Abstract
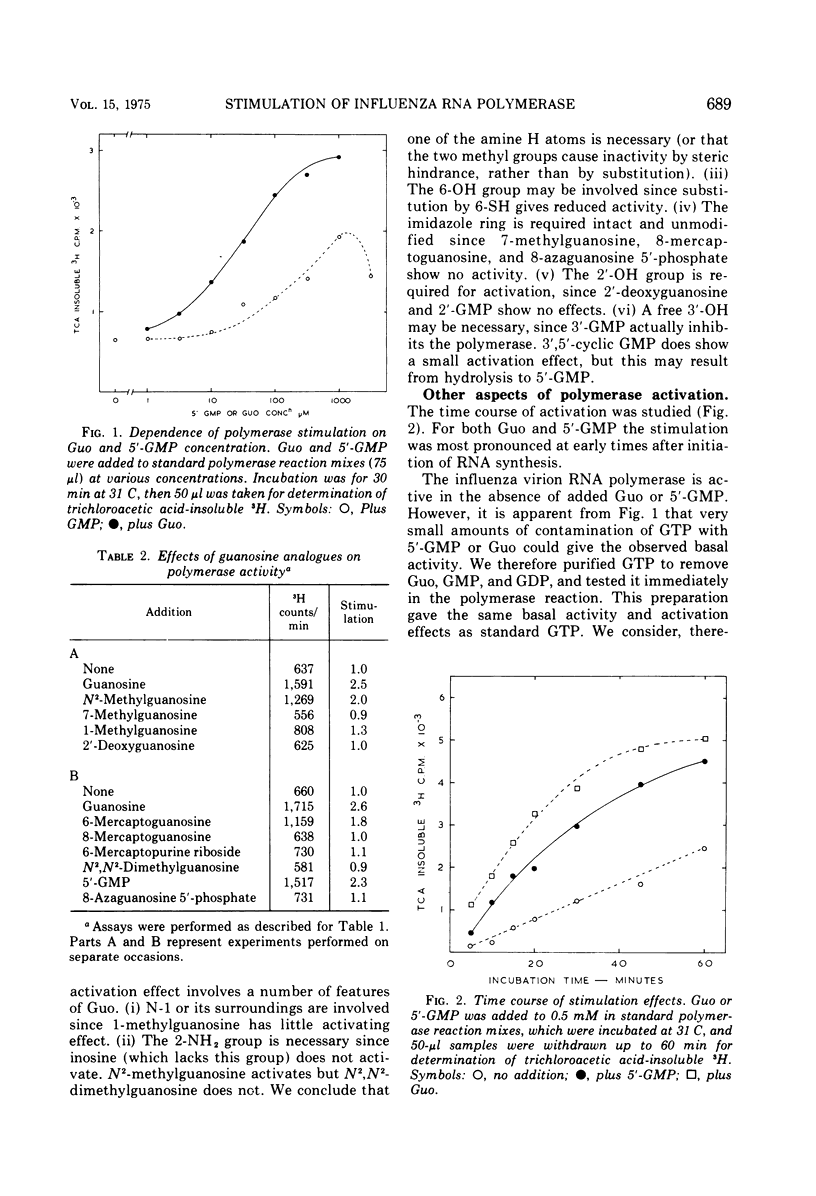
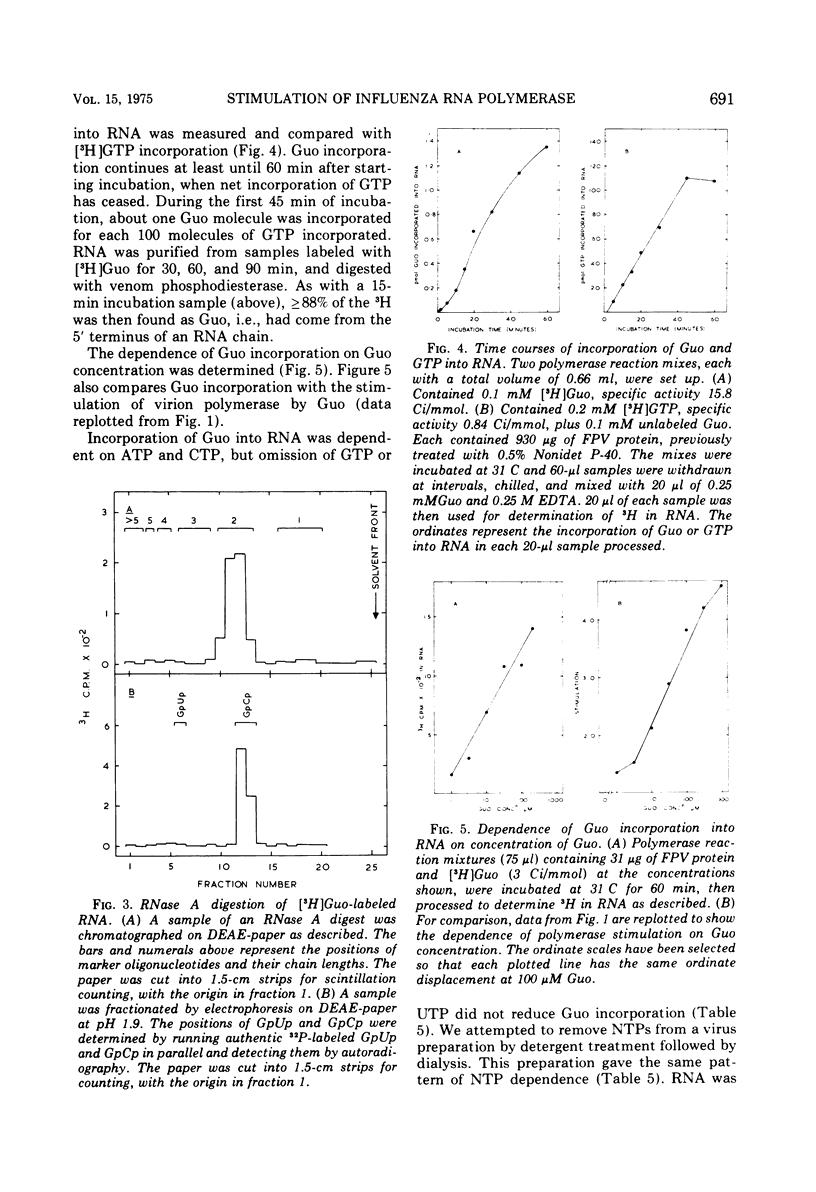
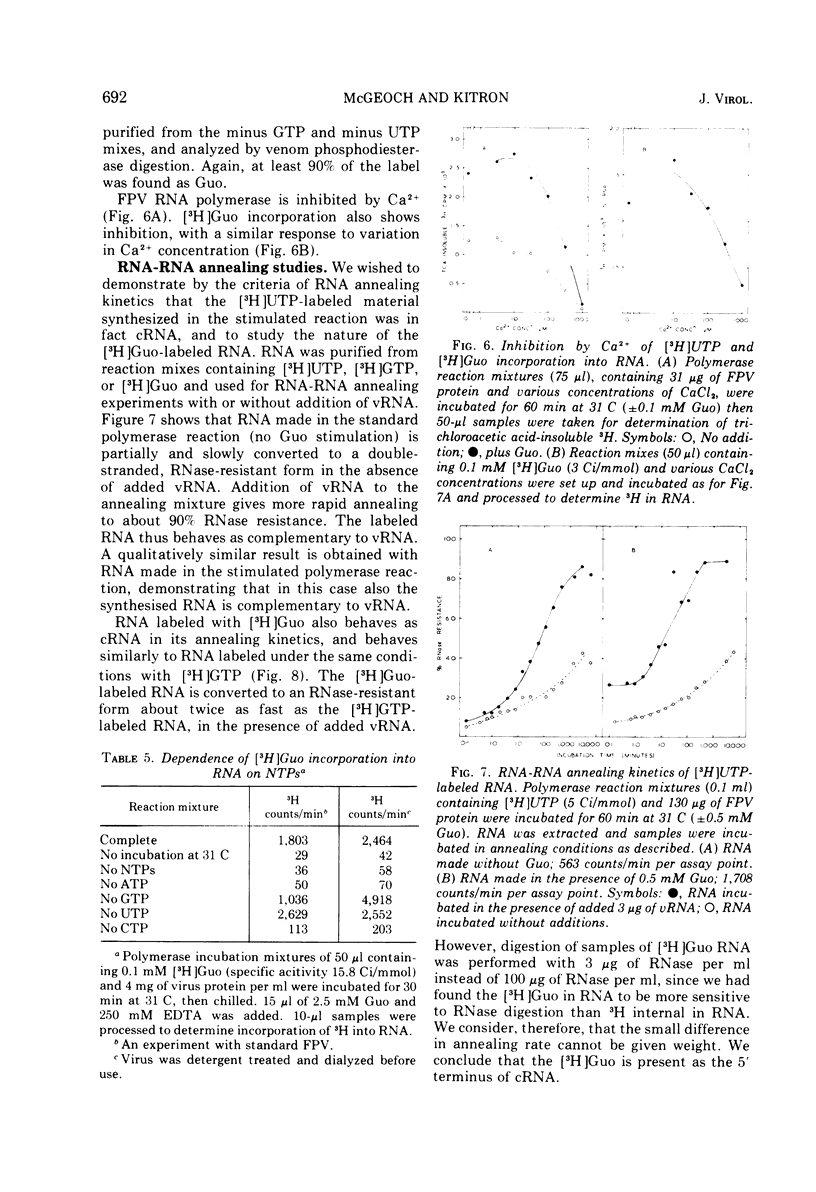
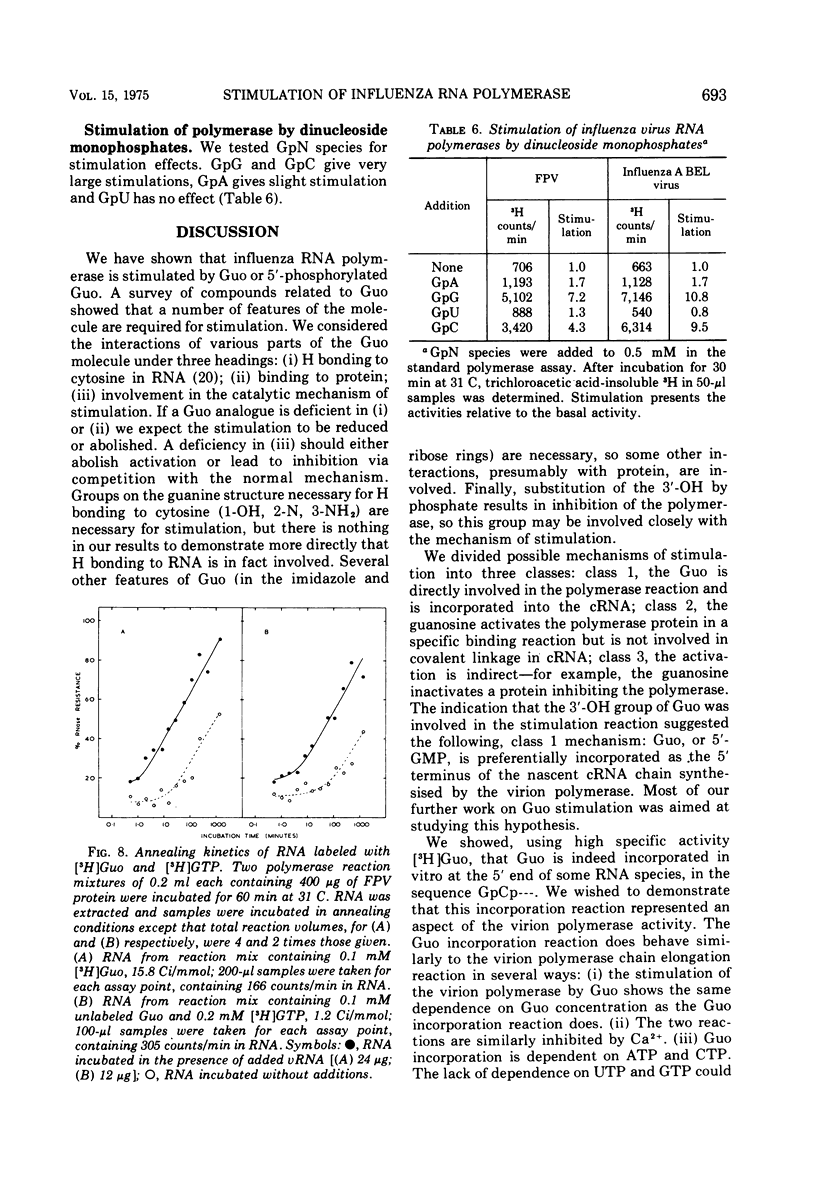
The activity of RNA-dependent RNA polymerase of several influenza viruses is stimulated by guanosine. Depending upon the virus strain used, the stimulation of initial reaction rate is up to 10-fold. 5'-GMP, 3',5'-cyclic GMP, and 5'-GDP show lesser stimulation effects. No other nucleosides of 5'-NMPs stimulate, but the dinucleoside monophosphates GpG and GpC show large stimulations. We present evidence that the stimulation represents preferential initiation of genome complementary RNA chains with guanosine: (i) [3-H] guanosine is incorporated specifically at the 5'terminus of RNA in polymerase reaction mixes in vitro. (ii) This incorporation reaction has several properties similar to those of the virion polymerase elongation reaction. (iii) RNA made in the stimulated reaction behaves as complementary RNA in annealing kinetic studies, as does RNA labeled with [3-H]guanosine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. II. Nature of the in vitro polymerase product. J Virol. 1971 Jul;8(1):74–80. doi: 10.1128/jvi.8.1.74-80.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M. 5'-Terminal nucleotide sequences of the double-stranded RNA of silkworm cytoplasmic polyhedrosis virus. J Mol Biol. 1974 Jun 15;86(1):31–48. doi: 10.1016/s0022-2836(74)80005-7. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Studies on polynucleotides. III. Enzymic degradation; substrate specificity and properties of snake venom phosphodiesterase. J Biol Chem. 1959 Aug;234(8):2105–2113. [PubMed] [Google Scholar]
- Shatkin A. J. Methylated messenger RNA synthesis in vitro by purified reovirus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3204–3207. doi: 10.1073/pnas.71.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- WILKINS M. H. Molecular configuration of nucleic acids. Science. 1963 May 31;140(3570):941–950. doi: 10.1126/science.140.3570.941. [DOI] [PubMed] [Google Scholar]
- Waterfield W. R., Spanner J. A., Stanford F. G. Tritium exchange from compounds in dilute aqueous solutions. Nature. 1968 May 4;218(5140):472–473. doi: 10.1038/218472a0. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylation of newly synthesized viral messenger RNA by an enzyme in vaccinia virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3014–3018. doi: 10.1073/pnas.71.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]



