Abstract
The current study investigated if the Big 5 personality traits predicted interleukin-6 (IL-6) levels in a national sample over the course of 5 years. In addition, interactions among the Big 5 were tested to provide a more accurate understanding of how personality traits may influence an inflammatory biomarker. Data included 1,054 participants in the Midlife Development in the U.S. (MIDUS) biomarkers subproject. The Big 5 personality traits were assessed in 2005-06 as part of the main MIDUS survey. Medication use, comorbid conditions, smoking behavior, alcohol use, body mass index, and serum levels of IL-6 were assessed in 2005-2009 as part of the biomarkers subproject. Linear regression analyses examined personality associations with IL-6. A significant Conscientiousness*Neuroticism interaction revealed that those high in both Conscientiousness and Neuroticism had lower circulating IL-6 levels than people with all other configurations of Conscientiousness and Neuroticism. Adjustment for health behaviors diminished the magnitude of this association but did not eliminate it, suggesting that lower comorbid conditions and obesity may partly explain the lower inflammation of those high in both Conscientiousness and Neuroticism. Our findings suggest, consistent with prior speculation, that average to higher levels of Neuroticism can in some cases be associated with health benefits—in this case when it is accompanied by high Conscientiousness. Using personality to identify those at risk may lead to greater personalization in the prevention and remediation of chronic inflammation.
Keywords: Personality, interactions, inflammation, interleukin 6, IL-6, health behaviors
Introduction
Inflammation, Personality, and Health
The public health relevance of inflammatory markers is now well-established (Harris et al., 1999), but the psychosocial conditions associated with inflammation are not yet well understood. Early evidence suggested that personality traits are one such factor. Some of the earliest work focused on how relatively specific personality traits (i.e., dispositional depression, anxiety, hostility) had a positive association with interleuken-6 (IL-6) and C-reactive protein (CRP) in both clinically depressed and community based samples (Coccaro, 2006; Graham et al., 2006; Howren, Lamkin, & Suls, 2009; Ladwig et al., 2003; Marsland et al., 2008).
More recent investigations specifically utilizing the Big 5 taxonomy of broad personality dimensions have extended these earlier findings. For example, in a Sardinian population sample, higher Neuroticism (composed of traits reflecting chronic negative affect such as depression, anxiety, and poor self-esteem) and lower Conscientiousness (composed of traits reflecting self-regulation and goal pursuit) predicted higher levels of both IL-6 and CRP (Sutin et al., 2010). Others have also noted that higher Conscientiousness predicted lower levels of IL-6 over 32 months in older community-dwelling persons (Chapman et al., 2011b), and that higher levels of self-directedness, a trait related to Conscientiousness, were also associated with lower levels of CRP (Henningsson et al., 2008). Other reported Big 5 correlates of higher levels of inflammation include low Openness to Experience for IL-6 longitudinally (Chapman et al., 2011b), CRP in African Americans cross-sectionally (Jonaissant, 2010), and lower levels of Extraversion cross-sectionally (Chapman et al., 2009). The Type D personality style, reflecting high Neuroticism and low Extraversion, has also been tied to tumor necrosis factor (TNF)–alpha (Dennollet et al., 2008; 2009) in heart disease patients. Importantly, the magnitude of personality-inflammation associations is non-trivial, with a 2 standard deviation difference in personality linked to odds ratios up to 1.40 (Sutin et al., 2010) for scoring above the high-risk IL-6 cut point of 3.19 pg/ml associated with a doubling of mortality risk (Harris et al., 1999).
There are many reasons why personality may be associated with inflammation, but one pathway which we test in the current study involves health behaviors. According to the Health Behavior Model of personality, levels of certain personality traits (particularly Conscientiousness and Neuroticism) are associated with either engagement in health promoting or health debilitating behaviors (Bogg & Roberts, 2004; Smith, 2006). In turn, behaviors such as smoking and excessive alcohol use are associated with higher levels of inflammation (Bermudez, Rifai, Buring, Manson, & Ridker, 2002; Wannamethee et al., 2005). Many such behaviors also influence adiposity levels, which induces inflammation and engenders chronic diseases with inflammatory components, such as cardiovascular disease (CVD) (Dandona, Aljada, & Bandyopadhyay, 2004; Guzik, Mangalat, & Korbut, 2006; Yudkin, Kumari, Humphries, & Mohamed-Ali, 2000). Prior investigations support the Health Behavior Model, in that adjusting for BMI and other health behaviors does partially attenuate the personality-inflammation link (Chapman et al., 2011b; Howren et al., 2009; Sesso et al., 2007; Sutin et al., 2010).
Current Study
We tested whether any of the Big 5 personality traits predicted IL-6 levels in a national sample of adults spanning the majority of the adult life span. Based on prior research, we hypothesized that higher levels of Neuroticism and lower levels of Conscientiousness would predict higher levels of circulating IL-6. Moreover, we were particularly interested in the interaction of Conscientiousness and Neuroticism. Although interactions are typically screened as a standard model specification procedure, there was also reason to explore them substantively in this case because recent work has noted several interactions involving Conscientiousness and/or Neuroticism in their relation to health outcomes (Chapman et al., 2010; Friedman, Kern, & Reynolds, 2010; Turiano, et al., 2012). Finally, to extend prior findings, medication use, chronic health conditions, health behaviors, and levels of adiposity were included as covariates to test whether they explained personality-inflammation associations. Although personality assessment preceded the measurement of these health factors by approximately 2 years, the chronic conditions, health behaviors, and adiposity were measured contemporaneously with IL-6. Lack of clear temporal sequencing therefore prevented definitive analysis of mediation. Thus, we examine only the general question of whether any health factors statistically explained personality-inflammation associations to some degree—this would be a necessary, but not sufficient condition for concluding that they are mediators.
Methods
Participants
The National Survey of Midlife Development in the U.S. (MIDUS) began in 1995-96 (MIDUS 1) as a national random digit dial sample of non-institutionalized, English-speaking adults living in the United States. A final sample of 7,108 participants aged 25-74 completed telephone and mail surveys. A longitudinal follow-up of the original sample was conducted in 2004-06 (MIDUS 2). From MIDUS 1, 4,963 (75% response rate adjusted for mortality) were successfully contacted to participate in another phone interview and self-administered questionnaire. A more complete discussion of selective attrition among the full MIDUS longitudinal sample is available elsewhere (Radler & Ryff, 2010).
Participants completing both MIDUS 1 and MIDUS 2 were invited to be part of the biomarker project by completing a detailed clinic-based assessment of health, disease-related biomarkers, and physiological function (see Love, Seeman, Weinstein, & Ryff, 2010 for full study description). Eligible participants were recruited by letter and with a follow-up telephone call. The final sample with complete data relevant to the current study numbered 1,054 participants. Data were collected between 2004 and 2009, with an average time of 2.80 years (SD = 1.33) between MIDUS 2 and completion of the biomarker subproject. Consenting participants were invited to stay overnight at one of three regional General Clinical Research Centers (GCRCs) at UCLA, Georgetown, or the University of Wisconsin. The study was approved by the institutional review board at each GCRC and informed written consent was obtained from all participants.
Study Measures
Covariates
All models were adjusted for potential confounds: age, sex, race, and education. Age ranged from 34 to 84 (M = 54.61; SD = 11.67) and the sex distribution of participants was 56% female and 44% male. Educational attainment was assigned a number ranging from 1 (no school/some grade school) to 12 (graduate or professional degree), corresponding to educational milestones or degrees. Mean level of education was approximately some college to college graduate (M = 7.75; SD = 2.46). Approximately 93% of the sample was Caucasian.
Medication Use
Since certain medication use can alter inflammation levels, all models were also adjusted for current use of antihypertensive, cholesterol-lowering, and steroid medication usage.
Comorbidity
Participants indicated if they were ever diagnosed with any of 18 chronic conditions in their lifetime: heart disease, high blood pressure, circulation problems, blood clots, heart murmur, stroke, anemia or other blood disorder, cholesterol problems, diabetes, asthma, emphysema, tuberculosis, thyroid disorder, peptic ulcer, cancer, colon polyps, arthritis, or liver disease. A count was created so that a higher score reflected greater level of comorbidity. Due to strong right skew, the number of chronic conditions was capped at 5 (M = 2.16; SD = 1.65).”
Personality Traits
The key predictor variables were assessed via the self-administered adjectival measures of the Big 5 assessed at MIDUS 2 (Zimprich, Allemand, & Lachman, 2012). The scale was developed from a combination of existing personality trait lists and inventories (Lachman & Weaver, 1997). Respondents were asked how much each of 26 adjectives described themselves on a scale ranging from 1 (not at all) to 4 (a lot). The adjectives were: moody, worrying, nervous, calm (Neuroticism); outgoing, friendly, lively, active, talkative (Extraversion); creative, imaginative, intelligent, curious, broad-minded, sophisticated, adventurous (Openness); organized, responsible, hardworking, careless, thorough (Conscientiousness); helpful, warm, caring, softhearted, sympathetic (Agreeableness). The mean was calculated from the adjectives for each trait, after reverse scoring the appropriate items. This scale has good construct validity (Mroczek & Kolarz, 1998) and significantly correlates with the NEO trait scales (Prenda & Lachman, 2001). Reliability alphas are as follows: agreeableness = .80; conscientiousness = .68; extraversion = .76; neuroticism = .74; openness = .77.
Interleukin-6
Fasting blood samples were collected from each participant’s non-dominant arm at approximately 7:00 am on the second day of their GCRC visit. Samples were frozen and stored in a -60 degree Celsius to -80 degree Celsius freezer until shipped on dry ice to the MIDUS Biocore Lab on a monthly basis. Samples were subsequently stored in a -65° Celsius freezer until assayed. Serum IL-6 was measured using high-sensitivity enzyme-linked immunosorbent assay according to manufacturer guidelines (R&D Systems, Minneapolis, MN). The laboratory intra- and inter-assay coefficients of variance were in acceptable ranges (< 10%). The median IL-6 level was 2.15 pg/ml and the interquartile range (IQR) was 1.36-3.47 pg/ml. Since the distribution for IL-6 had the typical positive skew, all values were natural log-transformed.
Health Behaviors
Participants indicated if they had ever smoked cigarettes regularly (at least a few cigarettes every day) and whether they were currently still smoking or quit. Dummy codes were created to contrast former smokers (33%) and current smokers (15%) with never smokers as the referent group (52%). Participants reported the average number of alcohol drinks consumed on days that they drank (Med = 1.00; IQR = 0-2).
Body Mass Index
Height and weight were assessed by clinical staff at the GCRC and a continuous measure of BMI was computed by dividing weight by height squared. Approximately 35% of the sample was overweight (BMI greater than or equal to 25) and 40% obese (BMI greater than or equal to 30; World Health organization, 2012).
Statistical Analyses
A series of multiple linear regression analyses were conducted in Mplus® 6.0 software (Muthén & Muthén, 1998-2010). The baseline model tested the association of the Big 5 personality traits with IL-6, and screened two-way interactions among traits, as well as age and gender as moderators. To statistically adjust for the ten two-way personality interaction terms, we applied the False Discovery Rate (FDR; Benjamini & Hotchberg, 1995). The FDR controls Type I error for multiple tests without the drastic inflation in Type II error seen in Bonferroni and other family-wise error corrections, and is particularly useful in early-stage or discovery phase research where Type II errors represent a major peril. However, we also consulted the Holm-Bonferoni adjustment, which controls Family-Wise Type I error. In addition to adjusting alpha levels based on the FDR, we tested the robustness of any observed interactions by examining DFbetas (the change in coefficients) for interaction terms against leverage (outliers on predictor variables) to identify whether interactions could be attributed to a few unusual cases.
The baseline model included all of the Big 5 personality traits and all significant interactions. In Model 2, key demographic variables (age, gender, race, education) were added. These represented likely confounders, or variables potentially linked to both personality and IL-6 but not on the theoretical causal path between personality and IL-6 (i.e., possible mediators). Adjustment for these factors yielded an estimate of the total association between personality and IL-6, unbiased by demographic confounding. In Model 3, medication use and comorbidity count were included. Model 4 included the following health behavior related variables: alcohol use, smoking behavior, and BMI. Model 3 and 4 thus added factors that, in theory, might mediate links between personality and IL-6. In models 3 and 4, the personality coefficient represent only the residual direct association of personality and IL-6 after controlling for potential mediators, not the total (i.e., direct + indirect) association. To further investigate whether these health behaviors met necessary but not sufficient criterion for mediators, we utilized linear regression analyses to test if personality was associated with each of the potential mediating variables as well as whether each putative mediator was associated with IL-6.
Results
First, a logit model was fit to determine if persons who participated in the main MIDUS survey differed in personality from those who completed the biomarkers subproject. Analysis of attrition revealed that those who did not participate in the subproject were more likely to score lower on Openness (OR = 0.86; CI = 0.79-0.93) and higher in Agreeableness (OR = 1.10; CI = 1.01-1.20).
Table 1 displays the regression models for IL-6. In the baseline model, Conscientiousness, Neuroticism, and Openness were each negatively associated with IL-6 and there was a positive association with Agreeableness. There was no evidence that age or gender interacted with any of the personality traits. Examining the two-way interactions among traits, a significant Conscientiousness*Neuroticism interaction emerged after adjustment of the FDR (p < 0.10). Using methods outlined by Aiken and West (1991), the interaction is plotted in Figure 1. The relationship between Neuroticism and IL-6 was flat at low levels of Conscientiousness, while the relationship was strongly negative at high levels of Conscientiousness (see Figure 1). Specifically, those with high levels of Conscientiousness and high levels of Neuroticism had the lowest levels of IL-6. In other words, the negative association between Neuroticism and IL-6 increased as the level of Conscientiousness increased. To test the regions of significance for the plotted interaction, we utilized the Johnson-Neyman technique (Hayes & Matthes, 2009; Johnson & Neyman, 1936) to identify the level of Conscientiousness where the effect of Neuroticism on IL-6 levels was statistically significant and non-significant. We found that the interaction was a significant predictor of IL-6 only at Conscientiousness levels greater than 3.32 (1-4 scale) and greater than 1.70 on Neuroticism (1-4 scale). Figure 2 displays the region of significance in the context of the joint distribution of Neuroticism and Conscientiousness. Lastly, examination of the DFbetas indicated that no outliers appeared to play a role in the interaction1.
Table 1.
Summary of Regression Analysis for Variables Predicting Log Transformed Interleukin 6 (N = 1,054)
| Variable | Correlation With IL-6 |
Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| B | SE B | P | B | SE B | P | B | SE B | P | B | SE(B) | P | ||
| Neuroticism | −.03 | −0.06 | 0.02 | .019 | −0.03 | 0.02 | .257 | −0.05 | 0.02 | .035 | −0.04 | 0.02 | .101 |
| Conscientiousness | −.07* | −0.08 | 0.02 | .001 | −0.08 | 0.02 | .001 | −0.07 | 0.02 | .003 | −0.06 | 0.02 | .010 |
| Extraversion | .01 | 0.01 | 0.03 | .797 | − 0.01 | 0.03 | .975 | −0.01 | 0.03 | .999 | 0.02 | 0.03 | .405 |
| Agreeableness | .06* | 0.06 | 0.03 | .020 | 0.04 | 0.03 | .156 | 0.03 | 0.03 | .279 | 0.01 | 0.03 | .933 |
| Openness | −.04 | −0.05 | 0.03 | .044 | −0.04 | 0.03 | .169 | −0.03 | 0.03 | .187 | −0.03 | 0.03 | .181 |
| C x N1 | −09*** | −0.06 | 0.02 | .007 | −0.05 | 0.02 | .013 | −0.05 | 0.02 | .024 | −0.04 | 0.02 | .058 |
| Age | .13*** | 0.10 | 0.02 | .001 | 0.09 | 0.02 | .001 | 0.13 | 0.02 | .001 | |||
| Male | −.06* | −0.04 | 0.05 | .368 | −0.03 | 0.05 | .484 | −0.08 | 0.05 | .094 | |||
| Minority | −.01 | 0.10 | 0.09 | .255 | 0.06 | 0.09 | .523 | −0.01 | 0.08 | .991 | |||
| Education | −.07* | −0.04 | 0.02 | .078 | −0.03 | 0.02 | .130 | −0.01 | 0.02 | .687 | |||
| Blood Thinner | 21 *** | 0.20 | 0.06 | .001 | 0.14 | 0.05 | .011 | ||||||
| Statin | .06* | 0.02 | 0.05 | .664 | 0.01 | 0.05 | .837 | ||||||
| Steroid | .01 | −0.03 | 0.07 | .701 | 0.03 | 0.06 | .636 | ||||||
| Comorbidity Count | .21*** | 0.06 | 0.02 | .001 | 0.03 | 0.02 | .103 | ||||||
| Average Alcohol Use | .06* | −0.01 | 0.03 | .767 | |||||||||
| Former Smoker | .03 | 0.05 | 0.05 | .324 | |||||||||
| Current Smoker | .07** | 0.20 | 0.07 | .004 | |||||||||
| BMI | .21*** | 0.24 | 0.02 | .001 | |||||||||
| R 2 | .03 | .09 | .13 | .21 | |||||||||
| F for change in R2 | 5.40*** | 9.60*** | 10.72*** | 14.76*** | |||||||||
Note. Personality traits, age, education, alcohol use, and BMI are in standard deviation units. Former and current smoking status was referenced to those who never smoked. All models were adjusted for the time interval between personality assessment and completion of biomarkers subproject questionnaires.
FDR adjustment for interaction term = p < 0.10.
Figure 1.
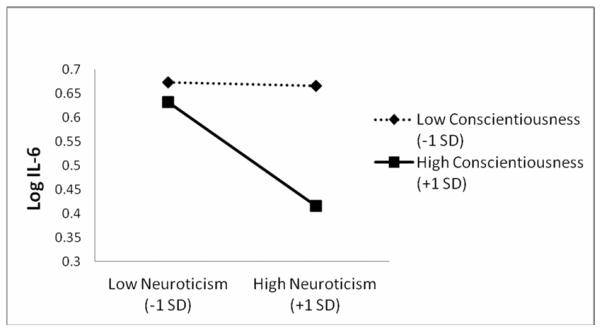
Conscientiousness by Neuroticism interaction predicting logged IL-6.
Figure 2.
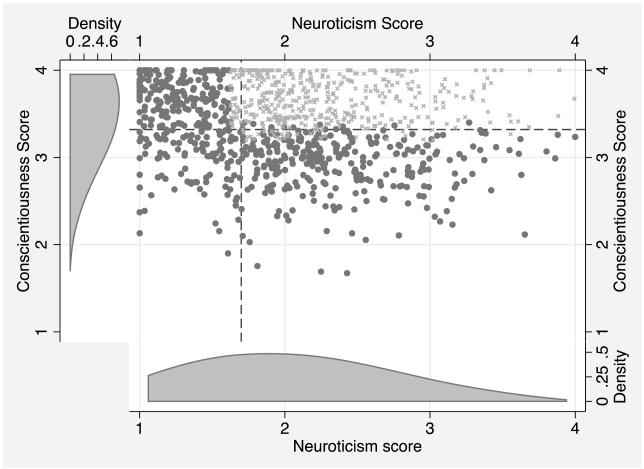
Joint distribution of Neuroticism and Conscientiousness with region of significance for interaction. The scores correspond to the average response to trait descriptive adjectives on the following Likert scale: 1: very unlike me; 2: a little unlike me; 3: a little like me; 4: very like me. In the scatter plot, the top right panel denoted by dashed lines indicates the region of significance, with observations marked with an “x” rather than a dot. The univariate distribution of Conscientiousness appears on the left of the scatter plot, and the univariate distribution of Neuroticism appears below the scatter plot. As can be seen, these distributions are not centered at the mean of the Likert scale (2.5).
High Conscientiousness and Neuroticism are related to low levels of IL-6, indicating higher Neuroticism may not inevitably lead to greater inflammation in all persons
After adjusting for demographic factors (Model 2), the interaction remained significant, with roughly 10% attenuation in magnitude. The agreeableness and openness associations were attenuated by roughly 50% and 32% respectively and rendered non-significant, suggesting they were primarily an artifact of uncontrolled demographic confounding. Increasing age predicted an increased level of IL-6. After adjusting for medication use and comorbidity in Model 3, the Conscientiousness*Neuroticism remained significant with roughly an additional 10% reduction in magnitude. Adjusting for health behaviors and BMI 2 (Model 4) reduced the magnitude of the Conscientiousness*Neuroticism interaction further by about 20%, with current smoking and higher BMI predicting higher levels of IL-6. The full set of mediators reduced the magnitude of the Conscientiousness*Neuroticism effect by roughly 30%.
To further examine the potential mediating pathways between personality, comorbidity, health behaviors, and BMI, a series of regression analyses were tested. Currently taking a blood thinning medication and higher levels of comorbidity were associated with increased levels of IL-6, as was higher BMI. Adjusting for demographic factors, the Conscientiousness*Neuroticism interaction was not statistically significant for either alcohol use or smoking status, but was in the expected directions. These associations, regardless of their significance, still represent covariance that leads to some reduction in point-estimate of the Conscientiousness*Neuroticism interaction term for IL-6, however. The interaction more closely approached statistical significance for predicting comorbidity (b = -0.09; p = .090) and BMI (b = -0.27; p = .147).
Discussion
The current findings are the first study of the Big 5 and inflammation in a national US sample, and are consistent with our hypothesis that Conscientiousness and Neuroticism are associated with IL-6. However, counter to our hypotheses, higher levels of Neuroticism were related to lower levels of inflammation under a particular circumstance: when Conscientiousness was also higher. This conditional negative association with IL-6 differs from prior evidence documenting a positive association with Neuroticism (Sutin et al., 2010) and closely related constructs such as depression and hostility (Howren et al., 2009; Suarez, 2003) because those studies reported main effects for Neuroticism. By contrast, our results suggest that Neuroticism associations with inflammation must be examined in the moderating context of Conscientiousness.
At first glance it may seem counterintuitive that an individual would score high on both Conscientiousness and Neuroticism because of the reported negative correlation between the two traits. However, the correlation between these traits in the current study was only -0.17, meaning that one trait only explains approximately 3% variation in the other and they are virtually independent. This small correlation leaves little doubt that individuals can score high on both traits as depicted in Figure 2.
Moreover, utilizing the regions of significance test for the interaction effect, we determined that the interaction was statistically significant among the 441 individuals (42% of sample) that scored both 3.32 or higher on Conscientiousness and 1.70 or higher on Neuroticism (also depicted in Figure 2). The healthy neuroticism effect thus appears to span a large range of Neuroticism from “moderate” to “high” levels, relative to the mean of the distribution. Relative to the Likert scale score metric, the Neuroticism boundary for the region of significance of 1.70 was well below the scale the midpoint of 2.5 (which, as Figure 2 shows, does not correspond to the mean of the Neuroticism distribution). Thus, one must interpret the trait ranges in which the interaction is significant as “medium”, “high,” etc. in relation to either the distribution itself or the Likert scale score range, which are rather different referents in this case. In broad strokes, and as seen in Figure 1, our results indicated that the higher an individual scored both in Conscientiousness and Neuroticism — the lower the level of IL-6.
What does this interaction between traits really mean? A likely reason for this interaction lies in the concept of “healthy neuroticism” first described by Friedman (2000). In essence, health anxiety generated by Neuroticism may be adaptive when it is accompanied by higher Conscientiousness: Conscientiousness provides the foundation of self-discipline and planning needed to take adaptive action, such as reducing behavior like smoking or overeating that has direct and indirect consequences for inflammation. Indeed, evidence suggests individuals scoring higher in Neuroticism are more likely to drink and smoke, but when these individuals are also high in Conscientiousness they are more likely to refrain from such behaviors (Terracciano & Costa, 2004; Turiano et al., 2012; Vollrath & Torgersen, 2002). In contrast, the neurotic person who is also low in Conscientiousness may not have healthy avenues to deal with stress and negative affect, and resorts to alcohol, cigarettes, and overeating, all behaviors tied to low Conscientiousness (Bogg & Roberts, 2004).
Our supplementary analyses attempted to examine if “healthy neurotics” had lower levels of inflammation because they were less likely to engage in smoking or heavier alcohol use. The direction of associations were similar to those in a previously published report utilizing a larger, and thus more powerful, sample size (N = 4,476) from this same study (Turiano et al., 2012). Persons higher in Conscientiousness and Neuroticism simultaneously had lower levels of BMI and chronic disease. This pattern of associations is consistent with the literal definition of healthy Neuroticism—i.e., neurotic persons achieving higher levels of health in various domains. According to Friedman (2000), some neurotic individuals may be hyper vigilant about health symptoms needing attention, detecting and thus preventing or treating disease/illness before long-term damage accumulates. It may also be the case that these individuals maintain more appropriate eating and exercise routines resulting in a healthier weight. This scenario is in parallel with the lower rates of chronic disease and lower BMI for individuals scoring high in both Conscientiousness and Neuroticism in the current study. That being said, since the potential mediators included in the current study explained only 30% of the variation in the healthy Neuroticism effect, this effect is largely attributable to unmeasured variables not tested in the current study. As stated by Friedman (2000; pg. 1102), “healthy neurotics are a confound to be reckoned with” and the only way to better understand why higher levels of Neuroticism are in some cases health protective will be through future studies including multiple behavioral, emotional, and physiologic mediators in their models.
At a broader level, the current study suggests that labeling certain Big 5 dimensions as either “good” versus “bad” or “related” versus “unrelated” to health is not sufficient for a full understanding of personality-health associations (Hampson, 2008). Instead, researchers should attempt to determine under what conditions, or for whom, or when a trait is adaptive or maladaptive (Nettle, 2006). Such scenarios are likely more realistic than simple linear main effects (Smith & Spiro, 2002; Cloninger, 2005), and consistent with the notion that neither high nor low levels of the Big 5 have universal benefit across all areas of adaptation (Nettle, 2006). Future investigations might attend more closely to such interactions.
Study findings must be weighed by a careful consideration of strengths and limitations. Although our outcome was measured on average a few years subsequent to personality, baseline levels of IL-6 were not available. Thus, we are unable to determine whether personality predicts prospective increases in IL-6 or is simply associated contemporaneously with IL-6 because we did not have a baseline measure of IL-6 in the current study. Future studies may disentangle this issue by collecting repeated measures of IL-6 as well as investigating whether a similar pattern of results would occur with other markers of the inflammatory response (e.g., CRP, TNF-alpha). Such investigations would be beneficial because recent research suggests that psychosocial variables may vary in the strength in their associations with different biomarkers (O’Donovan et al., 2010). In interpreting findings across the literature, not only the personality trait and the biomarker, but the size and composition of the sample will inevitably vary, so caution appears warranted in drawing unqualified or global conclusions about personality and inflammation.
Second, health behaviors and BMI were measured contemporaneously with IL-6. This prohibits strict mediation analyses. Nevertheless, implicating health behaviors as explanatory mechanisms (either mediators or confounders) for personality-IL-6 associations provides more information scientifically than merely omitting them. Future work may wish to pursue the temporal measurement structure necessary to explicitly test mediation. Finally, we did not examine non-linear or three way interactions, which would require a sample size well beyond ours, nor did we study the complete assortment of all possible health behaviors that might be involved in personality-inflammation associations.
It must also be noted that interaction effects are notoriously difficult to extend across study populations. However, we utilized an alpha correction and conducted sensitivity analyses to ensure the robustness of the interaction effect. In addition, other studies have noted a similar interaction using different samples, personality measures, and outcomes (Friedman et al., 2010; Terracciano & Costa, 2004; Vollrath & Torgersen, 2002). As well, the same interactions are not likely to exist for all outcomes (i.e., biomarkers vs. behaviors), and study population itself may be an additional moderator of personality trait interactions. In fact the measure of Neuroticism utilized in the current study was quite different from other investigations because it primarily taps the anxiety component of this trait. This may partly explain why others have found unmoderated positive associations between different Neuroticism-related scales measuring factors like depressive symptoms and IL-6 (Howren et al., 2009; Suarez, 2003). It will be important to test the interaction effects found in the current study with alternate, preferable multi-dimensional measures of Neuroticism to determine if the anxiety component of this Big 5 domain is driving the negative association of Neuroticism with IL-6. In sum, in addition to examining the most prominent behavioral contributors to health and disease, major strengths of the current study also include the longitudinal span (exceeding that of other Big 5 work in the US (Chapman et al., 2011b)), an objective biological outcome, sensitivity analysis to ensure the robustness of results, the use of a comprehensive personality taxonomy, and a large national US sample.
Overall, our findings suggest eventual clinical implications, since it is desirable to identify and address upstream or origin factors that may lead to elevated inflammation. As IL-6 may presage health deterioration and has been recently suggested as a clinical target (Nishimoto, 2010), personality factors to which it is linked may help personalize risk-prediction models used to identify candidates for prevention and early intervention (Chapman et al., 2011a). For example, it is possible approximate levels on the personality continuum that would be associated with IL-6 levels greater than 3.19 pg/ml that have been linked with increased mortality risk (Harris et al., 1999). Based on our findings, on average (setting all covariates to mean levels), a person scoring just 1 standard deviation below the mean on Neuroticism and 2 standard deviations below the mean in this measure of Conscientiousness will exceed the 3.19 pg/ml cut point. There is thus a possibility to develop measures for use in health care settings that can screen or phenotypically identify persons in whom chronic inflammation may develop.
In addition to simply identifying high-risk patients early, specific health-damaging personality tendencies themselves may constitute points of intervention which, over the long-term, can be partially ameliorated in some individuals. Before such clinical applications, however, more work on behavioral and physiological pathways linking personality to inflammation is needed.
Acknowledgements
This research was supported by Grant T32-MH018911-23 from the National Institute of Mental Health and by Grant T32-AG025671-02 from the National Institute of Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To examine whether the role of Neuroticism in the interaction might be confounded by age, we also examined a Conscientiousness*Age interaction term in our models. This added parameter did not appreciably affect the Conscientiousness*Neuroticism interaction.
Sensitivity analyses were conducted using waist to hip ratio instead of BMI. Findings were analogous with this measure of adiposity.
References
- Aiken LS, West SG. Sage; Newbury Park, CA: 1991. Multiple Regression: Testing and interpreting interactions. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Bermudez EA,, Rifai N, Buring JE, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler., Thromb., Vasc. Biol. 2002;106:1930–1937. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Fiscella K, Kawachi I, Duberstein PR. Personality, socioeconomic status, all-cause mortality in the United States. American Journal of Epidemiology. 2010;171:83–92. doi: 10.1093/aje/kwp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, Moynihan J. Gender, race/ethnicity, personality, and I nterleukin-6 in urban primary care patients. Brain, Behav., Immun. 2009;23:636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Roberts B, Duberstein P. Personality and longevity: Knowns, unknowns, and implications for public health and personalized medicine. Journal of Aging Research. 2011a doi: 10.4061/2011/759170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, van Wijngaarden E, Seplaki CL, Talbot N, Duberstein P, Moynihan J. Openness and conscientiousness predict 34-week patterns of interleukin-6 in older persons. Brain, Behav., Immun. 2011b;25:667–673. doi: 10.1016/j.bbi.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger R. How does personality affect mortality in the elderly? Psychosom. Med. 2005;67:839–840. doi: 10.1097/01.psy.0000189130.15870.93. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Association of C-reactive protein elevation with trait aggression and hostility in personality disorder subjects: A pilot study. J. Psychiatr. Res. 2006;40:460–465. doi: 10.1016/j.jpsychires.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Dennollett J, Schiffer AA, Kwaijtaal M, Hooijjkaas H, Hendriks EH, Widdershoven JW, Kupper N. Usefullness of Type D personality and kidney dysfunction as predictors of interpatient variability in inflammatory activation in chronic heart failure. Am. J. Cardiol. 2009;103:399–404. doi: 10.1016/j.amjcard.2008.09.096. [DOI] [PubMed] [Google Scholar]
- Dennollet J, Vrints CJ, Conraads VM. Comparing Type D personality and older age as correlates of tumor necrosis factor-alpha dysregulation in chronic heart failure. Brain, Behav., Immun. 2008;22:736–743. doi: 10.1016/j.bbi.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Friedman HS. Long-term relations of personality and health: Dynamisms, mechanisms, tropisms. Journal of Personality. 2000;68:1089–1108. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kern M, Reynolds CA. Personality and health, subjective well-being, and longevity. Journal of Personality. 2010;78:179–216. doi: 10.1111/j.1467-6494.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- Graham JF, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain, Behav., Immun. 2006;20:389–40. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? Journal of Physiological Pharmacology. 2006;57:505–528. [PubMed] [Google Scholar]
- Hampson SE. Mechanisms by which childhood personality traits influence adult well-being. Current Directions in Psychological Science. 2008;17:264–268. doi: 10.1111/j.1467-8721.2008.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hayes A, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Henningsson S, Baghaei F, Rosmond R, Holm G, Landen M, Anckarsater H, Ekman A. Association between serum levels of C-reactive protein and personality traits in women. Behav. Brain Funct. 2008;4:4–16. doi: 10.1186/1744-9081-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Johnson P, Neyman J. Tests of certain linear hypotheses and their applications to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Jonassaint CR, Boyle SH, Kuhn CM, Siegler IC, Siegler IC, Copeland WE, Williams R. Personality and inflammation: The protective effect of openness to expereince. Ethn Dis. 2010;20:11–144. [PMC free article] [PubMed] [Google Scholar]
- Lachman M, Weaver SL. Brandeis University, Department of Psychology; Waltham, MA: 1997. The Midlife Development Inventory (MIDI) personality scales: Scale construction and scoring (Tech. Rep. No.1) [Google Scholar]
- Ladwig KH, Mittag BW, Lowel H, Doring A, Wolfgang K. Influence of depressive mood on the association of CRP and obesity in 3205 middle aged healthy men. Brain, Behav., Immun. 2003;17:268–275. doi: 10.1016/s0889-1591(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behav., Immun. 2008;22:753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Sixth Edition Muthén & Muthén; Los Angeles, CA: 1998-2010. [Google Scholar]
- Nettle D. The evolution of personality variation in humans and other animals. Am. Psychol. 2006;6:622–631. doi: 10.1037/0003-066X.61.6.622. [DOI] [PubMed] [Google Scholar]
- Nishimoto N. Interleukin-6 as a Therapeutic Target in Candidate Inflammatory Diseases. Clin. Pharmacol. Ther. 2010;87:483–487. doi: 10.1038/clpt.2009.313. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin M, O’Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: Evidence for specificity in emotion-biology relationships. Brain, Behav., Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenda K, Lachman ME. Planning for the future: A life management strategy for increasing control and life satisfaction in adulthood. Psychology and Aging. 2001;16:206–216. [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of Aging and Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Wang L, Buring JE, Ridker PM, Graziano M. Comparison of interleukin-6 and c-reactive protein for risk of developing hypertension in women. Hypertension. 2007;49:304–310. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- Smith TW. Personality as risk and resilience in physical health. Current Directions in Psychological Science. 2006;15:227–231. [Google Scholar]
- Smith TW, Spiro A. Personality, health, and aging: prolegomenon for the next generation. Journal of Research in Personality. 2002;36:363–394. [Google Scholar]
- Suarez EC. Joint effect of hostility and severeity of depressive symptoms on plasma interleukin-6 concentration. Psychosom. Med. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, Schlessinger D, Costa PT. High neuroticism and low conscientiousness are associated with interleukin-6. Psychol. Med. 2010;40:1485–1493. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Costa PT. Smoking and the five-factor model of personality. Addiction. 2004;99:472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Whiteman SD, Hampson SE, Roberts BW, Mroczek DK. Personality and substance use in midlife: Conscientiousness as a moderator and the effects of trait change. Journal of Research in Personality. 2012;46:295–305. doi: 10.1016/j.jrp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath M, Torgersen S. Who takes health risks? A probe into eight personality types. Personality and Individual Differences. 2002;32:1185–1197. [Google Scholar]
- Wannamethee SG, Lowe GD, Sharper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur. Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- World Health Organization The World Health Organization Fact Sheet. 2012 [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zimprich D, Allemand M, Lachman ME. Factorial structure and age-related psychometrics of the MIDUS personality adjective items across the life span. Psychological Assessment. 2012;24:173–186. doi: 10.1037/a0025265. [DOI] [PMC free article] [PubMed] [Google Scholar]


