Abstract
Polyubiquitin chains on proteins flag them for distinct fates depending on the type of polyubiquitin linkage. While lysine48-linked polyubiquitination directs proteins to proteosomal degradation, lysine63-linked polyubiquitination promotes different protein trafficking and is involved in autophagy. Here we show that postsynaptic density (PSD) fractions from adult rat brain contain deubiquitinase activity that targets both lysine48 and lysine63-linked polyubiquitins. Comparison of PSD fractions with parent subcellular fractions by Western immunoblotting reveals that CYLD, a deubiquitinase specific for lysine63-linked polyubiquitins, is highly enriched in the PSD fraction. Electron microscopic examination of hippocampal neurons in culture under basal conditions shows immunogold label for CYLD at the PSD complex in approximately one in four synapses. Following depolarization by exposure to high K+, the proportion of CYLD-labeled PSDs as well as the labeling intensity of CYLD at the PSD increased by more than eighty percent, indicating that neuronal activity promotes accumulation of CYLD at the PSD. An increase in postsynaptic CYLD following activity, would promote removal of lysine63-polyubiquitins from PSD proteins and thus could regulate their trafficking and prevent their autophagic degradation.
Keywords: Ubiquitin, CYLD, Deubiquitinase, K63-linked, PSD, postsynaptic density
Introduction
During the last decade, ubiquitination of synaptic proteins received increasing attention [1-8]. Many of these studies focused on ubiquitination as a tag for proteasomal degradation. The ubiquitin proteasome system (UPS) is responsible for the degradation of the main postsynaptic density (PSD) scaffolds PSD95 [2], Shank and GKAP [1, 6] and may promote endocytosis of AMPA receptors [2-4]. Degradation of synaptic proteins by the UPS is thought to be involved in aspects of synaptic plasticity [9]
While attachment of lysine48-linked polyubiquitin chains directs proteins to proteasomal breakdown, attachment of another type of ubiquitin chain, lysine63-linked polyubiquitin, promotes different trafficking of proteins and is involved in diverse functions such as DNA repair, endocytosis, NFkB signaling and most notably in the formation and autophagic clearance of protein aggregates [10, 11]. Loss or suppression of autophagy has been demonstrated to promote neurodegeneration [12, 13], Thus an impairment of autophagy, in addition to, or instead of, an impairment of proteasomal degradation may be a factor in the development of certain neurodegenerative diseases.
CYLD, originally identified as cylindromatosis tumor suppressor gene, encodes a deubiquitinase specific for lysine63-linked polyubiquitin chains [14]. It is expressed in high levels in the brain [15], although, so far, studies focusing on CYLD in brain have been lacking. A study on p62, a CYLD-binding protein, implied that inhibition of CYLD leads to accumulation of proteins with Lysine63-linked polyubiquitination in brains of p62-/- mice [16].
Mass spectrometric analysis of affinity-purified PSD preparations from rat forebrain revealed CYLD to be a prominent protein in affinity purified PSD fractions [17]. Here we investigate the presence of CYLD at PSDs by immunoblotting and immunoEM, and test whether there are changes in PSD-associated CYLD levels following synaptic activity.
Materials and Methods
Materials
Antibodies to CYLD: rabbit polyclonal (1:250 or 1:500 for Westerns, 1:100 for EM) from Sigma (SAB4200060) and mouse monoclonal (E-4) from Santa Cruz (sc-74434, 1:500). Antibody to PSD-95 (1: 5000): custom made rabbit polyclonal to residues 290-307 by New England Peptide (Gardener, MA). Antibody to alpha-CaMKII mouse monoclonal (clone 6G9, 1:500) Antibody to ubiquitin: mouse monoclonal (clone Ubi-1, aka 042691GS) from Millipore (MAB150).
Di-ubiquitins from BostonBiochem (Cambridge, MA) and poly-ubiquitins from Enzo Life Sciences (Farmingdale, NY). CYLD lysate (lysate from HEKT 293 cells expressing CYLD) and control cell lysate (same type of cells transfected with empty vector) from Novus Biologicals (Littleton, CO)
The protocols for obtaining brains for subcellular fractionation and hippocampal cultures were approved by NIH Animal Use and Care Committee and conformed to NIH guidelines.
Subcellular fractionation from rat cerebral cortices was carried out as described previously [18]. Brains from adult Sprague Dawley rats were collected and frozen in liquid nitrogen within 2 minutes of decapitation by Pel-Freeze Biologicals (Rogers, AR). Frozen brains were thawed for 1 min in 0.32M sucrose at 37°C and were immediately dissected and homogenized. For rapidly processed PSD preparations, cerebral cortices were dissected out from adult Sprague Dawley rats and homogenized within an average of 1.5 min after decapitation.
Electrophoresis and Immunoblotting
Samples were separated by SDS-PAGE on 4-15% gradient Tris-HCl gels from BioRAD or 4-15 % gradient Bis-Tris gels from Life Technologies and transferred to nitrocellulose membranes, blocked, incubated with specified primary antibodies and then with horseradish peroxidase-conjugated secondary antibodies (1:50,000 dilution), and the signal was finally visualized by chemiluminescence (SuperSignal West Pico, Thermo Scientific).
Deubiquitination assay
Di- or polyubiquitins (0.25μg in 1mg/ml BSA) were incubated at 37°C with PSD fractions (25μg) in medium containing 0.5mM EDTA, 0.5mM EGTA, 100mM HEPES pH 7.4 in a final volume of 25μl. Reactions were terminated by the addition of SDS-containing electrophoresis sample buffer. In controls, enzymes were heat-inactivated by 2 min incubation of the PSD fraction at 99°C.
Preparation and treatment of dissociated hippocampal cultures
Hippocampal cells from 21-day embryonic Sprague-Dawley rats were dissociated and grown on a glial cell layer as described previously [19] for 19-21 days. Cell cultures were treated as described previously [20]. Control incubation medium contained 124 mM NaCl, 2 mM KCl, 1.24 mM KH2PO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 30 mM glucose in 25 mM HEPES at pH 7.4. High K+ medium contained 90 mMKCl with the NaCl concentration reduced accordingly to preserve isotonicity.
Pre-embedding immunogold-labeling and electron microscopy
After treatment, neuronal cultures were processed for pre-embedding immunogold-labeling as described previously [20]. Briefly, cultures were fixed in 4% paraformaldehyde (EMS, Hatfield, PA) in PBS for 30-45 min at room temperature, permeabilized, incubated with primary and secondary antibodies (Nanogold, Nanoprobes, Yaphank, NY) for 1-1.5 hr, then fixed with 2% gluteraldehyde in PBS, silver enhanced (HQ kit, Nanoprobes), and processed for electron microscopy. Only parallel samples from the same experiment were directly compared because the overall labeling sensitivity may differ between experiments.
Morphometry and statistical analysis
Excitatory synapses are identified by structural characteristics of clustered synaptic vesicles in the presynaptic terminal, the uniform 20 nm separation of the pre- and postsynaptic membrane, and the postsynaptic density (PSD) of dense material underneath the postsynaptic membrane [21]. The PSD complex was defined as the postsynaptic specialization that comprises the electron dense PSD core and the contiguous network [20, 22]. The measurement area of the PSD complex was marked by the postsynaptic membrane, a parallel dashed line drawn at 120 nm to the postsynaptic membrane, and two vertical lines to demarcate the area (Figure 4, bottom right panel). The distance of this measurement area of the PSD complex was set at 120 nm based on previous studies [20, 23]. CYLD labels appear as individual black grains in the PSD complex, and labeling intensity was quantified as number of labels per μm length of the postsynaptic membrane.
Figure 4. CYLD labeling at PSDs increases upon depolarization.
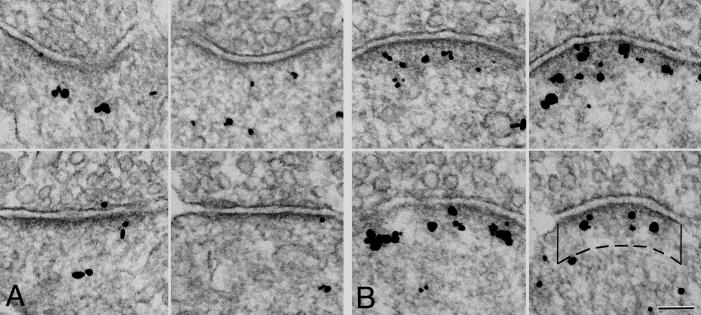
Electron micrographs comparing synapses from cultured hippocampal neurons in control (A) and depolarized (B., high K+ for 2 min) cultures. Immunogold label is for CYLD. More label for CYLD appears in the PSD complex after stimulation.
Bottom right panel: The area of measurement is delineated by the postsynaptic membrane, two vertical solid lines 120 nm long marking the lateral boundary of the PSD complex and a dashed line parallel to the postsynaptic membrane. Intensity of immunogold labeling intensity was calculated as the number of labels (black grains) inside the measurement area, divided by the length of the postsynaptic membrane Scale bar: 100nm
Every synaptic profile encountered was scored for CYLD antibody labeling on the microscope as negative (0-2 grains) or positive (3 or more grains), and the percentage of CYLD-labeled PSDs was calculated for each sample. Every cross-sectioned ,CYLD-positive PSD was photographed with a CCD camera (XR-100 from AMT, Danvers, MA, USA) for the calculation of labeling intensity. Statistical analysis (KaleidaGraph, Synergy Software) was carried out by Student's t test with confidence levels set at P <0.01 unless otherwise indicated.
Results
PSD fractions from rat brains contain deubiquitinase activity
Incubation of multi-ubiquitin chains (Diubiquitins or a mixture of polyubiquitins of differing chain lengths) linked through either lysine48 or lysine63 with PSD fractions, leads to their time-dependent breakdown (Figure 1). On the other hand, no loss of multi-ubiquitins is observed up to 60 minutes in control samples when PSD fractions are heat-inactivated prior the incubation. These results indicate the presence in isolated PSDs of deubiquitinases that target lysine48- as well as those that target lysine63-linked multi-ubiquitin chains. Biochemical and immunoEM experiments were carried out to test whether CYLD is one of the deubiquitinases integral to the PSD.
Figure 1. PSD fraction contains deubiquitinase activities that target lysine63- as well as lysine48-linked polyubiquitins.
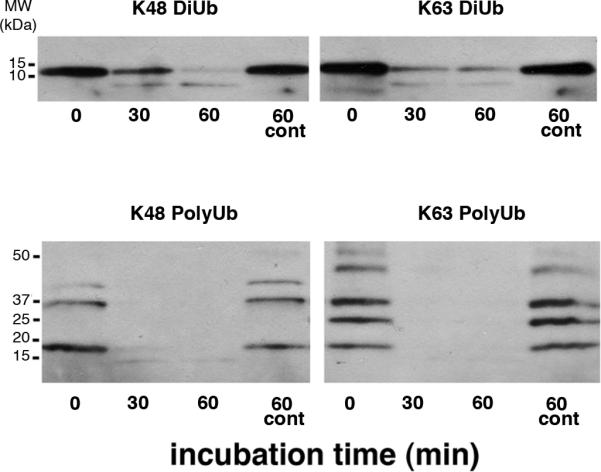
Lysine48- or lysine63-linked diubiquitins (above) and polyubiquitin mixtures of differing chain lengths (below) were incubated with the PSD fraction at 37°C. Controls (cont): PSD enzymes were heat-inactivated before co-incubation with polyubiquitins.
CYLD is highly enriched in the PSD fraction
Two antibodies raised against epitopes representing non-overlapping sequences of CYLD were used to probe the distribution of the protein in subcellular fractions from brain. The specificity of the antibodies was checked using lysates from HEK 293 cells overexpressing CYLD (Figure 2A). Both antibodies recognize a band around 110 KDa in PSD fractions, and a band of slightly lower mobility in lysates from cells overexpressing CYLD but not in control lysates. The lower mobility of the band in lysates is due to a DDK tag.
Figure 2. CYLD is highly enriched in PSD fractions.
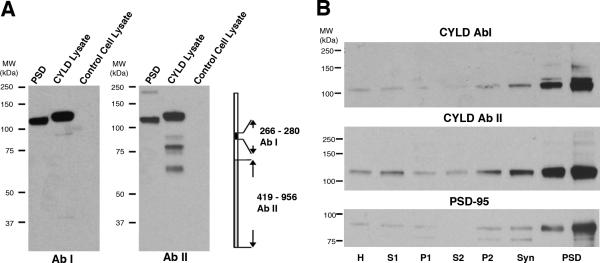
A: Westerns immunoblots using two antibodies (AbI and AbII), raised against peptides corresponding to non-overlapping sequences on the CYLD protein, recognized a band of ~110 KDa in PSD fractions and a band of slightly higher molecular weight in lysates from HEK293T cells overexpressing CYLD with a DDK tag. The band was absent in lysates from control (empty vector) cells.
B: Western immunoblots comparing levels of CYLD and PSD-95 in PSDs and parent subcellular fractions. Two CYLD antibodies described in (A) show a similar pattern of enrichment of CYLD in PSDs, in parallel to PSD-95, a marker for PSDs. Each lane contains 15 μg protein, except the first PSD lane that contains 5 μg. The molecular weight of CYLD is 106.7 KDa
H: homogenate; S: supernatant; P: pellet; Syn:synaptosome; PSD: postsynaptic density
Comparison of subcellular fractions from rat brain in western blots probed with these two CYLD antibodies show a drastic enrichment of the protein in the PSD fraction compared to parent fractions (Figure 2B). The enrichment of CYLD in the PSD fraction is comparable to that of PSD-95, a marker for PSDs.
CYLD is less prevalent in PSD fractions from rapidly processed brains
It is known that the relative amounts of CaMKII in the PSD fraction depend on the speed of processing of brains between decapitation and homogenization [24, 25]. Presumably, CaMKII accumulates on the PSD, during ischemic/excitatory conditions that prevail following the cessation of blood flow [26]. Thus a difference in levels of a protein in PSDs from rapidly vs. slowly processed brains is usually an indication of its activity-induced movement to/from the PSD. Comparison of PSD fractions from rapidly processed and commercially obtained brains (Figure 3) shows that CYLD has a similar pattern to CaMKII, suggesting that it may show activity-induced redistribution.
Figure 3. PSD fractions from rapidly processed brains contain less CYLD.

Westerns show relative quantities of three proteins in PSD fractions prepared from rapidly processed brains homogenized within 1.5 min after decapitation (1); or commercially obtained brains frozen and thawed as described in Methods (2). Similar to CaMKII, CYLD levels in PSD fractions vary according to the post-mortem processing of brains, suggesting translocation under excitatory conditions.
CYLD accumulates at the PSD upon depolarization
Possible activity-induced redistribution of CYLD was investigated by immuno-electron microscopy of dissociated hippocampal cultures under basal and depolarizing conditions. Under basal conditions, only about 1 in 4 synapses were scored as CYLD-positive (Ab 1) at the PSD (Table I).-Upon Depolarization with high K+ medium for 2 min, the percentage of CYLD-labeling of PSDs consistently increased (five experiments, Table I).
Table I.
Number of PSDs with CYLD label increases upon depolarization
| Percentage of PSDs with CYLD label | ||
|---|---|---|
| Basal | High K+ | |
| Experiment 1 | 15.9% (126) | 45.6% (68) |
| Experiment 2 | 27.8% (90) | 49.8% (215) |
| Experiment 3 | 27.8% (151) | 42.0% (69) |
| Experiment 4 | 30.4% (69) | 61.4% (88) |
| Experiment 5 | 28.2% (117) | 44.7% (132) |
| Mean±SEM | 26.0±2.4% | 48.7±3.4% |
(n): number of synapses scored
P<0.005, paired t-test for five experiments
Moreover, CYLD labeling intensity at the PSD complex also increased significantly after depolarization (Figure 4). Under basal conditions, the dense material underneath the postsynaptic membrane appeared thin, and only a few CYLD labels were present within the measurement area (Figure 4A). After depolarization, the PSD complex appeared thicker, and there were more CYLD labels within the measurement area (Figure 4B). We measured an average 81% increase in the intensity of labeling for CYLD at the PSD (five experiments, Table II).
Table II.
Intensity of CYLD label at the PSD increases upon depolarization
| Median number of gold labels/μm of PSD length | ||
|---|---|---|
| Basal | High K+ | |
| Experiment 1 | 9.6±1.2 (30) | 20.1±1.8 (33)** |
| Experiment 2 | 10.2±1.2 (22) | 20.6±2.2 (28)** |
| Experiment 3 | 8.6±1.6 (11) | 14.2±1.6 (22)* |
| Experiment 4 | 8.9±1.7 (16) | 15.5±1.7 (27)* |
| Experiment 5 | 9.0±1.2 (13) | 13.5±1.9 (11) N.S. |
| Mean±SEM | 9.3±0.3 | 16.8±1.5 |
(n): number of synapses CYLD-labeled PSDs measured.
***P<0.0005
P<0.05
N.S. non-significant, Student's t-test in individual experiments
P<0.005, paired t-test for five experiments
Discussion
Western immunoblotting using two antibodies that recognize distinct, non-overlapping sequences on CYLD reveal substantial enrichment of this deubiquitinase in PSD fractions compared to parent fractions. These results, together with previous mass spectrometric analyses of affinity-purified PSDs [17] suggest that CYLD is part of the PSD complex. The conclusion from biochemical results is validated by immunoEM studies that show CYLD labeling of PSDs.
Like CaMKII [24, 25], the amount of CYLD recovered in the PSD fraction varies according to post mortem processing of brains, presumably reflecting varying degrees of excitatory conditions. Similarly, in immunoEM experiments, CYLD labeling of PSDs increases significantly upon depolarization, confirming the correlation between activity and CYLD accumulation at the PSD. However, it should be noted that while CYLD labeling at PSDs increases significantly during activity, it is also detected under basal conditions, albeit to a lesser degree, implying that the presence of CYLD at the PSD does not necessarily require intense or pathological activity and might be promoted by normal levels of brain activity.
The activity-induced redistribution of CYLD places it into a group of dynamic protein components of the PSD . This group includes CaMKII and Shank1, proteins that move towards the PSD under excitatory conditions [23, 27] and contribute to the “thickening” of the PSD [21, 27]. Proteins that converge upon the PSD during activity may constitute a coordinated team that mediates activity-induced reorganization at the synapse.
Enzymatic activity of CYLD gives some clues as to the functional consequences of its accumulation at the PSD. CYLD is a deubiquitinase specific for lysine63-linked polyubiquitins. Proteins tagged with lysine63-linked polyubiquitins are not normally destined for proteasomal degradation because lysine63-linked polyubiquitination mediates trafficking of proteins into different subcellular compartments [11]. Most notably, lysine63-linked polyubiquitination targets proteins to aggresomes and promotes formation and autophagic clearance of protein inclusions [10, 28-30]. Thus accumulation of CYLD at the PSD following synaptic activity may remove lysine63-linked polyubiquitin chains from proteins and thus prevent trafficking and autophagic degradation of PSD components. Such a mechanism has been proposed for the sorting of an endosomal cargo protein, EGF receptor, where AMSH, a lysine63-linked deubiquitinase, is thought to rescue the cargo from lysosomal degradation and promote recycling of the receptor [31]. It is possible that CYLD may have a similar regulatory effect on the sorting of glutamate receptors at excitatory synapses.
Up to now ubiquitination of synaptic proteins has been largely considered to be a prelude to proteasomal degradation. Localization of CYLD, a deubiquitinase specific for lysine63-linked polyubiquitins, at the PSD suggests a more complex scenario of protein ubiquitination at the synapse where the type of ubiquitination is an important determinant of function. In this context CYLD could be a pivotal molecule at the cross-roads between the proteasomal and autophagic degradation pathways and could be a promising target for pharmacological intervention.
CYLD is a deubiquitinase specific for lysine63-linked polyubiquitins.
Presence of CYLD in PSDs is established by biochemistry and immunoEM.
CYLD accumulates on PSDs upon depolarization of neurons.
Accumulation of CYLD at PSDs may regulate trafficking/degradation of synaptic proteins
Acknowledgements
We thank Christine A. Winters for hippocampal neuronal cultures, Virginia Crocker and Rita Azzam for EM technical support, and Dr. Paul Gallant for teaching the rapid processing of brains. This research was supported by the Intramural Research Program of the NIH, NINDS.
Abbreviations
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- PSD
postsynaptic density
- UPS
ubiquitin proteasome system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nature Neuroscience. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 2.Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick GN, Bingol B, Weld HA, Schuman EM. Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr Biol. 2003;13:2073–2081. doi: 10.1016/j.cub.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Bingol B, Schuman EM. A proteasome-sensitive connection between PSD-95 and GluR1 endocytosis. Neuropharmacology. 2004;47:755–763. doi: 10.1016/j.neuropharm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Ang XL, Seeburg DP, Sheng M, Harper JW. Regulation of postsynaptic RapGAP SPAR by Polo-like kinase 2 and the SCFbeta-TRCP ubiquitin ligase in hippocampal neurons. J Biol Chem. 2008;283:29424–29432. doi: 10.1074/jbc.M802475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung AY, Sung CC, Brito IL, Sheng M. Degradation of postsynaptic scaffold GKAP and regulation of dendritic spine morphology by the TRIM3 ubiquitin ligase in rat hippocampal neurons. PLoS One. 2010;5:e9842. doi: 10.1371/journal.pone.0009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30:16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangash MA, Park JM, Melnikova T, Wang D, Jeon SK, Lee D, Syeda S, Kim J, Kouser M, Schwartz J, Cui Y, Zhao X, Speed HE, Kee SE, Tu JC, Hu JH, Petralia RS, Linden DJ, Powell CM, Savonenko A, Xiao B, Worley PF. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. doi: 10.1016/j.cell.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Tan JM, Wong ES, Kirkpatrick DS, Pletnikova O, Ko HS, Tay SP, Ho MW, Troncoso J, Gygi SP, Lee MK, Dawson VL, Dawson TM, Lim KL. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum Mol Genet. 2008;17:431–439. doi: 10.1093/hmg/ddm320. [DOI] [PubMed] [Google Scholar]
- 11.Lim KL, Lim GG. K63-linked ubiquitination and neurodegeneration. Neurobiol Dis. 2011;43:9–16. doi: 10.1016/j.nbd.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 14.Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 16.Wooten MW, Geetha T, Babu JR, Seibenhener ML, Peng J, Cox N, Diaz-Meco MT, Moscat J. Essential role of sequestosome 1/p62 in regulating accumulation of Lys63-ubiquitinated proteins. J Biol Chem. 2008;283:6783–6789. doi: 10.1074/jbc.M709496200. [DOI] [PubMed] [Google Scholar]
- 17.Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Mol Cell Proteomics. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca(2+)/calmodulin-dependent [correction of Ca(2+)/CaMKII-dependent] protein kinase II in neurons. J Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, McLaren RS, Winters CA, Ralston E. Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol Cell Neurosci. 1998;12:363–375. doi: 10.1006/mcne.1998.0723. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Tao-Cheng JH, Reese TS, Dosemeci A. SynGAP moves out of the core of the postsynaptic density upon depolarization. Neuroscience. 2011;192:132–139. doi: 10.1016/j.neuroscience.2011.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao-Cheng J-H. Activity-Induced Fine Structural Changes of Synapses in Mammalian Central Nervous System. In: Pickel V, Segal M, editors. Structure and Function of the Synapse. Neuroscience-net; 2012. [Google Scholar]
- 22.Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao-Cheng JH, Dosemeci A, Gallant PE, Smith C, Reese T. Activity Induced Changes in the Distribution of Shanks at Hippocampal Synapses. Neuroscience. doi: 10.1016/j.neuroscience.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994;63:1529–1537. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci U S A. 2005;102:11551–11556. doi: 10.1073/pnas.0505359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao-Cheng JH, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. J Comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- 27.Dosemeci A, Tao-Cheng JH, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM. Parkin mediate nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, Chin LS. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J Cell Biol. 2007;178:1025–1038. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin LS, Olzmann JA, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2010;38:144–149. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]


