Abstract
Using buprenorphine as a medication to treat opioid dependence is becoming more prevalent as illicit opiate use increases. Identifying the characteristics of opiate dependent individuals best suited to benefit from buprenorphine would improve guidelines for its administration. This study evaluates baseline and treatment participation variables for predicting positive response to short-term stabilization with buprenorphine. Data includes demographic, drug use, and other variables collected from participants undergoing stabilization over a 4-week period before being tapered off buprenorphine in a short-term detoxification process. Outcome variables include opioid use and retention. Logistic regression results indicate several characteristics associated with opioid use at the end of the stabilization period. These include being older, having no criminal history, and less opiate use. Criminal activity and opioid use in the last 30 days were significantly associated with shorter treatment stays. The benefits of identifying individual characteristics that may predict treatment response are discussed.
Introduction
Buprenorphine is an effective pharmacotherapy for opioid dependence (Fiellin et al., 2008; Ling et al., 2005) and many opiate-dependent patients begin treatment with a period of opioid detoxification (Blondell, Smith, Servoss, DeVaul, & Simons, 2007). It is likely that the use of buprenorphine in short-term treatment or detoxification will increase, as problems with prescription opioid and heroin abuse grow nationwide. Although some studies outline parameters for the use of buprenorphine in opiate-substitution or long-term maintenance treatment (Ling et al., 1998; 2005), few studies have addressed optimal practices in the use of buprenorphine for short-term treatment or detoxification purposes (Lanier, Umbricht, Harrison, Nuwayser, & Bigelow, 2008).
Research indicates that detoxification has limited long-term effectiveness (Amato, Davoli, Minozzi, Ali, & Ferri, 2005; Amato et al., 2008; Ling et al., 2009; Wesson & Smith, 2010), however, there may be some individuals for whom a short-term period of pharmacotherapy is sufficient for initiating and maintaining positive treatment outcome. In some cases, it may be the preferred treatment when compared with long-term maintenance treatment (Poirier et al., 2004; Stein, Cioe, & Friedmann, 2005; Sigmon, Dunn, Badger, Heil, & Higgins, 2009). For example, individuals who have a less severe opioid use history as measured by the quantity and frequency of use, years of use, and injection drug use, may be better candidates for short-term treatment or detoxification (Dunn, Sigmon, Strain, Heil, & Higgins, 2011). Predicting who will do well on this type of buprenorphine regimen may provide guidelines for its administration, and suggest more accurate prescription procedures to avoid a one-size-fits-all paradigm in detoxification and treatment interventions.
Detoxification regimes typically range from short 3–14 day inpatient hospitalizations (Berman, Kallmen, Barredal, & Lindqvist, 2007; Lanier et al., 2008), to combined stabilization/detoxification trials (Sigmon et al., 2009). In a recent pilot study of detoxification for prescription opioid dependence, Sigmon and colleagues (2009) examined abstinence at the end of a brief stabilization (mean = 12 days) and a 2-week taper trial in 12 study participants. No demographic or drug use differences were found between those who tested negative for all illicit opioids on the day following the taper and those who tested positive; however those who were positive had higher rates of alcohol problems. Another study assessed retention of opioid-dependent participants admitted for 5–7 day inpatient detoxification by comparing treatment completion versus drop-out (Berman et al., 2007). Characteristics of the individual more likely to drop out included no treatment planning at intake, no maintenance treatment, and a low score on a measure of positive aspects of drug use.
Using participant characteristics to predict performance and outcome variables, the current study assesses participants provided with buprenorphine pharmacotherapy for four weeks before beginning a taper off buprenorphine in a short-term detoxification procedure. Data collected in the larger study examined abstinence after random assignment to one of two taper schedules (Ling et al., 2009) and found that a relatively short taper (7 days) was as safe and effective as a longer taper (28 days). The current analyses includes multiple baseline variables examined as potential predictors of retention and opioid use to describe characteristics of individuals who are expected to do well in a short-term stabilization phase with buprenorphine.
Methods
Design
Data collection occurred from June 2003 through November 2005 as part of research funded by the National Institute on Drug Abuse’s Clinical Trials Network (CTN) addressing buprenorphine taper and implemented in 11 community treatment centers across the U.S. This was an open-label study providing four weeks of pharmacotherapy with Suboxone®, a combination product of buprenorphine and naloxone, for medication stabilization before participants were randomly assigned to, and began, one of two taper schedules (see Ling et al. 2009). The current analyses examines whether baseline and in-treatment variables predict treatment outcome, defined as retention and opioid use.
Participants
Participant recruitment occurred through word of mouth, public service announcements, newspaper advertisements, and referrals from local treatment and outreach programs, outpatient and inpatient alcohol and drug abuse clinics, primary care providers, local mental health centers, crisis clinics, and hospital emergency rooms. Participants interested in short-term detoxification with buprenorphine were assessed for eligibility. Inclusion criteria included being at least 15 years-of-age, and seeking detoxification for opiate dependence. Exclusion criteria included having a urine sample positive for methadone or benzodiazepine, poor general health, allergies to buprenorphine or naloxone, pregnant or nursing, or a medical condition that would make participation medically hazardous. Additional exclusion criteria included severe psychiatric condition, dependence on alcohol or any drug other than opiates, participation in an investigational drug study in the last 30 days, participation in methadone or Levo-Alpha Acetyl Methadol (LAAM) maintenance or detoxification in the last 30 days, pending legal action, or inability to remain in the area for the duration of the study. Non-pregnant or nursing females of childbearing potential were eligible to participate after agreeing to use an acceptable form of birth control. Ineligible individuals received referrals to local treatment facilities.
The study received approval from each of the participating site Institutional Review Boards and the UCLA Human Subjects Protection Committee. All participants were given complete descriptions of the study and each provided written informed consent prior to administration of any study procedures. Participant incentives included cash and grocery scrip based on site preferences or site Institutional Review Board requirements. Participants received $25 for each milestone visit (screening, start of induction, start of taper, follow-up visits) and $10 for each weekly clinic visit. Participants completing all post-taper visits received an additional $25. A total of 894 participants completed baseline assessment and 83.67% (748) were inducted onto buprenorphine. A total of 516 participants completed the four-week detoxification. Analyses indicate no differences in baseline demographic or drug use characteristics between those who were and were not retained through the 4-week medication stabilization phase, through to the taper.
Measures
The Adjective Rating Scale for Withdrawal (ARSW)
The ARSW collects information on the participant’s experience of opioid withdrawal such as muscle cramps, nausea, etc. (Amass, Kamien, & Mikulich, 2000; Bickel et al., 1988a, 1988b). Participants rate themselves on a scale ranging from 0 (none) to 9 (severe) for each of 16 signs and symptoms, with a maximum possible score of 144 indicating the most severe withdrawal experience. The ARSW was completed at screening, and weekly throughout the stabilization phase.
The Clinical Opiate Withdrawal Scale (COWS)
The 11-item questionnaire (Wesson & Ling, 2003) provides a description of signs and symptoms of opiate withdrawal that are clinically observed in the participant (e.g., sweating, runny nose, etc.) by an experienced clinician A total score ranges from 0 (none) to 48 (severe) withdrawal. The COWS was completed at screening, and weekly throughout the stabilization phase.
The Visual Analog Scale (VAS)
The VAS assesses the extent to which the participant feels any craving for opiates, the severity of their withdrawal symptoms, and the extent to which the study medication helps to ease drug cravings (if applicable). It consists of three 100-point lines anchored with “not at all” on one end and “extremely” on the other (Childress, McLellan, & O’Brien, 1986; Kaplan et al., 1985). The analyses used only responses to opiate craving. The VAS was completed at screening, and weekly throughout the stabilization phase.
The Addiction Severity Index-Lite (ASI-Lite)
The ASI-Lite is a standardized, multidimensional, semi-structured, comprehensive clinical interview designed to provide clinical information for formulating treatment plans as well as problem severity profiles in domains commonly affected by substance abuse (McLellan et al., 1985). The domains assessed are demographic information, alcohol use, drug use, medical, psychiatric, legal, family/social and employment/support. To collect additional information not included on the ASI, a separate 3-item assessment was constructed and administered in conjunction, and includes items assessing nicotine use and a distinction of illicit and prescribed methadone use. The ASI was administered at screening.
Toxicology Testing
Urine samples collected at screening and at weekly clinic visits were tested on-site for drug use using either Jant’s Accutest MultiDrug Screen-10 or ABI’s SureStep Drug Screen Card 10A. Urine drug toxicology results were coded qualitatively as positive or negative for morphine, methadone, oxycontin, cocaine, amphetamines, barbiturates, benzodiazepines, methamphetamines, phencyclidine (PCP), marijuana, and tri-cyclic antidepressants. In addition, all sites tested for the presence of oxycodone utilizing ABM’s Rapid One Oxycodone single dipstick.
Study Variables
Predictor Variables
Based on results from previous studies (Gerra et al., 2004; Marsch et al., 2005; Magura, Nwakeze, & Demsky, 1998; Simpson, Joe, & Rowan-Szal, 1997; Stein et al., 2005; Hillhouse, Marinelli-Casey, Gonzales, Ang, & Rawson, 2007) and from the association between baseline characteristics and outcomes of the current data, the following variables were potential predictors in separate analyses of retention and opioid use. (Frequencies presented in Table 1):
Table 1.
Characteristics of Participants at Baseline and at end of Stabilization Phase.
| Characteristics | Baseline N=894 | End of Stabilization Phase N=516 |
|---|---|---|
|
| ||
| Mean Age (SD) | 36.47 (10.46) | 35.89 (10.45) |
|
| ||
| Mean Number of years of education (SD) | 13 (2.08) | 13 (2.15) |
|
| ||
| Mean Number of days employed (in past 30 days) (SD) | 5 (3.13) | 4 (3.09) |
|
| ||
| Mean COWS (SD) | 8.40 (3.91) | 8.49 (3.96) |
|
| ||
| Mean ARSW (SD) | 61.74 (32.49) | 62.17 (32.15) |
|
| ||
| Mean VAS (SD) | 69.23 (24.08) | 69.63 (24.31) |
|
| ||
| % Gender (n) | ||
| Male | 67 (599) | 67.05 (346) |
| Female | 33 (295) | 32.95 (170) |
|
| ||
| % Race (n) | ||
| Caucasians | 73.88 (659) | 76.16 (393) |
| African-American | 13.68 (122) | 11.63 (60) |
| Hispanics | 9.64 (86) | 9.30 (48) |
| Others | 2.80 (25) | 2.91 (15) |
|
| ||
| % Marital Status (n) | ||
| Married/Remarried | 22.28 (199) | 24.22 (125) |
| Widowed/Separated/Divorced | 26.32 (235) | 24.22 (125) |
| Never Married | 51.40 (459) | 51.55 (266) |
|
| ||
| % Route of Administration (n) | ||
| Inject | 58.41 (521) | 54.26 (280) |
| Non-inject | 41.59 (371) | 45.74 (236) |
|
| ||
| % Arrested for Criminal Activity (lifetime) (n)* | ||
| Yes | 67 (599) | 61.24 (316) |
| No | 33 (295) | 38.76 (200) |
|
| ||
| % On Probation or Parole (n) | ||
| Yes | 10.53 (94) | 10.08 (52) |
| No | 89.47 (799) | 89.92 (464) |
|
| ||
| % Depressed (in past 30 days) (n) | ||
| Yes | 29.90 (267) | 28.88 (149) |
| No | 70.10 (626) | 71.12 (367) |
|
| ||
| % Previous treatment for drug abuse(lifetime) (n) | ||
| Yes | 73.57 (657) | 71.32 (368) |
| No | 26.43 (236) | 28.68 (148) |
|
| ||
| % Opiate use (In past 30 days) (n) | ||
| Less than 30 days | 24.83 (222) | 27.52 (142) |
| 30 days | 75.17 (672) | 72.48 (374) |
|
| ||
| % Cannabis use (In past 30 days) (n) | ||
| No use | 58.84 (526) | 58.14 (300) |
| Use 1+ days | 41.16 (368) | 41.86 (216) |
|
| ||
| % Methamphetamine use (In past 30 days) (n) | ||
| No use | 89.49 (800) | 89.53 (462) |
| Use 1+ days | 10.51 (94) | 10.47 (54) |
|
| ||
| % Nicotine usage (In past 30 days) (n) | ||
| No use | 14.99 (134) | 14.53 (75) |
| Use 1+ days | 85.01 (760) | 85.47 (441) |
|
| ||
| % UA positive for opioids | ||
| positive | 97.55 (795) | 62.98 (325) |
| negative | 2.45 (20) | 37.02 (191) |
|
| ||
| % UA positive for other drugs | ||
| positive | 98.02 (803) | 66.47 (341) |
| negative | 1.47 (12) | 33.53 (172) |
|
| ||
| % Daily Buprenorphine Dose (n) | ||
| 8 mg | -- | 9.30 (48) |
| 16 mg | -- | 27.33 (141) |
| 24 mg | -- | 63.37 (327) |
Significant difference between baseline and randomization time point (p<0.05)
Demographic Characteristics
Demographic information collected at baseline included age, gender, ethnicity, employment, marital status, education, and whether the participant reported living with other drug abusers in the household.
Drug Use Characteristics
Information about drug use was collected using the Addiction Severity Index (ASI) and a measure developed to add to, or expand, some information collected with the ASI. Opiate use in the last 30 days was categorized as either daily use, or less than daily use. Alcohol, cannabis, methamphetamine, and nicotine use were dichotomized separately into use on at least one day in the last 30 days or no use in the last 30 days. We also created a separate binary predictor for other drug use that were not previously dummy-coded as separate categories, and these other drugs include barbiturates, benzodiazepines, PCP, inhalants, psychedelic drugs, and tri-cyclic antidepressants. Years of drug use, polysubstance use, route of administration, and previous drug treatment experience were also examined from data collected with the ASI. Baseline opiate UA test results were also collected from participants.
Criminal Justice Characteristics
Two items assessing involvement in criminal activity collected with the ASI were included in the analyses. These include having ever been arrested for criminal activity, and being on probation or parole.
Psychological Status
One item from the ASI assessing depression (yes, no) in the last 30 days was included.
Withdrawal and Craving Scores
Summary scores from instruments that assess withdrawal symptoms and craving were included. The Clinical Opiate Withdrawal Scale (COWS) and the Adjective Rating Scale for Withdrawal (ARSW) both assess withdrawal symptoms, whereas the Visual Analog Scale (VAS) assesses craving.
Medication Dose
A single item reflecting stabilization/maintenance dose was included. Medication dose was flexible during weeks 1–3 and was determined by the study physician based on withdrawal symptoms, participant self-report of adverse events, and clinical expertise. Daily dose was fixed during week 4 and constrained in the main study to three levels of buprenorphine: 8mg, 16mg, and 24mg.
Outcome Variables
Retention and reduction in drug use are often identified as evidence of a positive treatment experience, and these outcomes are used in the current analyses to define a positive detoxification experience.
Retention
Measured in two ways: 1) whether an individual remained in treatment for 4 weeks from medication induction through the stabilization phase and 2) the number of weeks a participant remained in the stabilization phase.
Opioid Use
The main treatment outcome, defined as opioid use was measured in two ways: 1) an opiate-negative UA test result at the end of the stabilization phase, and 2) the Treatment Effectiveness Score (TES) or the percentage of opiate-negative UA tests over the total number of opiate tests possible during the stabilization phase.
Procedures
Study procedures were in accord with the standards of the human subjects protection committees at participating institutions and with the Helsinki Declaration of 1975. All participants received pharmacotherapy with the combination product of buprenorphine and naloxone (Suboxone®), as well as behavioral interventions across the duration of the study. Because study procedures were intended to mirror those occurring in “real-life” clinic settings, the behavioral treatment procedures in place at each treatment site were followed throughout the study with no attempt made to standardize or modify these site-specific procedures. All participants received a basic platform of substance abuse education, and all treatment sites provided self-help buprenorphine treatment booklets to research participants. No data were collected to assess engagement in the psychosocial treatment component.
Suboxone® was provided as a combination 4:1 ratio buprenorphine and naloxone sublingual tablet by Reckitt and Benckiser (Hull, UK) in two formulations (2 mg buprenorphine/0.5 naloxone and 8 mg buprenorphine/2 mg naloxone). Participants received weekly medication and explicit dosing instructions with induction occurring over the first three medication days. The 4-week stabilization phase included three weeks of flexible dosing to allow adjustments for individual responses to study drug, with daily dose fixed by the fourth week of 8 mg, 16 mg, or 24 mg of Suboxone. At the end of week four, participants were randomized to a 7 or 28 day medication taper schedule. (See Ling et al., 2009, for detailed descriptions of study procedures).
Data Analysis
Data collection occurred at screening/baseline, and throughout the 4-week stabilization phase. For the current analysis, we define as the stabilization phase the 4-weeks of pharmacotherapy lasting until the taper began.
List-wise deletion controlled for the small number of missing values for the variables in the analysis. After investigating and removing outliers, baseline data from 876 participants (out of 894; 97.9%) were included to examine retention up to the taper, and 732 participants inducted onto study drug (out of 748; 97.8%) were included in the analyses of weeks of retention. Data from 503 participants who completed the 4-week stabilization phase (out of 516; 97.48%) were used for both analyses of opioid use.
T-tests and Chi-square tests assessed the relationships between the baseline characteristics and outcome measures. Separate multiple logistic regressions, using backward elimination for variable selection, identified significant predictors for two of the outcome variables: retention as measured by whether a participant remained in the study through the four-week stabilization phase and opioid use as measured by opioid-negative UA results at the end of the taper. Discrete time survival using logistic regression (using indicator variables for each time point) analyzed the second retention variable: number of weeks a participant stayed in the study before dropping out. The Treatment Effectiveness Score (TES) results were categorized into two percentage groups (0–49, 50–100) and logistic regression with backward elimination for variable selection was used for analysis. All statistical tests were performed at 95% significance level. The Hosmer-Lemeshow (H-L) test analyzed the fit of the final model using binary logistic regression. Statistical analysis was performed using SAS 9.1 (SAS Institute Inc., Cary, NC) and STATA 10 (Statacorp LP). A collection of STATA commands (written by A. Dinno, Harvard School of Public Health) was used for discrete time survival analysis. Interactions among the measures were tested for all responses but the model with main effects was optimally based on either Likelihood ratio test or Akaike Information Criterion (AIC), whichever was appropriate. In the Odds Ratio analyses, odds for each outcome were obtained after controlling for the remaining significant characteristics in the model.
Results
Sample Characteristics
Table 1 shows participant characteristics for the baseline sample and the sample who completed the 4-week stabilization phase. There were no differences in characteristics between these groups except for lifetime criminal activity. At baseline, 67.0% of participants had a history of criminal activity compared to 61.2% of the stabilization completer group (p < 0.05).
Predictors
Table 2 shows the results of preliminary tests of association (Chi-squares and t-tests) between baseline characteristics, retention, and abstinence outcomes. There was a negative association between those arrested for criminal activities or on parole with weeks in treatment. Age, being married vs never married, non-daily opiate use vs daily use, and baseline negative opioid negative UA result were all positively associated with opiate negative urine result in the bivariate analysis. Scoring 50% or more on the TES was positively associated with being married vs single, non-daily opiate use (vs daily), opiate-negative baseline UA, alcohol use in the past 30 days, depression, route of administration (non-IV), and a history of previous drug treatment. Polydrug use, and arrested for criminal activities were negatively associated with TES score. Table 3 documents the variables that were significant in predicting each of the outcomes and the odds ratios at a 95% level of significance.
Table 2.
Associations between baseline characteristics, retention and abstinence.
| Retention | Opioid Use | |||||||
|---|---|---|---|---|---|---|---|---|
| Completed 4-Week Stabilization Phase | Weeks in Treatment | Opiate Negative Urinalysis | Treatment Effectiveness Score (TES) ≥ 50% | |||||
| Equality of Means | T | p-value | F | p-value | T | p-value | F | p-value |
| Age | 2.11 | 0.04* | 1.05 | 0.38 | 2.07 | 0.04* | 2.37 | 0.10 |
| Education | 0.31 | 0.76 | 0.64 | 0.64 | 1.21 | 0.23 | 0.15 | 0.86 |
| Days employed | −3.45 | 0.00* | 2.02 | 0.09 | −0.68 | 0.50 | 3.92 | 0.02 |
| COWS | −0.93 | 0.35 | 0.21 | 0.93 | −0.75 | 0.46 | 0.34 | 0.71 |
| ARSW | −0.33 | 0.74 | 0.37 | 0.83 | 1.55 | 0.12 | 3.05 | 0.05 |
| VAS | −0.51 | 0.61 | 0.35 | 0.84 | −0.27 | 0.79 | 0.55 | 0.58 |
| Opioid lifetime use, years | −1.67 | 0.06 | −1.51 | 0.13 | 0.15 | 0.88 | 0.90 | 0.57 |
| Test for Association | Unadjusted Odds Ratio | p-value | Unadjusted Odds Ratio | p-value | Unadjusted Odds Ratio | p-value | Unadjusted Odds Ratio | p-value |
| Gender, Female=1, male =0 | 1.00 | 0.83 | 5.65 | 0.23 | 0.15 | 0.7.0 | 0.84 | 0.66 |
| Race | 4.27 | 0.23 | 12.04 | 0.44 | 4.87 | 0.18 | 7.17 | 0.31 |
| Marital Status, married vs never married | 4.42 | 0.11 | 3.17 | 0.92 | 7.45 | 0.02* | 10.58 | 0.03* |
| Route of Administration, non-IV vs IV | 7.03 | 0.01* | 2.40 | 0.66 | 1.11 | 0.29 | 15.56 | 0.00* |
| Arrested for Criminal Activity | 0.53 | <0.00* | 0.21 | <0.00 | 0.78 | 0.08 | 0.52 | <0.00* |
| On Probation/Parole | 0.41 | 0.52 | 0.32 | 0.01* | 1.16 | 0.28 | 0.48 | 0.79 |
| Depressed, past 30 days | 0.67 | 0.41 | 4.26 | 0.37 | 1.38 | 0.24 | 6.30 | 0.04* |
| Previous drug treatment | 3.97 | 0.05* | 8.94 | 0.06 | 3.53 | 0.06 | 16.63 | 0.00* |
| Opiate use, non-daily vs daily, past 30 days | 1.49 | 0.01* | 1.31 | 0.21 | 1.61 | 0.02* | 1.94 | 0.00* |
| Cannabis use, past 30 days | 0.22 | 0.64 | 1.30 | 0.86 | 0.11 | 0.74 | 2.44 | 0.30 |
| Methamphetamine use, past 30 days | 0.99 | 0.62 | 0.98 | 0.47 | 0.99 | 0.95 | 1.12 | 0.57 |
| Nicotine use, past 30 days | 0.07 | 0.79 | 2.11 | 0.72 | 2.64 | 0.10 | 0.15 | 0.93 |
| Alcohol use, past 30 days | 1.05 | 0.31 | 1.32 | 0.25 | 0.58 | 0.45 | 4.47 | 0.03* |
| Other drug use, past 30 days | 3.02 | 0.08 | 1.20 | 0.27 | 1.27 | 0.24 | 2.33 | 0.13 |
| Polydrug use, past 30 days | 0.78 | 0.38 | 0.37 | .054 | 0.38 | 0.54 | 0.31 | 0.01* |
| Baseline UA negative | 1.28 | 0.26 | 1.05 | 0.31 | 81.12 | <.00* | 86.93 | <.00* |
| Currently living with other drug abuser | 0.39 | 0.53 | 1.34 | 0.25 | 0.47 | 0.49 | 0.18 | 0.67 |
| Dose | NA | NA | NA | NA | 4.06 | 0.03* | 18.90 | 0.00* |
Significantly associated with outcome at α=0.05
Table 3.
Possible Predictors of Retention and Opioid Use
| Retention | Opioid Use | |||
|---|---|---|---|---|
| Predictors | Completed Stabilization Phase | Weeks in Treatment | Opiate Negative Urinalysis | Treatment Effectiveness Score (TES) ≥ 50% |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Not arrested for Criminal Activity | 1.79** (1.33, 2.42) |
1.57** (1.16, 2.13) |
- | 1.83** (1.22, 2.77) |
| Opiate use, non-daily vs daily (past 30 days) | 1.46* (1.06, 2.02) |
1.45* (1.03, 2.02) |
1.79** (1.19, 2.70) |
2.16** (1.40, 3.35) |
| Days employed | 0.94** (0.90, 0.98) |
- | - | - |
| Age | - | - | - | 1.03** (1.01, 1.05) |
| Depressed | - | - | - | 1.54* (1.02, 2.33) |
| Previous drug treatment | - | - | 1.52* (1.02, 2.27) |
1.75* (1.11, 2.77) |
| Dose (8 mg vs. 24 mg) | - | - | - | 3.25* (1.51, 7.00) |
| Dose (16 mg vs. 24 mg) | - | - | - | 1.65* (1.07, 2.57) |
| ARSW | - | - | - | 1.01* (1.00, 1.01) |
| Polydrug use (past 30 days) | - | - | - | 0.36* (0.15, 0.90) |
| Baseline UA | - | - | 7.08** (4.33, 11.60) |
5.55** (3.98, 12.65) |
| Marital status (Married vs. Never married) | - | - | 1.96* (1.24, 3.08) |
- |
| Marital status (Widowed/Separated/Divorced vs Never married) | - | - | 1.61* (1.02, 2.54) |
- |
| Route of administration (non-inject vs inject) | - | - | - | 1.63* (1.10, 2.43) |
p<0.05;
p<0.01
OR = Odds Ratio; CI = Confidence Interval
Retention: Stabilization Completers
Baseline characteristics that played a significant role in predicting retention through the stabilization phase were non-daily opiate use in the past 30 days, lifetime arrest for criminal activity, and employment (past 30 days) (H-L p-value = 0.71). Controlling for the effects of opiate use and employment status for the past 30 days, the odds of a participant with no lifetime criminal arrest record remaining throughout the stabilization phase are 1.79 times that of a participant who was arrested for criminal activity at least once in his/her lifetime. Furthermore, after controlling for criminal activity and employment status, the odds of a person who did not use opiates daily in the last 30 days remaining in the stabilization phase until the taper are 1.46 times that of a person who used opiates daily. After controlling for opiate use and criminal activity, the odds of a participant with more days of employment remaining in the stabilization phase until the taper decreased by 6.1% compared to a participant with fewer days of employment.
Retention: Number of Weeks in Treatment
Retention, measured as the number of weeks a participant stayed in the study during the stabilization phase ranged from one to five weeks. Although the stabilization phase was defined as four weeks, there was a window for clinic visits which accounts for the extended weeks of participation. Past criminal history and opiate use in the last 30 days before the start of the study were significant in predicting this measure of retention. The results show that 81% of those who did not report any lifetime criminal history and who did not use opiates daily during the 30 days prior to the start of the study remained in the stabilization phase until the taper. Comparatively, only 63% of those who had a criminal history and used an opiate daily prior to the start of the study remained until the end of the stabilization phase (figure 1). The odds of a participant with no lifetime criminal history completing the stabilization phase are 1.57 times that of those who had a criminal history. Similarly, the odds of a participant who did not use an opiate daily prior to the start of the study completing the stabilization phase is 1.45 times that of the participant who used an opiate daily.
Figure 1.
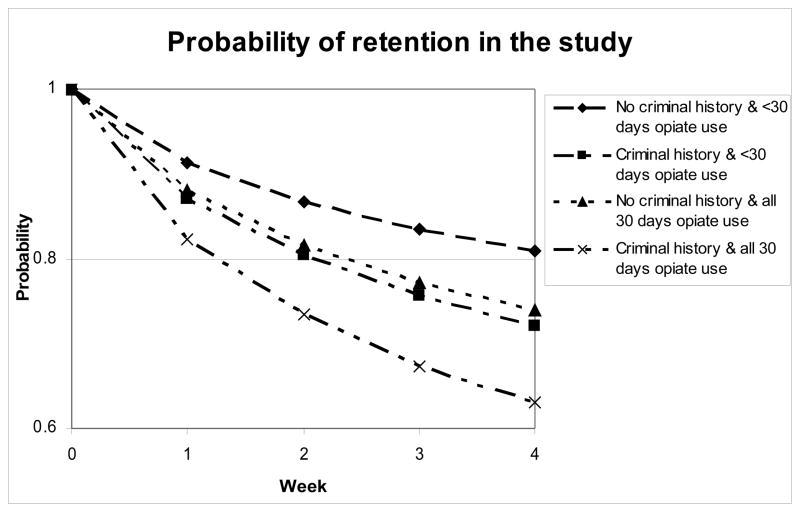
Retention (number of weeks until drop-out) by Predictor Variables.
Opioid Use: Opioid-negative UA Result at the end of the Stabilization Phase
Non-daily opioid use for the past 30 days at baseline, previous drug abuse treatment, and marital status are significant predictors of abstinence as measured by a single toxicology test at the end of the stabilization phase (H-L p-value = 0.54). Controlling for opiate use and marital status, the odds of a participant with previous drug abuse treatment being abstinent are 1.52 times higher than that of a participant who had no prior treatment. Controlling for prior drug treatment and marital status, the odds of a person who used opioids less than daily for the prior 30 days at baseline having an opioid-negative UA is 1.79 times that of a person who used opioids daily for the last 30 days at baseline. Finally, controlling for opiate use and prior drug treatment, the odds of a participant who was married having an opioid-negative UA result is 1.96 times that of a participant who was never married.
Opioid Use: Treatment Effectiveness Score (TES)
Opioid use during the entire stabilization period was also measured using the TES which is a percentage computed as the number of opioid-negative urine tests provided by each participant over the number of urine tests possible for each participant. The TES categorization was (1) those who had a TES percentage between 0% to 49%, and (2) those who had a TES score of 50% or more (reflecting at least 50% opioid-negative urine test results out of all possible tests). Logistic regression determined the predictors of opioid use defined by the TES. Route of drug administration, past criminal activity, non-daily opioid use in the past 30 days, previous drug abuse treatment, dose, age and withdrawal symptoms at baseline as measured with the ARSW were significant predictors of abstinence (H-L p-value = 0.40).
Participants who did not use drugs via injection had a 1.63 times higher odds of having a TES of at least 50% compared to those who used drugs via injection. The odds of a person not arrested for criminal activity to have a TES of at least 50% are 1.83 times higher than a person with was arrested for criminal activity. The odds of having a TES of at least 50% are 2.16 times higher for those who had non-daily opioid use compared to those who used opioids on each of the last 30 days. For participants who had previous drug abuse treatment, the odds of having a TES of at least 50% was 1.75 times higher than those with no previous drug abuse treatment. Participants who received 8 mg of buprenorphine daily had a 3.25 times higher odds of having a TES of at least 50% compared to those given 24 mg daily. Similarly, participants given 16 mg daily had 1.65 times higher odds of having a TES of at least 50% compared to those given 24 mg daily. For each year increase in age, the odds of having a TES of at least 50% increased by a factor of 1.03. Interestingly, for each one-point increase in ARSW (craving) score at baseline, the odds of having a TES of at least 50% increases 1.01 times.
Discussion
The results of our analyses illuminate some similarities and differences among and between the variables associated with positive outcome, retention and opioid use. For example, the results show the deleterious effect of having a history of criminal activity in terms of staying in treatment and maintaining abstinence. Not only was criminal activity the only variable differentiating the baseline and stabilization completer samples (see Table 1), but it was found to predict 3 of the 4 retention and opioid use variables, suggesting that following a standard treatment-as-usual format when providing treatment with buprenorphine may not be adequate for those with a criminal history. Our findings may also indicate that a 4-week medication period is not sufficient for those with a criminal history. Long-term pharmacotherapy with buprenorphine may provide a more appropriate period of stabilization for this population.
Recent research on criminal justice populations demonstrates that treatment with buprenorphine may be effective when initiated prior to release (Awgu, Magura, & Rosenblum, 2010; Cropsey et al., 2011; Springer, Chen, & Altice, 2010), and is equally effective in primary care settings for those with and without criminal justice histories (Wang, Moore, Sullivan, & Fiellin, 2009). A more recent publication accessed public databases to compare rates of criminal charges for those given at least one prescription for buprenorphine. There was no statistically significant change in the proportion of participants with at least one criminal charge in the two years before (42.9%) and after (38.5%) buprenorphine treatment. These findings suggest that for those who have been involved in criminal activities, treatment with buprenorphine may be most effective when it is initiated while incarcerated.
It is not surprising that previous drug use predicts future drug use. This is a regular finding in studies utilizing a pre- and post-treatment design (Hillhouse et al., 2007). In the current study, it was identified as a predictor in all four of the outcome variables examined.
The TES identified the most predictor variables. Given that the retention measures and opioid use measured by a single UA test at the end of the detoxification period are based on a single event, it either happened or not, it is reasonable that a measure that collects information across the entire study period would result in a more complicated picture. A participant can shift from periods of opioid use to non-use multiple times over the study duration, which may represent failed attempts to maintain abstinence. The TES assesses multiple time-points of possible drug use during treatment episodes rather than a single assessment, and more accurately acknowledges the complicated patterns of drug use seen during recovery attempts.
Ironically, one of the strengths of this study is matched by an associated limitation. None of the outcome variables are based on self-report data: the outcome measures of retention and drug use come from laboratory results and clinic records. While not suggesting that laboratory results and clinic records are never inaccurate, these findings do not rely on participants’ self-perceptions of behaviors and related performance variables. Conversely, the predictor variables used for these analyses are based on self-reported data and as such, rely on the honesty and accuracy of the participants. As with most studies assessing previous behaviors and events, our concern is not with dishonest responses, as with faulty memories. We assume, however, that the predictor variables used in this study are of suitably recent occurrence (in the past 30 days) or importance (criminal activity, previous drug treatment), that we have captured reasonably accurate data.
Although we do not address participant status at the 1- and 3-month follow-up in the current analyses, it may be important to note that, in looking at UA test results at baseline and at follow-up, the effectiveness of the 4-week stabilization phase provided to participants may be limited for long-term outcomes (Amato et al., 2005, 2008; Ling et al., 2009; Wesson & Smith, 2010), however, some participants may do well after short-term stabilization and detoxification regimes. UA test results at baseline were 97.6% positive for opioids, whereas subsequent UA opioid positive results were 64.8% at 1-month follow-up and 67.8% at 3-month follow-up. Future analyses of the follow-up data may help to identify for whom treatment with short-term detoxification may be sufficient for instilling long-term abstinence.
The findings from this study have important clinical applications for treatment providers, specifically providing information for those who offer buprenorphine treatment to opiate-dependent patients. Treatment providers and private practice clinicians may find it useful to know that positive outcomes may be likely when using buprenorphine for short-term treatment in some patients. For example, these findings show that those with more severe withdrawal symptoms at baseline do not experience higher levels of drop out or continued opiate use. Conversely, these findings have identified those for whom this short-term treatment with buprenorphine may not be successful such as those who use opioids more often, have a previous treatment experience, and have a criminal justice history. These findings support previous study results demonstrating that those with more severe drug use and less social stability are less likely to have positive treatment outcomes.
Although short-term treatment and detoxification regimes have not been found to be successful for many opioid-dependent patients, the findings from this study suggest that some individuals may do fine with limited-duration pharmacotherapy. Extrapolating from our findings, patients who are married, provide an opiate-negative urine test at baseline, and report no IV drug use may do well with a short-term stabilization on buprenophrine. Understanding who does well with what kinds of treatment is the next step in developing successful treatments for everyone, and research should continue to investigate the patient characteristics best suited for specific treatment characteristics.
The ability to predict who will do well given a specific treatment modality, ideology, component, or tool, is at the heart of research aimed at improving treatment effectiveness. We know that treatment is only effective for a percentage of those who participate, and that the effectiveness of a particular treatment may differ across participants. The challenge is identifying the individual characteristics associated with successful outcome for each treatment variable. In this way, clinicians can develop the most effective treatment components for each individual client from a variety of choices.
Acknowledgments
This work was supported by a series of grants from the National Institute on Drug Abuse as part of the Cooperative Agreement of the Clinical Trials Network (University of California, Los Angeles, U10 DA13045; New York University, U10 DA 013046; University of Washington, U10 DA 013714; Duke University, U10DA13711; Yale University, U10 DA 013038; New York State Psychiatric Institute, U10 DA 013035; University of Colorado Health Sciences Center, U10 DA13716; Oregon Health Sciences University, U10 DA 013036; The John Hopkins University, U10DA13034.) Thanks to the following for their contributions to this research: Bill Swafford, M.D., Lisa Darton, M.D., Irene Aguilar, M.D., Paula Riggs, M.D. (Rocky Mountain Node); Bill Dickenson, D.O., Andrew Saxon, M.D., Dennis Donovan, Ph.D. (Washington Node); Joshua Boverman, M.D., Dennis McCarty, Ph.D. (Oregon Node); Hansa Shah, M.D., Peter Strong, M.D., Thomas Kosten, M.D., Kathleen Carroll, Ph.D. (New England Node); Paul Casadonte, M.D., John Rotrosen, M.D. (New York Node); Jeremy Stowell, M.D., Elinore McCance-Katz, M.D., Maxine Stitzer, Ph.D. (Mid-Atlantic Node); Jeffry Selzer, M.D., Eric Collins, M.D., Edward Nunes, M.D. (Long Island Node); Thomas Mathew, M.D., James Finch, M.D., Len Handelsman, M.D., Robert Hubbard, Ph.D. (North Carolina Node).
Footnotes
Trial Registration: On Clinicaltrials.gov, Identifier: NCT00078117
Conflict of Interest Statement: No author contributing to this manuscript reports a conflict of interest, financial or otherwise.
No author reports a conflict of interest, financial or otherwise, relevant to this manuscript. All persons listed in the acknowledgement section above were investigators (site, protocol, or node PI) at the participating sites/treatment programs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug and Alcohol Dependence. 2000;58:143–52. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Amato L, Davoli M, Minozzi S, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2005:CD003409. doi: 10.1002/14651858.CD003409.pub3. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2008:CD005031. doi: 10.1002/14651858.CD005031. [DOI] [PubMed] [Google Scholar]
- Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine orm ethadone maintenance. Journal of Psychoactive Drugs. 2010;42:339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AH, Kallmen H, Barredal E, Lindqvist P. Hopeless patients? A study of illicit opiate users who drop out from in-patient detoxification. Journal of Substance Use. 2007;13:121–130. [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clinical Pharmacology and Therapeutics. 1988a;43:72–8. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. Journal of Pharmacology and Experimental Therapeutics. 1988b;247:47–53. [PubMed] [Google Scholar]
- Blondell RD, Smith SJ, Servoss TJ, DeVaul SK, Simons RL. Buprenorphine and methadone: a comparison of patient completion rates during inpatient detoxification. J Addict Dis. 2007;26:3–11. doi: 10.1300/J069v26n02_02. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal on Addictions. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Lane PS, Hale GJ, Jackson DO, Clark CB, Ingersoll KS, Islam MA, Stitzer ML. Results of a pilot randomized controlled trial of buprenorphine for opioid dependent women in the criminal justice system. Drug and Alcohol Dependence. 2011;119:172–178. doi: 10.1016/j.drugalcdep.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: A review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bubici C, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75:37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102(Suppl 1):84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol. 1985;46:267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Lanier RK, Umbricht A, Harrison JA, Nuwayser ES, Bigelow GE. Opioid detoxification via single 7-day application of a buprenorphine transdermal patch: an open-label evaluation. Psychopharmacology (Berl) 2008;198:149–158. doi: 10.1007/s00213-008-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, et al. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104:256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93:51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE. Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Exp Clin Psychopharmacol. 2005;13:293–302. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disorders. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Poirier MF, Laqueille X, Jalfre V, Willard D, Bourdel MC, Fermanian J, et al. Clinical profile of responders to buprenorphine as a substitution treatment in heroin addicts: results of a multicenter study of 73 patients. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:267–272. doi: 10.1016/j.pnpbp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug Alcohol Depend. 1997;47:227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: The impact of buprenorphine treatment. Journal of Urban Health. 2010;87:592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. Journal of General Internal Medicine. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EA, Moore BA, Sullivan LE, Fiellin DA. Effect of incarceration history on outcomes of primary care office-based buprenorphine/naloxone. Journal of General Internal Medicine. 2010;25:670–674. doi: 10.1007/s11606-010-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Smith DE. Buprenorphine in the treatment of opiate dependence. J Psychoactive Drugs. 2010;42:161–175. doi: 10.1080/02791072.2010.10400689. [DOI] [PubMed] [Google Scholar]


