Abstract
Although neuroactive steroids exert neuroprotective actions in different experimental models of neurodegenerative diseases, including those of Alzheimer's disease (AD), their relationships with aged related physiological and pathological brain changes remain to be clarified. In this study the levels of pregnenolone, dehydroepiandrosterone, progesterone, dihydroprogesterone, tetrahydroprogesterone, isopregnanolone, testosterone, dihydrotestosterone, 5α-androstane-3α,17β-diol, 5α-androstane-3β,17β-diol, 17α-estradiol and 17βestradiol were assessed in the limbic region of young adult (7 months) and aged (24 months) male wild type and triple transgenic AD mice. Age related neuropathological changes in AD brains, such as β-amyloid accumulation and gliosis, were associated with modified levels of specific neuroactive steroids and particularly with changes in the levels of progesterone and testosterone metabolites. The altered levels of neuroactive steroids in aged AD brains may impact on the activation of neuroprotective signaling mediated by classical and non-classical steroid receptors, like the GABA-A receptor.
Keywords: progesterone, testosterone, metabolic pathways, Alzheimer's disease, limbic region, GABA-A receptors
INTRODUCTION
The neuroactive steroid family includes steroid hormones produced in peripheral glands and steroids directly synthesized in the nervous system (i.e., neurosteroids)(Melcangi et al., 2008) They act as important physiological regulators of nervous function, affecting mood, behaviour, reproduction and cognition, as well as acting as protective agents in models of injury and disease, including experimental models of Alzheimer's disease (AD), Parkinson's disease, multiple sclerosis, traumatic brain injury, stroke, autism, schizophrenia, mood disorders and peripheral neuropathy (Melcangi et al., 2008; Panzica et al., 2012; Schumacher et al., 2012). Although neuroactive steroids have well established neuroprotective roles, their relationships with normal brain aging and age-related neurodegenerative diseases remain incompletely understood. Recent studies have shown that levels of neuroactive steroids can be affected by pathology or injury, as demonstrated in experimental models of multiple sclerosis, diabetic neuropathy, Parkinson's disease and trauma (Caruso et al., 2010a; Caruso et al., 2008; Giatti et al., 2010; Meffre et al., 2007; Melcangi et al., 2012; Melcangi and Garcia-Segura, 2010; Pesaresi et al., 2010), using highly sensitive and specific analytical methods, such as liquid chromatography tandem mass spectrometry.
Still unclear is the relationship between neuroactive steroids and AD. Evidence from a limited number of studies in post-mortem human brain suggests that age-related depletion of at least some neuroactive steroids may contribute to development of AD. For example, brain levels of testosterone in men are inversely associated with AD risk (Rosario et al., 2011; Rosario et al., 2004). Similarly, AD in women is linked to low brain levels of 17β-estradiol and estrone (Rosario et al., 2011; Yue et al., 2005). Further, tetrahydroprogesterone, also know as allopregnanolone, is also reported to be significantly lower in AD (Marx, et al., 2006). Examination of androgens (Rosario et al., 2010; Rosario et al., 2006), estrogens and progesterone (Carroll et al., 2007; Carroll et al., 2010; Rosario et al., 2010; Yue et al., 2005) and allopregnanolone (Singh et al., 2011; Wang et al., 2010) in transgenic mouse models of AD has largely supported a protective role of these neuroactive hormones against the progression of the disease. In addition to age-related losses of neuroactive steroids contributing to AD pathogenesis, other evidence indicates that AD neuropathology may alter neuroactive steroid levels. For instance, neurosteroidogenesis is impaired in cell lines exposed to β-amyloid (Aβ) peptide and oxidative stress (Schaeffer et al., 2008a; Schaeffer et al., 2006; Schaeffer et al., 2008b). Interestingly, increased mRNA levels of neurosteroidogenic enzymes have been reported in AD brain (Luchetti et al., 2011a; Luchetti et al., 2011b), suggesting the possibility of a compensatory response to AD-related changes in neuroactive steroids. Further investigation is necessary to clarify the relationships between aging, AD, and neuroactive steroids.
In the current study, the effects of aging and AD-like neuropathology on neuroactive steroid concentrations were characterized in triple transgenic AD (3xTg-AD) mice. Specifically, we have analyzed limbic regions of young adult (7 months) and senescent (24 months) male wild type (WT) and 3xTg-AD mice for levels of several neuroactive steroids: pregnenolone (PREG); dehydroepiandrosterone (DHEA); progesterone (PROG) and its metabolites dihydroprogesterone (DHP), tetrahydroprogesterone (THP) and isopregnanolone; testosterone and its metabolites dihydrotestosterone (DHT), 5α-androstane-3α,17β-diol (3α-diol), 5α-androstane-3β,17β-diol (3β-diol); and 17α-estradiol (17α-E) and 17β-E. Age-related development of neuropathology in 3xTg-AD mice was assessed by quantifying immunohistochemical indices of Aβ accumulation and gliosis.
Materials and Methods
Animals
Fifteen male homozygous 3xTg-AD mice (Oddo et al., 2003) and thirteen background strain, wild-type (WT) mice (C57BL/6/129S; The Jackson Laboratory, Bar Harbor, ME) were bred and maintained at the University of Southern California (USC) vivarium facilities with food and water available ad libitum. At 7 and 24 months of age mice were anesthetized (80mg/kg ketamine 5mg/kg xylazine, i.p.) and perfused with ice-cold saline. Brains were bisected, with one hemisphere immersion fixed in 4% paraformaldehyde/0.1 M PBS for 48 hrs. The other hemisphere was dissected into limbic regions and then frozen on dry ice for neurosteroid extraction and assessment. All experimentation was approved by the USC Institutional Animal Care and Use Committee and carried out in accordance with National Institutes of Health guidelines.
Immunohistochemistry
Fixed hemibrains were sectioned exhaustively in the horizontal plane at 40 µm. Every eighth section was pretreated with formic acid (99%) for 5 minutes, then immunolabeled using antibodies directed against Aβ (#71-58000, 1:300 dilution, Zymed, San Francisco, CA). Immunoreactivity was visualized using an avidin: biotinylated enzyme complex immunoperoxidase method (ABC Elite, Vector; Burlingame, CA, USA) as previously described (Rosario et al., 2006). For quantification of Aβ immunoreactivity load, grayscale images of high magnification fields (420 × 330 µm) were digitally captured (CCD camera coupled to Olympus Optical BX40 microscope) then filtered with a predetermined threshold using NIH Image 1.61 to create a binary image identifying positive and negative immunolabeling. Load was calculated as percentage of total pixel area positively labeled as previously described (Rosario et al., 2006). Mean load values were averaged from two to three non-overlapping fields from each brain region in five sections per animal.
Other sections were labeled using antibodies directed against ionized calcium binding adaptor molecule-1 (IBA-1, Wako Chemicals, Neuss, Germany) as a marker of microglial activation (Ito et al., 1998) and glial fibrillary acidic protein (GFAP, clone GA5; Sigma-Aldrich, Tres Cantos, Spain) as a marker of astrocyte reactivity (Middeldorp and Hol, 2011), as previously described (Barreto et al., 2009, Ito et al., 1998). IBA-1 and GFAP immunoreactivities were quantified by volume density morphometric analysis (Weibel, 1979). Experimenters were blinded to treatment conditions during quantification.
Assessment of neuroactive steroids by liquid chromatography tandem mass spectrometry
5-pregnen-3β-ol-20-one (PREG), progesterone (PROG), 5α-pregnane-3, 20-dione (DHP), 3α-hydroxy-5α-pregnen-20 one (THP), 3β-hydroxy-5α-pregnen-20 one (isopregnanolone), testosterone (T), 5α-androstane-17β-ol-3-one (DHT), 5α-androstane-3α,17β-diol (3α-diol), 5α-androstane-3β,17β-diol (3β-diol), dehydroepiandrosterone (DHEA), 17α-estradiol (17α-E) and 17β-estradiol (17β-E) were purchased from Sigma Aldrich. 17,21,21,21-D4-PREG (D4-PREG) was kindly synthesized by Dr. P. Ferraboschi (Dept. of Medical Chemistry, Biochemistry and Biotechnology, University of Milano, Italy); 2,2,4,6,6-17α,21,21,21-D9-PROG (D9-PROG) was obtained from Medical Isotopes, (Pelham, NH, USA); 2,4,16,16-D4-17β-estradiol (D4-17β-E) was obtained from CDN Isotope Pointe-Claire (Quebec-Canada). SPE cartridges (Discovery DS-C18 500 mg) were from Supelco, Italy. All solvents and reagents were HPLC grade (Sigma Aldrich, Italy).
Samples were extracted and purified according to Caruso et al. (Caruso et al., 2010b; Caruso et al., 2008). Briefly, samples were added with internal standards and homogenized in 3 ml of MeOH/acetic acid (99:1, v/v) using a tissue lyser (Qiagen, Italy). After an overnight incubation at 4°C, samples were centrifuged at 15300 g for 5 min and the pellet was extracted twice with 1 ml of MeOH/acetic acid (99:1, v/v). The organic phases were combined and dried with a gentle stream of nitrogen in a 40°C water bath. Samples were resuspended in 3 ml of MeOH/H2O (10:90, v/v) and passed through a SPE cartridge, previously activated with MeOH (5 ml) and MeOH:H2O 10:90 (v/v) (5 ml). Steroids were eluted in MeOH, concentrated and transferred into auto-sampler vials before LC–MS/MS analysis. Quantitative analysis was performed on the basis of calibration curves prepared and analyzed using deuterated internal standards. Calibration curves were extracted and analyzed as described above for samples.
Positive atmospheric pressure chemical ionization (APCI+) experiments were performed using a linear ion trap-mass spectrometer (LTQ, ThermoElectron Co., San Jose, CA, USA) using nitrogen as sheath, auxiliary and sweep gas and equipped with a Surveyor liquid chromatography (LC) Pump Plus and a Surveyor Autosampler Plus (ThermoElectron Co., San Jose, CA, USA). The mass spectrometer was employed in MS/MS mode using helium as collision gas. Samples were analyzed employing the transitions as previously reported (Pesaresi et al., 2010). The LC mobile phases were (A) H2O/0.1% formic acid and (B) methanol (MeOH)/0.1% formic acid. The gradient (flow rate 0.5 ml/min) was as follows: T0.0 70%A, T1.5 70%A, T2.0 55%A, T3.0 55%A, T35.0 36%A, T40.0 25%A, T41.0 1%A, T45.0 1%A, T45.2 70%A, T55.0 70%A. The split valve was set at 0–6.99 min to waste, 6.99–43.93 min to source and 43.93–55 min to waste. The Hypersil Gold column (100×3 mm, 3 µm; ThermoElectron Co, San Jose, CA, USA) was maintained at 40°C. The injection volume was 25µl and the injector needle was washed with MeOH/H2O 1/1 (v/v). Peaks of the LC–MS/MS were evaluated using a Dell workstation by means of the software Excalibur release 2.0 SR2 (ThermoElectron Co, San Jose, CA, USA).
Statistical Analysis
Unpaired Student's t test was applied to couples of independent variables. Data from experiments with more than two groups were analyzed by two-way analysis of variance (ANOVA), with sex and genotype as two independent variables, followed by the Bonferroni post-test. All analyses were performed using GraphPad PRISM (version 5).
Results
Development of neuropathology in male 3xTg-AD mice
Aβ immunoreactivity was not detectable in the hippocampal formation of WT mice. Therefore, Aβ immunoreactivity was quantitatively assessed in 3xTg-AD mice only (Fig. 1). Aβ immunoreactivity was most prominent in the subiculum region of both young and aged mice (Fig. 1 C and D). In the hippocampus of the young adult mice, Aβ immunoreactivity was restricted to the CA1 region, primarily observed in the pyramidal cell layer (Fig. 1 A). In the aged mice, hippocampal Aβ immunoreactivity was observed throughout CA1-3 regions and the dentate gyrus (Fig. 1 B). In the aged mice, Aβ immunoreactive load was over 5-fold higher in the hippocampus CA1 (P<0.005) and subiculum (P<0.001) regions compared to young adult 3xTgAD mice (Fig. 1 E).
Figure 1.
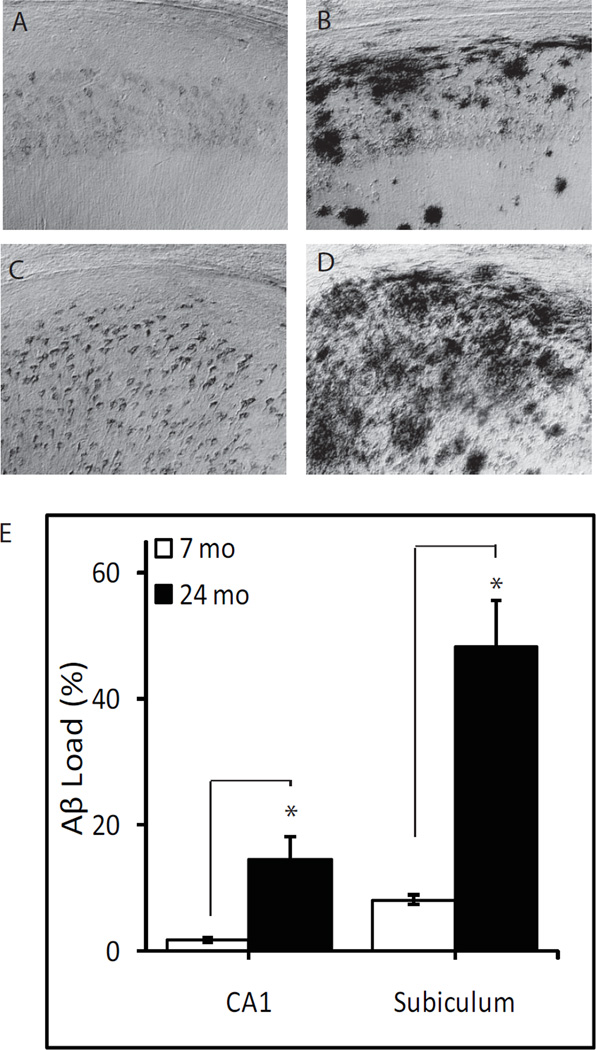
Age-related increase in Aβ immunoreactivity in 3xTg-AD mice. Representative photomicrographs show Aβ immunoreactivity in hippocampus CA1 (A, B) and subiculum (C, D) from 7 month-old (A, C) and 24 month-old (B, D) 3xTg-AD mice. E) Aβ immunoreactive load values in the hippocampus CA1 and subiculum of 7 month- (n=7) and 24 month-old (n=7) 3xTg-AD mice. * P<0.005.
GFAP and IBA-1 immunoreactivities were assessed as markers of gliosis in the hippocampus CA1 (Fig. 2). A significant effect of genotype was observed on both GFAP (P<0.001) and IBA-1 immunoreactivities (P<0.001), with over two-fold higher levels of GFAP and IBA-1 in both young adult and aged 3xTg-AD mice compared to WT mice of the same age. A significant effect of age was also observed on GFAP (P<0.001) and IBA-1 (P<0.001) immunoreactivities, with an age-related increase in the markers of gliosis observed in both WT and 3xTg-AD mice at 24 months of age. No significant interaction was observed between genotype and age.
Figure 2.
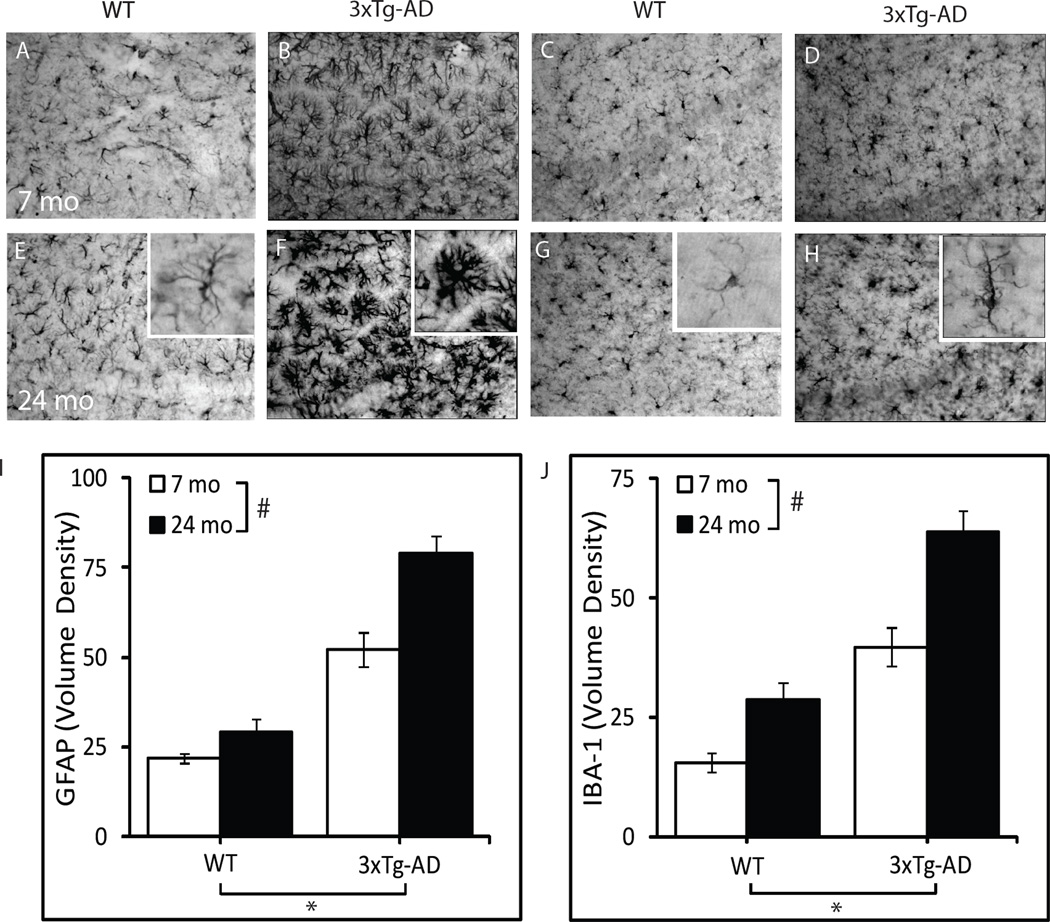
Age-related increase in markers of gliosis in WT and 3xTg-AD mice. Representative photomicrographs show GFAP (A, B, E, F) and IBA-1 (C, D, G, H) immunoreactivity in hippocampus CA1 of 7 month-old WT (A, C), 7 month-old 3xTg-AD (B, D), 24 month-old WT (E, G), and 24 month old 3xTg-AD mice (F, H). I) GFAP immunoreactivity volume density values in the CA1 region. J) IBA-1 immunoreactivity volume density values in the CA1 region. WT-7mo, n=6; WT-24mo, n=5; 3xTg-AD-7mo, n=7; 3xTg-AD-24mo, n=7. *P<0.001.
Neuroactive steroid levels in the limbic region of male 3xTg-AD mice
Levels of neuroactive steroids were measured by LC-MS/MS in the limbic region of young adult and aged senescent male 3xTg-AD mice. Levels were compared to those measured in WT animals. A significant effect of genotype (P<0.01) was observed on two PROG metabolites - DHP and isopregnanolone (Fig. 3), and on 17β-E (Fig. 4). Increased levels of DHP, isopregnanolone and 17β-E were observed in both young adult and aged 3xTg-AD mice compared to WT mice of the same age (Figs. 3 and 4). In case of DHP, a significant effect of age (P<0.001) also occurred (Fig. 3), with an age-related increase in DHP levels evident in both WT and 3xTg-AD mice at 24 months of age. However, no significant interaction was observed between genotype and age. Age also significantly affected levels of the precursors of DHP, PREG (P<0.01) and PROG (P<0.001), as well as T (P<0.01) and its derivatives, DHT (P<0.01) and 3α-diol (P<0.01) (Figs. 3 and 4). For all these neuroactive steroids, with the exception of 3α-diol, an age-related decrease of their levels occurred in both WT and 3xTg-AD mice at 24 months of age (Figs. 3 and 4). At variance, 3α-diol levels were up-regulated with aging in WT and 3xTg-AD mice (Fig. 4).
Figure 3.
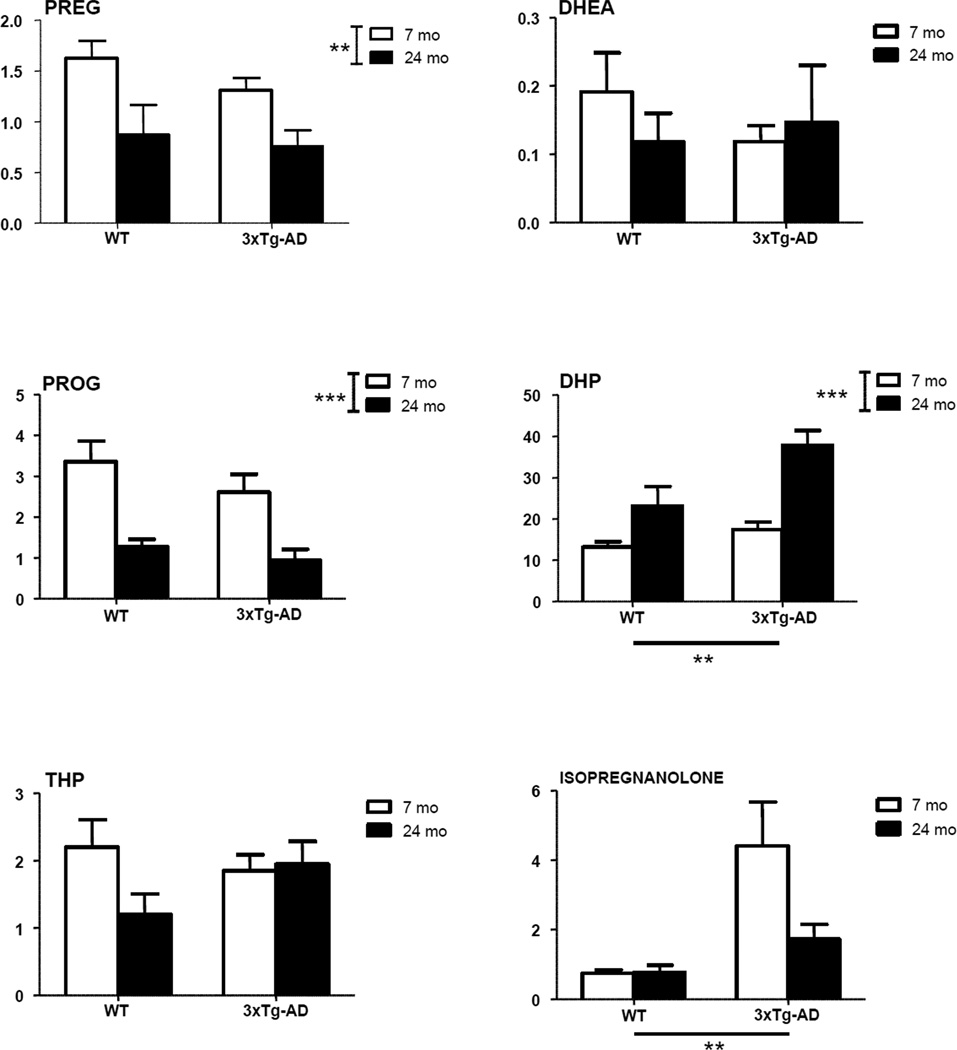
Levels of pregnenolone (PREG), dehydroepiandrosterone (DHEA), progesterone (PROG) and its metabolites, dihydroprogesterone (DHP), tetrahydroprogesterone (THP) and isopregnanolone in the limbic region of male wild type and 3xTg-AD mice at 7 and 24 months. Data are expressed as pg/mg tissue±SEM. The effects of age and genotype and the interaction age-genotype have been analyzed using the two-way ANOVA test. WT-7mo, n=8; WT-24mo, n=5; 3xTg-AD-7mo, n=8; 3xTg-AD-24mo, n=5. * P< 0.05; ** P< 0.01; *** P<0.001.
Figure 4.
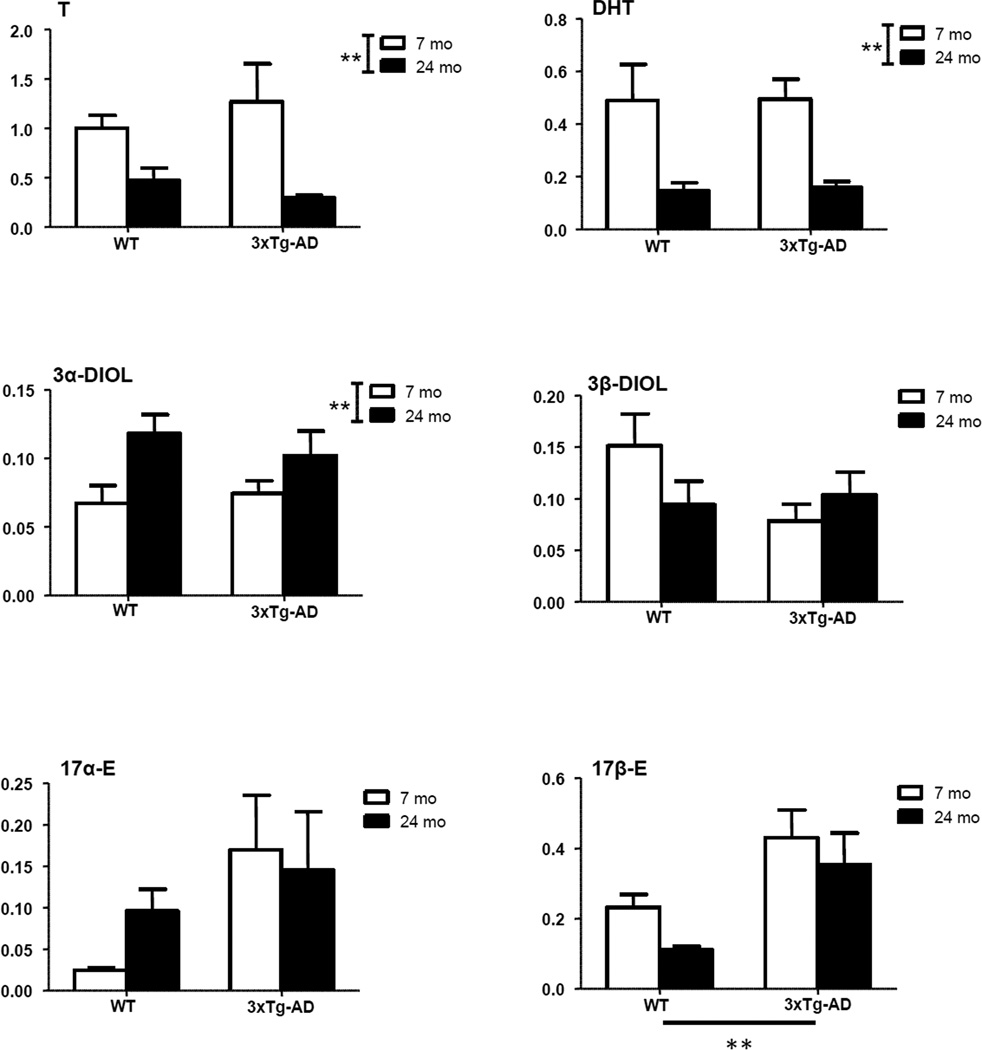
Levels of testosterone (T) and its metabolites, dihydrotestosterone (DHT), 5α-androstane-3α,17β-diol (3α-diol), 5α-androstane-3β,17β-diol (3β-diol), 17α-estradiol (17α-E) and 17β-E in the limbic region of male wild type and 3xTg-AD mice at 7 and 24 months. Data are expressed as pg/mg tissue±SEM. The effects of age and genotype and the interaction age-genotype have been analyzed using the two-way ANOVA test. WT-7mo, n=8; WT-24mo, n=5; 3xTg-AD-7mo, n=8; 3xTg-AD-24mo, n=5. * P< 0.05; ** P< 0.01.
To investigate potential relationships between precursors and metabolites we performed correlations between neuroactive steroid levels. In particular, on the basis of the classical steroidogenic pathways shown in Figure 5, we considered correlations among PREG, PROG and its metabolites (i.e., DHP, THP and isopregnanolone) in Table 1; and correlations among PREG, DHEA, T and its metabolites (i.e., DHT, 3α-diol and 3β-diol) as well as estrogens (i.e., 17α-E and 17β-E) in Table 2. As reported in Table 1, a positive correlation was observed between PREG and DHP in young WT animals (P<0.01). No significant correlation was observed between PREG and DHP in 3xTg-AD mice of the same age or with either aging WT or 3xTg-AD mice. Levels of DHP and THP were positively correlated in young 3xTg-AD mice (P<0.05), while the levels of DHP and isopregnanolone were negatively correlated in aged 3xTg-AD mice (P<0.01). The levels of THP and isopregnanolone were positively correlated in young and aged WT (P<0.05). This did not occur in 3xTg-AD mice.
Figure 5.
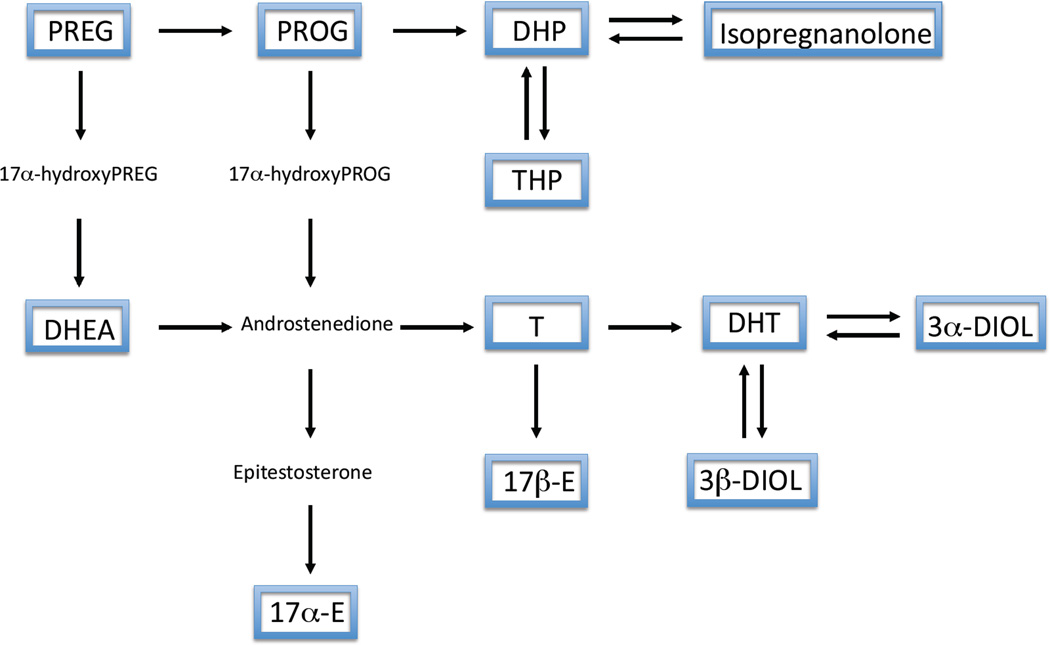
Schematic representation of neurosteroidogenesis. Framed neuroactive steroids reported have been assessed by LC-MS/MS.
Table 1.
Correlation (r value) between the levels of pregnenolone, progesterone and its metabolites in limbic area of young (7months) and aged (24 months) wild type (WT) or 3xTg-AD (TG) rats.
| Young | PREG | PROG | DHP | THP | Isopregnanolone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | |
| PREG | - | - | ||||||||
| PROG | NS | NS | - | - | ||||||
| DHP | 0.75** | NS | NS | NS | - | NS | ||||
| THP | NS | NS | NS | NS | NS | 0.66* | - | - | ||
| Isopregnanolone | NS | NS | NS | NS | NS | NS | 0.50* | NS | - | - |
| Aged | PREG | PROG | DHP | THP | Isopregnanolone | |||||
| WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | |
| PREG | - | - | ||||||||
| PROG | NS | NS | - | - | ||||||
| DHP | NS | NS | NS | NS | - | - | ||||
| THP | NS | NS | NS | NS | NS | NS | - | - | ||
| Isopregnanolone | NS | NS | NS | NS | NS | −0.87** | 0.76* | NS | - | - |
Young n= 8; Aged n= 5. NS = not significant.
p<0.05;
p<0.01;
p<0.001
Table 2.
Correlation (r value) between the levels of pregnenolone, dehydroepiandrosterone, testosterone and its metabolites and estrogens in limbic area of young (7months) and aged (24 months) wild type (WT) or 3xTg-AD (TG) rats.
| Young | PREG | DHEA | T | DHT | 3α-DIOL | 3β-DIOL | 17α-E | 17β-E | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | |
| PREG | - | - | ||||||||||||||
| DHEA | NS | NS | - | - | ||||||||||||
| T | 0.77** | NS | NS | NS | - | - | ||||||||||
| DHT | NS | NS | NS | NS | NS | NS | - | - | ||||||||
| 3α-DIOL | 0.57* | NS | 0.50* | NS | NS | NS | NS | NS | - | - | ||||||
| 3β-DIOL | 0.64* | NS | 0.51* | NS | 0.54* | NS | 0.55* | NS | 0.55 | NS | - | - | ||||
| 17α-E | NS | NS | 0.63* | NS | NS | NS | NS | NS | 0.89*** | NS | 0.75** | NS | - | - | ||
| 17β-E | 0.60* | NS | NS | NS | 0.73** | NS | NS | NS | NS | NS | 0.56* | 0.66* | NS | NS | - | - |
| Aged | PREG | DHEA | T | DHT | 3α-DIOL | 3β-DIOL | 17α-E | 17β-E | ||||||||
| WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | |
| PREG | - | - | ||||||||||||||
| DHEA | NS | NS | - | - | ||||||||||||
| T | NS | NS | NS | 0.75* | - | - | ||||||||||
| DHT | NS | NS | NS | NS | 0.99*** | NS | - | - | ||||||||
| 3α-DIOL | NS | NS | NS | NS | NS | NS | NS | NS | - | - | ||||||
| 3β-DIOL | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | - | - | ||||
| 17α-E | NS | NS | NS | 0.89** | NS | 0.84* | NS | NS | NS | NS | NS | NS | - | - | ||
| 17β-E | NS | NS | NS | 0.74* | NS | 0.74* | NS | NS | NS | NS | NS | NS | NS | 0.90** | - | - |
Young n=8; Aged n=5. NS = not significant.
p<0.05;
p<0.01;
p<0.001
As reported in Table 2, PREG levels positively correlated with T (P<0.01), 3α-diol, 3β-diol and 17β-E (P<0.05) levels in young WT animals. This correlation was lost in 3xTgAD mice of the same age and with aging in both WT and 3xTg-AD mice. In young WT animals, DHEA levels were positively correlated with the levels of 3α-diol, 3β-diol and 17α-E (P<0.05). In 3xTg-AD aged animals, DHEA levels were positively correlated with the levels of T, 17α-E and 17β-E (P<0.05). T levels in young WT mice were positively correlated to levels of 3β-diol (p<0.05) and of 17β-E (P<0.01). In aged WT animals, the levels of T and DHT were positively correlated (P<0.001), while in aged 3xTg-AD mice, the levels of T, 17α-E and 17β-E showed a positive correlation (P<0.05). In young WT animals, the levels of the first metabolite of T, DHT, were significantly correlated with those of 3β-diol (P<0.05), while the levels of the other metabolite of DHT, 3α-diol, were positively correlated with those of 17α-E (P<0.001). These correlations were lost in 3xTg-AD mice of the same age and with aging in both WT and 3xTg-AD mice. The levels of 3β-diol showed a positive correlation with the levels of 17α-E (P<0.01) and 17β-E (P<0.05) in young WT animals. 3β-diol levels maintained a positive correlation with the levels of 17β-E (P<0.05) in young 3xTg-AD mice. The levels of 17α-E and 17β-E showed a positive correlation (P<0.05) in aged 3xTg-AD mice.
Discussion
Here we have characterized the effect of normal and pathological aging on neuroactive steroid levels in the learning and memory centre of WT and 3xTg-AD mice. Our findings demonstrate that both aging and AD-related neuropathology have important effects on neuroactive steroid homeostasis in the region of the brain most severely affected in AD, the limbic region. The observed effects of age and AD-neuropathology on neuroactive steroid levels may contribute to age-related susceptibility to AD pathogenesis and age-dependent cognitive decline.
For this study we have selected male mice of 7 and 24 months of age, since the degree of pathological alterations is known to be affected by age in 3xTg-AD mice (Bittner et al., 2010; Caccamo et al., 2010; Ghosh et al., 2012; Olobarria et al., 2011; Overk et al., 2009). In fact, the pathological examination of the brain of 3xTg-AD mice revealed a 5-fold higher Aβ immunoreactive load in CA1 and subiculum at 24 months of age in comparison to 7 months of age. The volume density of GFAP immunoreactive astrocytes and IBA-1 immunoreactive microglia was also increased at 24 months of age versus 7 months of age in both 3xTg-AD and WT mice. Furthermore, the volume density of GFAP immunoreactive astrocytes and IBA-1 immunoreactive microglia was increased in 3xTg-AD mice compared to WT at both ages. Therefore, Aβ immunoreactive load and gliosis were affected both by age and genotype. Interestingly, neuroactive steroid levels, analyzed by LC-MS/MS in the limbic region, were also affected by age and genotype.
Our data extend previous observations showing that neuroactive steroid levels are affected by aging in the CNS of non-pathological animals (Schumacher et al., 2003). We observed an age-related decrease in the levels of PROG, together with an increase in the levels of its metabolite DHP, in the limbic region of WT and 3xTg-AD male mice. The levels of other neuroactive steroids were also affected by aging. For instance, in both WT and 3xTg-AD aged mice, the levels of T and its direct metabolite, DHT, were both decreased. Previous studies have similarly reported an age-related depletion in androgens in male rats (Rosario et al., 2009) and human frontal cortex (Rosario et al., 2011). Interestingly, in men, circulating levels of DHT do not change with age (Kaufman and Vermeulen, 2005), despite depleted brain levels of DHT (Rosario et al., 2011), highlighting the importance of characterizing age-related neuractive steroid changes directly in the brain.
These age-related changes in neuroactive steroid levels may contribute to susceptibility to cognitive decline and AD-related neuropathology with advancing age. Furthermore, previous studies have demonstrated that androgens elicit anti-inflammatory effects, for example testosterone reduces reactive gliosis following a stab wound in castrated rats (Arevalo et al., 2010; Barreto et al., 2007). Therefore, the age-related decline in androgens may contribute to the increased levels of gliosis observed in aged mice. Indeed, age-related depletion of androgens in males is believed to be an important risk factor for AD and may promote Aβ accumulation, with previous studies demonstrating that testosterone exerts a therapeutic effect in 3xTg-AD mice (Rosario et al., 2010).
The decrease in the levels of T and its direct metabolite, DHT, was associated with an increase in the levels of the further metabolite, 3α-diol. It is interesting to note that at variance to T and DHT, which bind to androgen receptor, 3α-diol interacts with GABA-A receptor (Melcangi et al., 2008). Thus, the increase in 3α-diol levels with aging may affect activation of GABA-A receptor.
In addition to being affected by aging, our findings suggest that AD neuropathology may also alter neuroactive steroid levels. Prior observations in post-mortem brain tissue of AD patients have demonstrated altered neuroactive steroid levels in brain regions affected by AD neuropathology (Luchetti et al., 2011a; Luchetti et al., 2011b; Marx et al., 2006; Rosario et al., 2011; Yue et al., 2005). However, these studies have not been able to clearly determine if altered neuroactive steroid levels are a risk factor for AD or an outcome of AD pathogenesis. In the 3xTg-AD mice, an age-dependent increase in Aβ pathology was associated with increased markers of gliosis compared to WT mice. Furthermore, increased levels of the neuroactive steroids DHP, isopregnanolone and 17β-E were also observed in 3xTg-AD compared to WT mice. In the current study, androgens were affected by aging but not AD neuropathology in the 3xTg-AD mice, supporting the notion that in the male brain, depleted androgens is a risk factor for AD, not a consequence of AD neuropathology.
Concerning PROG metabolites in the limbic region, we detected a significant increase in the levels of DHP and isopregnanolone, 3xTg-AD compared to WT mice. In addition, DHP positively correlated with THP in young 3xTg-AD mice, and negatively correlated isopregnanolone in aged 3xTg-AD mice. These correlations were not detected in WT mice. In contrast, 3xTg-AD mice did not show the positive correlations in the levels of PREG and DHP and in the levels of THP and isopregnanolone that were detected in young and older WT mice, respectively. These findings suggest that the metabolism of PROG is altered in the limbic region of 3xTg-AD mice. This alteration is of relevance, since PROG metabolites have been shown to exert neuroprotective actions (Brinton et al., 2008; Brinton and Wang, 2006; Sun et al., 2012). Indeed, treatment of 3xTg-AD mice with THP decreases Aβ accumulation (Chen et al., 2011), reverses cognitive deficits and promotes neurogenesis (Singh et al., 2011; Wang et al., 2010). The protective action of THP may be related with its ability to activate the GABA-A receptor (Melcangi et al., 2008). In contrast, isopregnanolone, which as shown here is increased in the limbic region of 3xTg-AD mice, does not bind directly to the GABA-A receptor (Bitran et al., 1991), but it antagonizes the effect of THP on the GABA-A receptor (Wang et al., 2002). Moreover, in this context, it is also important to highlight that, as recently reported (Luchetti et al., 2011a; Luchetti et al., 2011b), the expression of several GABA-A receptor subunits is significantly reduced in post-mortem brain tissue of AD patients. Indeed, alterations of GABA signals may play an important role in the cognitive and behavioral alterations occurring in AD (Birzniece et al., 2006; Lanctot et al., 2007). Therefore, brain alterations in PROG metabolites that modulate GABA-A receptor function, may contribute to the cognitive alterations of AD patients.
Another important modification detected in the limbic region of 3xTg-AD mice was a significant increase in the levels of 17β-E. This may represent an endogenous neuroprotective response to Aβ accumulation in the 3xTg-AD mice. Indeed, aromatase, the enzyme responsible for the synthesis of 17β-E from T, shows increased expression under neurodegenerative conditions (Azcoitia et al., 2001; Garcia-Segura and Balthazart, 2009; Garcia-Segura et al., 2003), including within the AD brain (Luchetti et al., 2011a; Luchetti et al., 2011b). In the aged 3xTg-AD mice, DHEA and T levels were positively correlated with 17β-E levels, supporting the notion that the elevated 17β-E levels observed in the 3xTg-AD mice are endogenously synthesized in the limbic region from T. Furthermore, 3β-diol levels showed also a positive correlation with 17β-E levels in young 3xTg-AD mice. These findings suggest a role in AD pathology for androgen receptor, target of the action of T and possibly of DHEA, and for estrogen receptors, target of the action of 17β-E and 3β-diol. Indeed, androgens as well as estrogens have been already shown to exert a therapeutic effect in 3xTg-AD mice (Carroll et al., 2007; Rosario et al., 2010; Rosario et al., 2006).
In conclusion, the present results show age-related modification in specific neuroactive steroid levels in the limbic region of 3xTg-AD mice brains in association with age-related pathological changes (i.e., Aβ immunoreactive load and gliosis). Due to the well-ascertained neuroprotective capacity of neuroactive steroids, the present findings may represent a preclinical background for a therapy based on these molecules to be applied in AD. In particular, the fact that some neuroactive steroids observed to be modified in 3xTg-AD mice are able to interact with classical steroid receptors (i.e., androgen, progesterone or estrogen receptors), while others interact with non-classical steroid receptors (i.e., GABA-A receptor), may open new therapeutic strategies based on specific synthetic ligands for classical and non-classical steroid receptors.
Acknowledgements
The financial support of Fondazione San Paolo (Progetto Neuroscienze PF-2009.1180) to R. C. Melcangi and NIH (AG05142) to C.J. Pike is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: There are no actual or potential conflicts of interest.
REFERENCES
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J. Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernia O, Carrero P, Azcoitia I, Garcia-Segura LM. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Endocrinology. 2009;150:5010–5015. doi: 10.1210/en.2009-0352. [DOI] [PubMed] [Google Scholar]
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 2007;25:3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Backstrom T, Johansson IM, Lindblad C, Lundgren P, Lofgren M, Olsson T, Ragagnin G, Taube M, Turkmen S, Wahlstrom G, Wang MD, Wihlback AC, Zhu D. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res. Rev. 2006;51:212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy- 5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bittner T, Fuhrmann M, Burgold S, Ochs SM, Hoffmann N, Mitteregger G, Kretzschmar H, LaFerla FM, Herms J. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer's disease mice. PLoS One. 2010;5:e15477. doi: 10.1371/journal.pone.0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front. Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr. Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Magri A, Oddo S. Age-dependent changes in TDP-43 levels in a mouse model of Alzheimer disease are linked to Abeta oligomers accumulation. Mol. Neurodegener. 2010;5:51. doi: 10.1186/1750-1326-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer's disease mice. Endocrinology. 2010;151:2713–2722. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, D'Intino G, Giatti S, Maschi O, Pesaresi M, Calabrese D, Garcia- Segura LM, Calza L, Melcangi RC. Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis. J. Neurochem. 2010a;114:921–932. doi: 10.1111/j.1471-4159.2010.06825.x. [DOI] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Melcangi RC. Effects of Short- and Long-Term Gonadectomy on Neuroactive Steroid Levels in the Central and Peripheral Nervous System of Male and Female Rats. J. Neuroendocrinol. 2010b;22:1137–1147. doi: 10.1111/j.1365-2826.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem. Int. 2008;52:560–568. doi: 10.1016/j.neuint.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang JM, Irwin RW, Yao J, Liu L, Brinton RD. Allopregnanolone promotes regeneration and reduces beta-amyloid burden in a preclinical model of Alzheimer's disease. PLoS One. 2011;6:e24293. doi: 10.1371/journal.pone.0024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front. Neuroendocrinol. 2009;30:v–ix. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog. Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Levault KR, Barnett AJ, Brewer GJ. A Reversible Early Oxidized Redox State That Precedes Macromolecular ROS Damage in Aging Nontransgenic and 3xTg-AD Mouse Neurons. J. Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatti S, D'Intino G, Maschi O, Pesaresi M, Garcia-Segura LM, Calza L, Caruso D, Melcangi RC. Acute experimental autoimmune encephalomyelitis induces sex dimorphic changes in neuroactive steroid levels. Neurochem. Int. 2010;56:118–127. doi: 10.1016/j.neuint.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Rothenburg L, Eryavec G. Behavioral correlates of GABAergic disruption in Alzheimer's disease. Int. Psychogeriatr. 2007;19:151–158. doi: 10.1017/S1041610206003899. [DOI] [PubMed] [Google Scholar]
- Luchetti S, Bossers K, Van de Bilt S, Agrapart V, Morales RR, Frajese GV, Swaab DF. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer's disease. Neurobiol. Aging. 2011a;32:1964–1976. doi: 10.1016/j.neurobiolaging.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer's disease, Parkinson's disease and multiple sclerosis. Neuroscience. 2011b;191:6–21. doi: 10.1016/j.neuroscience.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol. Psychiatry. 2006;60:1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–2517. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Caruso D, Levandis G, Abbiati F, Armentero MT, Blandini F. Modifications of Neuroactive Steroid Levels in an Experimental Model of Nigrostriatal Degeneration: Potential Relevance to the Pathophysiology of Parkinson's Disease. J. Mol. Neurosci. 2012;46:177–183. doi: 10.1007/s12031-011-9570-y. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Garcia-Segura LM. Sex-specific therapeutic strategies based on neuroactive steroids: In search for innovative tools for neuroprotection. Horm. Behav. 2010;57:2–11. doi: 10.1016/j.yhbeh.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell. Mol. Life Sci. 2008;65:777–797. doi: 10.1007/s00018-007-7403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J, Hol EM. GFAP in health and disease. Prog. Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Tripletransgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Agedependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer's disease mouse model: mechanism for deficient glutamatergic transmission? Mol. Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, Kelley CM, Mufson EJ. Brainstem Alzheimer's-like pathology in the triple transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2009;35:415–425. doi: 10.1016/j.nbd.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica GC, Balthazart J, Frye CA, Garcia-Segura LM, Herbison AE, Mensah-Nyagan AG, McCarthy MM, Melcangi RC. Milestones on Steroids and the Nervous System: 10 years of basic and translational research. J. Neuroendocrinol. 2012;24:1–15. doi: 10.1111/j.1365-2826.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm. Behav. 2010;57:46–55. doi: 10.1016/j.yhbeh.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimerlike neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J. Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Beckett TL, Carroll JC, Paul Murphy M, Stanczyk FZ, Pike CJ. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20:1534–1537. doi: 10.1097/WNR.0b013e328331f968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol. Aging. 2011;32:604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Dose-dependent and sequence-sensitive effects of amyloid-beta peptide on neurosteroidogenesis in human neuroblastoma cells. Neurochem. Int. 2008a;52:948–955. doi: 10.1016/j.neuint.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Modulation of neurosteroid production in human neuroblastoma cells by Alzheimer's disease key proteins. J. Neurobiol. 2006;66:868–881. doi: 10.1002/neu.20267. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Selective regulation of neurosteroid biosynthesis in human neuroblastoma cells under hydrogen peroxide-induced oxidative stress condition. Neuroscience. 2008;151:758–770. doi: 10.1016/j.neuroscience.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front. Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia- Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Singh C, Liu L, Wang JM, Irwin RW, Yao J, Chen S, Henry S, Thompson RF, Brinton RD. Allopregnanolone restores hippocampaldependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Ou X, Farley JM, Stockmeier C, Bigler S, Brinton RD, Wang JM. Allopregnanolone increases the number of dopaminergic neurons in substantia nigra of a triple transgenic mouse model of Alzheimer's disease. Curr. Alzheimer Res. 2012;9:473–480. doi: 10.2174/156720512800492567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods, Vol 1. Practical Methods for Biological Morphometry. London: Academic Press; 1979. [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc. Natl. Acad. Sci. U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]


