Abstract
The cysteinyl leukotrienes (cys-LTs) LTC4, LTD4, and LTE4 are a class of peptide-conjugated lipids formed from arachidonic acid and released during activation of mast cells (MCs). We now report that human cord-blood-derived MCs (hMCs) express the CysLT1 receptor, which responds not only to inflammation-derived cys-LTs, but also to a pyrimidinergic ligand, UDP. hMCs express both CysLT1 protein and transcript, and respond to LTC4, LTD4, and UDP with concentration-dependent calcium fluxes, each of which is blocked by a competitive CysLT1 receptor antagonist, MK571. Stably transfected Chinese hamster ovary cells expressing the CysLT1 receptor also exhibit MK571-sensitive calcium flux to all three agonists. Both hMCs and CysLT1 transfectants stimulated with UDP are desensitized to LTC4, but only partially to LTD4. Priming of hMCs with IL-4 for 5 days enhances their sensitivity to each agonist, but preferentially lowers their threshold for activation by LTC4 and UDP (≈3 log10-fold shifts in dose-response for each agonist) over LTD4 (1.3 log10-fold shift), without altering CysLT1 receptor mRNA or surface protein expression, implying the likely induction of a second receptor with CysLT1-like dual ligand specificity. hMCs thus express the CysLT1 receptor, and possibly a closely related IL-4-inducible receptor, which mediate dual activation responses to cys-LTs and UDP, providing an apparent intersection linking the inflammatory and neurogenic elements of bronchial asthma.
The cysteinyl leukotrienes (cys-LTs) elaborated by mast cells (MCs) and eosinophils are established mediators of bronchial asthma, on the basis of the efficacy of drugs that attenuate their biosynthesis or interfere with their receptors (1, 2). The cellular generation of the primary cys-LT, leukotriene (LT)C4, requires the sequential functions of 5-lipoxygenase (5-LO) (3) in the presence of 5-lipoxygenase-activating protein (FLAP) (4), and leukotriene C4 synthase (LTC4S) (5, 6). LTC4S conjugates LTA4, a product of arachidonic acid metabolism by 5-LO, to reduced glutathione, forming LTC4. LTC4 is released by a distinct cellular export mechanism (7), and is converted sequentially to LTD4 and LTE4 by extracellular γ-glutamyltransferase and dipeptidase, respectively (8, 9). LTC4, LTD4, and LTE4 then act at specific, seven transmembrane domain, G protein-coupled receptors to mediate bronchoconstriction, vasodilation, vascular leakage, and leukocyte emigration (10–14). To date, two cys-LT receptors, the CysLT1 receptor and the CysLT2 receptor, have been cloned and characterized (15–18). CysLT1 receptor mRNA is expressed in human spleen, peripheral blood leukocytes, and lung, where it resides in bronchial smooth muscle cells and alveolar macrophages (15). The gene for the CysLT1 receptor maps to the X chromosome (15) near a region (Xq13–21) containing candidate genes for bronchial asthma (19). When expressed in transfected cell lines, the CysLT1 receptor binds LTD4 with roughly 10-fold greater affinity than it binds LTC4 (EC50, 10−9 versus 10−8 M, respectively) (15, 16). The CysLT2 receptor, which is not blocked by currently available therapeutic cys-LT receptor antagonists, is expressed by peripheral blood leukocytes, lymph nodes, spleen, heart, and brain (17, 18). The gene for the CysLT2 receptor resides on chromosome 13q14 near the D13S153 locus, which has been linked to asthma (20). Unlike the CysLT1 receptor, the CysLT2 receptor binds LTC4 and LTD4 equally, with an EC50 of ≈10−8 M (17, 18) when expressed heterologously.
Human lung MCs are present in perivascular and perineural locations, as well as in the muscular, submucosal, and intraepithelial compartments of the bronchi, where they are increased in numbers in patients with asthma (21). When stimulated ex vivo by cross-linkage of their high-affinity Fc receptors for IgE (FcɛRI), dispersed human lung MCs generate cys-LTs (22). When human MCs (hMCs), derived in vitro from cord blood, are primed with recombinant human IL-4 (10 ng/ml), they express both the terminal biosynthetic enzyme, LTC4S, and FcɛRI, thereby markedly up-regulating their generation of cys-LTs in response to FcɛRI cross-linkage (23). Thus, the pivotal Th2 cytokine IL-4 regulates functions of MCs that are directly pertinent to their effector role in allergic inflammation. Because both the CysLT1 and CysLT2 receptors are expressed by hematopoietic cells with proinflammatory functions, we speculated that hMCs might also express these receptors, and that their expression and function might be modulated in parallel with the biosynthetic system for the generation of cys-LTs. We therefore examined the responses of hMCs to exogenous cys-LTs, and studied the modulation of these responses by IL-4 priming. In the present study, we demonstrate that hMCs express the CysLT1 receptor, which unexpectedly serves as a dual-specific receptor for both the cys-LTs and for the pyrimidine nucleotide UDP. Additionally, hMCs that are primed with IL-4 exhibit markedly enhanced calcium flux to LTC4 and UDP. Notably, IL-4 priming does not alter the levels of CysLT1 protein or RNA expression by hMCs, suggesting the possible induction of a second, CysLT1-like receptor on hMCs.
Materials and Methods
Culture of hMCs.
hMCs were derived from cord blood mononuclear cells cultured in the presence of stem cell factor (SCF; 100 ng/ml; Amgen Biologicals), IL-6 (50 ng/ml, R & D Systems), and IL-10 (10 ng/ml, R & D Systems), as described (24). Cells were harvested at 6–8 wk, at which time >95% stained positively with toluidine blue. For experiments in which the effects of IL-4 were examined, the hMCs were further cultured with either SCF alone or with SCF and IL-4 (10 ng/ml, R & D Systems) for 5 days at 37°C and 5% CO2.
Calcium Mobilization Assays.
Changes in the cytosolic concentration of free Ca2+ were measured with Fura-2-loaded hMCs or stably transfected Chinese hamster ovary (CHO) cells, at 0.5–1 × 106 cells per sample. The cells were washed and resuspended in Hank's balanced salt solution (HBSS) containing 1 mM CaCl2, 1 mM MgCl2, and 0.1% BSA, and subsequently labeled with Fura-2-AM (Molecular Probes) for 30 min at 37°C. The labeled cells were washed and resuspended in the same buffer. In some samples, the cells were pretreated with MK571 (Biomol, Plymouth Meeting, PA) for 1 min, pertussis toxin (Sigma) for 20 min, EGTA for 15 min, or serine borate for 1 min. The changes in intracellular Ca2+ were measured by using excitation at 340 and 380 nm in a fluorescence spectrophotometer (Hitachi F-4500) after stimulation with cys-LTs (Cayman Chemicals, Ann Arbor, MI), UDP (Sigma), or BAY u9773 (Biomol). The relative ratios of fluorescence emitted at 510 nm were recorded and displayed as a reflection of intracellular concentrations of Ca2+. The agonist concentration eliciting the half-maximal ratio was defined as the EC50 for each and was used to calculate IL-4-induced alterations in agonist responsiveness.
Northern Blotting and Reverse Transcriptase (RT)-PCR.
Total RNA was extracted from hMCs at 6–8 wk of culture with TRI Reagent (Molecular Research, Cincinnati), and RNA blotting was performed under high stringency conditions as previously described (23). For RT-PCR, RNA samples were primed with oligo(dT) and reverse transcribed in accordance with the manufacturer's protocol in an RT kit (CLONTECH). The following primers were designed to amplify the full-length coding sequences of the CysLT1 receptor, the CysLT2 receptor, and the P2Y2, P2Y4, and P2Y6 receptors, from either reverse-transcribed RNA or from human leukocyte cDNA (CLONTECH), reading from 5′ to 3′: CysLT1A (sense strand), ATGGATGAAACAGGAAATCTGAC; CysLT1B (antisense strand), TACTTTACATATTTCTTCTCCTTT; CysLT2A (sense strand), CAGCATGGAGAGAAAATTTATGT; CysLT2B (antisense strand), TTATACTCTTGTTTCCTTTCTCAACC; P2Y2A (sense strand), ATGGCAGCAGACCTGGGCC; P2Y2B (antisense strand), CTACAGCCGAATGTCCTTAGTG; P2Y4A (sense strand), ATGGCCAGTACAGAGTCCTCCCT; P2Y4B (antisense strand), TTACAATCTATCTGCCCTAGGAGT; P2Y6A (sense strand), GGCTTTGGAAGGCGGAGTTCA; and P2Y6B (antisense strand), TCCATGCCCAGCTGAGCTGAA. PCRs were performed with 5 units of Taq polymerase (Perkin-Elmer) for 35 cycles with the following parameters: 94°C × 1 min; 55°C × 1 min, 72°C × 1 min for 35 cycles of amplification in a Perkin–Elmer Thermal Cycler. Specific primers for human glyceraldehyde–3-phosphate dehydrogenase (CLONTECH) were run as positive controls. The PCR products were resolved on ethidium bromide-stained gels containing 0.5% agarose/0.5% Synergel (Diversified Biotech). The identities of the resultant PCR products were confirmed with an automated sequencing reaction (Dana-Farber Cancer Institute Molecular Biology Core Facility).
Stable Transfection of CHO Cells.
CHO cells stably transfected with the long isoform of mouse CysLT1 receptor cDNA were prepared as described (25). PCR-generated coding sequences of human P2Y4 receptor (amplified from IL-4-primed hMC RNA) and P2Y6 receptor (amplified from human leukocyte cDNA) were subcloned into the BamHI/EcoRV or BamHI/NotI sites of the pIRESneo expression vector (CLONTECH). One day before transfection, 2 × 105 CHO cells per 6-cm dish were seeded in growth medium [DMEM/F-12 (Life Technologies, Gaithersburg, MD) and 10% FCS (Sigma), with 100 μg/ml streptomycin and 100 units/ml penicillin (Sigma)], and were incubated at 37°C in 5% CO2. The transfection was performed with 5 μg of DNA, using SuperFect Transfection reagent (Qiagen, Valencia, CA), in accordance with the manufacturer's protocol. After 24 h, the cells were treated with trypsin/EDTA and incubated in growth medium for an additional 24 h, at which point the medium was changed to include G418 (800 μg/ml, Life Technologies). The medium was then changed every 24–48 h until colonies were visible (10–14 days), at which point they were picked and transferred to a 24-well plate. After confluency was reached, the cells were treated with trypsin, split, and then analyzed for the transduced gene with Northern blotting, as described above. The cells were expanded in G418-containing growth medium and used for further analyses. At least three positive clones were analyzed for each receptor. Nontransfected CHO cells were used as negative controls for all analyses.
Flow Cytometry.
Samples of 1 × 105 hMC were suspended in cold HBSS containing 2% FBS and 0.1% human serum (FACS buffer), and incubated on ice for 45 min with 20 μg/ml of an affinity-purified rabbit antibody raised against the peptide sequence FPVQNINLVTQKKAR corresponding to amino acids 127–141 of the human CysLT1 receptor. Replicate negative control samples were incubated with an equal concentration of a rabbit IgG of irrelevant specificity. The samples were washed in cold FACS buffer and incubated on ice for 45 min with a FITC-conjugated sheep anti-rabbit antibody (Jackson Immunoresearch) and then analyzed using FACSort (Becton Dickinson) as described (24). Results are presented as overlaid histograms.
Results and Discussion
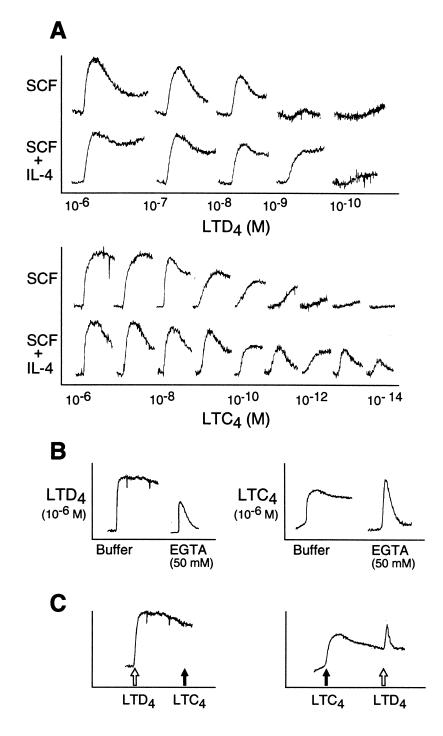
To determine whether mature hMCs expressed functional receptors for cys-LTs, we stimulated fura-2-loaded hMCs with exogenous LTC4 and LTD4 (10−6 to 10−14 M) after 5 days of maintenance with SCF with or without priming by IL-4, and assayed calcium flux (Fig. 1A). In hMCs maintained in SCF alone before stimulation, both agonists elicited rapid, sustained elevations in the levels of intracellular calcium, with 50% maximal responses to LTD4 at doses of 10−8 to 10−10 M and to LTC4 at higher concentrations (10−7 to 10−9 M). Priming of hMCs for 5 days with IL-4 shifted the dose–response curves for both agonists, with the concentration of LTD4 required to elicit the 50% maximal signal from hMCs being enhanced by log10 1.3 ± 0.4, compared with log10 3.8 ± 1.9 for LTC4 (n = 3 for each). The IL-4 priming effect for the hMC response to LTC4 exceeded that for the response to LTD4 by at least 2 log units in every experiment (Fig. 1A), resulting in equal agonist effects of LTC4 and LTD4 after priming in some experiments. Thus, all subsequent experiments focused on the characterization of the IL-4-up-regulated cys-LT response. At their maximally effective concentrations, LTD4 and LTC4 elicited calcium signals of comparable magnitude. Cell responses to LTC4 were essentially unaffected by the presence of 50 mM serine borate, which inhibits the extracellular conversion of LTC4 to LTD4 (n = 2, data not shown). The sustained phase, but not the initial phase, of the calcium response to each agonist was completely inhibited by the addition of the chelating agent EGTA (Fig. 1B), indicating that this latter phase depended on extracellular calcium, whereas the initial phase reflected mobilization of intracellular calcium stores. Although stimulation with LTD4 at concentrations of 100 nM to 1 μM always desensitized hMCs to subsequent stimulation with LTC4 at 1 μM, only partial desensitization to LTD4 (10 nM-1 μM) followed stimulation with 1 μM LTC4 (Fig. 1C). The hMCs also responded to LTE4 (data not shown), which functions as a weak partial agonist for both the CysLT1 and CysLT2 receptors (15, 18). The priming effect of IL-4, which was markedly preferential for LTC4-mediated calcium flux, combined with the lack of complete reciprocal cross-desensitization between LTC4 and LTD4 at saturating concentrations, suggested that the cys-LT-mediated calcium signals likely originated from more than one receptor on IL-4-primed hMCs.
Figure 1.
Calcium responses of hMCs to stimulation with LTD4 and LTC4, and preferential up-regulation by IL-4 of the response to LTC4. (A) hMCs maintained in SCF alone (100 ng/ml) or primed for 5 days with IL-4 (10 ng/ml) in the continued presence of SCF exhibit dose-dependent calcium responses to LTD4 (Upper) or LTC4 (Lower). The depicted results reflect separate experiments for LTD4 and LTC4 performed with hMCs from different donors. Priming the hMCs with IL-4 shifted the 50% maximal response to LTD4 (1.33 ± 0.41 log-fold increment, mean ± SEM, n = 3), and to LTC4 (mean, 3.83 ± 1.94 log-fold increment, n = 3) in every experiment performed. (B) The sustained phase of the cys-LT-induced calcium signal to each agonist was abolished by EGTA. (C) At 1 μM concentrations, LTD4 completely desensitized the hMCs to LTC4, whereas LTC4 partly desensitized them to LTD4. The depicted results for IL-4-primed hMCs were replicated three times for A and C, and two and three times for B.
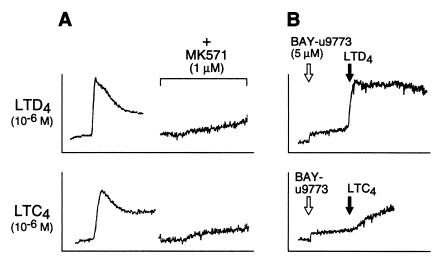
To determine whether the profile of cys-LT receptor(s) on IL-4-primed hMCs was compatible with the known cys-LT receptors, pharmacologic inhibitors and agonists were used for further characterization. The competitive antagonist MK571 (1 μM), which blocks access to the CysLT1 receptor but not to the CysLT2 receptor (15), completely blocked the responses of hMCs to both LTC4 and LTD4 at 1 μM to 100 nM in every experiment, with or without IL-4 priming (Fig. 2A). hMCs treated with 5 μM BAY-u9773, a compound that blocks the CysLT1 receptor and acts as a desensitizing agonist at the CysLT2 receptor (18), yielded a weak calcium signal that was resistant to MK571 (data not shown) and partially blocked subsequent reactivity to LTC4 but not to LTD4 (Fig. 2B). The complete blockade of hMC responses to either LTD4 or LTC4 by MK571, combined with the partial antagonistic effect of BAY-u9773 on LTC4- but not on LTD4-mediated calcium flux, indicated that the receptor(s) responsible for the IL-4-dependent LTC4 response of hMCs did not completely fit the profile of either the CysLT1 receptor or the CysLT2 receptor, alone or in combination.
Figure 2.
Effect of pharmacologic receptor inhibitors on cys-LT-mediated calcium signals of IL-4-primed hMCs. (A) The competitive CysLT1 receptor antagonist MK571 (1 μM) completely blocked the responses of IL-4-primed hMCs to 1 μM LTD4 (Upper) and LTC4 (Lower). (B) BAY-u9773 (5 μM), which blocks the CysLT1 receptor but activates and desensitizes the CysLT2 receptor, yielded a small calcium signal in IL-4-primed hMCs that did not attenuate the subsequent calcium response to LTD4 (Upper), but partly blocked the agonist effect of LTC4 (Lower). The data depicted in A and B were replicated in two additional experiments, each with hMCs of different donors.
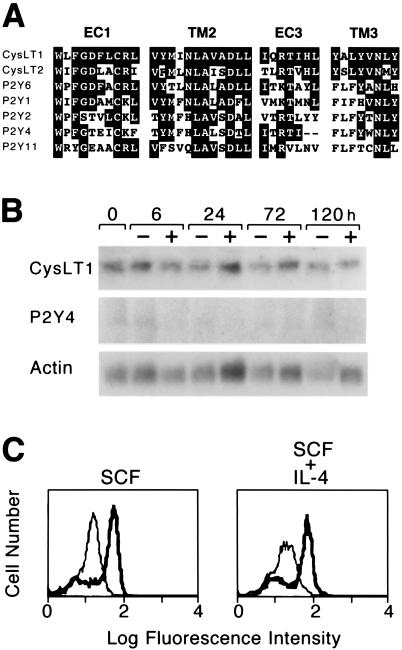
The unique pharmacologic profile of hMC responses to exogenous cys-LTs prompted a search of the existing GenBank databases for homologues of the known cys-LT receptors that might be previously unrecognized members of this family. The cDNA encoding the CysLT1 receptor is minimally homologous to that encoding the CysLT2 receptor, and their respective amino acid sequences are only 38% identical. The amino acid sequences of both the CysLT1 and CysLT2 receptors have 24–32% identity to members of the purinergic (P2Y) receptor family composed of G protein-coupled receptors that mediate responses to extracellular nucleotides. Domains that are strongly conserved between the CysLT1 receptor and the CysLT2 receptor include a WXFGDXCRX motif in the first extracellular loop (EC1) of each receptor, a hydrophobic motif (VXMXNLAXXDLL) in the second transmembrane domain (TM2), a positionally conserved XXRTXHL motif at the juxtamembrane region of the third extracellular loop (EC3), and a YXLYVNXY motif in the third transmembrane domain (TM3) (Fig. 3A). A search for proteins with similarity to the CysLT1 and CysLT2 receptors within these domains revealed particularly strong conservation with the P2Y6 receptor, which preferentially binds UDP, in EC1, where 8 of 10 amino acids are identical with the CysLT1 receptor. The P2Y6 receptor also exhibits homology to the CysLT1 receptor in TM2 (9 of 12 residues), EC3 (3 of 7), and TM3 (3 of 8), with conservative substitutions at some of the nonidentical residues. The P2Y4 receptor, which preferentially binds UTP, is identical to the CysLT1 receptor at 4 of 7 residues in EC3 and also exhibits homology in EC1, TM2, and TM3 (Fig. 3A), with some conserved substitutions as well. Lesser degrees of conservation in these domains were also found in the published amino acid sequences of three other known human P2Y receptors: P2Y1, P2Y2, and P2Y11 (Fig. 3A). Of the known human P2Y receptors, only the P2Y4 receptor could be amplified by PCR from reverse transcribed RNA from IL-4-primed MCs using specific primers. CysLT1 receptor transcripts were also present in these samples, but the CysLT2 receptor could not be amplified. In contrast, both the CysLT1 and CysLT2 receptors were amplified from RNA samples of human peripheral blood eosinophils (data not shown). RNA blot analysis confirmed weak steady-state expression of P2Y4 receptor mRNA by hMCs, as well as more robust levels of CysLT1 receptor mRNA (n = 2; representative blot shown in Fig. 3B). Neither transcript was up-regulated during 5 days of IL-4 priming, nor were detectable steady-state transcripts encoding the P2Y2, P2Y6, or CysLT2 receptors induced during that period (data not shown). Flow cytometry confirmed that cell membrane expression of the CysLT1 receptor was not altered on hMCs by 5 days of priming with IL-4 (Fig. 3C).
Figure 3.
Expression of the CysLT1 and P2Y4 receptors by hMC. (A) Homology domains in the amino acid sequences of the CysLT1 receptor, the CysLT2 receptor, and the known existing human P2Y receptors, in the first (EC1) and third (EC3) extracellular loops, and second (TM2) and third (TM3) transmembrane domains. Shaded residues indicate identity to the CysLT1 receptor. (B) RNA blot analysis detecting transcripts encoding the cys-LT and P24 receptors in RNA extracted from 107 hMCs maintained over a 5-day period in the presence of SCF alone (−) or with IL-4 (10 ng/ml) (+). Hybridization signals reflecting steady-state levels of mRNA encoding the CysLT1 and P2Y4 receptors are displayed, whereas the CysLT2 and P2Y6 receptors were not detected and are not shown. (C) Membrane expression of the CysLT1 receptor is not altered by 5 days of priming with IL-4. Bold tracings indicate staining with an anti-CysLT1 polyclonal antibody, while lighter tracings depict staining with a rabbit IgG of irrelevant specificity. The depicted cytofluorographs are representative of two experiments performed.
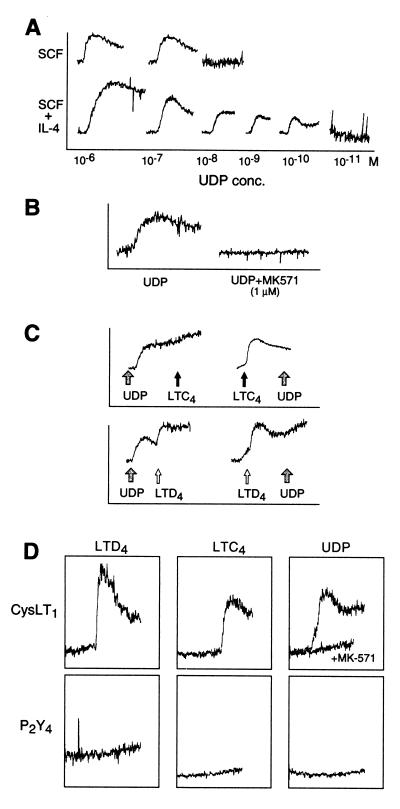
We used several strategies to determine whether hMCs might express a previously unrecognized cys-LT receptor with additional specificity for extracellular nucleotides. The P2Y receptors showing the greatest homology to the CysLT1 receptor in EC1 (P2Y6) and in EC3 (P2Y4) are pyrimidinergic based on their marked preference for nucleotides containing uracil (UDP and UTP, respectively) over adenine (ADP and ATP) (26). Stimulation of hMCs with 1 μM UDP yielded a strong, sustained calcium flux, and priming with IL-4 enhanced the sensitivity of hMCs to UDP by 3 log units in both experiments performed, reaching the low nanomolar range (Fig. 4A). UTP and ATP at 1 μM each failed to elicit a calcium response in hMCs, although responses to these agonists were elicited at 50 and 500 μM, respectively (data not shown). Like the responses to LTC4 and LTD4, the calcium signal elicited in hMCs by 1 μM UDP was not blocked by pertussis toxin (data not shown), but was blocked by 1 μM MK571 (Fig. 4B). Stimulation with either LTC4 or LTD4 at concentrations of 100 nM to 1 μM completely desensitized the IL-4-primed hMCs to subsequent UDP-induced calcium flux at 1 μM (Fig. 4C). Conversely, although 1 μM UDP completely desensitized the hMCs to a subsequent calcium flux mediated by 100 nM to 1 μM LTC4, it did not desensitize the hMCs to a subsequent LTD4-mediated calcium flux (Fig. 4C). The complete cross-desensitization of the hMCs by UDP to LTC4, but not to LTD4, indicates that LTC4 and UDP share an MK571-sensitive receptor on hMCs, the function of which is up-regulated by IL-4.
Figure 4.
LTC4 and UDP share receptors on hMC. (A) Effect of IL-4 priming of hMCs for 5 days compared with maintenance in SCF alone on dose-dependent calcium flux in response to UDP (10−11 M to 10−6 M). (B) The calcium signal induced by UDP (1 μM) is completely inhibited by MK571 (1 μM). (C) Cross-desensitization between UDP and LTC4 was complete at 1 μM of each agonist. In contrast, UDP did not desensitize the hMCs to LTD4, although LTD4 did desensitize the cells to UDP. (D) Stably transfected CHO cells expressing the human P2Y4 receptor do not respond to cys-LTs or UDP. CHO cells expressing the long isoform of the mouse CysLT1 receptor respond to LTD4 (10−9 M), LTC4 (10−7 M), and UDP (10−6 M, tracings shown with and without the addition of an equimolar concentration of MK571). The depicted results were replicated two times each for A, B, and C, with hMCs from different donors, and three times for D.
To determine whether the known CysLT receptors or uridine nucleotide receptors could account for the observed cross-desensitization between LTC4 and UDP on hMCs, CHO cells were stably transfected with cDNAs encoding the human P2Y6 and P2Y4 receptors, and the mouse CysLT1 receptor (25). As expected, CHO cells transfected with the P2Y4 receptor responded to UTP at 1 nM to 1 μM (data not shown), but did not respond to UDP or cys-LTs at the same concentration ranges (Fig. 4D). Transfectants expressing the P2Y6 receptor responded with a calcium flux to UDP stimulation, but did not respond to cys-LTs at 1 μM (data not shown). The calcium flux elicited by the pyrimidinergic ligands on both the P2Y6 and P2Y4 receptor transfectants were unaffected by MK571. As anticipated, the mouse CysLT1 receptor transfectants responded to LTC4 and LTD4 with strong calcium flux (Fig. 4D) that was previously shown to be inhibited by MK571 (25). Unexpectedly, however, the CysLT1 receptor transfectants responded strongly to UDP, with a calcium flux at 5 μM to 1 nM that was inhibited by equimolar concentrations of MK571 (Fig. 4D). UDP cross-desensitized the cells to 100 nM to 1 μM LTC4 and, to a lesser extent, to LTD4 (data not shown). Non-transfected control CHO cells did not respond to either cys-LTs or UDP. Thus CysLT1 is a dual-specificity receptor that is at least partly responsible for the shared responses of hMCs to cys-LTs and UDP, particularly in the absence of IL-4 priming. The preferential up-regulation of responsiveness to LTC4 and UDP over LTD4 in the IL-4-primed hMCs, which occurs without an effect on CysLT1 receptor transcript and protein levels, suggests the possibility of a separate CysLT1-like receptor that is inducible on hMCs. This putative receptor differs functionally from the CysLT1 receptor in its preference for LTC4, but shares the features of dual cys-LT/UDP specificity and inhibition by MK571.
Our study identifies the CysLT1 receptor on hMCs and defines dual-specificity for cys-LTs and UDP. Although the amino acid residues critical for cys-LT binding to their known receptors have not yet been identified, the critical residues for the selectivity and binding of extracellular nucleotides in the P2Y receptor family reside in their third, fifth, sixth, and seventh transmembrane domains (27, 28). Interestingly, these residues are not positionally conserved in the CysLT1 or CysLT2 receptors. Similarly, critical meta-binding sites for nucleotides in the P2Y1 receptor, which preferentially binds adenine nucleotides, have been proposed in the second and third extracellular loops on the basis of molecular modeling (28); again, these residues are not conserved in the known cys-LT receptors. The putative nucleotide-binding pocket in all P2Y family members is flanked by a pair of disulfide bridges between positionally conserved cysteine residues in the N termini and the third extracellular loop, and the first and third extracellular loops, respectively. Although these cysteine residues are conserved in the CysLT1 receptor, the CysLT2 receptor lacks the cysteine residues forming the second bridge. Because the competitive antagonist MK571 interfered with calcium flux in response to both cys-LTs and to UDP, it is likely that the respective binding sites for the two agonists are in close proximity to one another. The genes encoding the P2Y6 receptor and the P2Y2 receptor, which also binds uridine nucleotides, are closely linked on human chromosome 11q13 (29), a region containing genes linked to the inheritance of asthma (30, 31). The respective genes encoding the CysLT1 receptor and the P2Y4 receptor, both of which are expressed by hMCs, are closely linked on human chromosome Xq13–21, another region containing candidate asthma genes (19).
Variation in the rank order of the response of different contractile tissues to the family of cys-LTs had implied the existence of more than one possible receptor (32). The recent cloning of the human CysLT1 and CysLT2 receptors provided a rationale for such tissue-based diversity and added a hematopoietic cell location for these receptors to the expected smooth muscle distribution (15, 18). The homology between the P2Y and CysLT classes of receptors has been noted previously (18). Our study defines the CysLT1 receptor as one with dual cys-LT/UDP specificity, and suggests that an additional cys-LT/UDP receptor (CysLT3 receptor) may be induced on hMCs by IL-4. These observations implicate extracellular nucleotides and cys-LTs in a convergent pathway through which both neurogenic and immunologic mechanisms would intersect via hMCs in the Th2 polarized inflammatory diseases, such as bronchial asthma.
Acknowledgments
This work was supported by National Institutes of Health Grants AI-01305, AI-31599, AI-22531, and HL-36110, and by a grant from the Hyde and Watson Foundation.
Abbreviations
- cys-LT
cysteinyl leukotriene
- LT
leukotriene
- MCs
mast cells
- hMCs
human MCs
- 5-LO
5-lipoxygenase
- FLAP
5-LO-activating protein
- LTC4S
leukotriene C4 synthase
- FcɛRI
high-affinity Fc receptor for IgE
- RT
reverse transcriptase
- CHO
Chinese hamster ovary
- SCF
stem cell factor
References
- 1.Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, Reiss T F. Eur Resp J. 1998;11:1232–1239. doi: 10.1183/09031936.98.11061232. [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Dennis R, Ernst P, Sheehy O, Wood-Dauphinee S. Ann Intern Med. 1997;126:177–183. doi: 10.7326/0003-4819-126-3-199702010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dixon R A, Diehl R E, Opas E, Rands E, Vickers P J, Evans J F, Gillard J W, Miller D K. Nature (London) 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 4.Reid G K, Kargman S, Vickers P J, Mancini J A, Leveille C, Ethier D, Miller D K, Gillard J W, Dixon R A, Evans J F. J Biol Chem. 1990;265:19818–19823. [PubMed] [Google Scholar]
- 5.Lam B K, Penrose J F, Freedman G J, Austen K F. Proc Natl Acad Sci USA. 1994;91:7663–7669. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsch D J, Creely D P, Hauser S D, Mathis K J, Krivi G G, Isakson P C. Proc Natl Acad Sci USA. 1994;91:9745–9749. doi: 10.1073/pnas.91.21.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam B K, Owen W F, Austen K F, Soberman R J. J Biol Chem. 1989;264:12885–12891. [PubMed] [Google Scholar]
- 8.Anderson M E, Allison R D, Meister A. Proc Natl Acad Sci USA. 1982;79:1088–1091. doi: 10.1073/pnas.79.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C W, Lewis R A, Corey E J, Austen K F. Immunology. 1983;48:27–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Soter N A, Lewis R A, Corey E J, Austen K F. J Invest Dermatol. 1983;80:115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 11.Davidson A B, Lee T H, Scanlon P D, Solway J, McFadden E R, Jr, Ingram R H, Jr, Corey E J, Austen K F, Drazen J M. Am Rev Respir Dis. 1987;135:333–337. doi: 10.1164/arrd.1987.135.2.333. [DOI] [PubMed] [Google Scholar]
- 12.Griffin M, Weiss J W, Leitch A G, McFadden E R, Jr, Corey E J, Austen K F, Drazen J M. N Engl J Med. 1983;308:436–439. doi: 10.1056/NEJM198302243080807. [DOI] [PubMed] [Google Scholar]
- 13.Marom Z, Shelhamer J H, Bach M K, Morton D R, Kaliner M. Am Rev Respir Dis. 1982;126:449–451. doi: 10.1164/arrd.1982.126.3.449. [DOI] [PubMed] [Google Scholar]
- 14.Laitinen L A, Laitinen A, Haahtela T, Vilkka V, Spur B W, Lee T H. Lancet. 1993;341:989–990. doi: 10.1016/0140-6736(93)91073-u. [DOI] [PubMed] [Google Scholar]
- 15.Lynch K R, O'Neill G P, Liu Q, Im D S, Sawyer N, Metters K M, Coulombe N, Abramovitz M, Figueroa D J, Zeng Z, et al. Nature (London) 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 16.Sarau H M, Ames R S, Chambers J, Ellis C, Elshourbagy N, Foley J J, Schmidt D B, Muccitelli R M, Jenkins O, Murdock P R, et al. Mol Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- 17.Nothacker H P, Wang Z, Zhu Y, Reinscheid R K, Lin S H S, Civelli P. Mol Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- 18.Heise C E, O' Dowd B F, Figueroa D J, Sawyer N, Nguyen T, Im D-S, Stocco R, Bellefeuille J N, Abramovitz M, Cheng R, et al. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 19.Holroyd K J, Martinati L C, Trabetti E, Scherpbier T, Eleff S M, Boner A L, Pignatti P F, Kiser M B, Dragwa C R, Hubbard F, et al. Genomics. 1998;52:233–235. doi: 10.1006/geno.1998.5445. [DOI] [PubMed] [Google Scholar]
- 20.Kimura K, Noguchi E, Shibasaki M, Arinami T, Yokouchi Y, Takeda K, Yamakawa-Kobayashi K, Matsui A, Hamaguchi H. Hum Mol Genet. 1999;8:1487–1490. doi: 10.1093/hmg/8.8.1487. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen L A, Laitinen A, Haahtela T. Am Rev Respir Dis. 1993;147:697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan D W, Jr, Schleimer R P, Peters S P, Schulman E S, Adams G K, III, Newball H H, Lichtenstein L M. J Clin Invest. 1982;70:747–751. doi: 10.1172/JCI110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh F H, Lam B K, Penrose J F, Austen K F, Boyce J A. J Exp Med. 2001;193:123–133. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi H, Hirani W M, Yuan Q, Friend D, Austen K F, Boyce J A. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maekawa A, Kanaoka Y, Lam B K, Austen K F. Proc Natl Acad Sci USA. 2001;98:2256–2261. doi: 10.1073/pnas.041624398. . (First Published February 13, 2001; 10.1073/pnas.041624398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Kugelgen I, Wetter A. Naunyn-Schmiedeberg's Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 27.Moro S, Guo D, Camaioni E, Boyer J L, Harden T K, Jacobson K A. J Med Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moro S, Hoffmann C, Jacobson K A. Biochemistry. 1999;38:3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somers G R, Hammet F, Woollatt E, Richards R I, Southey M C, Venter D J. Genomics. 1997;44:127–130. doi: 10.1006/geno.1997.4841. [DOI] [PubMed] [Google Scholar]
- 30.Dizier M H, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, Charpin D, Degioanni A, Gormand F, Grimfeld A, et al. Am J Resp Crit Care Med. 2000;162:1812–1818. doi: 10.1164/ajrccm.162.5.2002113. [DOI] [PubMed] [Google Scholar]
- 31.Adra C N, Mao X Q, Kawada H, Gao P S, Korzycka B, Donate J L, Shaldon S R, Coull P, Dubowitz M, Enomoto T, et al. Clin Genet. 1999;55:431–437. doi: 10.1034/j.1399-0004.1999.550606.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee T H, Austen K F, Corey E J, Drazen J M. Proc Natl Acad Sci USA. 1984;81:4922–4925. doi: 10.1073/pnas.81.15.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]