflicts of Interest
For all the authors no conflicts of interest were declared.
References
- 1.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: A decade of progress. Chest. 2002;122:1037–1057. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 2.Mino-Kenudson M, Mark EJ. Reflex testing for epidermal growth factor receptor mutation and anaplastic lymphoma kinase fluorescence in situ hybridization in non-small cell lung cancer. Arch Pathol Lab Med. 2011;135:655–664. doi: 10.5858/2011-0029-RAI.1. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 5.Rena O, Papalia E, Ruffini E, et al. Stage i pure bronchioloalveolar carcinoma: Recurrences, survival and comparison with adenocarcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:409–414. doi: 10.1016/s1010-7940(02)00830-8. [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Nishio W, Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: Time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg. 2004;77:1926–1930. doi: 10.1016/j.athoracsur.2004.01.002. discussion 1931. [DOI] [PubMed] [Google Scholar]
- 7.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 8.Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new international association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 9.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: Modification of the 2004 who mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, egfr mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 10.Thunnissen E, Beasly M, Borczuk A, et al. Reproducibility of histopathological subtypes in pulmonary adenocarcinoma. An interobserver study. Mod Pathol. 2010;23:S415. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: A distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein a expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol. 2007;20:638–647. doi: 10.1038/modpathol.3800780. [DOI] [PubMed] [Google Scholar]
- 13.Onozato ML, Klepeis VE, Yagi Y, et al. A role of three-dimensional (3d)-reconstruction in the classification of lung adenocarcinoma. Anal Cell Pathol (Amst) 2012;35:79–84. doi: 10.3233/ACP-2011-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer. 2010;116:659–669. doi: 10.1002/cncr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami T, Nabeshima K, Makimoto Y, et al. Micropapillary pattern and grade of stromal invasion in pt1 adenocarcinoma of the lung: Usefulness as prognostic factors. Mod Pathol. 2007;20:514–521. doi: 10.1038/modpathol.3800765. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda N, Hamaguchi N, Takeuchi E, et al. Lung adenocarcinoma with a micropapillary pattern: A clinicopathological study of 25 cases. APMIS. 2006;114:381–385. doi: 10.1111/j.1600-0463.2006.apm_340.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda N, Hamauzu T, Toi M, et al. Pulmonary adenocarcinoma with micropapillary component: An immunohistochemical study. Case report. APMIS. 2005;113:550–554. doi: 10.1111/j.1600-0463.2005.apm_151.x. [DOI] [PubMed] [Google Scholar]
- 21.Maeda R, Isowa N, Onuma H, et al. Lung adenocarcinomas with micropapillary components. Gen Thorac Cardiovasc Surg. 2009;57:534–539. doi: 10.1007/s11748-009-0436-y. [DOI] [PubMed] [Google Scholar]
- 22.Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: A distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi’s type C tumours) Histopathology. 2005;46:677–684. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi T, Shirakusa T, Ishikawa Y, et al. Possible mechanism of metastasis in lung adenocarcinomas with a micropapillary pattern. Pathol Int. 2005;55:419–424. doi: 10.1111/j.1440-1827.2005.01847.x. [DOI] [PubMed] [Google Scholar]
- 24.Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology. 2004;45:580–586. doi: 10.1111/j.1365-2559.2004.01953.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: A distinctive histologic subtype with prognostic significance. Case series. Hum Pathol. 2008;39:324–330. doi: 10.1016/j.humpath.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Nitadori J, Bograd AJ, Kadota K, et al. Micropapillary morphology is an independent predictor of recurrence in patients undergoing limited resection for lung adenocarcinoma (LAC) ≤ 2cm. J Thorac Oncol. 2011;6:S564. [Google Scholar]
- 27.Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol. 2007;212:287–294. doi: 10.1002/path.2165. [DOI] [PubMed] [Google Scholar]


