Abstract
Background
This study aimed to determine whether the properties of the late negative responses (LNRs) of the electroretinogram (ERG) elicited by sawtooth flicker are consistent with the characteristics of the photopic negative response generated by a light pulse (PhNRpulse).
Methods
ERG recordings were obtained from 10 visually normal individuals and from 6 patients with optic atrophy (OA) in response to 8-Hz rapid-on and rapid-off sawtooth flicker and to brief (4 ms) light pulses. All stimuli were either long-wavelength (R), middle-wavelength (G), or a combination of equal luminances of long and middle-wavelengths (Y) presented on a short-wavelength, rod-saturating adapting field. Amplitudes of LNRs were obtained in response to rapid-on (LNRon) and rapid-off (LNRoff) sawtooth flicker, and were also derived from the sum of the ERG waveforms to the two sawtooth phases (LNRadd).
Results
For the control subjects, PhNRpulse amplitude varied with stimulus wavelength, being largest in response to a long-wavelength pulse, as expected. However, the amplitudes of LNRon, LNRoff, and LNRadd were not significantly different for R, Y, and G sawtooth flicker. Despite the absence of a chromatic effect, LNRoff and LNRadd amplitudes were significantly smaller in the OA patients than in the controls, similar to the results for the PhNRpulse, implying an inner retinal origin for the LNRoff and LNRadd. However, LNRon amplitudes did not differ significantly between the OA patients and controls, although there was a significant correlation between the LNRon and PhNRpulse for R stimuli.
Conclusion
We conclude that LNRoff and LNRadd but not LNRon can be useful measures to assess the integrity of the inner retina that can complement the PhNRpulse.
Keywords: electroretinography, ERG, photopic negative response, chromatic, flicker, optic atrophy
Introduction
The photopic negative response (PhNR) of the light-adapted full-field ERG is a slow negative component that follows the b-wave. Previous studies have demonstrated that the PhNR elicited by a brief light pulse (here termed the PhNRpulse) originates from the spiking activity of inner retinal neurons, primarily ganglion cells [1, 2]. For example, the PhNRpulse is reduced or absent in macaques following intravitreal injection of tetrodotoxin (TTX) as well as in macaques with experimental glaucoma [1, 2]. In addition, the PhNRpulse is reduced in patients with glaucoma [3] and optic atrophy (OA) [4-7], as well as in patients with retinal vascular diseases that affect primarily the inner retina [8-10].
Given that the pulse stimulus is so brief, the PhNRpulse contains overlapping responses to both light onset (i.e., luminance increment) and light offset (i.e., luminance decrement). The PhNR onset and offset responses have often been elicited separately by using a discrete luminance step that is usually 200 ms in duration [1-3, 11], or by using square-wave flicker, which is typically presented at a temporal frequency of approximately 2 Hz [12-14]. The response to a luminance step contains a late negative component (PhNRon) that follows the b-wave response to the onset of the luminance increment and also a late negative component (PhNRoff) that follows the d-wave response to the increment offset. The ERG response to square-wave flicker shows similar late negative components [12-14].
Pharmacologic studies have demonstrated that the late negative responses observed using luminance steps and square-wave flicker are reduced in macaques following the intravitreal injection of TTX and in animals with experimental glaucoma [1, 2, 12-14]. Moreover, there is a reduction in both the PhNRon and PhNRoff to a luminance step in patients with glaucoma [3, 11]. Therefore, it is apparent that both the PhNRon and PhNRoff in response to luminance steps and to square-wave flicker have a major contribution from inner retinal neurons.
An alternative approach to segregating ERG onset and offset responses is to use sawtooth flicker, which consists of a series of luminance ramps that are either rapid-on (abrupt onset and gradual offset) or rapid-off (gradual onset and abrupt offset) [15-17]. The response to rapid-on sawtooth flicker contains a fairly large negative component that follows the b-wave, and the response to rapid-off sawtooth flicker has a small negative component following the d-wave [15-17]. A recent study investigated the nature of these late negative responses (LNRs) elicited by achromatic rapid-on and rapid-off sawteeth [17] (in the present study, the term LNR is used rather than PhNR in order to avoid implications as to the retinal origin of the response). The LNR elicited by rapid-off sawtooth flicker (LNRoff) was reduced in glaucoma patients compared to controls and was correlated significantly with the PhNRpulse [17], suggesting an inner retinal origin for the LNRoff. However, the LNR elicited by rapid-on sawtooth flicker (LNRon) was not significantly different between glaucoma patients and control subjects. Moreover, there was not a significant correlation between the LNRon and the PhNRpulse [17]. Thus, there appears to be a fundamental difference between the LNRon elicited by rapid-on sawtooth flicker and the PhNRon elicited by luminance increments and square-wave flicker.
In the present study, we investigated further the characteristics of the LNRs to rapid-on and rapid-off sawtooth flicker in order to better understand the nature of these responses. Instead of the broadband, achromatic stimuli used previously [17], we presented sawtooth flicker with different chromatic properties. Previous studies have shown that the wavelength composition of the stimulus can influence the amplitude of the PhNRpulse [2, 18, 19] as well as the amplitudes of the PhNRon and PhNRoff elicited by luminance steps [2]. In general, the largest amplitude of PhNR is obtained using a long-wavelength stimulus presented against a short-wavelength adapting field [2, 18]. We examined the effect of stimulus chromatic properties on the LNRs to sawtooth flicker in order to determine if their response characteristics vary with wavelength similar to previous reports of the PhNRpulse and of the PhNRon and PhNRoff elicited by luminance steps. Specifically, we used stimuli that had different degrees of excitation for the long-wavelength (L) and middle-wavelength (M) cones, ranging from approximately equal L and M cone excitation to excitation that was strongly biased toward L cones.
We also compared the ERG responses obtained from individuals with optic atrophy (OA) and presumed inner retinal damage to those obtained from visually normal subjects. As noted above, OA patients can have reduced PhNRpulse amplitudes [4-7], but responses to luminance increments and decrements have not been reported previously in such patients. A reduced amplitude of the LNRs elicited by sawtooth flicker in OA patients would indicate a probable inner retinal origin for these ERG response components.
In addition, we examined the late negative component of the ERG waveform that is obtained by summing the responses to rapid-on and rapid-off sawtooth flicker (LNRadd). It has been proposed that the addition of rapid-on and rapid-off waveforms minimizes the linear components of the ERG responses and emphasizes the nonlinear components, which are presumed to originate from within the inner retina [17]. A similar approach has been used previously to demonstrate that the addition of ERG responses to increments and decrements using full-field square-wave flicker can simulate the pattern ERG, which is presumed to reflect the integrity of retinal ganglion cells [12-14]. In the case of achromatic sawtooth flicker [17], it was observed that the LNRadd was significantly different in glaucoma patients than in control subjects, and there was a significant correlation between the LNRadd and the LNRpulse, thus indicating an inner retinal origin for the LNRadd. We examined this relationship further using sawtooth flicker with different chromatic characteristics and in OA patients compared to control subjects.
Method
Subjects
Ten visually normal control subjects, ages 22 to 61 years (mean age, 38 years) and six patients with OA, ages 26 to 63 years (mean age, 42.6 years) participated in the study. The study protocol was approved by an institutional review board at the University of Illinois at Chicago and informed consent was obtained from all participants prior to testing. Control subjects had best-corrected visual acuity of 20/20 or better in each eye and no history of ocular disease. The clinical characteristics of the OA patients are given in Table 1. All patients had clear ocular media and normal intraocular pressures. Molecular genetic analysis indicated that patients 1 and 6 each had a mitochondrial point mutation at base pair 11778 (G→A), which corresponds to the NADH dehydrogenase 4 (MTND4) gene. Patient 4 had a c.1090C→T (p.R364W) mutation in the mitofusin 2 (MFN2) gene. No molecular genetic information was available for the other three OA patients; their diagnosis was based on clinical findings.
Table 1.
Patient characteristics
| Patient No. | Age (years) | Gender | Diagnosis | Eye tested | Visual acuity | Optic disc pallor |
|---|---|---|---|---|---|---|
| 1 | 26 | M | LHON | OS | 6/100 | Severe |
| 2 | 35 | M | LHON | OD | 6/160 | Severe |
| 3 | 43 | M | LHON | OD | 3/400 | Severe |
| 4 | 44 | M | HMSN VI with optic atrophy | OD | 20/25 | Mild |
| 5 | 45 | F | DOA | OS | 20/400 | Moderate |
| 6 | 63 | M | LHON | OD | 3/110 | Severe |
LHON, Leber hereditary optic neuropathy; HMSN VI, hereditary motor and sensory neuropathy type VI; DOA, dominant optic atrophy.
Measurements of retinal nerve fiber layer thickness (RNFLT) in the OA patients were obtained using spectral domain optical coherence tomography (SD-OCT; OPKO Instrumentation, Miami, FL). The peripapillary RNFLT was measured along a circle of 3.45-mm diameter centered on the optic nerve. The RNFLT was calculated for the superior (46-135 degrees), nasal (136-225 degrees), inferior (226-315 degrees), and temporal (316-45 degrees) quadrants using the OPKO software. The RNFLT measurements obtained from the four quadrants of each of the six OA patients are given in Table 2, together with the mean RNFLT for each subject. These measurements were compared to normative data obtained from 245 eyes of 123 visually normal subjects in the age range of 19 to 85 years (mean age, 43 years), provided by the manufacturer. The RNFL was considered abnormally thin if the thickness value was less than the 5th percentile of the normative data. According to this criterion, all patients had an abnormally thin RNFL in at least two of the four quadrants, and the mean RNFLT of each patient was reduced significantly.
Table 2.
Retinal nerve fiber layer thickness (RNFLT) of the optic atrophy patients
| Patient No. | RNFLT (μm) |
||||
|---|---|---|---|---|---|
| Superior Retina | Inferior Retina | Nasal Retina | Temporal Retina | Mean | |
| 1 | 59** | 47** | 52** | 47* | 51** |
| 2 | 51** | 50** | 41** | 52 | 48** |
| 3 | 68** | 92** | 59* | 55 | 68** |
| 4 | 99* | 119 | 75 | 47* | 87* |
| 5 | 78** | 58** | 55* | 38** | 57** |
| 6 | 82** | 60** | 57* | 62 | 66** |
Between the 1st and 5th percentile of controls
less than the 1st percentile of controls.
Stimuli and Recording System
The full-field stimuli for the ERG measurements were generated by LED arrays contained within a ColorDome stimulator (Diagnosys LLC, Littleton, MA) and were either middle-wavelength (516 nm [G]), long-wavelength (640 nm [R]), or a combination of equal luminances of long and middle wavelengths [Y]. Stimuli were presented either as G, Y, and R pulses that had a duration of 4 ms and a luminance of 3.0 cd•s/m2 or as 8-cycle trains of G, Y, and R rapid-on and rapid-off sawtooth flicker at a frequency of 8.06 Hz, with a contrast of 100% and a mean luminance of 200 cd/m2 (illustrations of single cycles of the rapid-on and rapid-off stimuli are given in Fig. 2). According to a previous study [15], the ERG responses to rapid-on and rapid-off sawtooth flicker have a similar waveform morphology at frequencies of 4 Hz and 8 Hz, and sawtooth flicker at a frequency of 8 Hz has been used previously to elicit onset and offset responses [16]. Of note, the time between the luminance transients in an 8.06-Hz sawtooth (124 ms) corresponds approximately to the time between luminance transients in a 4-Hz square-wave (125 ms).
Fig. 2.
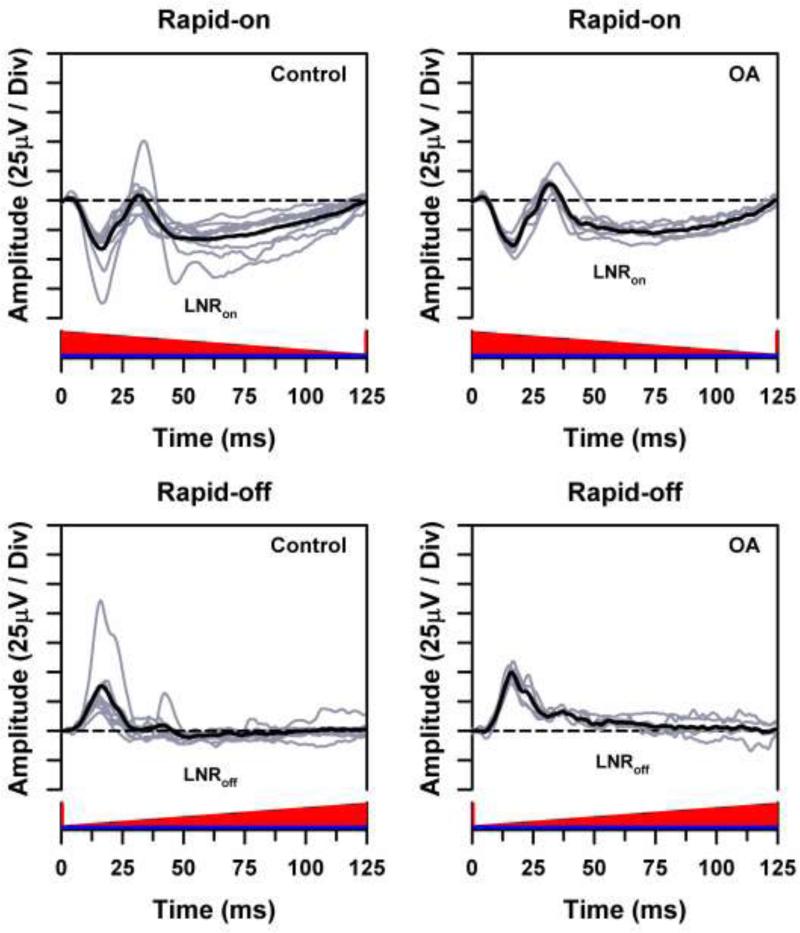
Individual ERG waveforms (gray traces) and overall mean ERG waveform (black traces) for the control subjects (left) and OA patients (right) in response to long-wavelength rapid-on (top) and rapid-off (bottom) sawtooth flicker presented against a short-wavelength adapting field, with the stimuli represented along the x-axes. Horizontal dashed lines represent the baseline from which the LNR was measured.
All stimuli were presented on a short-wavelength (464-nm), rod-saturating adapting field with a luminance of 12.5 photopic cd/m2 (41.1 scotopic cd/m2, which is equivalent to 3.3 log scotopic trolands, assuming an 8-mm dilated pupil) that was presented continuously. The spectral characteristics and the photopic and scotopic luminances of the full-field stimuli were calibrated using a PR-650 SpectraScan colorimeter (Photo Research, Inc., Chatsworth, CA). Photopic luminances were based on the 10-deg luminance efficiency function [V10(λ)], given that the non-foveal retina is the major contributor to the full-field ERG.
L-cone and M-cone excitation values were derived as described previously [20] and were converted to cone Weber contrasts for the pulses and to cone Michelson contrasts for the sawtooth flicker. Cone Weber contrast was defined as (ET – EB) / EB, where ET indicates the cone excitation produced by the test pulse plus short-wavelength adapting field and EB indicates the cone excitation produced by the adapting field alone. Cone Michelson contrast was defined as (Emax – Emin) / (Emax + Emin), where Emax indicates the maximum cone excitation (produced by the test stimulus plus adapting field) and Emin indicates the minimum cone excitation (produced by the adapting field alone). L-cone and M-cone Weber contrasts were nearly equal for the G pulse (69.29 vs. 49.57, respectively), were less similar for the Y pulse (89.54 vs. 30.81, respectively), and were most disparate for the R pulse (109.79 vs. 12.12, respectively). The L-cone and M-cone Michelson contrasts for sawtooth flicker were similar for the G stimulus (0.95 vs. 0.93, respectively), were slightly less similar for the Y stimulus (0.96 vs. 0.89, respectively), and were least similar for the R stimulus (0.97 vs. 0.78, respectively).
ERGs were recorded using a DTL electrode referenced to the forehead, with an earlobe ground electrode. ERG responses were acquired using a Diagnosys E2 console. Data were sampled at the rate of 2 kHz using an amplifier bandpass setting of 0.3 Hz to 500 Hz. No 60-Hz notch filter was used.
Procedure
One eye of each subject was tested, with the non-tested eye occluded using an eye patch. The pupil of the tested eye was dilated using 1% tropicamide and 2.5% phenylephrine hydrochloride drops. Following insertion of the electrode under room illumination, subjects were adapted to the short-wavelength adapting field for 3 min before the first pulse stimulus was presented. Pulses were presented with an inter-pulse interval of approximately 2 s until 5 reproducible ERG responses were obtained. Presentation of the rapid-on and rapid-off sawtooth stimuli followed the presentation of the pulse stimuli. Prior to the first sawtooth stimulus for each chromatic condition, subjects were adapted for 1 min to a uniform field of the same mean luminance and chromatic properties as the flicker. The sawtooth stimuli were repeated with an inter-stimulus interval of 5 s until 5 reproducible recordings were obtained. The five ERG responses obtained for each condition were averaged for each subject. For the rapid-on and rapid-off conditions, the response to the first stimulus cycle was omitted because the initial luminance change was from the mean level rather than being a full luminance excursion. There was no evidence of adaptation during the following 7 cycles of the waveform; the responses all had the same amplitude and timing characteristics. Therefore, these 7 response cycles were averaged, and measurements were obtained from the averaged waveforms.
Analysis
For the ERG responses to pulses and rapid-on sawtooth flicker, the a-wave amplitude was measured from the baseline (defined as the mean of the first 2.5 ms of the waveform) to the a-wave trough and the b-wave amplitude was measured from the a-wave trough to the b-wave peak. For the ERG responses to rapid-off sawtooth flicker, the d-wave amplitude was measured from the baseline to the d-wave peak.
The amplitudes of the PhNRpulse and the various forms of the LNR were measured as follows. For the control subjects, the PhNRpulse, LNRon, LNRoff, and LNRadd amplitudes were calculated as the difference between the baseline and the mean of 11 consecutive ERG data points centered at the trough of the LNR. The ERG waveforms of the patients with OA often did not have a defined trough. Therefore, to determine the appropriate measurement time, we first calculated the implicit times for the PhNRpulse, LNRon, LNRoff, and LNRadd components of the ERGs of the control subjects. The implicit times of the control subjects were not significantly different for the G, Y and R stimuli, so we averaged the mean implicit times for the three chromatic conditions. These mean implicit times (±1 standard deviation) were 72.1 ± 8.5 ms (PhNRpulse), 58.2 ± 5.6 ms (LNRon), 51.6 ± 4.2 ms (LNRoff), and 55.0 ± 7.9 ms (LNRadd). The PhNRpulse, LNRon, LNRoff, and LNRadd amplitudes of the OA patients were then calculated as the difference between the baseline and the mean of 11 consecutive ERG data points centered at the mean implicit times of the control subjects.
The response amplitudes for the PhNRpulse, LNRon, LNRoff, and LNRadd conditions were analyzed using two-way repeated-measures analyses of variance (ANOVAs). The analyses were performed separately for these four conditions. Post-hoc comparisons were performed using t-tests with a Bonferroni correction for multiple comparisons. Linear regression was used to evaluate the relationship between PhNRpulse amplitudes and the LNR amplitudes obtained using the sawtooth stimuli. Correlation coefficients with a p-value less than 0.05 were considered statistically significant.
Results
ERG waveforms
Figs. 1 through 3 present the ERG waveforms of the control subjects and OA patients in response to a long-wavelength (R) stimulus. In these figures, the gray ERG waveforms represent the responses of the individual subjects and the black trace represents the mean. The ERG responses to a pulse are shown in Fig. 1. The slow negative component following the b-wave in the control waveforms represents the PhNRpulse. The mean ERG waveform of the OA patients lacked the negative trough, and the response component following the b-wave remained above the baseline (dashed line).
Fig. 1.
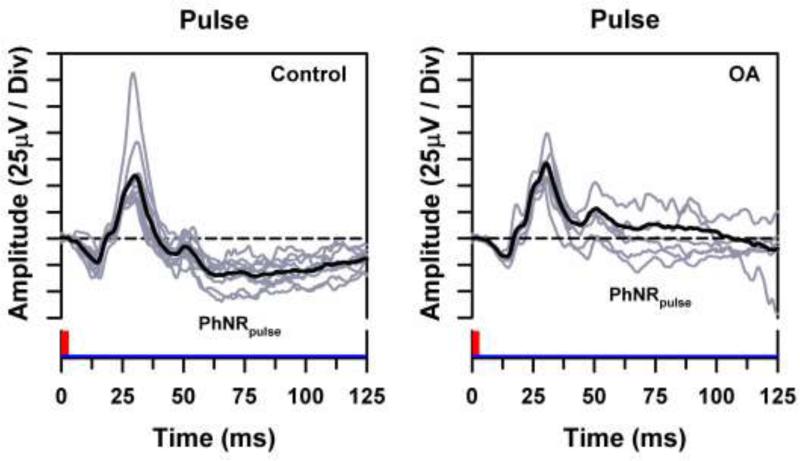
Individual ERG waveforms (gray traces) and overall mean ERG waveform (black traces) for the control subjects (left) and OA patients (right) in response to a long-wavelength luminance pulse presented against a short-wavelength adapting field, with the stimuli represented along the x-axes. Horizontal dashed lines represent the baseline from which the PhNRpulse was measured.
Fig. 3.
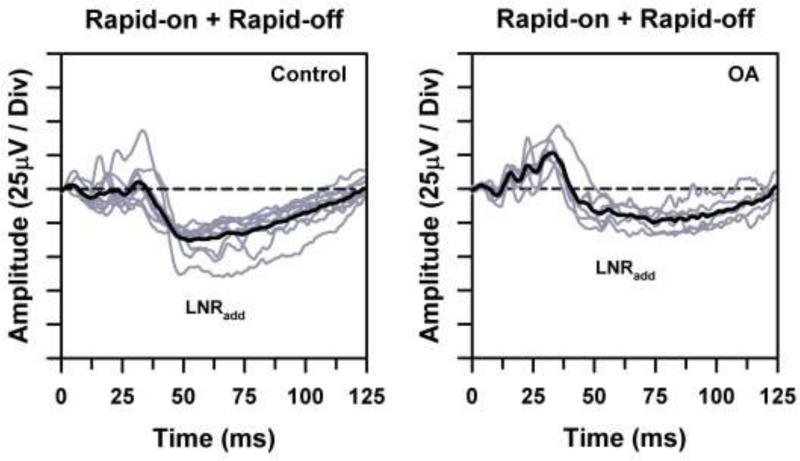
Individual ERG waveforms (gray traces) and overall mean ERG waveform (black traces) for the control subjects (left) and OA patients (right), obtained by summing the ERG waveforms obtained in response to the rapid-on and rapid-off sawtooth flicker shown in Fig. 2. Horizontal dashed lines represent the baseline from which the LNR was measured.
The ERG responses to rapid-on and rapid-off sawtooth stimuli are illustrated in Fig. 2. In response to the rapid-on stimulus, the waveforms of both the control subjects and the OA patients showed a late negative component that is labeled the LNRon. In response to the rapid-off stimulus, the mean ERG waveform of the control subjects showed an LNR of small amplitude (LNRoff). For the OA patients, this late component did not extend below the baseline.
The a-wave and b-wave amplitudes did not differ significantly between the OA patients and control subjects for any of the chromatic pulses. Similarly, the a-wave, b-wave, and d-wave amplitudes were not significantly different between the control subjects and OA patients for either the G, Y or R sawtooth flicker.
The waveforms obtained by adding the ERG responses to the rapid-on and rapid-off stimuli are illustrated in Fig. 3. The mean waveform of the control subjects was relatively flat over the first 35 ms, indicating that these regions of the rapid-on and rapid-off sawtooth responses approximately cancelled each other. This initial flat region was followed by a negative-going response component (LNRadd). Similarly, the waveforms of the OA patients had an LNRadd component that extended below the baseline. The OA patients also tended to show a relatively prominent positive-going component prior to the LNRadd (which is also apparent in the ERG of one of the control subjects). Of note, the summation waveforms presented previously using achromatic sawtooth flicker [17] also showed a fairly prominent positive-going response prior to the LNRadd, indicating that there can be non-cancelling (presumably nonlinear) response components at the time of the b-wave.
PhNRpulse amplitudes
The mean PhNRpulse amplitudes of the control subjects and OA patients for the G, Y, and R stimuli are shown in Fig. 4. The mean PhNRpulse amplitude for the control subjects was smallest for the G stimulus and largest for the R stimulus, with an intermediate value for the Y stimulus. The amplitude difference between the control results for the G and R stimuli was statistically significant (t = 3.34, p < 0.05), but the other comparisons among the chromatic stimuli were not statistically significant. There was not a significant effect of stimulus chromatic characteristics on the PhNRpulse amplitudes of the OA patients, who had near-zero mean PhNRpulse amplitudes for all three stimuli. The mean PhNRpulse amplitudes of the patients were reduced significantly below normal for the R (t = 5.57, p < 0.001) and Y (t = 4.24, p < 0.01) stimuli but not for the G stimulus.
Fig. 4.
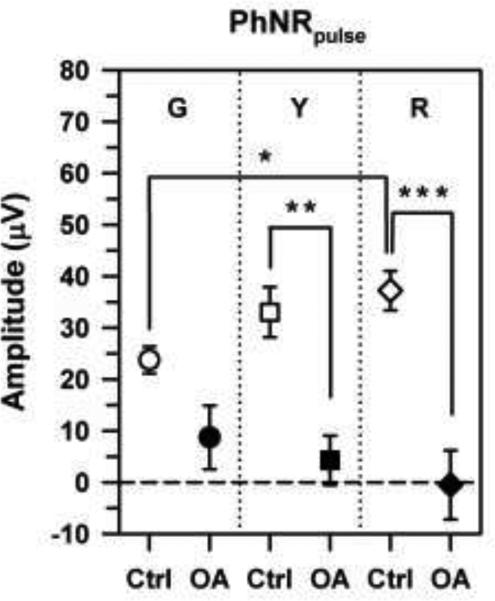
Mean PhNRpulse amplitudes of the control subjects (open symbols) and OA patients (filled symbols) in response to G (circles), Y (squares), and R (diamonds) pulses. The horizontal dashed line demarcates zero amplitude. Error bars represent ±1 standard error of the mean (sem). Brackets and asterisks indicate statistically significant comparisons, where * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001.
LNR amplitudes
The mean LNRon and LNRoff amplitudes of the control subjects and OA patients for G, Y, and R sawtooth flicker are shown in Fig. 5. The mean LNRon amplitudes of the control subjects were approximately the same as their mean PhNRpulse amplitudes (Fig. 4), and the mean LNRon amplitude was smallest for the G stimulus, as it was for PhNRpulse. However, the variability was greater for the LNRon amplitudes of the control subjects, so that there was no significant effect of stimulus chromatic characteristics on their LNRon amplitudes, in contrast to the results for the PhNRpulse.
Fig. 5.
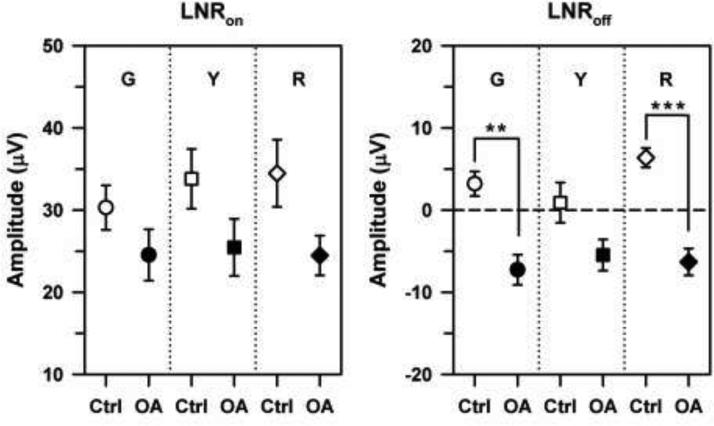
Mean LNRon (left) and LNRoff (right) amplitudes of the control subjects (open symbols) and OA patients (filled symbols) in response to G (circles), Y (squares), and R (diamonds) sawtooth flicker that was either rapid-on (left) or rapid-off (right). The horizontal dashed line demarcates zero amplitude; negative amplitudes indicate a response above the baseline. Error bars represent ±1 sem. Brackets and asterisks indicate statistically significant comparisons, where ** indicates p < 0.01 and *** indicates p < 0.001.
The LNRon amplitudes of the OA patients were equivalent for all three chromatic conditions and were substantially larger than their corresponding PhNRpulse amplitudes (Fig. 4), unlike the results for the control subjects. Although the LNRon amplitudes of the OA patients were lower overall than those of the control subjects, the differences between the patients and controls were not statistically significant, given the variability of the control results. Nevertheless, there was a modest but statistically significant correlation between PhNRpulse amplitude for the R pulse and LNRon amplitude for R rapid-on sawtooth flicker (see Table 3). The correlations between PhNRpulse amplitude for an R pulse and LNRon amplitude for G and Y rapid-on sawtooth flicker were not significant, however.
Table 3.
Correlation coefficients between PhNRpulse for a long-wavelength (R) stimulus and LNRs to sawtooth flicker with three chromatic properties
| LNRon | LNRoff | LNRadd | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G | Y | R | G | Y | R | G | Y | R | |
| PhNRpulse R | 0.48 | 0.34 | 0.54* | 0.67** | 0.33 | 0.78*** | 0.73*** | 0.64** | 0.80*** |
p < 0.05
p < 0.01
p < 0.001
In comparison to LNRon amplitudes, the mean LNRoff amplitudes were quite small for both the patients and control subjects (the dashed line in Fig. 5 indicates zero amplitude). The mean LNRoff amplitudes of the OA patients were all negative, which indicates that their responses were above the baseline for all three chromatic conditions. There was no statistically significant effect of stimulus chromatic properties on the LNRoff amplitudes of either the control subjects or the OA patients, as was also the case for LNRon amplitudes. However, the LNRoff amplitudes of the patients were significantly smaller than those of the control subjects for the G (t = 3.86, p < 0.01) and R (t = 4.68, p < 0.001) stimuli, although not for the Y stimulus. The correlations between the PhNRpulse for the R pulse and the LNRoff for G and R rapid-off sawtooth flicker were statistically significant (Table 3), but this was not the case for Y rapid-off sawtooth flicker.
The mean LNRadd amplitudes of the control subjects and OA patients for the G, Y, and R stimuli are presented in Fig. 6. Similar to the results for LNRon and LNRoff, there was no significant effect of stimulus chromatic properties on LNRadd amplitudes for either the control subjects or the OA patients, although the R stimulus elicited the largest LNRadd amplitude for the control subjects. The LNRadd amplitudes of the OA patients were reduced significantly below those of the control subjects for all three chromatic stimuli (t = 3.63, p < 0.05 [G], t = 3.50, p < 0.05 [Y]; t = 4.67, p < 0.01 [R]), with the largest difference for the R stimulus. Moreover, there were statistically significant correlations between PhNRpulse amplitude for the R pulse and LNRadd amplitudes for the G, Y, and R stimuli, with the highest correlation for R sawtooth flicker (Table 3).
Fig. 6.
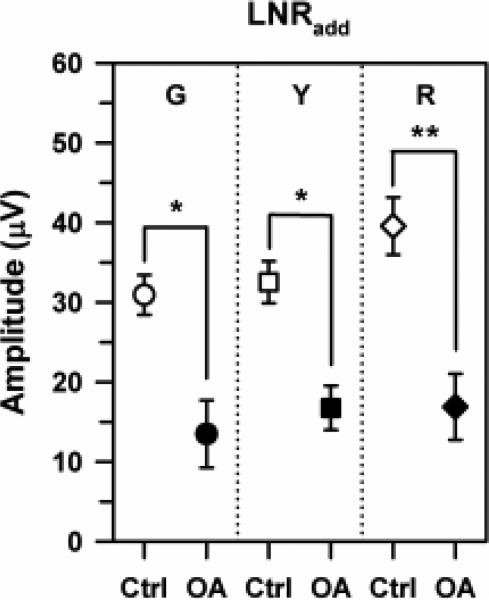
Mean LNRadd amplitudes for the control subjects (open symbols) and OA patients (filled symbols) derived from the summed responses to rapid-on and rapid-off sawtooth flicker that was either G (circles), Y (squares), or R (diamonds). Error bars represent ±1 sem. Brackets and asterisks indicate statistically significant comparisons, where * indicates p < 0.05 and ** indicates p < 0.01.
Discussion
This study investigated the characteristics of the late negative responses to rapid-on (LNRon) and rapid-off (LNRoff) sawtooth flicker using two approaches. First, we examined the effect of the chromatic characteristics of the stimuli to determine whether the LNRs showed the same wavelength dependence as has been described for the PhNRpulse [2, 18]. Second, we compared the LNRs of visually normal control subjects with those of patients with OA to determine whether the LNRs to sawtooth flicker are affected by inner retinal disease, as is the PhNRpulse [3-10].
Consistent with previous studies [2, 18], the PhNRpulse amplitudes of our control subjects were significantly larger for the R than for the G stimulus, with an intermediate amplitude in response to the Y stimulus. This result supports a previous proposal [2] that the PhNRpulse amplitude is largest for a pulse that excites predominantly one cone type, presumably because this stimulus produces the least spectral antagonism, which would tend to favor the inner retina. We show further that PhNRpulse amplitude is related to the ratio of L-cone to M-cone Weber contrast, which was greatest for the R stimulus, lowest for the G stimulus, and intermediate for the Y stimulus. In comparison, the amplitudes of LNRon, LNRoff, and LNRadd of the control subjects were not influenced significantly by the chromatic properties of the sawtooth flicker, although there was a tendency for the largest amplitudes to occur in response to R stimuli. A plausible explanation for the minimal chromatic effect observed using sawtooth flicker is that LNR amplitude was governed by L-cone and M-cone Michelson contrasts, which were fairly similar for G, Y, and R sawtooth flicker, with only a slightly higher contrast ratio for R sawtooth flicker.
Despite the absence of a significant chromatic effect using sawtooth flicker, the LNRoff amplitudes differed significantly between the OA patients and controls for the G and R stimuli, even though the amplitudes were relatively small overall. Moreover, the LNRadd amplitudes differed significantly between the OA patients and controls for the G, Y, and R stimuli. These results indicate that the inner retina likely makes a substantial contribution to the LNRoff and LNRadd, particularly for R stimuli, which showed the greatest difference between OA patients and controls.
However, LNRon amplitudes did not differ significantly between the OA patients and controls for either the G, Y, or R stimulus (although there was a tendency for the mean amplitudes of LNRon for the various chromatic stimuli to be smaller overall for the OA patients than for the control subjects). We note, however, that there was a significant, although modest, correlation between LNRon amplitudes and PhNRpulse amplitudes for the R stimuli (Table 3). The lack of a significant difference between the OA patients and controls for mean LNRon amplitudes is likely due in part to the larger intersubject variability for LNRon amplitudes compared to those for LNRoff or LNRadd.
Our finding that significant differences existed between the OA patients and control subjects for the LNRoff and LNRadd conditions, together with the lack of a significant difference for LNRon, are consistent with the results of a recent study in which achromatic sawtooth flicker at a frequency of 4 Hz was used to examine LNRs in glaucoma patients vs. control subjects [17]. That study reported that LNRoff and LNRadd but not LNRon differed significantly between glaucoma patients and controls.
Our results for LNRon using long-wavelength sawtooth flicker differ from previous findings for long-wavelength luminance steps. Both the PhNRon and PhNRoff in response to long-wavelength luminance steps were reduced following intravitreal TTX injections in macaques [1, 2] and they were also reduced in patients with glaucoma [3], indicating that both the PhNRon and PhNRoff have a substantial contribution from inner retinal neurons. In the present study, however, the mean LNRon amplitude of the OA patients for R rapid-on sawtooth flicker (Fig. 5, left) was not significantly different from that of the control subjects. The explanation for this apparent difference between the PhNRon measured using a long-wavelength step and the LNRon to long-wavelength rapid-on sawtooth flicker remains to be determined.
Of particular interest, LNRadd amplitudes differed significantly between the OA patients and controls for all chromatic stimuli. Similarly, in a previous study [17], LNRadd for achromatic sawtooth flicker discriminated best between glaucoma patients and controls, compared to LNRon and LNRoff. Therefore, the addition of ERG responses to rapid-on and rapid-off sawtooth flicker appears to emphasize the nonlinear (presumably inner retinal) contribution to the response, as has been described previously for the addition of ERG waveforms obtained in response to square-wave flicker with opposite phase relationships [12-14].
In conclusion, the LNRs elicited by 8-Hz sawtooth flicker do not show the same wavelength dependence as does the PhNRpulse. Moreover, the LNRon elicited by rapid-on sawtooth flicker did not differ significantly between the patients with OA and the control subjects for any chromatic condition, indicating that this ERG response component likely has a substantial contribution from retinal elements other than inner retinal neurons. Nevertheless, both the LNRoff and the LNRadd components of the sawtooth flicker ERG appear to have a major contribution from the inner retina, given the significant differences observed between the OA patients and controls, particularly for long-wavelength stimuli. Therefore, our results support the hypothesis [17] that the LNRoff and especially the LNRadd could serve as useful measures of inner retinal function that can complement the PhNRpulse in patients with retinal or optic nerve diseases.
Acknowledgments
This research was supported by NIH research grant 5R01EY008301, NIH ARRA grant 3R01EY008301-18S1, NIH core grant P30EY001792, a grant from the Foundation Fighting Blindness, Columbia, Maryland, and a Research to Prevent Blindness unrestricted departmental award. The authors thank Gerald A. Fishman, M.D., Chicago Lighthouse for People Who Are Blind or Visually Impaired and the Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, for his help in recruiting and evaluating the patients.
Footnotes
Present Address:
S. Gowrisankaran Vision Performance Institute, College of Optometry, Pacific University, Forest Grove, Oregon USA
M. A. Genead The Pangere Center for Hereditary Retinal Diseases, The Chicago Lighthouse for People Who Are Blind or Visually Impaired, Chicago, Illinois, USA
A. Anastasakis Department of Ophthalmology, University of Crete, Crete, Greece
References
- 1.Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(6):1124–1136. [PubMed] [Google Scholar]
- 2.Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ. Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007;48(10):4818–4828. doi: 10.1167/iovs.07-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42(2):514–522. [PubMed] [Google Scholar]
- 4.Gotoh Y, Machida S, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004;122(3):341–346. doi: 10.1001/archopht.122.3.341. [DOI] [PubMed] [Google Scholar]
- 5.Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA. Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci. 2004;45(10):3827–3837. doi: 10.1167/iovs.04-0458. [DOI] [PubMed] [Google Scholar]
- 6.Tamada K, Machida S, Yokoyama D, Kurosaka D. Photopic negative response of full-field and focal macular electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol. 2009;53(6):608–614. doi: 10.1007/s10384-009-0731-2. [DOI] [PubMed] [Google Scholar]
- 7.Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, Terasaki H. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007;48(2):820–824. doi: 10.1167/iovs.06-0845. [DOI] [PubMed] [Google Scholar]
- 8.Machida S, Gotoh Y, Tanaka M, Tazawa Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol. 2004;137(5):938–940. doi: 10.1016/j.ajo.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006;50(4):367–373. doi: 10.1007/s10384-006-0326-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Wu D, Huang S, Yan H. The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol. 2006;113(1):53–59. doi: 10.1007/s10633-006-9015-z. [DOI] [PubMed] [Google Scholar]
- 11.Horn FK, Gottschalk K, Mardin CY, Pangeni G, Junemann AG, Kremers J. On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc Ophthalmol. 2011;122(1):53–62. doi: 10.1007/s10633-011-9258-1. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci. 2000;41(9):2797–2810. [PubMed] [Google Scholar]
- 13.Luo X, Frishman LJ. Retinal pathway origins of the pattern electroretinogram (PERG). Invest Ophthalmol Vis Sci. 2011;52(12):8571–8584. doi: 10.1167/iovs.11-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson MC, Viswanathan S. Comparison of uniform field and pattern electroretinograms of humans. J Mod Opt. 2007;54(9):1281–1288. [Google Scholar]
- 15.Khan NW, Kondo M, Hiriyanna KT, Jamison JA, Bush RA, Sieving PA. Primate retinal signaling pathways: suppressing on-pathway activity in monkey with glutamate analogues mimics human CSNB1-NYX genetic night blindness. J Neurophysiol. 2005;93(1):481–492. doi: 10.1152/jn.00365.2004. [DOI] [PubMed] [Google Scholar]
- 16.Alexander KR, Fishman GA, Barnes CS, Grover S. On-response deficit in the electroretinogram of the cone system in X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2001;42(2):453–459. [PubMed] [Google Scholar]
- 17.Pangeni G, Lämmer R, Tornow RP, Horn FK, Kremers J. On- and off-response ERGs elicited by sawtooth stimuli in normal subjects and glaucoma patients. Doc Ophthalmol. 2012;124(3):237–48. doi: 10.1007/s10633-012-9323-4. [DOI] [PubMed] [Google Scholar]
- 18.Sustar M, Cvenkel B, Brecelj J. The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol. 2009;118(3):167–177. doi: 10.1007/s10633-008-9150-9. [DOI] [PubMed] [Google Scholar]
- 19.Kremers J, Jertila M, Link B, Pangeni G, Horn FK. Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol. 2012;124(2):79–90. doi: 10.1007/s10633-011-9304-z. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Pokorny J, Smith VC. Associating color appearance with the cone chromaticity space. Vision Res. 2005;45(15):1929–1934. doi: 10.1016/j.visres.2005.01.033. [DOI] [PubMed] [Google Scholar]


