Abstract
We tested whether daily exercise modulates immune and neuroimmune cytokines, hippocampus-dependent behavior and hippocampal neurogenesis in aging male F344 rats (18 mo upon arrival). Twelve weeks after conditioned running or control group assignment (n = 6 per group), the rats were trained and tested in a rapid water maze followed by an inhibitory avoidance task. The rats were BrdU-injected beginning 12 days after behavioral testing and killed 3 weeks later to quantify cytokines and neurogenesis. Daily exercise increased neurogenesis and improved immediate and 24 h water maze discrimination index (DI) scores and 24 h inhibitory avoidance retention latencies. Daily exercise decreased cortical VEGF, hippocampal IL-1β and serum MCP-1, GRO-KC and leptin levels but increased hippocampal GRO-KC and IL-18 concentrations. Serum leptin concentration correlated negatively with new neuron number and both DI scores while hippocampal IL-1β concentration correlated negatively with memory scores in both tasks. Cortical VEGF, serum GRO-KC and serum MCP-1 levels correlated negatively with immediate DI score and we found a novel positive correlation between hippocampal IL-18 and GRO-KC levels and new neuron number. Pathway analyses revealed distinct serum, hippocampal and cortical compartment cytokine relationships. Our results suggest that daily exercise potentially improves cognition in aging rats by modulating hippocampal neurogenesis and immune and neuroimmune cytokine signaling.
Keywords: adult neurogenesis, hippocampus, running, cytokine, chemokine, biomarker, learning, memory, water maze, Fisher 344, Bio-Plex
1. Introduction
Developing novel strategies to protect cognition in our burgeoning elderly population is critical for managing the burden and cost of its care. Hippocampal neurogenesis is a form of plasticity that declines significantly with age in rodents (Bizon et al., 2004; Dupret et al., 2008; Kuhn et al., 1996), dogs (Siwak-Tapp et al., 2007) and non-human primates (Aizawa et al., 2009; Gould et al., 1999b) primarily because the neural progenitor cell (NPC) precursors of new neurons and glia become increasingly quiescent with age (Cameron and McKay, 1999). The abundance of neurons added daily to the young mammalian hippocampus (Cameron and McKay, 2001) suggests that neurogenesis contributes to hippocampal integrity and indeed, measures of neurogenesis and ability in hippocampus-dependent tasks generally relate in young mammals (Deng et al., 2010; but see Epp et al., 2011; Gould et al., 1999a). Measures of neurogenesis have been related to measures of performance in hippocampus-dependent tasks among aged dogs (Siwak-Tapp et al., 2007), aged non-human primates (Aizawa et al., 2009) and when an experimental manipulation introduces enough variability into both measures to detect the relationship in aged rats (Bizon et al., 2004; Dupret et al., 2008; Kempermann et al., 2002; Speisman et al., 2012). Combined, these data suggest that protecting hippocampal neurogenesis from the effects of age may also protect some forms of cognition.
Experimental manipulations that produce neuroimmune responses can impair hippocampal neurogenesis and cognition. For example, systemic or central bacterial lipopolysaccharide (LPS) injections activate microglia, potently block neuronal differentiation (Ekdahl et al., 2003; Monje et al., 2003), and disrupt the integration of young neurons into existing hippocampal circuitry (Belarbi et al., 2012). Of the cytokines known to be stimulated by LPS (see Erickson and Banks, 2011), only a handful have been shown to affect in vivo and in vitro neurogenesis (Ben-Hur et al., 2003; Buckwalter et al., 2006; Grotendorst et al., 1989; Liu et al., 2009; Lum et al., 2009; Monje et al., 2003; Qin et al., 2008; Turrin et al., 2001; Vallieres et al., 2002; Villeda et al., 2011). In humans, experimental LPS impairs verbal and non-verbal memory (Reichenberg et al., 2001), but confirming its effects on neurogenesis awaits technology that permits the visualization of neurogenesis in the living brain. However little, if any, evidence of hippocampal neurogenesis is detected in the post-mortem tissue of patients who exhibited profound memory loss after γ-irradiation therapy, which also stimulates neuroimmune signaling (Coras et al., 2010; Correa et al., 2004; Crossen et al., 1994; Monje et al., 2007). The deleterious effects of LPS and γ-irradiation on hippocampal neurogenesis in rodents can be blocked by non-steroidal anti-inflammatory treatment (Monje et al., 2003; Rola et al., 2008; Tan et al., 2011), confirming a role for downstream immune and/or neuroimmune signaling cascades in mediating the effects of these treatments on neurogenesis.
In aged rodents, systemic or central LPS administration stimulates exaggerated microglial responses, cytokine levels and memory impairment (Barrientos et al., 2006; Chen et al., 2008; Godbout et al., 2005; Xu et al., 2010). In fact, the transcription of neuroimmune molecules is upregulated categorically with age but most robustly in aged rodents that exhibit impaired performances across hippocampus-dependent tasks (Blalock et al., 2003; Kohman et al., 2011a). Whole brain preparations have revealed that the concentrations of some cytokines that increase with age in rodents also associate negatively with measures of long-term potentiation and spatial ability (Felzien et al., 2001; Griffin et al., 2006; Prechel et al., 1996; Ye and Johnson, 1999). In aged and aging humans, increased circulating immune cytokine concentrations have been linked to cognitive impairments (Gimeno et al., 2008; Krabbe et al., 2009; Krabbe et al., 2004; Magaki et al., 2007; Rachal Pugh et al., 2001; Rafnsson et al., 2007; Weaver et al., 2002). In a recent study, Villeda and colleagues elegantly narrowed a list of 17 potential circulating cytokines (of 66 examined) down to 6 that related to age-impaired in neurogenesis and cognition. They then showed that increased circulating eotaxin concentrations alone compromise neurogenesis, synaptic plasticity and memory across hippocampus-dependent tasks (Villeda et al., 2011). These data highlight that the systematic testing of circulating and central cytokine biomarker correlates of neurogenesis and cognition can reveal mechanistic candidates. Importantly, these candidates can include hypoactive or senescent immune and neuroimmune cytokine signaling, particularly in aged rats (Conde and Streit, 2006; Ziv et al., 2006).
Elderly humans who exercise regularly exhibit better scores on cognitive tests and have larger hippocampal volumes relative to sedentary elderly humans (Christensen and Mackinnon, 1993; Churchill et al., 2002; Colcombe and Kramer, 2003; Erickson et al., 2010). Young and aged rodents that exercise daily on a running wheel exhibit enhanced measures of plasticity that include neurogenesis and long-term potentiation and better performances on hippocampus-dependent tasks (Brown et al., 2003; Creer et al.; Kronenberg et al., 2003; Kumar et al., 2012; Lambert et al., 2005; Lugert et al.; Madronal et al., 2010; Steiner et al., 2008; Suh et al., 2007; van Praag et al., 1999; van Praag et al., 2002; van Praag et al., 2005). In young rats that run voluntarily, increased levels of neurogenesis are associated with reduced hippocampal IL-1β levels (Chennaoui et al., 2008; Farmer et al., 2004; Leasure and Decker, 2009; Stranahan et al., 2006), suggesting that physical activity may stimulate plasticity and improve cognition by modulating neuroimmune signaling pathways, possibly through attenuation of microglia proliferation (Kohman et al., 2011b) and/or altering immune related gene expression (Kohman et al., 2011a) as previously demonstrated in aged mice. Therefore, we tested the effects of conditioned wheel running on the rapid acquisition and retention of a water maze hidden platform, inhibitory avoidance acquisition and retention, hippocampal neurogenesis and 24 immune and neuroimmune cytokine concentrations in aging F344 rats. We expected that conditioned runners would exhibit better learning and memory indices and have higher rates of neurogenesis than control rats. We also expected that conditioned runners might have altered levels of immune and/or neuroimmune cytokines that may relate to measures of hippocampal integrity and/or hippocampal neurogenesis.
2. Methods
2.1. Subjects
All rat subjects were treated in accordance with University of Florida and federal policies regarding the humane care and use of laboratory animals. Upon arrival, sexually naïve male Fischer 344 rats (18 mo; n = 12) purchased from the National Institute of Aging colony at Harlan Sprague Dawley Laboratories (Indianapolis, IA) were housed individually in corn cob bedding-lined hanging shoebox cages located in a colony room maintained on a 12:12 h light:dark cycle at 24±1°C. The rats were given access to Harlan Teklad Rodent Diet #8604 and water ad libitum. All rats were weighed weekly and checked daily to ensure that they did not exhibit age-related health problems including (but not limited to) poor grooming, reduced food and water intake, excessive porphyrin secretion or weight loss.
One week after arrival, the rats were assigned randomly to the conditioned runner or control group (n = 6 per group). Control rats were maintained individually in standard laboratory cages with access to food and water ad libitum for the 18 weeks-long duration of the experiment while runners were conditioned to run for food to prevent the well-documented decreases in running behavior exhibited by aged rats across weeks of an experiment (Cui et al. 2009; Holloszy et al., 1985; Kumar et al., 2012). Therefore, runners rats were housed individually in a chamber containing a running wheel (model H10-38R, Coulbourn Instruments, Allentown, PA) on which they could run for unlimited food (Kumar et al., 2012). A Graphic State Notation computer program (Version 3.02, Coulbourn Instruments, Allentown, PA) recorded wheel rotations and was programmed to deliver 45 mg food pellets (Harlan Teklad Rodent Diet #8604) based upon wheel rotations. The frequency of 45 mg food pellet delivery was decreased from 1 pellet per rotation at the beginning of conditioning to 1 pellet per 3–4 meters by ~4 weeks. By the 8th week of conditioning, all runners consistently ran ~ 4 km per week. If a conditioned runner rat lost more than 10% of the weight expected based on their pre-conditioning baseline and the weight changes of the control rats, the number of wheel rotations required for food delivery was reduced. Note that the body masses of conditioned runners (418.52±5.12 g) were similar to controls (414.26±5.26 g) at the beginning of the experiment (t(10) = −0.45; p = 0.66) and tended to be smaller (357.97±12.79 and 417.50±33.41 g, respectively) at the end of the experiment (t(10) = 1.97; p = 0.08). The experiment timeline is depicted in Fig. 1.
Figure 1. Experiment timeline.
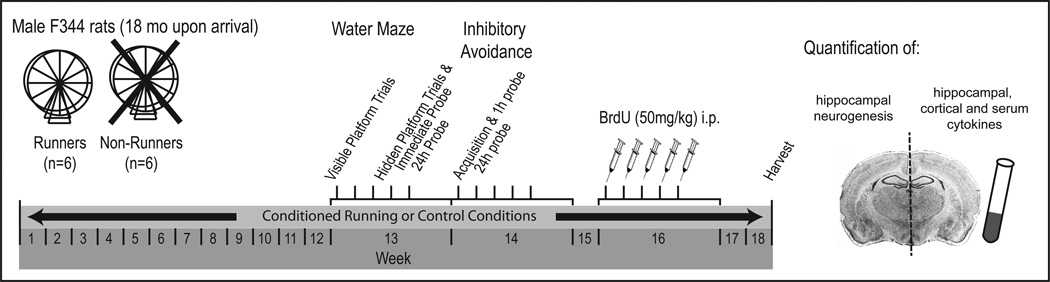
Male F344 rats (18 mo) were assigned randomly to either a conditioned running (n = 6) group that voluntarily ran for food for the entire 18 weeks-long experiment or a sedentary control (n = 6) group fed ad libitum. All rats underwent water maze training and testing during the 13th week followed by inhibitory avoidance training and testing during the 14th week. During the 16th week, the rats were BrdU-injected (50 mg/kg/day; i.p.) daily for 5 days and then killed at the end of the 18th week to quantify 24 immune and neuroimmune cytokine simultaneously with hippocampal neurogenesis.
2.2. Water maze training and testing
Each rat was trained and tested in a black water maze tank (1.7 m diameter) housed in a well-lit room. The tank was filled with water (27±2°C) to a depth of 8 cm below the tank rim. A Columbus Instruments tracking system (Columbus, OH) was used to record latencies (s), pathlengths (cm), % time spent in the outer annulus of the maze and platform crossings (see Fig. 2). Rats were initially habituated to the pool on 3 trials during which they were released from different pool locations and allowed to climb onto a visible platform. Rats were dried with towels and warm air between blocks and before being returned to their home cages.
Figure 2. Runners and controls perform similarly on the visible platform task.
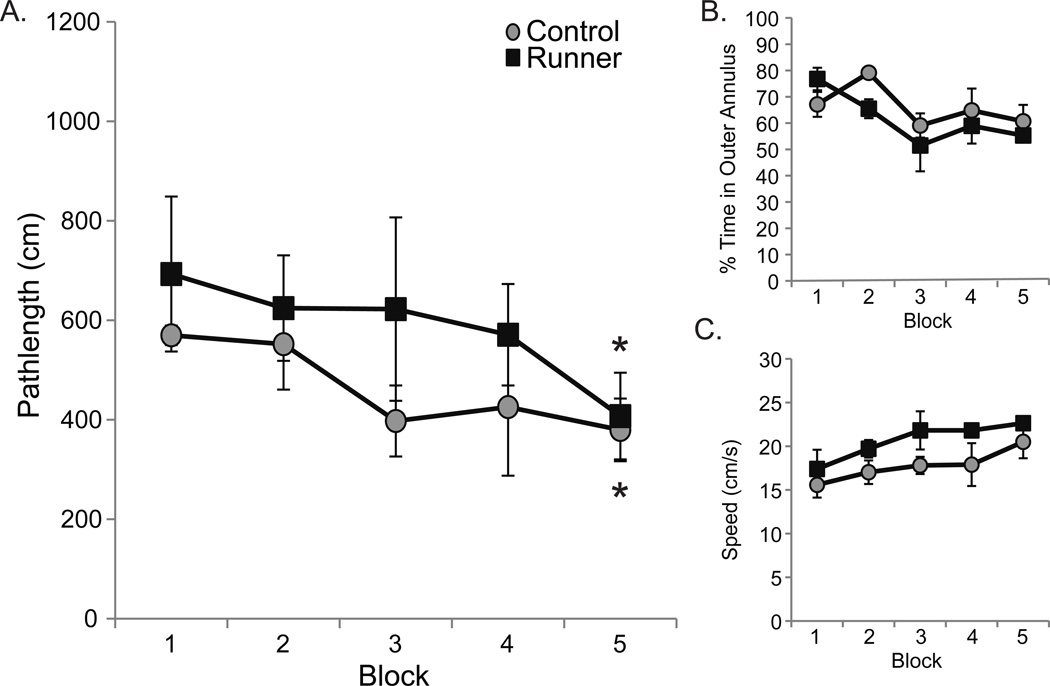
Data are shown as group means (±S.E.M.). Gray circles represent control values and black squares represent conditioned runner values. (A) Conditioned runners and controls located and escaped to a visible water maze platform with equal proficiency (interaction effect: p=0.90). Planned comparisons confirmed decreased pathlengths on the 5th block relative to the 1st block for control rats (*p< 0.05) and conditioned runner rats (*p< 0.05), confirming similar sensorimotor and procedural abilities between groups. (B) Regardless of exercise history (interaction effect: p=0.20), rats decreased the amount of time in a block spent swimming around the outer annulus of the water maze as training commenced (blocks 1, 2 > 3 and 5 and block 2 > 4; all p values <0.05). (C) Although conditioned runners tended to swim more quickly on all visible platform training blocks combined (p = 0.08), all rats (interaction effect: p = 0.83) significantly decreased their swim speeds across trials (p < 0.01). Specifically, the aged rats swam more quickly on Block 1 versus 3, 4 and 5 and on Block 2 versus 5 (all p values < 0.05).
2.2.1. Visible platform training
Beginning the 13th week of the experiment, the rats were trained in 5 blocks of 3 60 s visible platform trials (15 min inter-block interval [IBI] and 20 s inter-trial interval [ITI]) that require intact procedural and sensorimotor ability (Vorhees and Williams, 2006). The flagged platform (29 cm diameter) protruded 1.5 cm from the water surface and the pool was surrounded by a black curtain to mask distal cues. The platform location and N, S, E and W release points were randomized across trials. Rats failing to locate and climb onto the platform within the allotted 60 s were guided to the platform by the experimenter. One control rat was removed from the experiment after failing to locate the visible platform on ≥ 2 trials over the last 2 blocks. Latencies (s) and pathlengths (cm) served as measures of procedural and sensorimotor ability, % time spent in the outer annulus served as a measure of anxiety and swim speed (cm/s) served as a measure of locomotor ability.
2.2.2. Hidden platform training
Three days after visible platform training, the rats were trained on 5 blocks of 3 60 s hidden platform trials (15 min IBI and 20 s ITI) that require intact spatial ability (Vorhees and Williams, 2006). This rapid water maze training protocol is sensitive to age-related cognitive decline and the effects of differential experience on spatial ability in aged rats (Carter et al., 2009; Foster and Kumar, 2007; Foster et al., 2003; Kumar et al., 2012; Speisman et al., 2012). The platform was hidden 1.5 cm below the water surface in the center of the NE quadrant of the water maze now surrounded by highly visible distal cues. N, S, E and W release points were randomized across each trial. Rats that failed to locate and climb onto the platform within the allotted 60 seconds were guided to the platform by the experimenter before being removed from the maze. Latencies (s) and pathlengths (cm) served as measures of spatial ability, % time spent in the outer annulus served as a measure of anxiety and swim speed (cm/s) served as a measure of locomotor ability.
2.2.3. Immediate and delayed probe trials
The escape platform was removed from the water maze in probe trials administered immediately or 24 h after the last hidden platform training trial to test strength of learning and memory, respectively, for the platform location. In both probe trials, rats were released from the quadrant opposite to the goal quadrant for a 60 second free swim. A hidden platform trial block was administered after the first probe trial to reinforce the association between the platform localization and escape from the pool. The time (s) spent in each quadrant, platform location crossings and discrimination index (DI) scores [(t(G)−t(O))/(t(G)+t(O)), where t(O) is time spent in the opposite quadrant and t(G) is time spent in the goal quadrant] served as measures of strength of learning and memory in probe trials. DI scores take into account the quadrant to be approached (the “goal quadrant” [G]) and the quadrant to be avoided (the “opposite quadrant” [O]), and often produces a higher fidelity memory index for aged rats that often make wide sweeping turns while navigating by swimming.
2.3. Inhibitory avoidance training and testing
Beginning the 14th week, the rats were trained and tested in an inhibitory avoidance apparatus (Coulbourn Instruments, Allentown, PA) consisting of dark and lighted chambers with a shockable metal grid floor separated by a sliding door. During acquisition, the rat was placed in the lighted compartment for 90 s before the sliding door opened and latency to enter the dark compartment was recorded. Upon entry to the dark compartment, the door closed and a mild foot shock (0.21 mA for 3 s) was delivered 10 s later. The animal’s behavioral responses (i.e. a jump or rapid movement) confirmed that they had experienced the shock. The rat was then returned its home cage before being returned to the lighted chamber for 90 s both 1 and 24 h later, and the time taken to enter the dark side after the door opened was recorded as a measure of memory. Retention latencies were set at 900 s for rats not entering the dark compartment within 15 min. Door opening, shock delivery and data acquisition was computer controlled.
2.4. Bromodeoxyuridine injections
We waited 16 weeks after the experiment onset and 3 weeks after spatial learning before labeling dividing NPCs with the DNA synthesis marker bromodeoxyuridine (BrdU; Sigma Aldrich, St. Louis, MO) to measure the effects of long-term daily exercise on neurogenesis while minimizing the well-known effects of spatial behavior on neurogenesis (Gould et al., 1999a, Epp et al., 2010). NPC proliferation is unaffected when BrdU is administered at the end of hippocampus-dependent learning (Gould et al., 1999a) and any latent effects of hippocampus-dependent behavior on new neurons produced 3 weeks are possible but unexpected. Rats were injected intraperitoneally once per day over 5 days beginning 16 weeks after the experiment onset to label dividing cells. BrdU was dissolved in freshly prepared 0.9% isotonic sterile saline at a concentration of 20 mg/ml (w/v) just prior to use at a volume of 2.5 ml/kg (50 mg/kg/injection). This dose of BrdU labels dividing hippocampal NPCs safely and effectively in adult rodents (Cameron and McKay, 2001; Kolb et al., 1999).
2.5. Histology
At the end of the 18th week (21 d after the first BrdU injection), the rats were anaesthetized deeply with a ketamine (90 mg/kg)/xylazine (10 mg/kg) cocktail (Webster Veterinary Supply, Sterling, MA). Blood was collected from the left ventricle of the heart before rats were decapitated and their brains extracted rapidly. Ventral hippocampi and frontal cortices were rapidly dissected from the left hemisphere, flash frozen and then stored at −86°C until protein harvest for cytokine quantification. Although central cytokine levels in these unperfused rats could reflect circulating levels of diffusible cytokines we neither detected immune-to-brain cytokine clusters nor concentrations of individual cytokines that were affected by running similarly in the blood and brain that would validate this hypothesis. Similar masses of hippocampal (t(10) = 1.00; p = 0.34) and cortical (t(10) = −0.01; p = 1.00) tissue were collected from controls (79.70±11.30 and 246.40±21.95 mg, respectively) and conditioned runners (65.90±8.05 and 246.60±15.69 mg, respectively). Serum supernatant was collected from blood samples after refrigeration for 24 h at 4°C and centrifugation at 1,000×g for 10 min at RT and then stored −86°C until cytokine quantification. The right hemisphere of the brain was post-fixed overnight in freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA) and then equilibrated in 30% sucrose (~ 4 days) at 4°C, before being sectioned coronally through the dentate gyrus, beginning between ~ −1.72 and −1.92 mm posterior to bregma according to Paxinos and Watson (82) at 40 µm intervals on a freezing stage sledge microtome (Model 860; American Optical Corporation; IMEB Inc., San Marcos, CA). The six sets of every sixth section collected through the left side of the dentate gyrus were stored at −20°C in a cryoprotectant solution of 30% ethylene glycol, 25% glycerin and 45% 0.1 M sodium phosphate buffer until processed immunohistochemically to quantify neurogenesis.
2.6. Protein harvest from brain tissue
Hippocampi and frontal cortices were thawed at 4°C in 0.1 M tris-buffered saline (TBS) containing 0.1% Igepal and 1 µl/ml each of 2 protease inhibitor cocktails added just prior to use. The first protease inhibitor cocktail contained 0.5 M phenylmethylsulfonyl fluoride, 5 mg pepstatin A and 1 mg chymostatin/ml DMSO and the second contained 1 M G-aminocapfroic acid, 1 M P-aminobenzidine, 1 mg leupeptin and 1 mg aprotinin/ml sterile water. Tissue was mashed manually and then sonicated using a dismembrator (ThermoFisher Scientific; Pittsburgh, PA). Tissue supernatant was collected by centrifugation (12,000 rpm for 10 min at 4°C) and its protein concentration quantified using a Bradford protein assay and a Bio-Rad SmartSpec Plus Spectrophotometer (Hercules, CA). Similar total protein concentrations were harvested from the hippocampi (t(10) = −1.28; p = 0.23) and cortices (t(10) = −0.07; p = 0.94) of controls (0.93±0.19 and 1.05±0.06 mg/mL, respectively) and conditioned runners (1.20±0.09 and 1.06±0.09 mg/mL, respectively). Protein samples were stored at −86°C until cytokine concentrations were quantified using Bio-Plex technology.
2.7. Immunohistochemistry
Hippocampal sections were stained immunohistochemically to quantify and phenotype new (BrdU+) cells using methods previously described (Ormerod et al., 2003; Palmer et al., 2000; Speisman et al., 2012).
2.7.1. Enzyme substrate immunostaining
Before processing and between steps, free-floating hippocampal sections were washed repeatedly in tris-buffered saline (TBS; pH 7.4). The sections were incubated in 0.3% H2O2 in TBS for 10 min at RT to quench endogenous peroxidase, rinsed in 0.9% NaCl and then incubated in 2 N HCl for 20 min at 37°C to denature DNA. The sections were then blocked in a solution of 3% normal donkey serum (NDS) and 0.1% triton-x in TBS (v/v) for 20 min and then incubated overnight in rat anti-BrdU (1:500; AbD Serotec, Raleigh, NC) at 4°C. The next day, they sections were incubated in biotinylated donkey anti-rat IgG (Jackson ImmunoResearch, West Grove, PA; 1:500) for 4 h and then avidin-biotin horseradish peroxidase (PK-6100: Vector Laboratories, Burlingame, CA) for 2 h at RT. The horseradish peroxidase complex was then revealed by reaction with 0.02% 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma Aldrich, St. Louis, MO) and 0.5% H2O2 in TBS. Sections were mounted on glass slides, dried overnight and dehydrated in an alcohol series prior to being cover-slipped under permount (Thermo Fisher Scientific, Pittsburgh, PA).
2.7.2. Fluorescent immunostaining
Sections were washed repeatedly between steps in TBS (pH 7.4). The sections were blocked in NDS solution and then incubated overnight at 4°C in primary antibodies raised against the mature neuronal protein neuronal nuclei (mouse anti-NeuN, 1:500; Chemicon, Temecula, CA) and the immature neuronal protein doublecortin (goat anti-DCX, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) or the oligodendrocyte precursor marker chondroitin sulfate proteoglycan (rabbit anti-NG2, 1:500; Chemicon, Temecula, CA) and the astrocyte/neural stem cell protein glial fibrillary acidic protein (chicken anti-GFAP, EnCor Biotech, Alachua, FL). The following day sections were incubated with maximally cross-adsorbed fluoroscien isothiocyanate (FITC)-conjugated anti-mouse and cyanine (Cy) 5-conjugated anti-goat secondary antibodies to reveal neurons or FITC-conjugated anti-rabbit and Cy5-conjugated anti-chicken secondary antibodies to reveal glia for 4 h at RT (all secondaries diluted at 1:500; Jackson ImmunoResearch, West Grove, PA). Sections were then fixed with 4% paraformaldehyde, rinsed in 0.9% NaCl, incubated in 2N HCl and then incubated in rat anti-BrdU (1:500; AbD Serotec, Raleigh, NC) overnight at 4°C followed by Cy3-conjugated anti-rat secondary for 4 h at RT. Finally, nuclei were labeled by incubation in 4', 6-diamidino-2-phenylindole (DAPI; 1:10,000; Calbiochem, San Diego, CA) for 10 minutes. Sections were mounted on glass slides under the anti-fading agent PVA-DABCO (2.5% diazobicyclooctane, 10% polyvinyl alcohol and 20% glycerol in TBS; Sigma Aldrich).
2.8. Cell quantification
2.8.1. Total new cell number
The total number of new (BrdU+) cells was estimated on one 1-in-6 series of systematically uniform sections (spaced 240 µm apart) taken through the rostral-caudal extent of the dentate gyrus in the left hemisphere of each rat using stereological principles (Boyce et al., 2010; Cameron and McKay, 1999; Kempermann et al., 2002; Ormerod et al., 2003; West et al., 1991). We randomly selected which of the 6 collected sets of sections to process immunohistochemically to ensure that the first section in each rats’ set was randomly the 1st – 6th section taken from dentate gyrus. New cells produced in the hippocampal subgranular zone (SGZ) presumably migrate deeper into the granule cell layer (GCL) over the 16–21 d survival period employed. We therefore counted round or oval BrdU+ cells (revealed by DAB staining) in both the SGZ and GCL on each section taken through the rostral-caudal extent of the dentate gyrus in the left hemisphere of each aged rat (~12 sections per rat) using a Zeiss Axio Observer Z1 inverted microscope under a 40X objective. Because new cells are often situated irregularly through the SGZ and GCL, we counted BrdU+ cells exhaustively on each systematically uniform series of sections per rat. The mean (±SEM) number of 131±12 and 191±12 BrdU+ cells in the dentate gyri of control and conditioned runner groups, respectively, is considered a sufficient number of events to insure precision among stereological estimates of total events (Boyce et al., 2010).
The total number of BrdU+ cells counted in the dentate gyrus of each rat was multiplied by 6 (the section interval in each set) and by 2 (to account for the other half of the brain) to produce a stereological estimate of total number of new cells surviving in the dentate gyrus (Kempermann et al., 2002; West et al., 1991). Because age and exercise may influence vascular volumes (Fabel et al., 2003; Hattiangady and Shetty, 2008), SGZ and GCL areas (mm2) on which new cells were counted were measured under a 20X objective using AxioVision software (Carl Zeiss, Thornwood, NY) and then GCL volumes obtained using Cavalieri’s principle for calculating the volume of a truncated cone (Galea et al., 2000; Uylings et al., 1986): Volume = ∑(sections) * ⅓I (h1 + √h1 * √h2 + h2), where I is the distance between sections (240 µm) and h1 and h2 are the two section areas between which the volume was calculated. We also confirmed that new cell densities reflected total new cell estimates because of potential changes in vascular volumes and because we quantified neurogenesis on only ½ of the hippocampus.
2.8.2. New cell phenotypes
At least 100 BrdU+ cells on quadruple fluorescent-stained sections were scanned through their x, y and z-planes using a Zeiss LSM 710 fully spectral laser scanning confocal microscope equipped with 405 (used to excite DAPI), 488 (used to excite FITC), 510, 543 (used to excite Cy3) and 633 (used to excite Cy5) laser lines under a 40X objective (with 2.3X digital zoom) to quantify the proportion that expressed neuronal or glial proteins. BrdU+ cells were considered to express neuronal or glial protein when a full “z-dimension” scan revealed that its BrdU/DAPI+ nucleus clearly expressed the neuronal proteins DCX and/or NeuN, the oligodendrocyte precursor protein NG2 or the astrocyte protein GFAP. The total number of BrdU+ cells was multiplied by the % of BrdU+ expressing each cell phenotype to determine the total number of new neurons and glia produced in the aging brain and this number was related to water maze probe trial performance.
2.9. Multiplex quantification of cytokines
Concentrations of immune cytokines in blood serum and neuroimmune cytokine concentrations in hippocampal and cortical protein samples were quantified using a Bio-Rad Bio-Plex 2000 Suspension Array system and an EMD Millipore Rat Cytokine/Chemokine kit (#RCYTO-80K-PMX; Billerica, MA) according to kit instructions. This kit simultaneously detects the following concentrations of 24 analytes simultaneously in a single sample: IL-1α (6.23–20,000 pg/mL), IL-1β (2.32–20,000 pg/mL), IL-2 (3.67–20,000 pg/mL), IL-4 (2.30–20,000 pg/mL), IL-5 (2.89–20,000 pg/mL), IL-6 (9.80–20,000 pg/mL), IL-9 (12.85–20,000 pg/mL), IL-10 (5.41–20,000 pg/mL), IL-12 (4.13–20,000 pg/mL), IL-13 (23.2–20,000 pg/mL), IL-17 (1.61–20,000 pg/mL), IL-18 (4.78–20,000 pg/mL), eotaxin (3.27–20,000 pg/mL), G-CSF (1.31–20,000 pg/mL), GM-CSF (13.11–20,000 pg/mL), IP-10 (3.78–20,000 pg/mL), leptin (21.50–100,000 pg/mL), GRO/KC (2.06–20,000 pg/mL), IFN-γ (4.88–20,000 pg/mL), MCP-1 (3.81–20,000 pg/mL), TNF-α (4.44–20,000 pg/mL), MIP-1α (1.94–20,000 pg/mL), RANTES (54.42–20,000 pg/mL), VEGF (4.93–20,000 pg/mL).
All standards, controls and samples were prepared on ice and serum and tissue samples were run in separate plates. Seven standards (with expected concentrations of 20,000, 5,000, 1,250, 312.5, 78.13, 19.53 and 4.88 pg/mL of each analyte except leptin that had expected concentrations of 100,000, 25,000, 12,500, 6250, 1562.5, 390.63 and 24.41 pg/mL) were prepared by serial dilution with kit assay buffer. Serum samples were diluted 1:5 with kit assay buffer while tissue supernatant samples were kept neat and 25 µl volumes of each standard, blank, vendor-supplied known control and sample were loaded in duplicate into a 96-well filter plate (EMD Millipore; Billerica, MA). Kit serum matrix (25 µl) was added to each standard, control and sample in the serum quantification plate while kit assay buffer (25 µl) was added to each standard, control and sample in the tissue sample plates final volume of 50 µl. Approximately 100 polystyrene beads each of 24 different color addresses were added to each well and incubated for 18 h on a shaker at 4°C. Each primary antibody raised against an analyte to be quantified was adsorbed to 1 of the 24 unique sets of color addressed beads. After several washes in kit wash buffer under vacuum filtration, the beads were incubated in biotinylated secondary antibodies for 2 h at RT and then after several washes kit wash buffer under vacuum filtration, in streptavidin-phycoerythrin reporter for 30 min at RT before being resuspended in sheath fluid (Bio-Rad; Hercules, CA). Analytes were identified by color address and analyte concentrations were quantified by phycoerythrin emission intensity using a dual laser Bio-Rad BioPlex 2000 system with Luminex xMAP technology (Bio-Rad; Hercules, CA). Data were collected using BioPlex Manager Software version 4.1.
A standard curve for each analyte was generated using a five-parameter logistic non-linear regression model on averaged duplicate observed standard concentrations. Single standard concentrations were employed in cases that its duplicate % coefficient of variation (CV) was >10% and its % recovery (observed/expected concentration) fell outside of the accepted 70–130% range. Once the positive control concentrations were confirmed to fall within the expected ranges, sample concentrations were compared against the standard curve.
Prior to statistical analysis, duplicate sample concentrations with % CV < 10 were averaged. If the % CV for a set of duplicates was > 10% and a concentration fell ±2 standard deviations from the group mean the outlying concentration was discarded. We discarded the outlying data point of one conditioned runner rat serum leptin analysis and an outlying data point from a different conditioned runner from the serum MCP-1 analysis. Cytokine concentrations below the threshold of detection were set to 0 and concentrations that exceeded the maximum expected concentration were set to 20,000 pg/ml (or 100,000 pg/ml for leptin). Data were expressed in pg/mL serum or pg/mg of hippocampal or cortical tissue.
2.10. Cytokine cluster analysis
Pathway or ‘Cluster’ analyses were conducted as described previously (Baron and Kenny, 1986; Erickson and Banks, 2011) to identify groups of cytokines with concentrations that may change in a coordinated fashion (i.e. in clusters) and therefore represent known or novel signaling pathways. First, cluster analyses were conducted on cytokine concentrations detected within blood, hippocampal and cortical compartments independently to both confirm and expand upon immune and neuroimmune cytokine signaling clusters in the aged rat. Second, cluster analyses were conducted on cytokine concentrations between blood and cortical compartments and between blood and hippocampal compartments to confirm and potentially reveal immune-to-brain signaling pathways in the aged rat. Third, we ran analyses on cytokine concentrations between cortical and hippocampal compartments to ask whether running modulates neuroimmune cytokines locally or regionally. Bonferroni-corrected alpha levels were set for each analysis based upon the number of analytes exceeding the threshold of detection.
Pairs of cytokines with concentrations deemed statistically related by Spearman rank correlation coefficients (r-values) after Bonferroni corrections were ranked and plotted in descending order connected with a solid line. If one cytokine in a pair to be plotted was already plotted in a cluster, then a decision point was reached and we employed a modification to the previously reported procedure (Baron and Kenny, 1986; Erickson and Banks, 2011). The unplotted cytokine was added to the cluster if it correlated significantly with all of the cytokines already in the cluster. If the unplotted cytokine was not statistically related to one or more of the already clustered cytokines, then the cytokine pair about to be plotted was plotted as a new cluster. If a cytokine pair about to be plotted was already linked through potential mediators in the already plotted cluster, then the residual of its r-value minus the product of the r-values of the plotted pairs between the cytokines about to be plotted was compared against the Bonferroni corrected p-value. If the residual r-value of the cytokine pair about to be plotted remained statistically significant, the cytokines were connected in the existing cluster with a dotted line (no mediators were detected in the current study).
2.11. Statistical analyses
All statistical analyses were conducted using STATISTICA software (Version 10; StatSoft; Tulsa, OK) and all data are represented in figures as the group average (± S.E.M.). Student’s t-tests were used to test the effect of the independent variable (conditioned running) on dependent measures of general health (body mass, swim speeds), strength of spatial learning and memory (probe trial discrimination index scores, number of platform crossings), neurogenesis (new cell number, total new neuron number, total new glia number) and cytokine concentration (for each of the 24 analytes). Non-parametric Mann Whitney U tests were used to test the effects of the independent variable (conditioned running) on categorical percentages of BrdU+ cells expressing neuronal or glial phenotypes and on inhibitory avoidance acquisition and retention latencies that were set to 900 s for animals that did not enter the shock-paired side of the chamber by the end of the session. Repeated measures analyses of variance (ANOVAs) tested the effect of the independent variable (conditioned running) on dependent measures collected repeatedly, such as spatial and non-spatial acquisition of a platform location (latencies and path lengths). Newman Keuls post-hoc tests were used to reveal significant differences. Spearman rank correlations were run to test the relationship between the concentration of cytokine analytes modulated by running, behavioral measures and measures of neurogenesis because some analyte concentrations fell below the threshold of detection. The α-level was set at 0.05.
3. Results
3.1. Aging rats that run daily locate a visible platform as well as controls but swim faster
Since path lengths correlated positively with latencies between visible (all r values ≥ 0.69; all p values < 0.05) and hidden (all r values ≥ 0.85; all p values < 0.01) platform trials, we report analyses on path lengths to avoid redundancy. Figure 2A shows pathlengths across visible platform training blocks. An ANOVA exploring the effects of conditioned running and training block on visible platform path lengths revealed that conditioned runners and controls swam similar distances to the visible platform (F(1,9) = 1.38; p = 0.27) across training blocks (F(4,36) = 1.56; p = 0.20 and interaction effect: F(4,36) = 0.26; p = 0.90). Because visible platform learning curves can be relatively shallow in aged rats (see Kumar et al., 2012; Speisman et al., 2012), planned comparisons were used to confirm that control rats and conditioned runner rats swam shorter path lengths on the 5th relative to the 1st block (p values < 0.05, respectively). These data suggest that both conditioned runners and controls are similarly capable of learning to locate and escape to a visible water maze platform.
The % of time spent swimming in the outer annulus of the pool by controls and conditioned runners was calculated as a measure of anxiety (Fig. 2B). An ANOVA revealed a significant effect of training block (F(4,36) = 5.32; p < 0.01) but not conditioned running (F(1,9) = 0.67; p = 0.44 and interaction effect: F(4,36) = 1.59; p = 0.20) on this measure. Specifically, all rats spent significantly less time in the outer annulus as training commenced (blocks 1, 2 > 3, 5 and block 2 > 4; all p values < 0.05), suggesting that anxiety levels decreased in aged rats with training, regardless of exercise history.
Swim speeds exhibited across blocks by controls and conditioned runners were recorded as a measure of locomotor ability (Fig. 2C). Although an ANOVA revealed a statistically significant effects of training block (F(4,36) = 5.55; p < 0.01) and a tendency for conditioned running to affect swim speed (F(1,9) = 3.84; p = 0.08), these effects did not statistically significantly interact (F(4,36) = 0.37; p = 0.83). Although conditioned runners tended to swim faster than controls on all blocks combined, all aged rats swam more quickly as training progressed (blocks 1 > 3, 4 and 5 and block 2 > 5; all p values < 0.05). These data suggest that daily exercise may potentiate the increased swimming proficiency or reduce the floating tendencies exhibited by aging rats across visible platform training blocks. Consistent with our previous finding (59) and the idea that running-induced fitness rather than mild food deprivation associated with the operant delivery of food for running affects swim speeds, the body masses of conditioned runners was similar to those of the controls at the beginning of the experiment and only tended to be smaller at the end of the experiment (see Section 2.1 for body masses). .
3.2. Daily exercise improves spatial ability in aging rats
We compared pathlengths to the hidden platform across training blocks as a measure of spatial ability (Fig. 3A). An ANOVA revealed that pathlengths were significantly affected by conditioned running (F(1,9) = 20.89; p < 0.01), training block (F(4,36) = 6.55; p < 0.01) and the interaction between conditioned running and training block (F(4,36) = 4.05; p < 0.01). All rats swam more directly to the hidden platform as training commenced (blocks 1, 2 > 3, 4 and 5, p values < 0.05), but conditioned runners swam more directly across all blocks combined than controls (p < 0.01). Conditioned runners exhibited shorter pathlengths than controls on the 1st, 4th and 5th training blocks (p values < 0.05), indicating that they solved the spatial task more proficiently than the controls. However, their better performances on the 1st hidden platform training block could also indicate that runners better learned, remembered and/or applied procedural information obtained during visible platform training conducted first (Gerlai, 2001; Ormerod and Beninger, 2002). Therefore, we confirmed that runners (−130.22±37.53 cm/block) exhibited steeper average pathlength slopes than controls (−5.36±35.65 cm/block) across hidden training blocks 2–5 (gray dotted lines in Fig. 3A; t(10) = 2.29; p < 0.05).
Figure 3. Conditioned runners outperformed controls on the water maze hidden platform task.
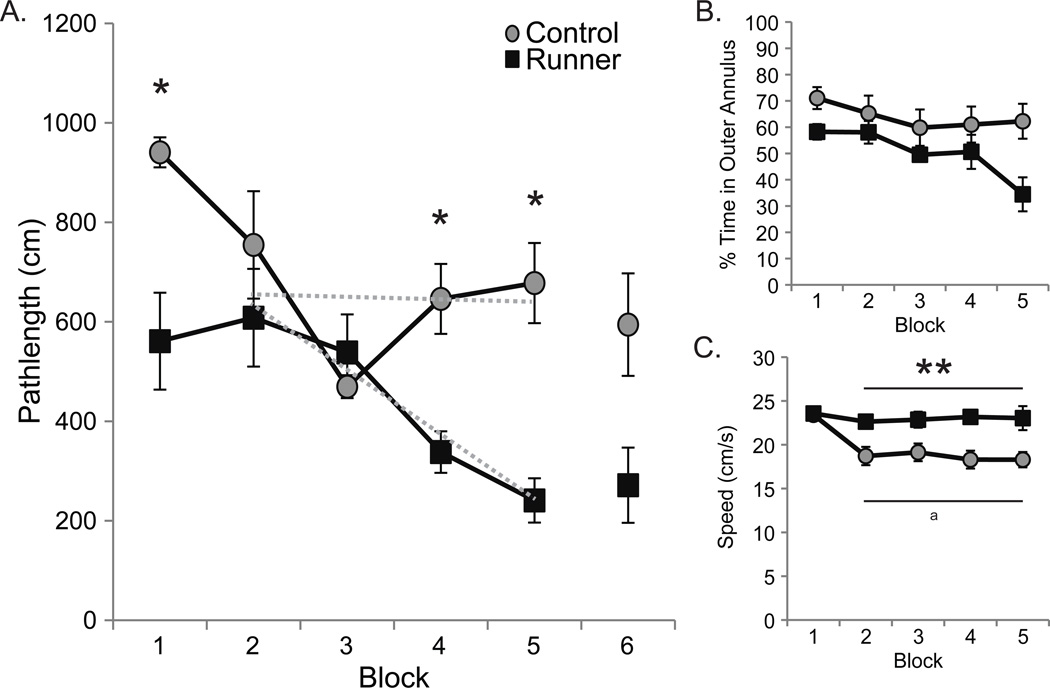
Data are shown as group means (±S.E.M.). Gray circles represent control values and black squares represent conditioned runner values. (A) All rats combined swam more directly to the hidden water maze platform as training commenced (block 1 > 3 and 4, all p values < 0.05), but conditioned runners swam more directly than controls, regardless of training block (p < 0.01), particularly on the 1st, 4th and 5th training blocks (*all p values < 0.05). Because runners outperformed controls on the 1st training block, we confirmed average pathlength slopes were steeper for runners (−130.22±37.53 cm/block) versus controls (−5.36±35.65 cm/block) across hidden training blocks 2–5 (gray dotted lines; p < 0.05). A paired t-test confirmed that the rats exhibited similar pathlengths on the 5th and a 6th training block administered after the 1 h probe trial to reinforce the association between locating the platform and escape from the pool (p=0.55). (B) Although conditioned runners spent significantly less time in the outer annulus than controls on all blocks combined (p < 0.05), all rats combined reduced the % of time spent in the outer annulus of the maze (Blocks 1, 2 > 5; all p values <0.05). (C) Conditioned runners swam significantly faster to the hidden platform than controls on all blocks combined (p < 0.01) and while they maintained their faster swim speeds across trials, control rats swam significantly slower than conditioned runners on Blocks 2–5 (all p values < 0.01) and slower than they swam on the first training block (Block 1 > 2, 3, 4 and 5; aall p values < 0.01).
We calculated the % of time spent in the outer annulus of the maze on hidden platform trials by conditioned runners and controls to determine if anxiety was differentially affected by previous training (Fig 3B). An ANOVA revealed significant effects of conditioned running (F(1,9) = 6.45; p < 0.05) and training block (F(4,36) = 3.98; p < 0.01) but no interaction effect (F(4,36) = 1.62; p = 0.19). Specifically, all rats combined spent less time in the outer annulus as training progressed (blocks 1, 2 > 5, p values < 0.05), but conditioned runners spent less time than controls on all blocks combined. These data suggest that although anxiety decreases with training in all aged rats, prior training may potentiate this effect in rats that exercise daily.
We calculated swim speeds on hidden platform training blocks as a measure of locomotor ability in aged conditioned runners and controls (Fig. 3C). Swim speeds were significantly affected by conditioned running (F(1,9) = 13.07; p < 0.01), training block (F(4,36) = 6.27; p < 0.01), and the interaction between running and training block (F(4,36) = 3.87; p < 0.01). Conditioned runners swam significantly faster to the hidden platform than controls on all blocks combined (p < 0.01) and maintained the swim speeds that they achieved on later visible platform trials across all hidden platform training blocks (see above). In all rats combined, swim speeds decreased after the first block (all p values < 0.01), but this effect was because while conditioned runners maintained their speeds across blocks, control rats swam significantly slower after the first training block (block 1 > 2, 3, 4 and 5; all p values < 0.01) These data support the notion that daily exercise can potentiate the effects of water maze training on the swimming proficiency of aging rats, potentially by improving their stamina.
3.3. Aging rats that exercise exhibit better memory for the platform location on probe trials
A 60 s probe trial was conducted immediately after the final hidden platform trial (Fig. 4). An ANOVA revealed that all rats combined exhibited a significant quadrant preference (F(3,27) = 24.99; p < 0.0001) and that quadrant preference significantly interacted with group (F(3,27) = 7.54; p < 0.001) on the immediate probe. Specifically, conditioned runners spent significantly more time in the goal quadrant (p=0.0003; Fig. 4A) and less time in the opposite quadrant (p=0.045) but similar amounts of time in the left (p=0.32) and the right quadrants (p=0.96) relative to controls. Similarly, conditioned runners exhibited significantly better DI scores than controls (t(9)= 4.17, p < 0.01; Fig. 4B) and tended to cross over the location that housed the platform on training trials significantly more frequently (4.33±0.71 crossings) than controls (2.60±0.51 crossings) did (t(9) = 1.90; p = 0.09). A refresher block of hidden platform trials was administered after the immediate probe to minimize the probability that the association between platform localization and escape from the pool was extinguished by the immediate probe trial. A paired t-test on the 5th and 6th hidden platform blocks confirmed that the rats exhibited similar path lengths before and after the probe trial (t(10) = 0.29, p = 0.78; see Fig. 3A).
Figure 4. Conditioned runners exhibit better memory in the water maze and on an inhibitory avoidance task.
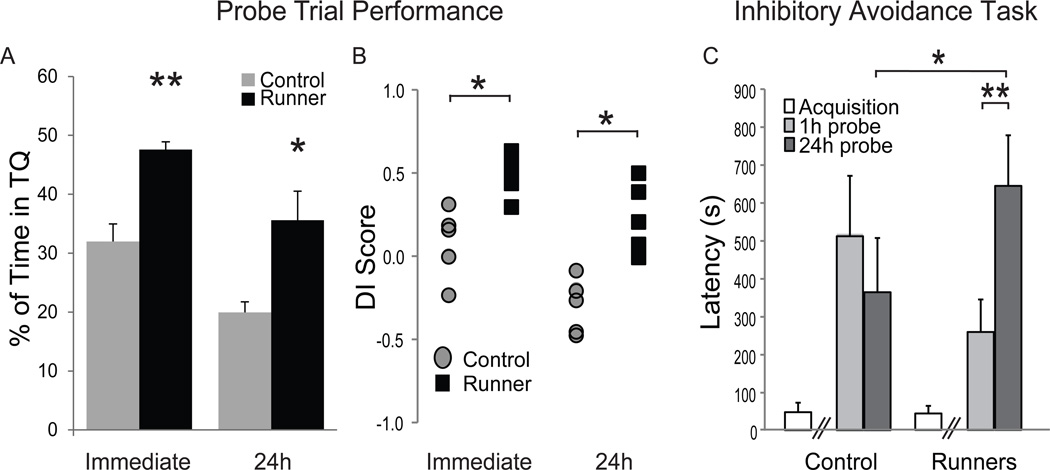
(A). A 60 s probe trial was conducted immediately after or 24 h after the final hidden platform trial and mean % time spent in the goal quadrant (±S.E.M.) is depicted for the controls (gray bars) and conditioned runners (black bars). On the immediate probe (**p=0.0003) and on the 24 h probe (*p=0.026), conditioned runners spent significantly more time in the goal quadrant than control rats did. (B) Individual discrimination index (DI) scores were calculated for control (gray circles) and conditioned runner rats (black squares) and then plotted. Positive scores represent better goal (versus opposite) quadrant discrimination. Conditioned runners exhibited better DI scores on both the immediate (*p < 0.01) and 24 h (*p < 0.01) water maze probe trials than control rats. (C) Finally, rats were trained (white bars) and then tested 1 h (light gray bars) and 24 h (dark gray bars) after training in an inhibitory avoidance task. Conditioned runners and controls entered the dark side of the inhibitory avoidance chamber that delivered shock equally as quickly during the acquisition phase of the task (p=0.90). Although both control and conditioned runners exhibited similar 1 h retention latencies, conditioned runners took significantly longer than controls to re-enter the dark side 24 h after training (p<0.05).
A second probe trial was administered 24 h after the 5th hidden platform block. An ANOVA revealed that all rats combined exhibited a significant quadrant preference (F(3,27) = 3.56; p = 0.027) and that quadrant preference interacted with group (F(3,27) = 5.16; p = 0.006) on the 24 h probe trial (Fig. 4A). Specifically, conditioned runners spent significantly more time in the goal quadrant (p=0.026), tended to spend less time in the opposite quadrant (p=0.052) and spent similar amounts of time in the left (p=0.416) and right quadrants (p=0.498) relative to controls. Similarly, conditioned runners exhibited significantly better DI scores than controls (t(9) = 4.39; p < 0.01; Fig. 4B) and crossed the location that housed the hidden platform on training trials significantly more frequently than controls (5.17±0.40 versus 1.20±0.20 crossings, respectively; t(9) = 8.28, p < 0.01) on the delayed probe trial. These data suggest that conditioned runners both learned and remembered the hidden platform location better than controls.
Finally, a regression analysis of the distance to escape the pool on block 5 of cue discrimination training was compared to the distance to escape for block 5 of the spatial discrimination task as well as the discrimination index score obtained on the immediate probe. No association was observed indicating that acquisition of the spatial discrimination was not linked to the acquisition performance for cue discrimination.
3.4 Inhibitory avoidance scores
One week after the onset of visible platform water maze training, the rats were trained and tested in an inhibitory avoidance task (Fig. 4C). Mann-Whitney U tests confirmed that conditioned runners and controls entered the shock-paired dark side of the inhibitory avoidance chamber equally as quickly during the acquisition phase of the task (U = 0.01; Z = −1.19; p = 0.92). Although 1 h latencies were similar between groups (U = 8.00; Z = −1.19; p = 0.24), conditioned runners tended to have longer 24 h latencies than controls (U = 5.00; Z = 1.73; p = 0.08). Spearman Rank Correlation was used to compare learning and memory on the water maze discrimination index scores and 1 and 24 h inhibitory avoidance retention latencies. The results indicated a relationship between the 24 h retention scores on the water maze and inhibitory avoidance (r = 0.63, p < 0.05).
3.5. Daily exercise increases neurogenesis in aged rats by increasing new cell number
The total number of new cells was estimated in the dentate gyri of all rats using stereological principles (Fig. 5A, B and E). A student’s t-test confirmed that the total number of BrdU+ cells was higher in the dentate gyri of conditioned runners relative to controls (t(9) = 3.44, p < 0.01; Fig. 5E). Although exercise could potentially increase vascular volume within the neurogenic niche, dentate gyri volumes that new cells were estimated through were similar between controls (4.32±0.17 mm3) and conditioned runners (4.53±0.30 mm3; t(9) = −0.57, p = 0.58). As expected from these data sets, new cell densities were also higher in conditioned runners (514.77±40.95 cells/mm3) versus controls (370.35±46.14 cells/mm3; t(9) = 2.35, p < 0.05). Because the rats survived several weeks after BrdU was injected, these differences could reflect effects on NPC division and/or the survival of new cells, but are consistent with the well-known effects of physical exercise on NPC division.
Figure 5. Conditioned running potentiated hippocampal neurogenesis in aging rats.
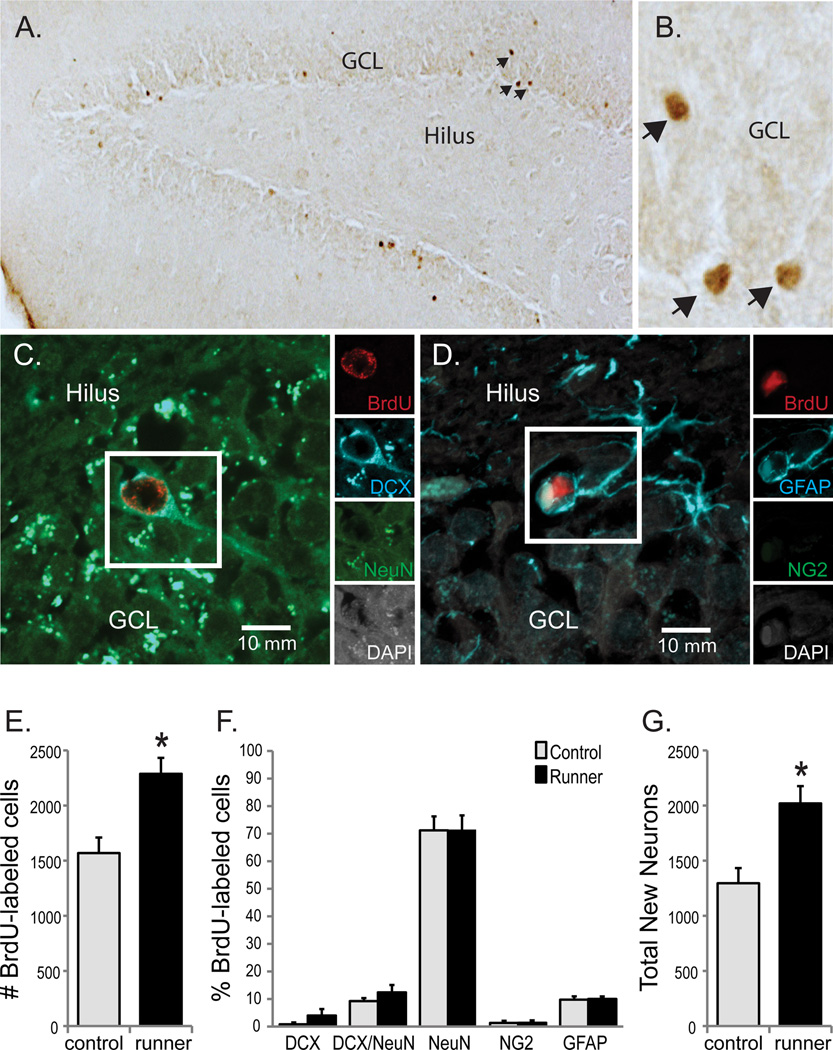
(A) Transmitted light micrograph showing new (BrdU+; in brown) cells located through the GCL and SGZ of aged rats under a 10X objective revealed by DAB. (B) Shows a subset of BrdU+ cells depicted in (A) under the 40X objective used for counting. (C and D) Confocal images of samples of new cells (BrdU/DAPI+; in white and red) expressing the neuronal proteins DCX (in blue) and NeuN (in green; [C]) or the astrocyte protein GFAP (in blue; [D]). Insets show each channel independently and scale bars represent 10 µm. (A, B and E) More BrdU+ cells were detected in the dentate gyri of conditioned runners vs. controls (*p < 0.01). (C, D and F) Similar percentages of new cells in the dentate gyri of conditioned runners and controls expressed immature neuronal (DCX+), transitioning neuronal (DCX/NeuN+), mature neuronal (NeuN+), oligodendroglial (NG2+) or astroglial (GFAP+) proteins. Consistent with the ~2 week survival period, most new cells expressed mature neuronal phenotypes, followed by astrocyte and transitioning neuronal phenotypes. (G) The total estimated new neuron number was significantly higher in conditioned runners versus controls (*p < 0.01). Data are group means ±S.E.M obtained from conditioned runners (black bars) and controls (gray bars).
We confirmed that new cell differentiation was unaffected by conditioned running by quantifying the percentage of BrdU+ cells expressing immature neuronal (DCX+), transitioning neuronal (DCX/NeuN+), mature neuronal (NeuN+), oligodendroglial (NG2+), or astroglial (GFAP+) phenotypes (Fig. 5C, D and F). Mann Whitney U tests (nrunner = 6 and ncontrol =5 in all comparisons) confirmed that the percentages of BrdU+ cells expressed immature neuronal (U=8, Z=1.187, p=0.024), transitioning neuronal (U=14.5, Z=0.0, p=1.0), mature neuronal (U=15.0, Z=0.0, p=1.00), GFAP+ (U=13.0, Z= −0.274, p=0.784) and oligodendrocyte precursor (U=14.5, Z=0.0, p=1.0) phenotypes was similar between conditioned runner and control rats. Consistent with a 2.5–3 week long survival period after BrdU, most new cells (~70%) expressed mature neuronal phenotypes followed by astrocyte and transitioning neuronal phenotypes (~10% each). Very few new cells expressed immature neuronal or or oligodendroglial phenotypes (<3%) in the dentate gyri of all rats combined (Fig. 5). Note that all of the BrdU/GFAP+ cells were detected outside of the subgranular zone and exhibited an astrocyte rather than radial glial (or neural stem cell)-like morphology.
The total new cell number (Fig. 5E) was multiplied by the % of neurons (immature, transitioning and mature), oligodendrocytes or astrocytes (Fig. 5F) for each rat to estimate total numbers of each new cell phenotype (Fig. 5G). Relative to controls, conditioned runners had significantly more new neurons (t(9) = 3.26; p < 0.01), tended to have more new astrocytes (157.41±31.27 and 227.59±22.06, respectively; t(9) = −1.88; p = 0.09), and had similar numbers of new oligodendrocyte precurors (26.31±16.55 and 31.43±19.89, respectively; t(9) = −0.19; p = 0.85). New neuron number tended to correlate positively with immediate (p = 0.08; Fig. 6A) and 24 h (p = 0.059; Fig. 6B) water maze probe discrimination index scores.
Figure 6. Probe trials scores relate to measures of neurogenesis in aging rats.
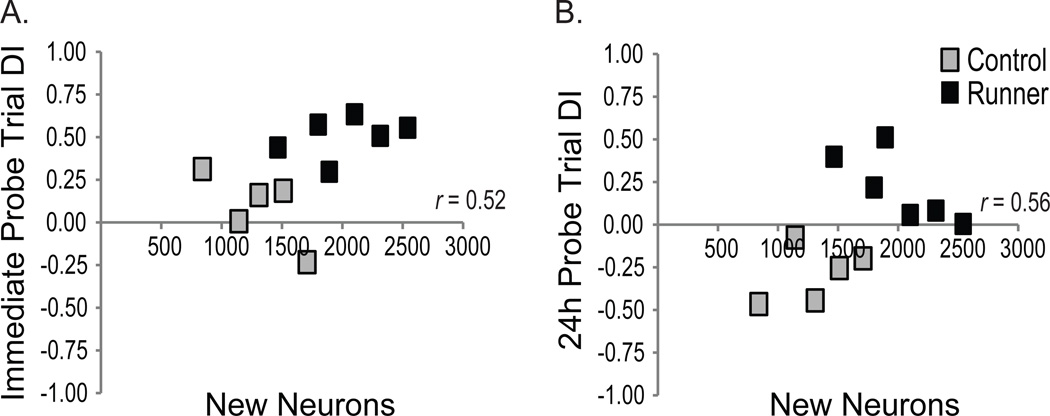
Spearman rank correlations were conducted on total new (BrdU+) DCX and/or NeuN+ neuron numbers and DI scores obtained from control rats (light gray squares) and conditioned runners (black squares). Total new neuron number tended to correlate with (A) DI scores obtained in the immediate probe trial (p=0.08) and (B) DI scores obtained from the 24 h probe trial (p = 0.059).
3.6. Distinct cytokine relationships were detected in serum, hippocampal and cortical compartments
Concentrations of 24 cytokines were quantified in blood serum and hippocampal and cortical protein samples of each behaviorally characterized rat that neurogenesis was also quantified in (Table 1). Note that concentrations of eotaxin, GRO-KC, IL-10, IL-13, IL-17, leptin, and RANTES were at least a magnitude higher in circulation versus the brain. IFNγ was only detected in blood serum whereas G-CSF, GM-CSF, IL1α, IL-2, IL-4, IL-5, IP-10 and TNFα were only detected in the brain. Interestingly, of the cytokines only detected in the brain, G-SCF, GM-CSF, IL-10, and IP-10 were detected in the cortex but not in the hippocampus. An ~2-fold higher concentration of IL-1β and MCP-1 was detected in the hippocampus versus cortex whereas an ~3-fold higher concentration of IL-12 and an ~2-fold higher concentration of IL-2 and IL-5 was detected in the cortex versus hippocampus. These data suggest that in aged rats, circulating cytokine concentrations do not appear to reflect central concentrations. In addition, there appear to be regional differences in the basal expression of central cytokines and therefore, likely their influence.
Table 1.
Some hippocampal (pg/mg), cortical (pg/mg) and circulating (pg/mL) cytokines are modulated by daily exercise in aging rats. Mean (±S.E.M.) values are reported.
| SERUM | HIPPOCAMPUS | CORTEX | ||||
|---|---|---|---|---|---|---|
| Controls | Runners | Controls | Runners | Controls | Runners | |
| Eotaxin | 55.06 ± 13.76 | 196.23 ± 147.02 | 2.37 ± 0.76 | 3.46 ± 0.87 | 1.90 ± 0.16 | 1.73 ± 0.27 |
| G-CSF | 0 ± 0 | 2.23 ± 2.23 | 0 ± 0 | 0 ± 0 | 0.25 ± 0.04 | 0.17 ± 0.07 |
| GM-CSF | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.09 ± 0.11 | 0.99 ± 0.33 |
| GRO-KC | 1322.52 ± 219.98 | 776.89 ± 154.63+ | 13.36 ± 2.57 | 25.83 ± 5.55+ | 9.82 ± 0.80 | 10.54 ± 2.28 |
| IFN-γ | 26.41 ± 18.51 | 415.65 ± 398.82 | 0 ± 0 | 1.52 ± 1.29 | 0 ± 0 | 0 ± 0 |
| IL-1α | 0 ± 0 | 233.95 ± 233.95 | 2.55 ± 2.55 | 14.81 ± 7.03 | 5.60 ± 1.06 | 4.96 ± 1.58 |
| IL-1β | 200.54 ± 174.94 | 222.88 ± 182.13 | 40.44 ± 3.46 | 22.27 ± 4.38** | 13.07 ± 2.38 | 13.56 ± 1.82 |
| IL-2 | 0 ± 0 | 0 ± 0 | 8.62 ± 5.09 | 13.73 ± 4.28 | 27.50 ± 1.75 | 22.47 ± 4.18 |
| IL-4 | 0 ± 0 | 47.56 ± 41.81 | 2.73 ± 0.90 | 4.88 ± 0.99 | 3.17 ± 0.33 | 2.63 ± 0.53 |
| IL-5 | 0 ± 0 | 18.32 ± 13.88 | 1.46 ± 1.01 | 1.73 ± 0.94 | 4.54 ± 0.56 | 3.84 ± 0.91 |
| IL-6 | 67.57 ± 21.94 | 537.54 ± 498.29 | 17.31 ± 8,12 | 30.14 ± 10.05 | 14.02 ± 1.82 | 16.28 ± 4.19 |
| IL-9 | 243.99 ± 137.04 | 190.51 ± 111.29 | 491.60 ± 301.67 | 588.73 ± 267.64 | 396.14 ± 50.08 | 385.10 ± 86.51 |
| IL-10 | 59.89 ± 59.89 | 59.98 ± 59.98 | 0 ± 0 | 0 ± 0 | 4.56 ± 0.37 | 4.52 ± 1.78 |
| IL-12 | 38.77 ± 21.73 | 68.30 ± 62.32 | 5.13 ± 1.50 | 7.60 ± 2.04 | 19.58 ± 3.29 | 17.99 ± 3.55 |
| IL-13 | 108.00 ± 38.54 | 332.52 ± 282.22 | 2.68 ± 2.06 | 9.42 ± 3.66 | 6.06 ± 0.25 | 5.13 ± 1.05 |
| IL-17 | 20.47 ± 8.29 | 29.24 ± 19.27 | 0.77 ± 0.40 | 0.68 ± 0.38 | 0.57 ± 0.09 | 0.48 ± 0.17 |
| IL-18 | 351.01 ± 189.13 | 789.49 ± 422.74 | 105.42 ± 31.38 | 209.72 ± 28.51* | 77.38 ± 3.90 | 70.00 ± 10.25 |
| IP-10 | 0 ± 0 | 53.56 ± 53.56 | 0 ± 0 | 5.72 ± 5.00 | 1.57 ± 0.11 | 1.41 ± 0.19 |
| Leptin | 10001.90 ± 850.70 | 5414.09 ± 743.34** | 17.84 ± 5.15 | 19.97 ± 6.43 | 10.39 ± 0.51 | 9.52 ± 1.86 |
| MCP-1 | 719.92 ± 109.32 | 323.09 ± 134.99* | 113.92 ± 68.04 | 138.22 ± 49.40 | 51.23 ± 3.85 | 39.93 ± 8.78 |
| MIP-1α | 5.67 ± 2.25 | 9.42 ± 6.61 | 0.61 ± 0.25 | 4.05 ± 3.09 | 0.34 ± 0.02 | 0.43 ± 0.09 |
| RANTES | 18364.54 ± 1635.46 | 11780.8 ± 3208.50 | 0 ± 0 | 13.49 ± 6.72 | 17.22 ± 1.86 | 42.96 ± 16.84 |
| TNF-α | 0 ± 0 | 2.40 ± 1.52 | 2.44 ± 1.09 | 3.27 ± 1.41 | 2.28 ± 0.06 | 2.20 ± 0.61 |
| VEGF | 21.80 ± 21.80 | 23.38 ± 23.38 | 3.46 ± 1.52 | 6.46 ± 2.68 | 1.91 ± 0.25 | 0.98 ± 0.33+ |
p<0.01,
p<0.05 and
0.05<p<0.10 vs. control values.
We next analyzed cytokine relationships within and between blood serum, hippocampal and cortical compartments to further explore the ideas that circulating concentrations may predict central cytokine signaling and that regional differences in central cytokine expression may reflect more local signaling. Pathway analyses (see Methods Section 2.10) revealed distinct clusters within but no clusters between compartments after Bonferroni adjustments for multiple comparisons (Table 2a–c and Fig. 7). In serum: 1) MCP-1 and GRO-KC and 2) IL-6 and IL-13 were identified as independent clusters. In the hippocampus: 1) IL-17 and VEGF, 2) IL-5 and VEGF, 3) MCP-1, IL-2 and VEGF, 4) MCP, IL-2, TNF-α, MIP-1α and IL-5, 5) IL-2 and GRO-KC, 6) eotaxin, TNFα and IL-12, 7) IL-2 and IL-4 and 8) IL-4 and IL-6 were identified as independent clusters. In the cortex: 1) IL-2 and GM-CSF, 2) GM-CSF and IL-18, 4) GM-CSF and IL-10, and 5) IL-13 and IL-4 were identified as independent clusters. While these results indicate strong relationships between cytokine concentrations within each brain region and within serum, no clusters emerged between these compartments. The lack of significant relationships between serum and brain cytokine concentrations may indicate that circulating factors neither diffuse nor are transported in detectable quantities into hippocampal and cortical regions in aging rats unchallenged by an inflammatory event (Erickson and Banks, 2011). The lack of significant relationships between hippocampal and cortical compartments suggests that basal neuroimmune signaling is a local event. Of course, the lack of significant between-compartments relationships could simply reflect the stringency inherent to Bonferroni adjustments, which increase the likelihood of type II errors.
Table 2.
Spearman rank correlation coefficients (rs) between cytokine pairs detected in (A) serum, (B) hippocampal and (C) cortical compartments reveal clusters (see Fig. 7).
| (A) SERUM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eotaxin | GRO-KC | IFN-γ | IL-1β | IL-6 | IL-9 | IL-10 | IL-12 | IL-13 | IL-17 | IL-18 | leptin | MCP-1 | MIP-1α | RANTES | VEGF | |
| eotaxin | −0.06 | 0.67 | −0.61 | 0.82 | −0.12 | −0.36 | 0.75 | 0.72 | 0.35 | −0.57 | −0.53 | −0.08 | −0.47 | −0.09 | −0.26 | |
| GRO-KC | −0.06 | 0.07 | −0.28 | 0.14 | 0.21 | 0.42 | −0.04 | −0.03 | 0.17 | −0.04 | 0.56 | 0.96* | 0.67 | 0.85 | −0.24 | |
| IFN-γ | 0.67 | 0.07 | −0.46 | 0.58 | −0.33 | 0.25 | 0.34 | 0.57 | 0.00 | −0.46 | −0.43 | 0.01 | −0.03 | 0.03 | −0.18 | |
| IL-1β | −0.61 | −0.28 | −0.46 | −0.52 | 0.44 | 0.14 | −0.51 | −0.55 | −0.36 | 0.83 | 0.22 | −0.27 | −0.01 | −0.48 | 0.51 | |
| IL-6 | 0.82 | 0.14 | 0.58 | −0.52 | −0.16 | −0.35 | 0.85 | 0.88* | 0.65 | −0.52 | −0.28 | 0.10 | −0.23 | 0.14 | −0.04 | |
| IL-9 | −0.12 | 0.21 | −0.33 | 0.44 | −0.16 | −0.04 | −0.15 | −0.47 | −0.18 | 0.26 | 0.29 | 0.30 | −0.11 | −0.01 | 0.03 | |
| IL-10 | −0.36 | 0.42 | 0.25 | 0.14 | −0.35 | −0.04 | −0.60 | −0.38 | −0.46 | 0.22 | 0.02 | 0.41 | 0.69 | 0.40 | −0.22 | |
| IL-12 | 0.75 | −0.04 | 0.34 | −0.51 | 0.85 | −0.15 | −0.60 | 0.85 | 0.69 | −0.47 | −0.15 | −0.09 | −0.55 | −0.08 | 0.12 | |
| IL-13 | 0.72 | −0.03 | 0.57 | −0.55 | 0.88* | −0.47 | −0.38 | 0.85 | 0.55 | −0.51 | −0.17 | −0.08 | −0.37 | 0.04 | 0.04 | |
| IL-17 | 0.35 | 0.17 | 0.00 | −0.36 | 0.65 | −0.18 | −0.46 | 0.69 | 0.55 | −0.38 | 0.03 | 0.12 | −0.05 | 0.21 | −0.07 | |
| IL-18 | −0.57 | −0.04 | −0.46 | 0.83 | −0.52 | 0.26 | 0.22 | −0.47 | −0.51 | −0.38 | 0.31 | 0.01 | 0.17 | −0.35 | 0.61 | |
| leptin | −0.53 | 0.56 | −0.43 | 0.22 | −0.28 | 0.29 | 0.02 | −0.15 | −0.17 | 0.03 | 0.31 | 0.65 | 0.26 | 0.44 | 0.29 | |
| MCP-1 | −0.08 | 0.96* | 0.01 | −0.27 | 0.10 | 0.30 | 0.41 | −0.09 | −0.08 | 0.12 | 0.01 | 0.65 | 0.73 | 0.83 | −0.20 | |
| MIP-1α | −0.47 | 0.67 | −0.03 | −0.01 | −0.23 | −0.11 | 0.69 | −0.55 | −0.37 | −0.05 | 0.17 | 0.26 | 0.73 | 0.68 | −0.16 | |
| RANTES | −0.09 | 0.85 | 0.03 | −0.48 | 0.14 | −0.01 | 0.40 | −0.08 | 0.04 | 0.21 | −0.35 | 0.44 | 0.83 | 0.68 | −0.50 | |
| VEGF | −0.26 | −0.24 | −0.18 | 0.51 | −0.04 | 0.03 | −0.22 | 0.12 | 0.04 | −0.07 | 0.61 | 0.29 | −0.20 | −0.16 | −0.50 | |
| (B) HIPPOCAMPUS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eotaxin | GRO-KC | IL-1α | IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-9 | IL-12 | IL-13 | IL-17 | IL-18 | leptin | MCP-1 | MIP-1α | RANTES | TNF-α | VEGF | |
| eotaxin | 0.79 | 0.77 | −0.10 | 0.84 | 0.84 | 0.81 | 0.88 | 0.85 | 0.89* | 0.86 | 0.68 | 0.70 | 0.69 | 0.79 | 0.78 | 0.36 | 0.89* | 0.82 | |
| GRO-KC | 0.79 | 0.78 | −0.25 | 0.90* | 0.86 | 0.72 | 0.88 | 0.70 | 0.74 | 0.77 | 0.63 | 0.85 | 0.42 | 0.82 | 0.75 | 0.79 | 0.80 | 0.87 | |
| IL-1α | 0.77 | 0.78 | −0.03 | 0.69 | 0.66 | 0.69 | 0.85 | 0.65 | 0.83 | 0.75 | 0.56 | 0.71 | 0.64 | 0.56 | 0.70 | 0.60 | 0.69 | 0.72 | |
| IL-1β | −0.10 | −0.25 | −0.03 | −0.10 | −0.30 | 0.13 | −0.18 | 0.14 | −0.05 | −0.27 | 0.28 | −0.60 | 0.01 | 0.06 | 0.04 | −0.36 | 0.03 | 0.07 | |
| IL-2 | 0.84 | 0.90* | 0.69 | −0.10 | 0.90* | 0.92* | 0.84 | 0.83 | 0.79 | 0.68 | 0.77 | 0.68 | 0.42 | 0.95* | 0.92* | 0.53 | 0.94* | 0.90* | |
| IL-4 | 0.84 | 0.86 | 0.66 | −0.30 | 0.90* | 0.83 | 0.90* | 0.79 | 0.78 | 0.66 | 0.68 | 0.70 | 0.47 | 0.86 | 0.80 | 0.56 | 0.86 | 0.87 | |
| IL-5 | 0.81 | 0.72 | 0.69 | 0.13 | 0.92* | 0.83 | 0.77 | 0.88 | 0.78 | 0.57 | 0.88 | 0.43 | 0.44 | 0.89* | 0.93* | 0.33 | 0.91* | 0.90* | |
| IL-6 | 0.88 | 0.88 | 0.85 | −0.18 | 0.84 | 0.90* | 0.77 | 0.80 | 0.87 | 0.70 | 0.59 | 0.75 | 0.67 | 0.80 | 0.79 | 0.56 | 0.83 | 0.81 | |
| IL-9 | 0.85 | 0.70 | 0.65 | 0.14 | 0.83 | 0.79 | 0.88 | 0.80 | 0.71 | 0.55 | 0.88 | 0.40 | 0.59 | 0.88 | 0.84 | 0.12 | 0.82 | 0.87 | |
| IL-12 | 0.89* | 0.74 | 0.83 | −0.05 | 0.79 | 0.78 | 0.78 | 0.87 | 0.71 | 0.74 | 0.58 | 0.68 | 0.81 | 0.71 | 0.84 | 0.40 | 0.91* | 0.72 | |
| IL-13 | 0.86 | 0.77 | 0.75 | −0.27 | 0.68 | 0.66 | 0.57 | 0.70 | 0.55 | 0.74 | 0.49 | 0.84 | 0.49 | 0.53 | 0.54 | 0.58 | 0.69 | 0.70 | |
| IL-17 | 0.68 | 0.63 | 0.56 | 0.28 | 0.77 | 0.68 | 0.88 | 0.59 | 0.88 | 0.58 | 0.49 | 0.26 | 0.33 | 0.82 | 0.80 | 0.08 | 0.75 | 0.90* | |
| IL-18 | 0.70 | 0.85 | 0.71 | −0.60 | 0.68 | 0.70 | 0.43 | 0.75 | 0.40 | 0.68 | 0.84 | 0.26 | 0.45 | 0.50 | 0.53 | 0.83 | 0.61 | 0.56 | |
| leptin | 0.69 | 0.42 | 0.64 | 0.01 | 0.42 | 0.47 | 0.44 | 0.67 | 0.59 | 0.81 | 0.49 | 0.33 | 0.45 | 0.41 | 0.59 | 0.05 | 0.62 | 0.38 | |
| MCP-1 | 0.79 | 0.82 | 0.56 | 0.06 | 0.95* | 0.86 | 0.89* | 0.80 | 0.88 | 0.71 | 0.53 | 0.82 | 0.50 | 0.41 | 0.89* | 0.38 | 0.91* | 0.90* | |
| MIP-1α | 0.78 | 0.75 | 0.70 | 0.04 | 0.92* | 0.80 | 0.93* | 0.79 | 0.84 | 0.84 | 0.54 | 0.80 | 0.53 | 0.59 | 0.89* | 0.31 | 0.93* | 0.80 | |
| RANTES | 0.36 | 0.79 | 0.60 | −0.36 | 0.53 | 0.56 | 0.33 | 0.56 | 0.12 | 0.40 | 0.58 | 0.08 | 0.83 | 0.05 | 0.38 | 0.31 | 0.36 | 0.49 | |
| TNF-α | 0.89* | 0.80 | 0.69 | 0.03 | 0.94* | 0.86 | 0.91* | 0.83 | 0.82 | 0.91* | 0.69 | 0.75 | 0.61 | 0.62 | 0.91* | 0.93* | 0.36 | 0.85 | |
| VEGF | 0.82 | 0.87 | 0.72 | 0.07 | 0.90* | 0.87 | 0.90* | 0.81 | 0.87 | 0.72 | 0.70 | 0.90* | 0.56 | 0.38 | 0.90* | 0.80 | 0.49 | 0.85 | |
| (C) CORTEX | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eotaxin | G-CSF | GM-CSF | GRO-KC | IL-1α | IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-9 | IL-10 | IL-12 | IL-13 | IL-17 | IL-18 | IP-10 | leptin | MCP-1 | MIP-1α | RANTES | TNF-α | VEGF | |
| eotaxin | 0.54 | 0.82 | 0.06 | 0.55 | 0.25 | 0.70 | 0.80 | 0.64 | 0.47 | 0.68 | 0.82 | 0.54 | 0.88 | 0.52 | 0.70 | 0.68 | 0.63 | 0.12 | 0.37 | −0.23 | 0.60 | 0.83 | |
| G-CSF | 0.54 | 0.41 | 0.62 | 0.63 | 0.15 | 0.58 | 0.62 | 0.48 | 0.58 | 0.17 | 0.44 | 0.63 | 0.57 | 0.48 | 0.30 | 0.68 | 0.71 | −0.31 | 0.53 | −0.12 | 0.63 | 0.72 | |
| GM-CSF | 0.82 | 0.41 | 0.09 | 0.71 | 0.19 | 0.91* | 0.84 | 0.45 | 0.55 | 0.62 | 0.93* | 0.61 | 0.87 | 0.69 | 0.92* | 0.73 | 0.55 | 0.03 | 0.18 | −0.33 | 0.72 | 0.65 | |
| GRO-KC | 0.06 | 0.62 | 0.09 | 0.29 | 0.16 | 0.19 | 0.31 | 0.24 | 0.05 | −0.15 | 0.05 | 0.42 | 0.27 | −0.09 | 0.14 | 0.56 | 0.55 | −0.55 | 0.71 | 0.16 | 0.61 | 0.19 | |
| IL-1α | 0.55 | 0.63 | 0.71 | 0.29 | −0.25 | 0.80 | 0.87 | 0.66 | 0.73 | 0.47 | 0.67 | 0.82 | 0.75 | 0.74 | 0.62 | 0.52 | 0.38 | 0.14 | 0.10 | −0.46 | 0.55 | 0.56 | |
| IL-1β | 0.25 | 0.15 | 0.19 | 0.16 | −0.25 | 0.10 | −0.06 | −0.28 | 0.03 | 0.19 | 0.35 | −0.13 | 0.15 | 0.08 | 0.16 | 0.25 | 0.51 | −0.50 | 0.50 | −0.16 | 0.35 | 0.15 | |
| IL-2 | 0.70 | 0.58 | 0.91* | 0.19 | 0.80 | 0.10 | 0.80 | 0.41 | 0.55 | 0.52 | 0.82 | 0.60 | 0.75 | 0.76 | 0.82 | 0.71 | 0.51 | 0.02 | 0.06 | −0.38 | 0.75 | 0.73 | |
| IL-4 | 0.80 | 0.62 | 0.84 | 0.31 | 0.87 | −0.06 | 0.80 | 0.82 | 0.59 | 0.65 | 0.83 | 0.83 | 0.95* | 0.56 | 0.71 | 0.67 | 0.57 | 0.20 | 0.33 | −0.44 | 0.67 | 0.68 | |
| IL-5 | 0.64 | 0.48 | 0.45 | 0.24 | 0.66 | −0.28 | 0.41 | 0.82 | 0.43 | 0.64 | 0.51 | 0.75 | 0.75 | 0.24 | 0.37 | 0.30 | 0.30 | 0.35 | 0.24 | −0.29 | 0.38 | 0.49 | |
| IL-6 | 0.47 | 0.58 | 0.55 | 0.05 | 0.73 | 0.03 | 0.55 | 0.59 | 0.43 | 0.34 | 0.64 | 0.72 | 0.60 | 0.80 | 0.45 | 0.33 | 0.41 | −0.19 | 0.12 | −0.29 | 0.32 | 0.38 | |
| IL-9 | 0.68 | 0.17 | 0.62 | −0.15 | 0.47 | 0.19 | 0.52 | 0.65 | 0.64 | 0.34 | 0.77 | 0.32 | 0.63 | 0.51 | 0.43 | 0.25 | 0.38 | 0.39 | 0.12 | −0.55 | 0.25 | 0.35 | |
| IL-10 | 0.82 | 0.44 | 0.93* | 0.05 | 0.67 | 0.35 | 0.82 | 0.83 | 0.51 | 0.64 | 0.77 | 0.59 | 0.87 | 0.72 | 0.76 | 0.63 | 0.65 | 0.05 | 0.29 | −0.51 | 0.61 | 0.56 | |
| IL-12 | 0.54 | 0.63 | 0.61 | 0.42 | 0.82 | −0.13 | 0.60 | 0.83 | 0.75 | 0.72 | 0.32 | 0.59 | 0.82 | 0.41 | 0.65 | 0.44 | 0.35 | −0.09 | 0.24 | −0.27 | 0.68 | 0.53 | |
| IL-13 | 0.88 | 0.57 | 0.87 | 0.27 | 0.75 | 0.15 | 0.75 | 0.95* | 0.75 | 0.60 | 0.63 | 0.87 | 0.82 | 0.51 | 0.80 | 0.68 | 0.61 | 0.05 | 0.42 | −0.36 | 0.74 | 0.71 | |
| IL-17 | 0.52 | 0.48 | 0.69 | −0.09 | 0.74 | 0.08 | 0.76 | 0.56 | 0.24 | 0.80 | 0.51 | 0.72 | 0.41 | 0.51 | 0.51 | 0.45 | 0.44 | −0.01 | −0.02 | −0.40 | 0.30 | 0.44 | |
| IL-18 | 0.70 | 0.30 | 0.92* | 0.14 | 0.62 | 0.16 | 0.82 | 0.71 | 0.37 | 0.45 | 0.43 | 0.76 | 0.65 | 0.80 | 0.51 | 0.61 | 0.34 | −0.12 | 0.07 | −0.15 | 0.80 | 0.60 | |
| IP-10 | 0.68 | 0.68 | 0.73 | 0.56 | 0.52 | 0.25 | 0.71 | 0.67 | 0.30 | 0.33 | 0.25 | 0.63 | 0.44 | 0.68 | 0.45 | 0.61 | 0.85 | −0.31 | 0.64 | 0.06 | 0.73 | 0.63 | |
| leptin | 0.63 | 0.71 | 0.55 | 0.55 | 0.38 | 0.51 | 0.51 | 0.57 | 0.30 | 0.41 | 0.38 | 0.65 | 0.35 | 0.61 | 0.44 | 0.34 | 0.85 | −0.37 | 0.83 | −0.09 | 0.55 | 0.46 | |
| MCP-1 | 0.12 | −0.31 | 0.03 | −0.55 | 0.14 | −0.50 | 0.02 | 0.20 | 0.35 | −0.19 | 0.39 | 0.05 | −0.09 | 0.05 | −0.01 | −0.12 | −0.31 | −0.37 | −0.45 | −0.51 | −0.36 | 0.08 | |
| MIP-1α | 0.37 | 0.53 | 0.18 | 0.71 | 0.10 | 0.50 | 0.06 | 0.33 | 0.24 | 0.12 | 0.12 | 0.29 | 0.24 | 0.42 | −0.02 | 0.07 | 0.64 | 0.83 | −0.45 | 0.07 | 0.40 | 0.23 | |
| RANTES | −0.23 | −0.12 | −0.33 | 0.16 | −0.46 | −0.16 | −0.38 | −0.44 | −0.29 | −0.29 | −0.55 | −0.51 | −0.27 | −0.36 | −0.40 | −0.15 | 0.06 | −0.09 | −0.51 | 0.07 | −0.13 | −0.24 | |
| TNF-α | 0.60 | 0.63 | 0.72 | 0.61 | 0.55 | 0.35 | 0.75 | 0.67 | 0.38 | 0.32 | 0.25 | 0.61 | 0.68 | 0.74 | 0.30 | 0.80 | 0.73 | 0.55 | −0.36 | 0.40 | −0.13 | 0.69 | |
| VEGF | 0.83 | 0.72 | 0.65 | 0.19 | 0.56 | 0.15 | 0.73 | 0.68 | 0.49 | 0.38 | 0.35 | 0.56 | 0.53 | 0.71 | 0.44 | 0.60 | 0.63 | 0.46 | 0.08 | 0.23 | −0.24 | 0.69 | |
After Bonferroni adjusted α-levels: *p<0.00042 in serum, *p<0.00029 in hippocampus and *p<0.00020 in cortex.
Note that no between compartments clusters emerged after Bonferroni corrections.
Figure 7. Cytokine clusters detected in the serum, hippocampal and cortical samples obtained from aging rats.
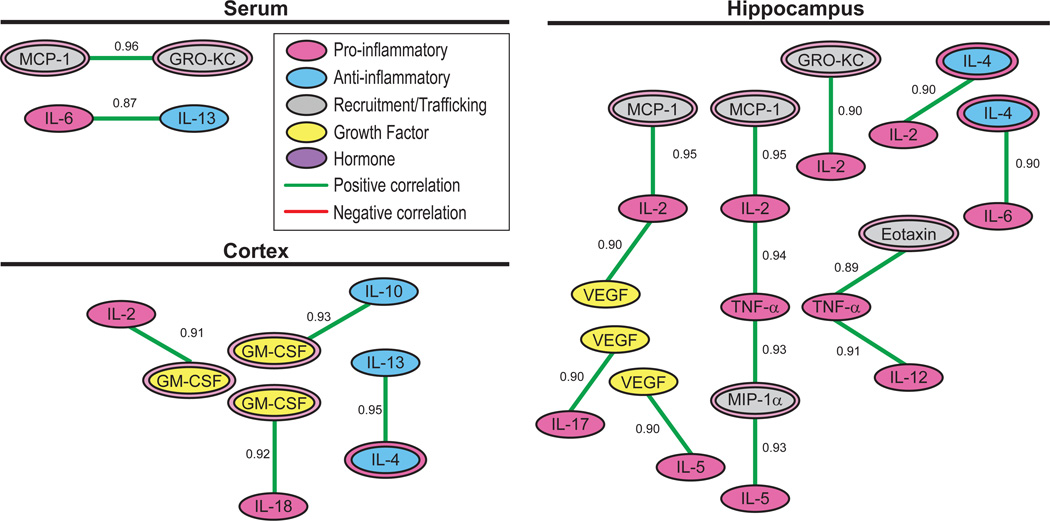
To confirm and expand upon known cytokine pathways, we examined cytokines with concentrations that changed in a coordinated fashion. Cytokine pairs were plotted in descending order based upon Spearman r values deemed statistically significant after Bonferroni corrections. If one cytokine in a correlated pair about to be plotted was already part of a plotted cluster, and the unplotted cytokine was correlated with all cytokines in the plotted cluster, then the new pair was added to the cluster. If the unplotted cytokine of the pair about to be plotted was not significantly correlated with all cytokines in the existing cluster, the pair was plotted as a new cluster. Proteins are color coded by their known primary function and green and red lines represent positive and negative correlations, respectively. We detected 2 serum cytokine clusters, 8 hippocampus cytokine clusters and 4 cortical clusters in aging rats. Note that no between-compartment clusters indicative of immune-to-brain signaling pathways modulated by running in aged rats were detected.
3.7. Measures of behavior and neurogenesis relate to concentrations of cytokines modulated by running
To identify cytokine candidates linked to behavior and neurogenesis, we first identified cytokines that were modulated by exercise using Student’s t-tests (see Table 1). Compared to controls, conditioned runners had significantly lower hippocampal IL-1β (t(9) = 3.14; p < 0.05), circulating MCP-1 (t(9) = 2.28; p ≤ 0.05) and circulating leptin (t(9) = 4.06; p < 0.01) but higher hippocampal IL-18 (t(9) = −2.46, p < 0.05) concentrations. Concentrations of circulating GRO-KC (t(9) = 2.08; p = 0.07) and cortical VEGF (t(9) = 2.16, p = 0.06) tended to be lower whereas hippocampal concentrations of GRO-KC (t(9) = −1.90, p = 0.09) tended to be higher in runners versus controls.
Of the cytokines with concentrations that were significantly modulated by conditioned running in aged rats, several were modulated in a correlated manner (see Fig. 7A and Table 3). For example serum MCP-1, which was decreased by conditioned running, correlated positively with serum GRO-KC (p < 0.01) and both concentrations tended to correlate positively with serum leptin (p = 0.06 and p = 0.08). Serum leptin was strongly decreased by conditioned running and correlated positively with hippocampal IL-1β (p < 0.05) but tended to correlate negatively with hippocampal IL-18 (p = 0.06) and hippocampal GRO-KC (p = 0.08). Hippocampal IL-1β was decreased by conditioned running and correlated negatively with hippocampal IL-18 (p < 0.05) and tended to correlate positively with cortical VEGF (p = 0.07). A strong positive correlation was detected between hippocampal IL-18, which was increased with running, and hippocampal GRO-KC (p < 0.01). These data suggest that conditioned running modulates subsets of cytokines within and between serum, hippocampal and cortical compartments.
Table 3.
Measures of several variables significantly modulated by daily exercise in aging rats correlate. Spearman rank correlation coefficients (rs) were calculated test the strength of the relationships between concentrations of serum (S), hippocampal (H) and cortical (C) cytokines, and measures of spatial and hippoocampal neurogenesis that were significantly modulated by conditioned running.
| SERUM | HIPPOCAMPUS | CORTEX | NEUROGENESIS & BEHAVIOR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCP-1 | leptin | GRO-KC | IL-1β | IL-18 | GRO-KC | VEGF | New Neuron # | Immed. DI | 24 hr DI | 24 hr IA | |
| MCP-1 (S) | 0.65+ | 0.96*** | 0.54 | −0.36 | −0.14 | 0.13 | −0.53 | −0.81** | −0.39 | −0.30 | |
| leptin (S) | 0.65+ | 0.56+ | 0.76* | −0.61+ | −0.58+ | 0.52 | −0.77** | −0.73* | −0.78** | −0.46 | |
| GRO-KC (S) | 0.96*** | 0.56+ | 0.42 | −0.29 | −0.20 | 0.07 | −0.41 | −0.75** | −0.48 | −0.18 | |
| IL-1β (H) | 0.54 | 0.76* | 0.42 | −0.60* | −0.25 | 0.57+ | −0.58+ | −0.58+ | −0.68* | −0.63* | |
| IL-18 (H) | −0.36 | −0.61+ | −0.29 | −0.60* | 0.85*** | −0.51 | 0.94*** | 0.51 | 0.54 | 0.16 | |
| GRO-KC (H) | −0.14 | −0.58+ | −0.20 | −0.25 | 0.85*** | −0.37 | 0.79** | 0.38 | 0.41 | −0.11 | |
| VEGF (C) | 0.13 | 0.52 | 0.07 | 0.57+ | −0.51 | −0.37 | −0.43 | −0.64* | −0.51 | −0.60* | |
| New Neuron # | −0.53 | −0.77** | −0.41 | −0.58+ | 0.94*** | 0.79** | −0.43 | 0.59+ | 0.55+ | 0.18 | |
| Immed. DI | −0.81** | −0.73* | −0.75** | −0.58+ | 0.51 | 0.38 | −0.64* | 0.59+ | 0.47 | 0.45 | |
| 24 hr DI | −0.39 | −0.78** | −0.48 | −0.68* | 0.54 | 0.41 | −0.51 | 0.55+ | 0.47 | 0.62* | |
| 24 hr IA | −0.30 | −0.46 | −0.18 | −0.63* | 0.16 | −0.11 | −0.60+ | 0.18 | 0.45 | 0.62* | |
p < 0.001,
p<0.01,
p < 0.05 and
0.05 < p < 0.10.
Next we examined the strength of relationships between variables significantly affected by conditioned running (total new neuron number, probe trial discrimination index scores and 24 h inhibitory avoidance retention latencies) using Spearman rank correlations (see Table 3). Interestingly, 24 h retention latencies on the inhibitory avoidance task correlated positively with water maze 24 h probe discrimination index scores (p < 0.05). These data suggest that these tasks are both similarly sensitive to age-related cognitive decline and the beneficial effects of conditioned running on spatial ability in aged rats. As mentioned previously, new neuron number tended to correlate positively with immediate (p = 0.08) and 24 h (p = 0.059) water maze probe discrimination index scores (see Fig. 8B and Table 3).
Figure 8. Some cytokines are modulated in a coordinated fashion by conditioned running in aging rats and relate to measures of hippocampus-dependent behavior and hippocampal neurogenesis.
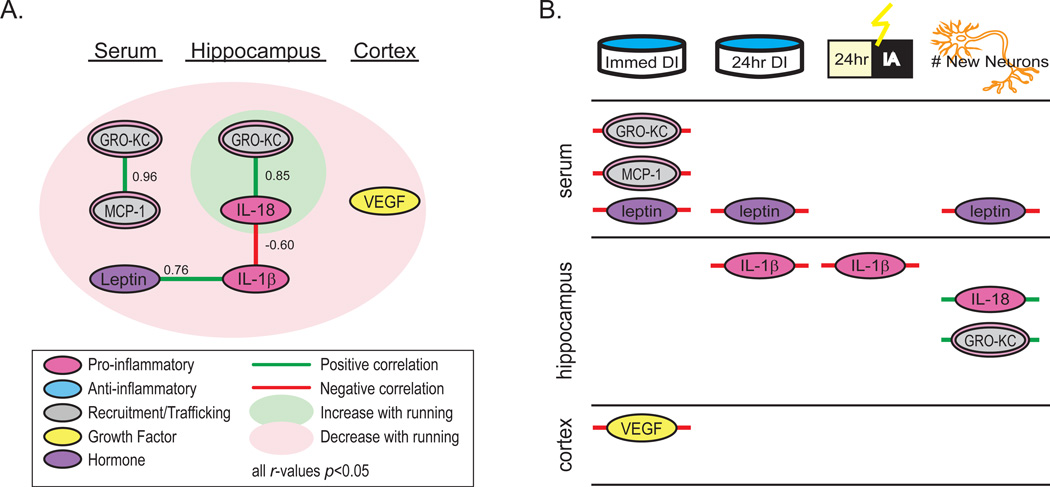
Spearman rank correlations were run on immune and neuroimmune cytokines with concentrations that were modulated by running (see Table 1), water maze DI scores, inhibitory avoidance retention latencies and total new neuron number. Of the cytokines altered by daily exercise, several were modulated in a coordinated fashion. Cytokines are color-coded to denote their primary, typically systemic, known function. Concentrations increased by running are plotted in the green circle while those that decrease are plotted in the red circle. Negatively correlated cytokines are linked with red lines while positively correlated cytokines are linked with green lines. (B) Depicts relationships between cytokines, behavioral measures and measures of neurogenesis that were modulated by running. Water maze discrimination index scores, inhibitory avoidance 24 h retention latencies and new neuron number were significantly affected by conditioned running. Note that only statistically significant correlations (p < 0.05) are shown.
Finally, we explored relationships between cytokine, behavioral and neurogenesis measures that were modulated by conditioned running (Table 3 and Fig. 8B). New neuron number, which was potentiated by running, correlated negatively with serum leptin level (p < 0.01) but positively with hippocampal IL-18 (p < 0.001) and hippocampal GRO-KC (p < 0.01) expression and tended to correlate negatively with hippocampal IL-1β expression (p = 0.06). Immediate probe trial discrimination index scores, which increased with running, correlated negatively with cortical VEGF levels (p < 0.05) and circulating levels of leptin (p < 0.05) MCP-1 (p < 0.01) and GRO-KC (p < 0.01) and tended to correlate negatively with hippocampal IL-1β (p = 0.06). Twenty-four hour discrimination index scores correlated negatively with circulating leptin levels (p < 0.01) and hippocampal IL-1β expression (p < 0.05). Interestingly, serum leptin levels correlated negatively with immediate discrimination index scores (p < 0.05), 24 h discrimination index scores (p < 0.01) and new neuron numbers (p < 0.01). Serum leptin level correlated positively with hippocampal IL-1β concentrations (p < 0.05), and hippocampal IL-1β concentrations correlated negatively with 24 h water maze (p < 0.05) and 24 inhibitory retention (p < 0.05) performances. Cortical VEGF tended to correlate negatively with 24 h inhibitory retention latencies (p = 0.06).
4. Discussion
An important goal for aging research is to identify markers of biological aging that predict cognitive decline. In the current study, we measured hippocampal neurogenesis and identified potential serum and central markers in rats after rejuvenating their cognition with a behavioral treatment. Several months of food-motivated wheel running improved aging rats’ abilities to rapidly learn a hidden platform location and retain or consolidate spatial/contextual information. Hippocampal neurogenesis is a well-characterized marker of brain aging that was potentiated by daily exercise in the current study along with correlated increases in hippocampal IL-18 and GRO-KC. Both central and peripheral markers of inflammation have been hypothesized to contribute to age-related decreases in cognitive function and we found that daily exercise decreased hippocampal IL-1β expression, which was correlated negatively with water maze and inhibitory avoidance memory scores. Due to the invasive nature of identifying markers in brain tissue, many researchers have focused upon identifying serum markers. In the aging rat, leptin emerged as a potential serum marker for age-related declines in cognition and plasticity because it correlated negatively with water maze performances and new neuron number. Moreover, daily exercise decreased serum leptin along with serum MCP-1 (CCL2) levels and tended to decrease serum GRO-KC (CXCL1) level. Interestingly, serum leptin, GRO-KC and MCP-1 levels along with cortical VEGF level (which tended to decrease with daily exercise) correlated negatively with the water maze learning index. Our data suggest that daily exercise may rejuvenate cognition and neurogenesis in aging rats by modulating immune and neuroimmune signaling pathways.
Our data suggest that the exercise protocol employed may rejuvenate cognition and neurogenesis in aging rats by modulating immune and neuroimmune signaling pathways. Importantly, the observed benefits may also be due to a caloric restriction associated with the exercise protocol. Exercise and caloric restriction beneficially affect a number of biological processes in aged rats that include the modulation of inflammatory signaling pathways (see Chung et al., 2009). However, in the current study any caloric restriction was voluntary and very mild producing body weight changes of less than 10% (compare masses in Section 2.1; Bondolfi et al., 2004; Lee et al., 2000; Van der Borght et al., 2007), which would also be consistent with an exercise-induced increase in fitness. Exercise is known to stimulate neurogenesis in young and aged animals (Albeck et al., 2006; Brown et al., 2003; Kobila et al., 2011; Parachikova et al., 2008; van Praag et al., 2005) while the effects of caloric restriction on neurogenesis may be limited to younger animals (Lee et al., 2000; Van der Borght, 2007; Bondolfi, 2004). Regardless, the inflammation associated with obesity and a sedentary lifestyle is thought to contribute to diseases of aging and an understanding how exercise with and without caloric restriction influences inflammatory signaling cascades will be important for the development of treatments.
Consistent with previous studies conducted using socially isolated animals, wheel running improved cognition (Albeck et al., 2006; Parachikova et al., 2008; van Praag et al., 2005) and amplified basal levels of hippocampal neurogenesis without altering the percentage of new cells that acquired neuronal or glial fates (Brown et al., 2003; Farmer et al., 2004; Kannangara et al., 2011; Kannangara et al., 2009; Kronenberg et al., 2006; Mustroph et al., 2012; Snyder et al., 2009). Although our multiple injection paradigm and ~16–21 d-long survival period cannot dissociate the between the effects of exercise on NPC proliferation versus the survivability of new cells, these data are consistent with those of several other studies showing that physical exercise increases NPC proliferation (Kempermann et al., 2010; van Praag et al,, 1999). New neuron number tended to correlate positively with immediate and delayed water maze probe trial discrimination index scores but not inhibitory avoidance retention latency scores. Importantly, the 900 s latency ceiling employed in the inhibitory avoidance task may have masked the relationship between the memory of contextual information and new neuron number. We recently reported that new neuron number strongly correlated with discrimination index scores in environmentally and socially enriched aged rats and their controls (Speisman et al., 2012). While physical activity is typically considered to induce neurogenesis, environmental enrichment is typically considered to promote the survival and potentially the integration of new neurons into active hippocampal networks (Deisseroth et al., 2004; Deng et al., 2010; Gould et al., 1999a; Leuner et al., 2006; Stephens et al., 2012 but see Kobilo et al., 2011), which may more profoundly impact hippocampal integrity (Kempermann et al., 2010).
Figure 8 illustrates a potential link between serum inflammatory markers and hippocampal cytokines associated with cognition and neurogenesis. Exercise-modulated circulating leptin level (Chennaoui et al., 2008; Novelli et al., 2004) correlated negatively with maze discrimination index scores, new neuron number and the hippocampal expression of IL-18 but positively with hippocampal IL-1β expression. Although leptin’s pleiotropic effects are typically associated with energy regulation, recent work suggests that leptin can directly stimulate the proliferation of neural progenitor cells both in vitro and in vivo (Garza et al., 2008; Perez-Gonzalez et al.). These data would suggest that serum leptin influences hippocampal neurogenesis in the aging rat through an intermediary signaling molecule or simply that the serum leptin levels detected in aged rats exceed those found in healthy young animals to the point that they become detrimental. Consistent with the latter notion, exercise decreased leptin levels in our aging rats. Indeed, leptin is emerging as a potential immune-to-brain signaling mediator (Hosoi et al., 2002) because leptin levels are elevated by peripheral inflammatory stimuli (Mastronardi et al., 2005; Sarraf et al., 1997) that incidentally decrease neurogenesis (Monje et al., 2003) and leptin treatment increases brain levels of hippocampal IL-1β (Hosoi et al., 2002). Alternatively, serum leptin concentration in the current study may simply be a marker for an immune signaling cascade containing the molecule that affects neurogenesis.
Indeed, serum GRO-KC and MCP-1 levels were also decreased by exercise, strongly correlated with one another, and independently (along with leptin) correlated with the acquisition of a spatial search strategy in the water maze (Tables 1 and 3 and Fig. 8). Previous work has shown that MCP-1 levels that are elevated by high fat diet-induced obesity in young mice, can be reduced by daily exercise (Kizaki et al., 2011). Recently, Villeda and colleagues found that circulating CCL2, along with eotaxin (CCL11), MCP-5 (CCL12), MIP-3β (CCL19), haptoglobin and β2 microglobulin levels were related to age-impaired neurogenesis and performance in a working/reference memory radial water maze task. They then showed that circulating eotaxin levels alone could impair neurogenesis, synaptic plasticity, working/reference memory and contextual fear conditioning (Villeda et al., 2011). We neither detected an effect of exercise on circulating eotaxin levels, nor relationships between circulating eotaxin levels and measures of neurogenesis or water maze performance in aging rats. However, age-related changes in circulating eotaxin may be species-dependent or relate to cognition and neurogenesis by interacting with another variable that differed between studies. Nonetheless, exploring the molecular mechanisms by which circulating factors, such as leptin, GRO-KC, MCP-1 and perhaps eotaxin relate to measures of cognition and plasticity are important future research avenues.
Another important finding of the current study is the compartmental specificity of exercise-associated changes in cytokine levels. After several months of daily exercise, leptin levels decreased only in serum and IL-1β was reduced and IL-18 increased only in the hippocampus (Table 1). A previous report also found that daily treadmill exercise decreases hippocampal, but not circulating, cortical, cerebellar or pituitary levels of IL-1β in young rats (Chennaoui et al., 2008). Our data extends these findings to aging rats, by showing that hippocampal, but not cortical or circulating IL-1β levels are reduced by exercise. Furthermore, hippocampal IL-1β expression was correlated positively with serum leptin and negatively correlated with both water maze and inhibitory avoidance memory scores (Table 3 and Fig. 8). Leptin is actively transported across the blood-brain-barrier (Morrison, 2009) and previous work has demonstrated that leptin treatment increases IL-1β in the hippocampus (Hosoi et al., 2002), providing one possible mechanism for the observed relationship. Moreover, the inverse relationship between IL-1β and cognition is consistent with a growing body of work indicating that elevated hippocampal IL-1β levels impair memory and synaptic plasticity in young and aged rats (Barrientos et al., 2009; Barrientos et al., 2006; Barrientos et al., 2003; Chennaoui et al., 2008; Hein et al., 2010; O'Callaghan et al., 2009). Together these data suggest that hippocampal IL-1β concentration may be a reliable biomarker of mnemonic decline and, along with circulating leptin, a target for nootropic drug development.
In contrast to IL-1β, IL-18 and GRO-KC levels were increased in the hippocampus of the exercise group. Hippocampal IL-18 and GRO-KC concentrations correlated positively with one another and independently with new neuron number, while hippocampal IL-18 concentration correlated negatively with hippocampal IL-1β concentration (Fig. 8). Daily exercise has been shown previously to potentiate hippocampal GROα (murine CXCL1) mRNA levels in aging Tg2576 mice that express human amyloid protein (Parachikova et al., 2008). Interestingly, these mice also exhibited improved water radial arm maze performance and decreased hippocampal IL-1β levels (Nichol et al., 2008; Parachikova et al., 2008). GRO-KC stimulates adult rat spinal cord oligodendrocyte (Robinson et al., 1998) and fetal ventral midbrain precursor (Edman et al., 2008) proliferation and IL-18 may attenuate neuronal differentiation in cultured fetal rat-derived neural progenitor cells (Liu et al., 2005), but this is the first report of a relationship between either factor and adult NPC behavior, to our knowledge. Conflicting reports suggest that IL-18 promotes neuroprotection and spatial ability (Ryu et al.; Yaguchi et al.) but also age-related cognitive decline (Blalock et al., 2003; Mawhinney et al.). Our data showing that exercise-increased hippocampal IL-18 levels correlate positively with new neuron number (which correlate positively with spatial ability in aging rats; Table 2 and Speisman et al., 2012) are consistent with the former notion. The question remains as to how IL-18 could be linked to improved hippocampal function. One possibility is that exercise may improve hippocampal health and stimulate neurogenesis in the aging brain by improving vascular health (Palmer et al., 2000). GRO-KC and IL-18 exhibit pro-angiogenic properties and are linked through ras-raf-ERK-MAPK signaling (Park et al., 2001; Zhong et al., 2008). Although we did not observe an exercise-induced shift in hippocampal VEGF levels, previous research indicates that running potentiates hippocampal neurogenesis through VEGF activity in young mice (Fabel et al., 2003; Tang et al., 2010) and increases hippocampal VEGF levels in middle-aged mice (Latimer et al., 2011). However, VEGF levels are known to decline in the aged brain (Shetty et al., 2005) and may require rejuvenation before exercise can potentiate neurogenesis beyond a basal level (Fig. 6 versus Speisman et al., 2012). Note that although every analyte measured was detected in the hippocampus or cortex (from which similar amounts of protein were harvested), the volume of protein obtained from ½ of the hippocampus or cortex could have been too small to quantify changes in the concentration low-level analytes, such as VEGF.
Our cluster analyses further suggest that cytokine signaling in aging rat runners and their controls is compartmentalized. In serum, we found strong positive correlations between GRO-KC and MCP-1 and between IL-6 and IL-13 concentrations. Coordinated monocyte/macrophage-derived serum MCP-1 and GRO-KC concentrations have been reported in models of wound repair-induced angiogenesis and in the serum of LPS-treated mice (Barcelos et al., 2004; Erickson and Banks, 2011) and coordinated circulating IL-13 and IL-6 concentrations may be consistent with the heightened Th2 response hypothesized to occur with age (Grolleau-Julius et al., 2010). Although IL-13 was detected in cortical clusters and GRO-KC and MCP-1 were detected hippocampal clusters we did not detect between-compartments correlations that would indicate direct diffusion or transport across the blood-brain-barrier to either location.
In the brain, we detected correlated concentrations of cytokines that were distinct in the cortex and hippocampus. In the cortex, concentrations of structurally and functionally homologous IL-13 and IL-4, which are expressed by microglia and typically associated with anti-inflammatory and neuroprotective effects in the brain were correlated (Opal and DePalo, 2000; Ponomarev et al., 2007; Shin et al., 2004). Cortical GM-CSF was detected in separate clusters with IL-2, IL-10 and IL-18. Astrocytic GM-CSF acts on its receptors expressed by microglia and oligodendrocytes (Kimura et al., 2000). IL-2 and its receptor protein is thought to be expressed by neurons, glia and microglia while IL-18 mRNA is expressed by microglia with its receptors being expressed by neurons, astrocytes and microglia throughout the brain (Hanisch and Quirion, 1995; Tambuyzer et al., 2009). While the relationship between brain IL-2 and GM-CSF is unclear, IL-10 decreases but IL-18 increases GM-CSF production by peripheral immune cells and IL-10 may suppress microglial inflammatory responses (Lee et al., 2010).
In the hippocampus, VEGF correlated independently with IL-2 and MCP-1, IL-5, and finally IL-17. Endothelial VEGF production can be stimulated by IL-2, and microglial, endothelial cell or smooth muscle cell MCP-1 expression in response to vascular injury (Parenti et al., 2004). IL-5 and IL-17, often associated with allergic reactions, can also stimulate VEGF production. Interestingly, the injury-induced expression of MIP-1α, MCP-1, GM-CSF, and TNF-α and in some cases IL-1β appears to recruit macrophages and induce phagocytosis (Ousman and David, 2001). The coordinated concentration of IL-2 with this cluster is interesting because IL-2 is often associated with self-recognition (Kolls and Linden, 2004). IL-2 and IL-4 are co-regulated by exhaustive acute exercise in muscle and in serum presumably to stimulate repair processes (Rosa Neto et al., 2011). Relationships between IL-2 and IL-4, IL-2 and GRO-KC, and between IL4 and IL-6 that we detected in the hippocampus have not yet been reported in the brain, to our knowledge. Future work that confirms and expands these regionally distinct neuroimmune signaling pathways and tests their effects in the aging brain will be critical for understanding their impact on cognition and plasticity. Certainly, regional changes in other signaling systems are under exploration in the aging brain (for example, see McQuail et al., 2012).
Implications
Daily exercise improved spatial/contextual ability, perhaps by stimulating hippocampal plasticity in the form of neurogenesis and by modulating immune and neuroimmune signaling. Daily exercise was associated with the decreased expression of factors that correlated negatively with learning, memory and neurogenesis measures but the increased expression of factors that correlated positively with our neurogenesis measure. The picture of how immune and neuroimmune signaling impacts cognition and plasticity is growing. We add to this picture by showing that exercise modulates factors distinctly in serum and in the brain, suggesting that immune factors do not appreciably diffuse or are transported into the brains of aging rats that exercise and their controls. We also found that exercise modulated neuroimmune factors distinctly in the cortex and hippocampus, which supports the notion that in the brain, neuroimmune signaling is region-specific. Serum leptin emerged as a biomarker for both brain and cognitive aging. Along with serum leptin, serum MCP-1 and GRO-KC levels may predict spatial ability. We confirmed that hippocampal IL-1β levels may be a marker of mnemonic ability and we discovered that hippocampal IL-18 and GRO-KC levels correlated positively with neurogenesis. In summary, our work suggests that engaging in physical activity may reverse some aspects of age-related cognitive decline, perhaps by stimulating neurogenesis and by modulating beneficial and detrimental aspects of immune and neuroimmune signaling. Our correlational data begin to provide a framework for systematically manipulating these immune and neuroimmune signaling molecules to test their effects on cognition and neurogenesis across lifespan in future experiments.
Highlights.
Daily exercise may rejuvenate age-impaired cognition and hippocampal neurogenesis by modulating immune and neuroimmune signaling pathways.
Acknowledgements
The authors thank Dr. Gerry Shaw for his gift of anti-chicken GFAP. The study was supported by grants from the National Institutes of Health (AG014979, AG037984) to TCF, the Evelyn F McKnight Brain Research Foundation and NIH grant AG038600 to TCF and BKO, and the Broad Foundation for Biomedical Research to BKO. RBS was the recipient of a National Science Foundation Graduate Research Fellowship (DGE-0802270).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that all research reported in this manuscript was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Barcelos LS, Talvani A, Teixeira AS, Cassali GD, Andrade SP, Teixeira MM. Production and in vivo effects of chemokines CXCL1-3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm Res. 2004;53:576–584. doi: 10.1007/s00011-004-1299-4. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Arellano C, Ferguson R, Jopson T, Rosi S. Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav Immun. 2012;26:18–23. doi: 10.1016/j.bbi.2011.07.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol.Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:33–40. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJ. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 2010;38:1011–1025. doi: 10.1177/0192623310385140. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154–164. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat.Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennaoui M, Drogou C, Gomez-Merino D. Effects of physical training on IL-1beta, IL-6 and IL-1ra concentrations in various brain areas of the rat. Eur Cytokine Netw. 2008;19:8–14. doi: 10.1684/ecn.2008.0115. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon A. The association between mental, social and physical activity and cognitive performance in young and old subjects. Age Ageing. 1993;22:175–182. doi: 10.1093/ageing/22.3.175. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blümcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133:3359–3372. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging. 2009;30:903–909. doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman LC, Mira H, Erices A, Malmersjö S, Andersson E, Uhlén P, Arenas E. Alpha-chemokines regulate proliferation, neurogenesis, and dopaminergic differentiation of ventral midbrain precursors and neurospheres. Stem Cells. 2008;26:1891–1900. doi: 10.1634/stemcells.2007-0753. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Haack AK, Galea LA. Task difficulty in the Morris water task influences the survival of new neurons in the dentate gyrus. Hippocampus. 2010;20:866–867. doi: 10.1002/hipo.20692. [DOI] [PubMed] [Google Scholar]
- Epp JR, Scott NA, Galea LA. Strain differences in neurogenesis and activation of new neurons in the dentate gyrus in response to spatial learning. Neuroscience. 2011;172:342–354. doi: 10.1016/j.neuroscience.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2010;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25:1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Felzien LK, McDonald JT, Gleason SM, Berman NE, Klein RM. Increased chemokine gene expression during aging in the murine brain. Brain Res. 2001;890:137–146. doi: 10.1016/s0006-8993(00)03090-0. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33:1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat.Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999b;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Grolleau-Julius A, Ray D, Yung RL. The role of epigenetics in aging and autoimmunity. Clin Rev Allergy Immunol. 2010;39:42–50. doi: 10.1007/s12016-009-8169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res Brain Res Rev. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y. Leptin regulates interleukin-1beta expression in the brain via the STAT3-independent mechanisms. Brain Res. 2002;949:139–146. doi: 10.1016/s0006-8993(02)02974-8. [DOI] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, van Praag H. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging. 2011;32:2279–2286. doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kodama T, Aguila MC, Zhang SQ, Inoue S. Granulocyte-macrophage colony-stimulating factor modulates rapid eye movement (REM) sleep and non-REM sleep in rats. J Neurosci. 2000;20:5544–5551. doi: 10.1523/JNEUROSCI.20-14-05544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizaki T, Maegawa T, Sakurai T, Ogasawara JE, Ookawara T, Oh-ishi S, Izawa T, Haga S, Ohno H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochem Biophys Res Commun. 2011;413:454–459. doi: 10.1016/j.bbrc.2011.08.117. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rodriguez-Zas SL, Southey BR, Kelley KW, Dantzer R, Rhodes JS. Voluntary wheel running reverses age-induced changes in hippocampal gene expression. PLoS One. 2011a;6:e22654. doi: 10.1371/journal.pone.0022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Deyoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2011b;26:803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19:2337–2346. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Mortensen EL, Avlund K, Pilegaard H, Christiansen L, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK, Bruunsgaard H. Genetic priming of a proinflammatory profile predicts low IQ in octogenarians. Neurobiol Aging. 2009;30:769–781. doi: 10.1016/j.neurobiolaging.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J.Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2012;33:828.e821–828.e817. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83:206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Latimer CS, Searcy JL, Bridges MT, Brewer LD, Popović J, Blalock EM, Landfield PW, Thibault O, Porter NM. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS One. 2011;6:e26812. doi: 10.1371/journal.pone.0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Liu H, Jia D, Fu J, Zhao S, He G, Ling EA, Gao J, Hao A. Effects of granulocyte colony-stimulating factor on the proliferation and cell-fate specification of neural stem cells. Neuroscience. 2009;164:1521–1530. doi: 10.1016/j.neuroscience.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lum M, Croze E, Wagner C, McLenachan S, Mitrovic B, Turnley AM. Inhibition of neurosphere proliferation by IFNgamma but not IFNbeta is coupled to neuronal differentiation. J.Neuroimmunol. 2009;206:32–38. doi: 10.1016/j.jneuroim.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Madronal N, Lopez-Aracil C, Rangel A, del Rio JA, Delgado-Garcia JM, Gruart A. Effects of enriched physical and social environments on motor performance, associative learning, and hippocampal neurogenesis in mice. PLoS One. 2010;5:e11130. doi: 10.1371/journal.pone.0011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi CA, Srivastava V, Yu WH, Les Dees W, McCann SM. Lipopolysaccharide-induced leptin synthesis and release are differentially controlled by alpha-melanocyte-stimulating hormone. Neuroimmunomodulation. 2005;12:182–188. doi: 10.1159/000084851. [DOI] [PubMed] [Google Scholar]
- Mawhinney LJ, de Vaccari Rivero JP, Dale GA, Keane RW, Bramlett HM. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 12:123. doi: 10.1186/1471-2202-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Banuelos C, LaSarge CL, Nicolle MM, Bizon JL. GABA(B) receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol Aging. 2012;33:1124 e1121–1112 e1121. doi: 10.1016/j.neurobiolaging.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, Deyoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli M, Pocai A, Skalicky M, Viidik A, Bergamini E, Masiello P. Effects of life-long exercise on circulating free fatty acids and muscle triglyceride content in ageing rats. Exp Gerontol. 2004;39:1333–1340. doi: 10.1016/j.exger.2004.06.014. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Beninger RJ. Water maze versus radial maze: differential performance of rats in a spatial delayed match-to-position task and response to scopolamine. Behav Brain Res. 2002;128:139–152. doi: 10.1016/s0166-4328(01)00316-3. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Ousman SS, David S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J Neurosci. 2001;21:4649–4656. doi: 10.1523/JNEUROSCI.21-13-04649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1978–H1984. doi: 10.1152/ajpheart.00414.2003. [DOI] [PubMed] [Google Scholar]
- Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer's disease. J Alzheimers Dis. 24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechel MM, Halbur L, Devata S, Vaidya AM, Young MR. Increased interleukin-6 production by cerebral cortical tissue of adult versus young mice. Mech Ageing Dev. 1996;92:185–194. doi: 10.1016/s0047-6374(96)01833-7. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci. 1998;18:10457–10463. doi: 10.1523/JNEUROSCI.18-24-10457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Fishman K, Baure J, Rosi S, Lamborn KR, Obenaus A, Nelson GA, Fike JR. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat Res. 2008;169:626–632. doi: 10.1667/RR1263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Neto JC, Lira FS, Zanchi NE, Oyama LM, Pimentel GD, Santos RV, Seelaender M, Oller do Nascimento CM. Acute exhaustive exercise regulates IL-2, IL-4 and MyoD in skeletal muscle but not adipose tissue in rats. Lipids Health Dis. 2011;10:97. doi: 10.1186/1476-511X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu HJ, Kim JE, Kim MJ, Kwon HJ, Suh SW, Song HK, Kang TC. The protective effects of interleukin-18 and interferon-gamma on neuronal damages in the rat hippocampus following status epilepticus. Neuroscience. 170:711–721. doi: 10.1016/j.neuroscience.2010.07.048. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shin WH, Lee DY, Park KW, Kim SU, Yang MS, Joe EH, Jin BK. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia. 2004;46:142–152. doi: 10.1002/glia.10357. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Pastoriza JM, Severance JE, Foster TC, Ormerod BK. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Zurborg S, Horster H, Fabel K, Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Stephens CL, Toda H, Palmer TD, DeMarse TB, Ormerod BK. Adult neural progenitor cells reactivate superbursting in mature neural networks. Exp Neurol. 2012;234:20–30. doi: 10.1016/j.expneurol.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- Tan YF, Rosenzweig S, Jaffray D, Wojtowicz JM. Depletion of new neurons by image guided irradiation. Front Neurosci. 2011;5:59. doi: 10.3389/fnins.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Xia FC, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol. 2010;170:16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J.Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin–6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Xu YZ, Nygard M, Kristensson K, Bentivoglio M. Regulation of cytokine signaling and T-cell recruitment in the aging mouse brain in response to central inflammatory challenge. Brain Behav Immun. 2010;24:138–152. doi: 10.1016/j.bbi.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Yaguchi T, Nagata T, Yang D, Nishizaki T. Interleukin-18 regulates motor activity, anxiety and spatial learning without affecting synaptic plasticity. Behav Brain Res. 206:47–51. doi: 10.1016/j.bbr.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Zhong L, Roybal J, Chaerkady R, Zhang W, Choi K, Alvarez CA, Tran H, Creighton CJ, Yan S, Strieter RM, Pandey A, Kurie JM. Identification of secreted proteins that mediate cell-cell interactions in an in vitro model of the lung cancer microenvironment. Cancer Res. 2008;68:7237–7245. doi: 10.1158/0008-5472.CAN-08-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]


