Abstract
High risk human papillomavirus (HPV) is an established cause of head and neck carcinomas arising in the oropharynx. The presence of HPV has also been reported in some carcinomas arising in sinonasal tract, but little is known about their overall incidence or their clinicopathologic profile. The surgical pathology archives of The Johns Hopkins Hospital were searched for all carcinomas arising in the sinonasal tract from 1995 to 2011, and tissue microarrays were constructed. P16 immunohistochemistry and DNA in situ hybridization for high-risk types of HPV were performed. Demographic and clinical outcomes data were extracted from patient medical records. Of 161 sinonasal carcinomas, 34 (21%) were positive for high risk HPV DNA, including type 16 (82%), type 31/33 (12%), and type 18 (6%). HPV-positive carcinomas consisted of 28 squamous cell carcinomas and variants (15 non- or partially-keratinizing, 4 papillary, 5 adenosquamous, 4 basaloid), 1 small cell carcinoma, 1 sinonasal undifferentiated carcinoma, and 4 carcinomas that were difficult to classify but exhibited adenoid cystic carcinoma-like features. Immunohistochemistry for p16 was positive in 59/161 (37%) cases, and p16 expression strongly correlated with the presence of HPV DNA: 33 of 34 (97%) HPV positive tumors exhibited high p16 expression, whereas only 26 of 127 (20%) HPV negative tumors were p16 positive (p < .0001). The HPV-related carcinomas occurred in 19 men and 15 women ranging in age from 33 to 87 years (mean 54). A trend toward improved survival was observed in the HPV-positive group (hazard ratio=0.58, 95% confidence interval [0.26, 1.28]). The presence of high risk HPV in 21% of sinonasal carcinomas confirms HPV as an important oncologic agent of carcinomas arising in the sinonasal tract. While non-keratinizing squamous cell carcinoma is the most common histologic type, there is a wide morphologic spectrum of HPV-related disease that includes a variant that resembles adenoid cystic carcinoma. The distinctiveness of these HPV-related carcinomas of the sinonasal tract with respect to risk factors, clinical behavior, and response to therapy remains to be clarified.
Keywords: Human papillomavirus, sinonasal carcinoma, squamous cell carcinoma, adenoid cystic carcinoma, sinonasal undifferentiated carcinoma
Introduction
Carcinomas of the sinonasal tract affect approximately 0.5 to 1.0 patients per 100,000 per year.(1,2) This group of malignancies is diverse, and includes tumors arising from the surface epithelium as well as the seromucinous glands.(3) The causative factors for the development sinonasal cancer are not well understood.(3) Wood dust and other occupational exposures are recognized risk factors, but only for a rare subtype, intestinal-type adenocarcinoma.(4–7) Cigarette smoking, an important risk factor for carcinomas of most head and neck locations, has only a weak association with sinonasal carcinomas.(8–10)
High risk types of human papillomavirus (HPV) are now well established as major etiologic factors of head and neck cancer.(11) HPV-related squamous cell carcinomas arise predominantly in the oropharynx where they account for up to 80% of cases.(12) Numerous studies have also identified HPV in tumors of the sinonasal tract, but widely disparate detection rates - ranging from 0 to 100% - have shrouded its role as a cancer causing agent at this site.(13–26) These inconsistencies in HPV detection reflect dissimilar detection assays and divergent study populations that have included both neoplastic and non-neoplastic processes with a particular focus on Schneiderian papillomas. Based on these widely disparate findings, the World Health Organization International Agency for Research on Cancer has acknowledged that the evidence establishing HPV as a causative agent for carcinomas of the sinonasal tract is currently inadequate.(27) The purpose of this study was: 1) to determine the prevalence of high-risk HPV in primary sinonasal carcinomas using a highly sensitive and specific DNA in-situ hybridization based approach combined with p16 immunohistochemistry; and 2) to define the histologic spectrum of HPV-related sinonasal carcinomas.
Methods
Cases
This study was approved by Institutional Review Board of The Johns Hopkins Medical Institutions. The surgical pathology archives of The Johns Hopkins Hospital were searched for all cases of primary carcinomas diagnosed from 1995 to 2011. Esthesioneuroblastomas and carcinomas arising from the nasopharynx, skin, or odontogenic apparatus and involving the sinonasal tract secondarily were excluded. Hematoxylin and eosin-stained sections were reviewed and the tumors were classified according to their histologic, and in some cases, immunohistochemical features. Pertinent clinical information was obtained from The Johns Hopkins Hospital’s electronic medical records. Additional death information was obtained from the publicly available United States Social Security Administration Death Index.
Tissue microarrays
Tissue microarrays (TMAs) were constructed from the tissue blocks of 140 primary sinonasal carcinomas. Two to three cores 1 mm in diameter were taken from each donor block to address tumor heterogeneity. Twenty-one additional cases that were too scant to include on the TMAs were tested on whole slides.
Immunohistochemistry
All sinonasal carcinomas were evaluated by immunohistochemistry for expression of the CDK-inhibitor p16, a biomarker of HPV E7 oncoprotein activity. Five-micrometer sections of formalin-fixed and paraffin embedded tissues were deparaffinized. Antigen retrieval was performed using heat induced (92°C for 30 minutes) epitope retrieval with 10mM citrate buffer. Sections were incubated with a mouse monoclonal antibody against p16 (MTM Laboratories, Heidelberg, Germany) which was visualized using the Ultra view polymer detection kit (Ventana Medical Systems Inc., Tucson, AZ) on a Ventana Benchmark XT autostainer (Ventana). P16 expression was scored as positive if strong and diffuse nuclear and cytoplasmic staining was detected in ≥70% of the tumor.(12)
DNA In situ hybridization
Five-micrometer sections from the formalin-fixed paraffin-embedded tumor blocks were evaluated for the presence of HPV DNA by in situ hybridization. Two different detection methods were used. Type-specific assays for type 16, 18, and 31/33 were performed using the in situ hybridization-catalyzed signal amplification method for biotinylated probes (DAKO GenPoint, Carpinteria, CA). Briefly, the 5-mm tissue sections underwent deparaffinization, heat-induced target retrieval in citrate buffer, and digestion using Proteinase K (Roche Diagnostics, Indianapolis, IN). Slides were subsequently hybridized with biotinylated HPV type-specific probes for types 16, 18, and 31/33 (DAKO, Carpintera, CA). Signal amplification was performed by consecutive application of a streptavidin-HRP complex and AQ2 biotinyl tyramide. Visualization of hybridization signals was performed by incubation with the chromogenic substrate diaminobenzidine.
For broader high-risk HPV detection, we also used the Ventana Inform HPV III Family 16 Probe (B) kit (Ventana Medical Systems, Tucson, AZ). For this assay, slides were conditioned using Ventana cell conditioner #2 and ISH-protease 3. Hybridization utilized the HPV III Family16 probe set that captures HPV genotypes 16, 18, 33, 35, 45, 51, 52, 56, and 66. Signals were detected using the in situ hybridization iView Blue Plus Detection Kit, which is an indirect biotin-streptavidin system that detects fluorescein-labeled probes. The kit uses an alkaline phosphatase enzyme and NBT/BCIP substrate chromogen reaction that provides an intense blue, permanent color as well as a red counter stain. All reagents are provided prediluted and ready-to-use on BenchMark Series automated slide stainers (Ventana Medical Systems, Tucson, AZ).
For both detection assays, punctate hybridization signals localized to the tumor cell nuclei defined an HPV-positive tumor (Figure 1D). HPV positive controls were cases of HPV-16, HPV-18, and HPV-31/33 positive oropharyngeal cancers, as well as the HPV16-positive SiHa and CaSki cell lines.
Figure 1.
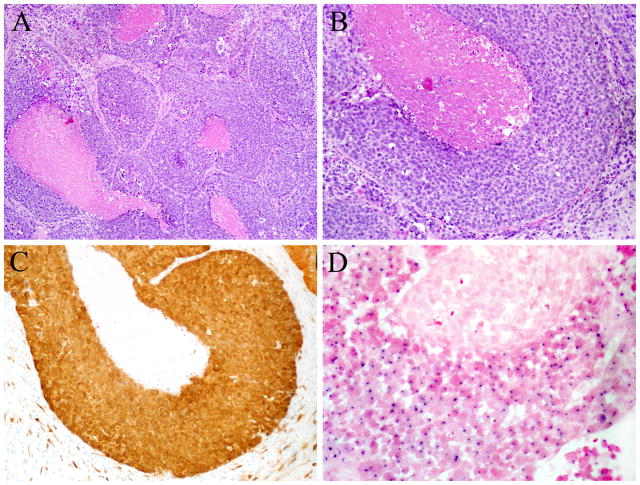
HPV-related sinonasal carcinomas. A–B. Most of the HPV-related sinonasal squamous cell carcinomas were morphologically identical to HPV-related oropharyngeal carcinomas, exhibiting little or no keratinization, tumor infiltration by lymphocytes, and limited desmoplastic stromal reaction (Hematoxylin and eosin, X100 (A) and X200 (B)). C. All but one of the HPV-positive cases was diffusely positive for p16 in a nuclear and cytoplasmic distribution (p16 immunohistochemistry, X200). D. HPV positivity was defined by the presence of dot-like in situ hybridization signals in tumor nuclei (High risk HPV in situ hybridization, X400).
Statistical Analysis
The Kaplan-Meier method was used to estimate the survival rate among the HPV positive and HPV negative patients, and log-rank test was used to compare the survival between them. Univariate and multivariable Cox regression models were applied to evaluate the association of HPV status and survival. All tests were two-sided, and p value < 0.05 was considered to indicate statistical significance. The analysis was carried out using statistical software SAS version 9.3 (Cary, NC).
Results
The results are summarized in Table 1. High risk HPV was identified in 34 of 161 (21%) cases. Each case was positive with one of the type-specific probes: 28 type 16, 4 type 31/33, 2 type 18. Twenty-nine cases, all of which were positive with one of the type specific probes, were positive for the high risk probe cocktail. Immunohistochemistry for p16 was positive in 59/161 (37%) cases overall, and p16 expression was strongly associated with the presence of HPV DNA: 33 of 34 HPV positive tumors exhibited high p16 expression, whereas only 26 of 127 HPV negative tumors were p16 positive (97% versus 20%, p < .0001).
Table 1.
HPV status of sinonasal carcinomas by tumor type
| Tumor type | p16 IHC (%) | HPV DNA in situ hybridization (%) | ||||
|---|---|---|---|---|---|---|
| type 16 | type 18 | type 31/33 | high risk cocktail | total | ||
|
| ||||||
| Squamous cell carcinoma | 42/91 (46) | 24/91 (26) | 2/91 (2) | 2/91 (2) | 24/91 (26) | 28/91 (31) |
| Keratinizing | 3/25 (12) | 0/25 (0) | 0/25 (0) | 0/25 (0) | 0/25 (0) | 0/25 (0) |
| Non/partially keratinizing | 24/44 (55) | 14/44 (32) | 1/44 (2) | 0/44 (0) | 12/44 (27) | 15/44 (34) |
| Basaloid squamous | 5/8 (63) | 3/8 (38) | 0/8 (0) | 1/8 (13) | 4/8 (50) | 4/8 (50) |
| Papillary | 5/5 (100) | 2/5 (40) | 1/5 (20) | 1/5 (20) | 3/5 (60) | 4/5 (80) |
| Adenosquamous | 5/6 (83) | 5/6 (83) | 0/6 (0) | 0/6 (0) | 5/6 (83) | 5/6 (83) |
| Sarcomatoid | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
|
| ||||||
| Adenoid cystic-like carcinomas | 4/4 (100) | 2/4 (50) | 0/4 (0) | 2/4 (50) | 4/4 (100) | 4/4 (100) |
|
| ||||||
| Salivary gland carcinoma | 4/34 (12) | 0/34 (0) | 0/34 (0) | 0/34 (0) | 0/34 (0) | 0/34 (0) |
| Adenoid cystic | 3/26 (12) | 0/26 (0) | 0/26 (0) | 0/26(0) | 0/26 (0) | 0/26 (0) |
| Myoepithelial | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| Mucoepidermoid | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Acinic cell | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Epithelial-myoepithelial | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Adenocarcinoma, NOS | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
|
| ||||||
| Sinonasal adenocarcinoma | 2/7 (29) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7(0) | 0/7 (0) |
|
| ||||||
| Intestinal type | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
|
| ||||||
| Non-intestinal type, low grade | 0/4 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) |
|
| ||||||
| Non-intestinal type, high grade | 2/2 (100) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
|
| ||||||
| Small cell carcinoma | 4/6 (67) | 1/6 (17) | 0/6 (0) | 0/6 (0) | 1/6 (17) | 1/6 (17) |
|
| ||||||
| SNUC | 4/16 (25) | 1/16 (6) | 0/16 (0) | 0/16 (0) | 0/16 (0) | 1/16 (6) |
|
| ||||||
| NUT midline carcinoma | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
|
| ||||||
| Total | 60/161 (37) | 28/161 (17) | 2/161 (1) | 4/161 (2) | 30/161 (19) | 34/161 (21) |
IHC, immunohistochemistry; HPV, human papillomavirus; NOS, not otherwise specified; SNUC, sinonasal undifferentiated carcinoma.
By histologic type, HPV was detected in 28 of 91 (31%) squamous cell carcinomas, 1 of 6 (17%) small cell carcinomas, 1 of 16 (6%) sinonasal undifferentiated carcinomas, and 4/4 (100%) peculiar carcinomas that were difficult to classify. HPV was not detected in any of the salivary type carcinomas (n=34), sinonasal adenocarcinomas (n=7), or NUT midline carcinomas (n=3). The presence of HPV was not uniform across all forms of squamous cell carcinomas. HPV was not detected in conventional keratinizing squamous cell carcinoma (0 of 25) or in the spindle cell variant (0 of 3); but it was detected in non-keratinizing or partially keratinizing squamous cell carcinoma (15 of 44, 34%) (Figure 1), the basaloid squamous cell variant, (4 of 8, 50%), the papillary variant (4 of 5, 80%) (Figure 2A), and adenosquamous cell carcinoma (5 of 6, 83%) (Figure 2B). Of the 16 squamous cell carcinomas arising in association with a Schneiderian papilloma, only 1 (6%) was HPV-positive. The positive case arose one year following the reported diagnosis of Schneiderian papilloma; however, the papilloma itself was not available for review or HPV testing. Although p16 immunoreactivity was frequent in the small cell carcinomas (4 of 6, 67%) and SNUCs (4 of 16, 25%), only 1 (17%) small cell carcinoma (Figure 2C) and 1 (6%) SNUC (Figure 2D) were positive for HPV positive by in situ hybridization.
Figure 2.
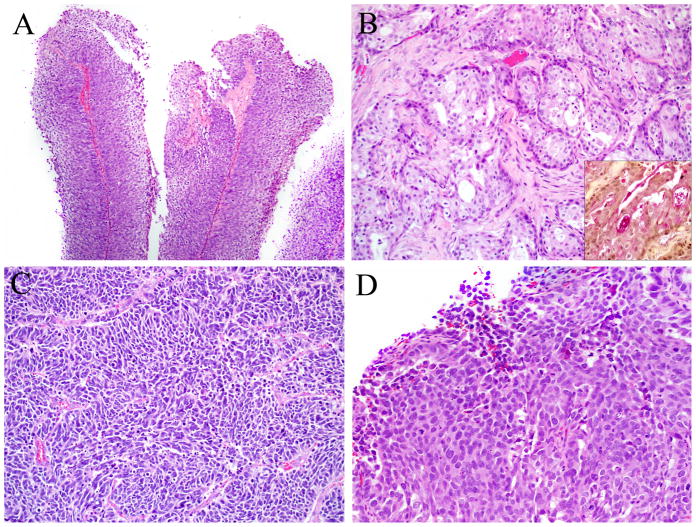
HPV-related sinonasal carcinomas. Histologic variants of HPV-related sinonasal carcinomas included: A. Five papillary squamous cell carcinomas (Hematoxylin and eosin, X100); B. Five adenosquamous carcinomas (Hematoxylin and eosin, and mucicarmine (inset), X200); C. One small cell carcinoma (Hematoxylin and eosin, X200); and D. One sinonasal undifferentiated carcinoma (Hematoxylin and eosin, X200).
Nine of the HPV-related carcinomas exhibited ductal differentiation. As mentioned above, five of them had distinct zones of overt squamous and glandular differentiation, and were therefore classified as adenosquamous carcinoma (Figure 2B). Each adenosquamous carcinoma had well-formed ducts and mucin (intraluminal and intracellular) as demonstrated by a mucicarmine stain. Like the HPV-positive squamous cell carcinomas, the squamous components of the HPV-positive adenosquamous carcinomas were non-keratinizing or partially keratinizing. They were not mucoepidermoid carcinomas because the glandular component was generally separate from squamous areas and most cases (4 of 5) contained squamous cell carcinoma in situ.
The four remaining HPV-related carcinomas with ductal differentiation had peculiar histologic features and were difficult to subtype by current classification schemes (Figure 3). They were morphologically most suggestive of salivary gland carcinomas, particularly solid variant of adenoid cystic carcinoma, which was the original diagnosis for three of these cases. These four carcinomas shared a basaloid appearance with a predominantly solid growth pattern. Each case exhibited ductal differentiation but also a myoepithelial phenotype with expression of 2 or more myoepithelial immunohistochemical stains (i.e., S100, calponin, actin, and p63). Unlike true adenoid cystic carcinomas, however, three of these HPV-related carcinomas were associated with in situ squamous cell carcinoma of the overlying epithelium. While these cases had some morphologic overlap with basaloid squamous cell carcinoma, they were distinguished from this variant by the absence of squamous differentiation in the invasive tumor and their biphasic nature (ducts and abluminal myoepithelial cells). Immunostains for p63 were particularly helpful in making this distinction since this marker was expressed in an abluminal distribution in the four unusual carcinomas, not diffusely as is uniformly seen in basaloid squamous cell carcinoma.(28,29) HPV types 31/33 were detected in 2 of the carcinomas, and type 16 was detected in the other 2 cases.
Figure 3.
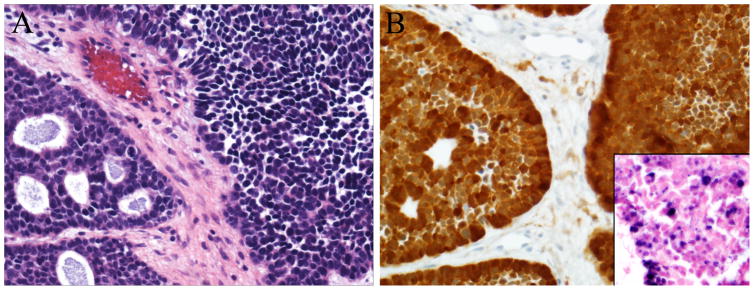
HPV-related sinonasal carcinomas. A. Four HPV-associated carcinomas exhibited features of salivary gland carcinomas, particularly adenoid cystic carcinoma. Although these tumors were predominantly solid (right), they also showed cribriform areas with duct formation (left) (Hematoxylin and eosin, X400). B. The tumors were strongly immunoreactive to p16 (p16 immunohistochemistry, X400), and punctate hybridization signals were seen in tumor nuclei on HPV in situ hybridization (inset, high-risk HPV in situ hybridization, X400).
Clinically, the HPV-positive carcinomas occurred in 19 men and 15 women (male:female ratio 1.3:1). The male:female ratio was similar to that of the HPV-negative carcinomas (1.2:1). Patients with HPV-positive carcinomas ranged in age from 33 to 87 years (mean 54), while patients with HPV-negative carcinomas ranged in age from 26 to 93 years (mean 59). The median follow-up was 3.5 years (range, 0.12 years to 15 years). For patients with HPV-negative carcinomas of any type, median survival time was 6.7 years (95% confidence interval [4.0, NA]), and 5-year and 10-year survival rates were 58.7% (95% CI [47.6%, 68.2%]) and 46.2% (95% CI [34.7%, 57.0%]), respectively. Median survival among patients with HPV positive carcinomas of any type was not reached, and their 5-year and 10-year survival was 71.2% (95% CI [45.6%, 86.3%]) and 61.0% (95% CI [31.8%, 80.8%]), respectively. The hazard rate among the patients with any HPV negative carcinoma was 72% higher than the patients with HPV positive carcinoma (hazard ratio =1.72 with 95% CI [0.78, 3.82]), and after adjusting for age and gender, the hazard ratio was 1.34 (95% CI [0.60, 3.02]) (Figure 4A). When restricted to squamous cell carcinomas, median survival for the HPV-negative cases was 5.5 years (95% confidence interval [2.7, NA]), and 5-year and 10-year survival rates were 50.6% (95% CI [35%, 64.3%]) and 42.7% (95% CI [27.6%, 57.0%]), respectively. Median survival among patients with HPV positive squamous cell carcinomas was not reached, and their 5-year and 10-year survival was 69.7% (95% CI [40.7%, 86.5%]) and 55.7% (95% CI [22.5%, 79.5%]), respectively. The hazard rate among the patients with HPV negative squamous cell carcinomas was 80% higher than those with HPV positive squamous cell carcinomas (hazard ratio =1.80 with 95% CI [0.74, 4.38]), and after adjusting for age and gender, the hazard ratio was 1.17 (95% CI [0.48, 2.89]). (Figure 4B).
Figure 4.
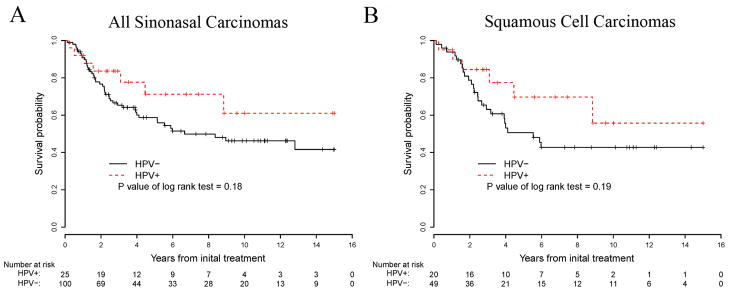
Kaplan-Meier plots showing the 15-year survival curves of all types of HPV positive and HPV negative sinonasal carcinomas (A), and squamous cell carcinomas only (B).
Discussion
Efforts to understand, prevent, and treat cancers of the sinonasal tracts have been blocked by a failure to recognize relevant etiologic agents. Unlike other areas of the head and neck (e.g. oral cavity and larynx) where tumorigenesis is largely attributed to the carcinogenic effects of tobacco use, cigarette smoking is not strongly associated with carcinomas arising in the sinonasal tract. Exposure to wood dust has been established as a causative agent, but this link is restricted to a subset of sinonasal adenocarcinomas and does not account for the vast majority carcinomas encountered in the sinonasal tract.(4–7) The frequent detection of human papillomavirus (HPV) has generally evoked “guilt by association”, but the mere detection of HPV in sinonasal cancers has not proven an active role in carcinogenesis. Highly sensitive PCR-based assays are able to detect trace amounts of HPV in various benign and malignant sinonasal lesions, but they have been unable to separate the incidental presence of virus (e.g. passenger virus and viral contaminants) from cancer causing HPV infection which requires nuclear transcription of the HPV genome and subsequent viral-induced alterations in cellular pathways.(14–20,22,24–26) Accordingly, the causes of sinonasal carcinomas continue to elude recognition, and the role of HPV has been particularly elusive.
A growing experience with HPV-related squamous cell carcinomas of the oropharynx has provided new insights into HPV-related tumorigenesis and improved methods for HPV detection. Integration of HPV DNA, expression of HPV E6/E7 mRNA transcripts, and disruption of critical regulatory host pathways (e.g. p53 and Retinoblastoma pathway) are sequential, coupled and compulsory steps that provide multiple targets for HPV detection. As one example, Rb inactivation by the viral oncoprotein E7 induces overexpression of p16 such that immunohistochemical detection of p16 serves as a reliable surrogate marker for high risk HPV in oropharyngeal cancers.(30) In the oropharynx, the dual presence of HPV DNA as visualized by HPV DNA in situ hybridization, together with high levels of p16 protein expression as detected by immunohistochemistry, is taken as compelling evidence for biologically active HPV.(31,32) In the sinonasal tract, p16 overexpression has likewise been correlated with the presence of high risk HPV.(19) Using an approach that combined direct visualization of high risk HPV DNA and p16 overexpression, we were able to establish the presence of biologically active HPV in 21% of sinonasal carcinomas. Although this rate does not approach that noted in the oropharynx where HPV is detected in up to 80% of oropharyngeal carcinomas,(12) our findings strongly suggest that high risk HPV is the most significant causative factor currently recognized for carcinomas arising in the sinonasal tract.
By tumor type, squamous cell carcinomas were most likely to be HPV-related. Confirming the findings of El-Mofty et al.,(19) we noted that about one-third of all sinonasal squamous cell carcinomas are both high risk HPV and p16 positive, and that HPV positivity consistently localizes to the non-keratinized and basaloid subgroups. Thus, the strong association between HPV and the non-keratinizing and basaloid phenotype that has been so well documented in the oropharynx is preserved in the sinonasal tract.(33) Although these non-keratinizing carcinomas of the sinonasal tract are sometimes designated as Schneiderian carcinomas, high risk HPV infection does not appear to represent a compulsory step in the carcinomatous transformation of a Schneiderian papilloma as has been so widely suspected.(17,18,26,34) Only 1 of 16 (6%) carcinomas arising in association with Schneiderian papillomas was HPV-positive.
Our comprehensive analysis of all types of sinonasal carcinomas revealed that the presence of high risk HPV is not entirely restricted to non-keratinizing squamous cell carcinoma, but it can be detected in other histologic patterns and other tumor types. Like the oropharynx, high risk HPV can sometimes be detected in specific variants of sinonasal squamous cell carcinomas including the papillary,(21) basaloid squamous,(35) and adenosquamous variants.(36) Furthermore, the finding of high risk HPV in a small cell carcinoma of the sinonasal tract parallels the experience in the oropharynx where small cell carcinomas are also sometimes HPV-related.(37) One novel association that does not have a counterpart in the oropharynx is a form of HPV-related carcinoma with features of a salivary gland neoplasm. These tumors usually carry a diagnosis of solid variant of adenoid cystic carcinoma, but they may in fact represent a unique type of HPV-related carcinoma that is restricted to the sinonasal tract. In their analysis of adenoid cystic carcinomas of the head and neck, Boland et al. were able to detect HPV in only 2 of 27 (7%) cases. Evocatively, the 2 HPV-positive tumors demonstrated solid growth and were located in the sinonasal tract.(38)
In the oropharynx, HPV-related squamous cell carcinoma has emerged as a distinct type of head and neck cancer with its own unique epidemiologic, demographic, histopathologic, and clinical profile.(39) It is not yet clear whether HPV-positivity likewise confers a distinctive set of epidemiologic and clinical characteristics for carcinomas of the sinonasal tract. High risk sexual practices (e.g. high lifetime number of sexual partners) have been associated with HPV-related carcinomas of the oropharynx.(40,41) Behavioral factors linking HPV transmission and cancer development have yet to be clarified for carcinomas of the sinonasal tract, but the detection of HPV in these tumors suggests that there may be important risk factors for HPV infection which have not been elucidated. HPV-related carcinoma of the oropharynx predominates in men,(42) but we found only a slight male predilection for HPV-related carcinomas of the sinonasal tract. In the oropharynx, HPV-related carcinomas are consistently diagnosed in patients in their 40s through 60s.(42) In the sinonasal tract, HPV-related carcinomas most frequently arise in patients who are in their 50s (mean 54 years), but they can occur across a very broad age range of ages (range, 33 to 87 years). In the oropharynx, HPV positivity is associated with improved clinical outcomes.(11,43,44) In the sinonasal tract, we also noted a trend towards improved clinical outcomes for patients with HPV positive carcinomas. However, the ability to fully appreciate the effect of HPV status on clinical outcomes in our retrospective analysis was limited by a nonuniform group of patients in regards to tumor type, tumor stage and mode of therapy. The value of HPV status as a prognostic indicator will require a comparison of a larger cohort of patients with similar demographic and clinicopathologic backgrounds.
In summary, this study draws attention to the sinonasal tract as a second anatomic subsite of the head and neck where carcinomas are often HPV-associated. High risk HPV is most commonly detected in non-keratinizing squamous cell carcinomas, but it can be detected in other types of sinonasal carcinomas including a salivary gland-like carcinoma that resembles the solid variant of adenoid cystic carcinoma. The identification of HPV-related sinonasal carcinomas sets the stage for prospective studies to determine whether HPV-positivity confers distinct biological and clinical characteristics and provides new opportunities for the development of targeted therapies for this patient population.
Acknowledgments
This study was funded by the Grover Hutchins Memorial Fund and by the NIDCR (P50 DE019032).
References
- 1.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck. 2012;34:877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 2.Ayiomamitis A, Parker L, Havas T. The epidemiology of malignant neoplasms of the nasal cavities, the paranasal sinuses and the middle ear in Canada. Arch Otorhinolaryngol. 1988;244:367–371. doi: 10.1007/BF00497467. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Tse LLY, Hunt JL, et al. Tumours of the nasal cavity and paranasal sinuses: introduction. In: Barnes L, Eveson JW, Reichart P, Sidranksy D, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Head and neck Tumours. Lyon, France: IARC Press; 2005. pp. 12–15. [Google Scholar]
- 4.Demers PA, Kogevinas M, Boffetta P, et al. Wood dust and sino-nasal cancer: pooled reanalysis of twelve case-control studies. Am J Ind Med. 1995;28:151–166. doi: 10.1002/ajim.4700280202. [DOI] [PubMed] [Google Scholar]
- 5.d’Errico A, Pasian S, Baratti A, et al. A case-control study on occupational risk factors for sino-nasal cancer. Occup Environ Med. 2009;66:448–455. doi: 10.1136/oem.2008.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224–231. doi: 10.1002/ijc.2910530209. [DOI] [PubMed] [Google Scholar]
- 7.Luce D, Leclerc A, Begin D, et al. Sinonasal cancer and occupational exposures: a pooled analysis of 12 case-control studies. Cancer Causes Control. 2002;13:147–157. doi: 10.1023/a:1014350004255. [DOI] [PubMed] [Google Scholar]
- 8.Hayes RB, Kardaun JW, de Bruyn A. Tobacco use and sinonasal cancer: a case-control study. Br J Cancer. 1987;56:843–846. doi: 10.1038/bjc.1987.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.‘t Mannetje A, Kogevinas M, Luce D, et al. Sinonasal cancer, occupation, and tobacco smoking in European women and men. Am J Ind Med. 1999;36:101–107. doi: 10.1002/(sici)1097-0274(199907)36:1<101::aid-ajim14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RT, Fitz-Hugh GS, Constable WC. Malignant neoplasms of the nasal cavities and paranasal sinuses: (a retrospective study) Laryngoscope. 1977;87:726–736. doi: 10.1002/lary.5540870508. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 12.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 13.Syrjanen S, Happonen RP, Virolainen E, et al. Detection of human papillomavirus (HPV) structural antigens and DNA types in inverted papillomas and squamous cell carcinomas of the nasal cavities and paranasal sinuses. Acta Otolaryngol. 1987;104:334–341. doi: 10.3109/00016488709107337. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, Tate JE, Crum CP, et al. Prevalence of human papillomaviruses (HPV) in benign and malignant tumors of the upper respiratory tract. Mod Pathol. 1996;9:15–20. [PubMed] [Google Scholar]
- 15.Saegusa M, Nitta H, Hashimura M, et al. Down-regulation of p27Kip1 expression is correlated with increased cell proliferation but not expression of p21waf1 and p53, and human papillomavirus infection in benign and malignant tumours of sinonasal regions. Histopathology. 1999;35:55–64. doi: 10.1046/j.1365-2559.1999.00688.x. [DOI] [PubMed] [Google Scholar]
- 16.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald C, Lindeberg H, Pedersen BL, et al. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish Epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–1110. doi: 10.1097/00005537-200106000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Cheung FM, Lau TW, Cheung LK, et al. Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J. 2010;89:E5–E12. doi: 10.1177/014556131008901002. [DOI] [PubMed] [Google Scholar]
- 19.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 20.Furuta Y, Takasu T, Asai T, et al. Detection of human papillomavirus DNA in carcinomas of the nasal cavities and paranasal sinuses by polymerase chain reaction. Cancer. 1992;69:353–357. doi: 10.1002/1097-0142(19920115)69:2<353::aid-cncr2820690213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 22.Kashima HK, Kessis T, Hruban RH, et al. Human papillomavirus in sinonasal papillomas and squamous cell carcinoma. Laryngoscope. 1992;102:973–976. doi: 10.1288/00005537-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Klemi PJ, Joensuu H, Siivonen L, et al. Association of DNA aneuploidy with human papillomavirus-induced malignant transformation of sinonasal transitional papillomas. Otolaryngol Head Neck Surg. 1989;100:563–567. doi: 10.1177/019459988910000607. [DOI] [PubMed] [Google Scholar]
- 24.McKay SP, Gregoire L, Lonardo F, et al. Human papillomavirus (HPV) transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope. 2005;115:1428–1431. doi: 10.1097/01.mlg.0000168091.50584.b4. [DOI] [PubMed] [Google Scholar]
- 25.Mineta H, Ogino T, Amano HM, et al. Human papilloma virus (HPV) type 16 and 18 detected in head and neck squamous cell carcinoma. Anticancer Res. 1998;18:4765–4768. [PubMed] [Google Scholar]
- 26.Syrjanen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174–181. doi: 10.1136/jcp.56.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, editor. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 90: Human Papillomaviruses. Lyon, France: IARC Press; 2007. Summary of Data reported and evaluation; pp. 465–476. [Google Scholar]
- 28.Serrano MF, El-Mofty SK, Gnepp DR, et al. Utility of high molecular weight cytokeratins, but not p63, in the differential diagnosis of neuroendocrine and basaloid carcinomas of the head and neck. Hum Pathol. 2008;39:591–598. doi: 10.1016/j.humpath.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Emanuel P, Wang B, Wu M, et al. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18:645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- 30.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann M, Ihloff AS, Gorogh T, et al. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–1602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 32.Begum S, Cao D, Gillison M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 33.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2:49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 36.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous Carcinoma of the Head and Neck: Relationship to Human Papillomavirus and Review of the Literature. Head Neck Pathol. 2011 doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boland JM, McPhail ED, Garcia JJ, et al. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 39.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D. C Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 40.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 41.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 42.Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 43.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]


