Abstract
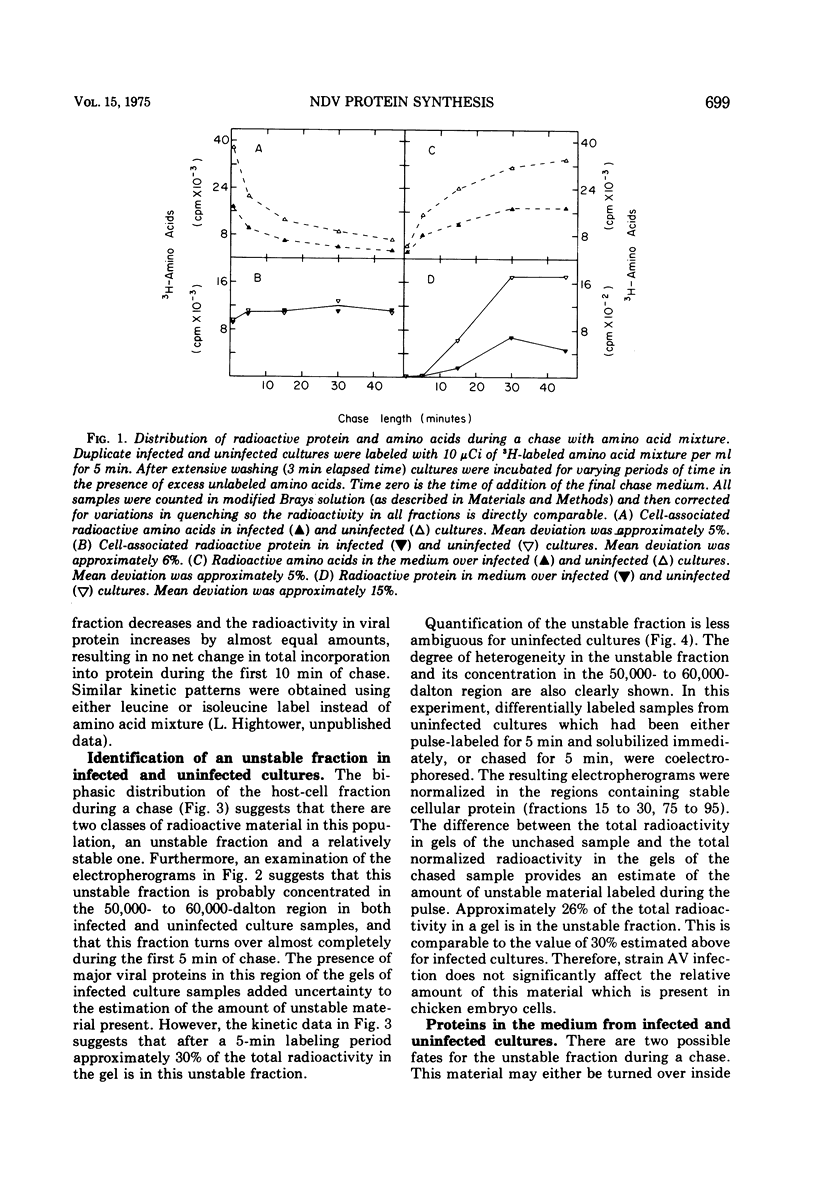
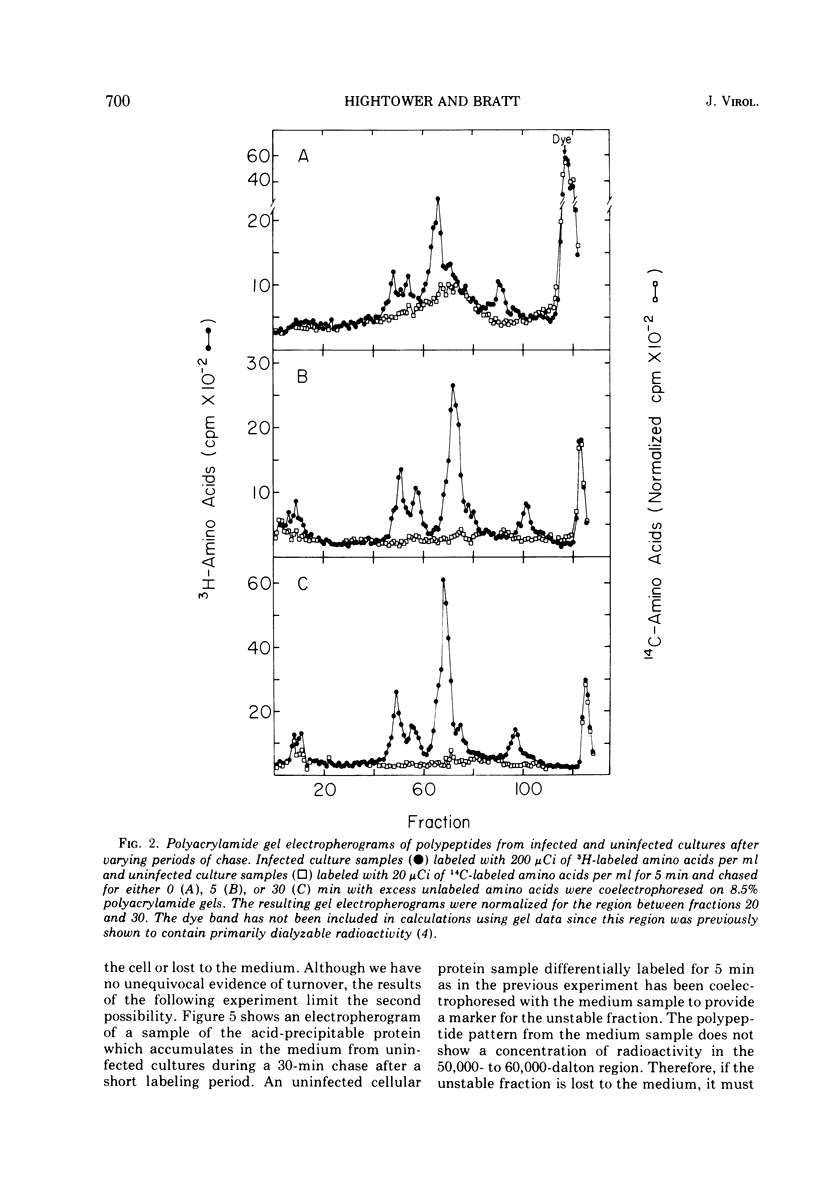
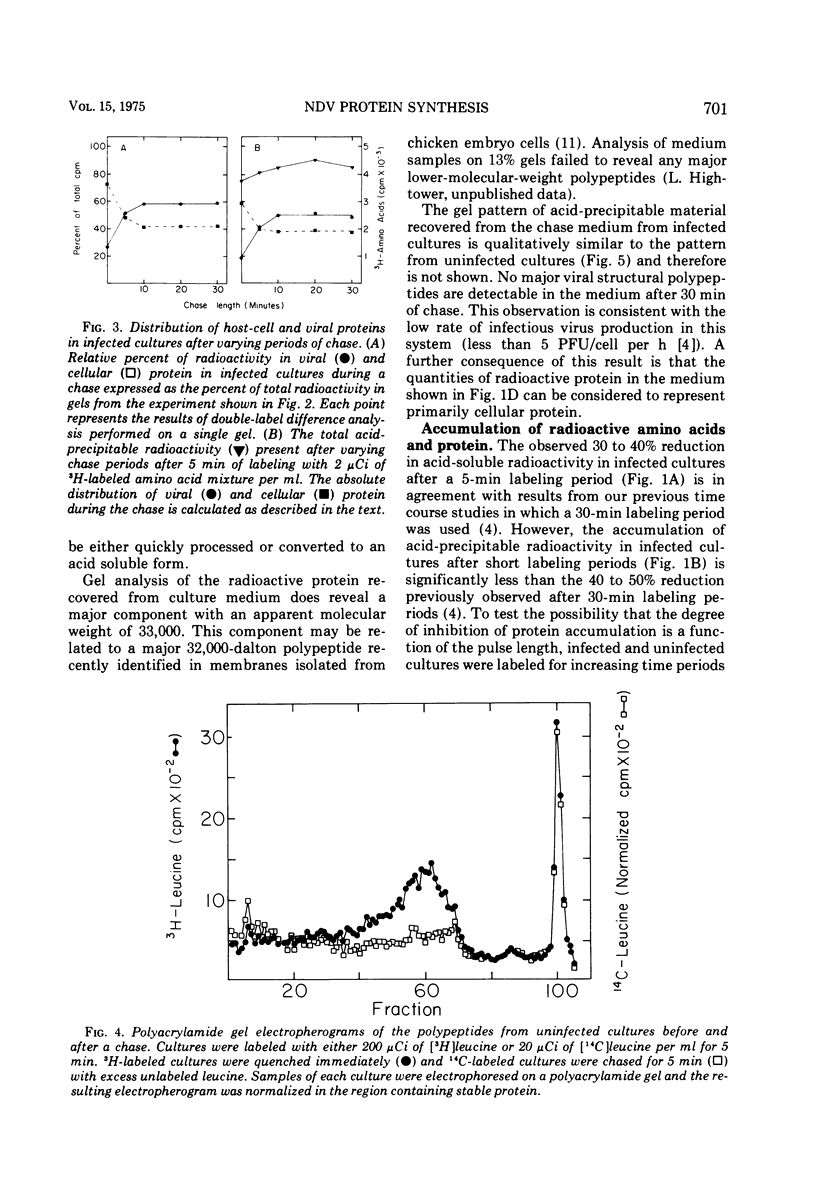
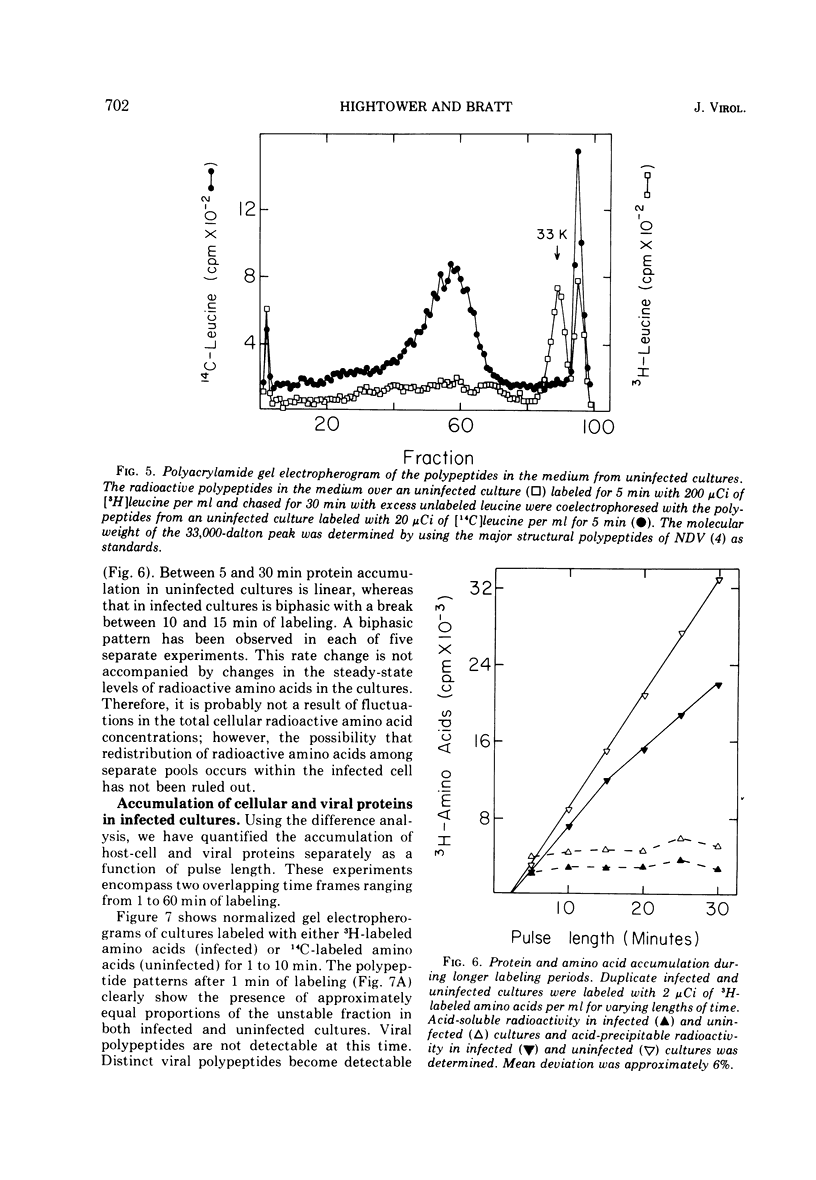
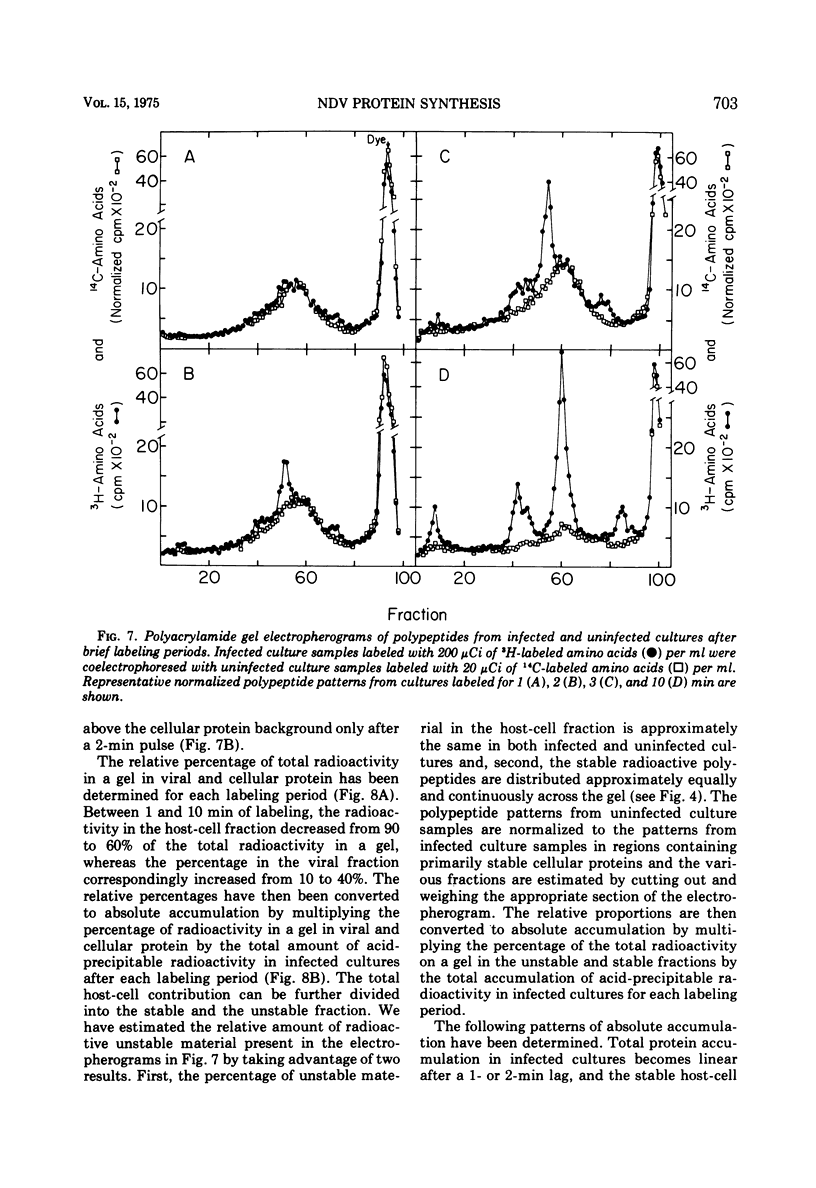
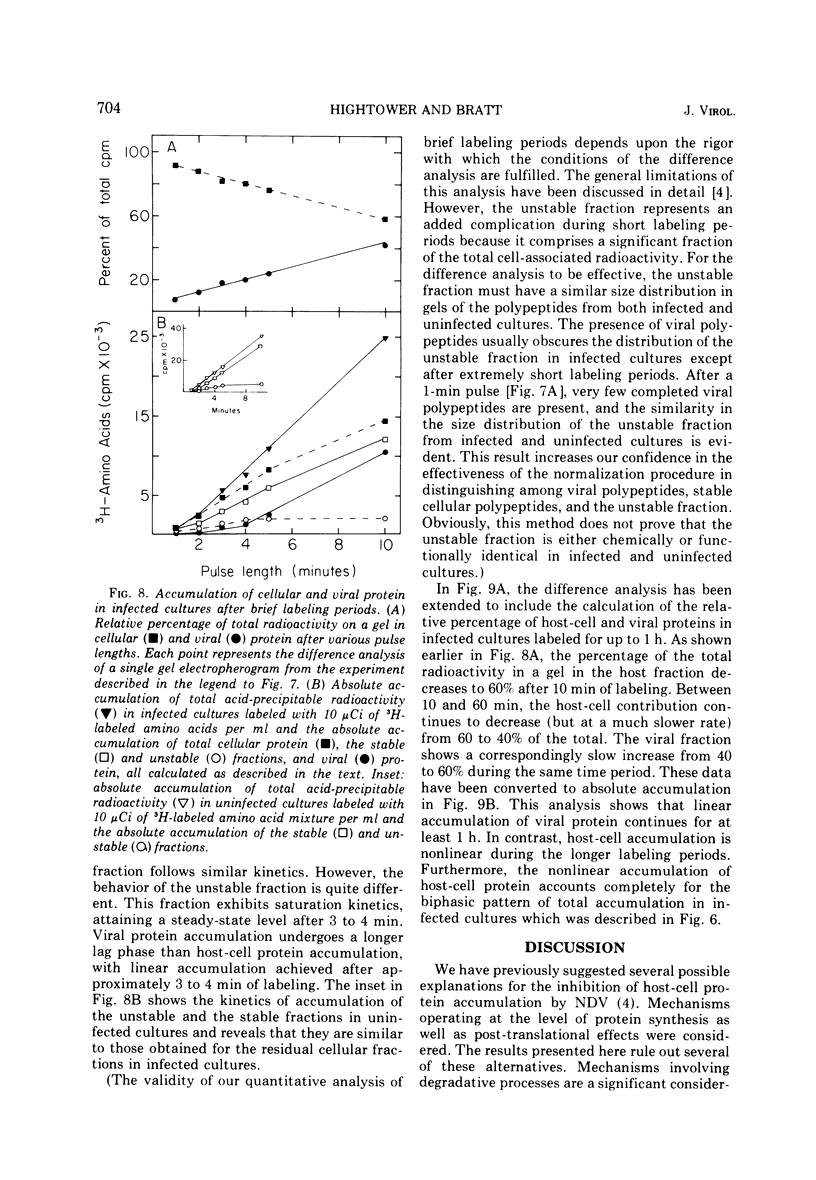
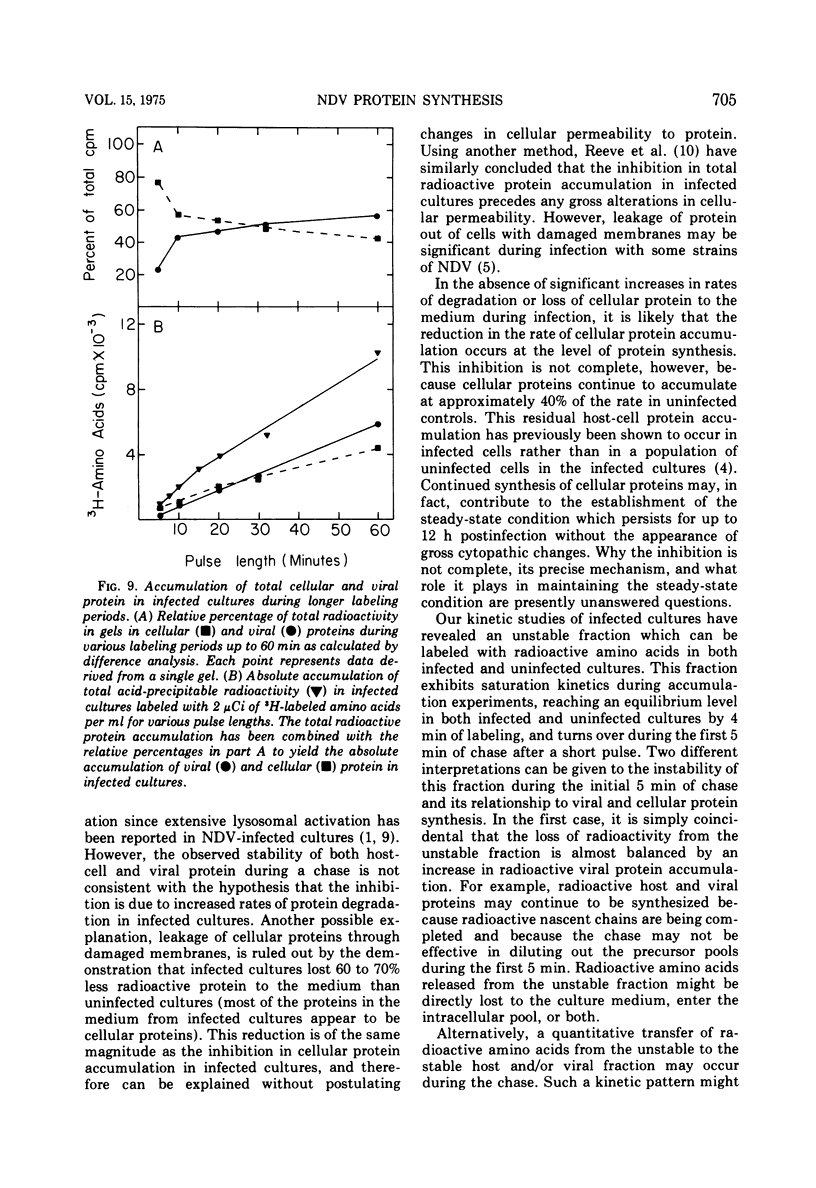
Pulse-chase experiments and studies on the effects of varying pulse lenghts on radioactive amino acid and protein accumulation have been carried out to evaluate several possible mechanisms for the inhibition in cellular protein accumulation during infection of chicken embryo cells by Newcastle disease virus. The inhibition is probably at the level of synthesis of cellular protein since no evidence for either increased degradation of protein or alterations in cellular permeability to protein was found in infected cultures. The magnitude of the reduction in the rate of cellular protein accumulation and consequently total protein accumulation depend upon the length of the radioisotopic labeling period. In contrast, the rate of viral protein accumulation is independent of the length of the labeling period. A double-label difference analysis of polyacrylamide gels was used in all of the kinetics studies to distinguish between viral and cellular protein accumulation. An unstable fraction which could be labeled with radioactive amino acids was detected in both infected and uninfected cultures. This material migrated mainly in the 50,000- to 60,000-dalton region of polyacrylamide gels, exhibited saturation kinetics during accumulation studies, and turned over rapidly during a chase. The relative amount of this fraction was not affected by infection. Gel analysis of the radioactive protein recovered from the medium from both infected and uninfected cultures revealed a major component with an apparent molecular weight of 33,000. None of the major viral polypeptides could be detected in the medium after a 30-min chase following a brief labeling period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman J., Wilson D. E. Newcastle disease virus-induced plasma membrane damage. J Gen Virol. 1974 Jul;24(1):101–113. doi: 10.1099/0022-1317-24-1-101. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Lomniczi B., Burke D. C. The effect of infection with different strains of Newcastle disease virus on cellular RNA and protein synthesis. J Gen Virol. 1972 Jan;14(1):99–101. doi: 10.1099/0022-1317-14-1-99. [DOI] [PubMed] [Google Scholar]
- Poste G. Virus-induced polykaryocytosis and the mechanism of cell fusion. Adv Virus Res. 1970;16:303–356. doi: 10.1016/s0065-3527(08)60026-3. [DOI] [PubMed] [Google Scholar]
- Reeve P., Alexander D. J., Pope G., Poste G. Studies on the cytopathic effects of Newcastle disease virus: metabolic requirements. J Gen Virol. 1971 Apr;11(1):25–34. doi: 10.1099/0022-1317-11-1-25. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELOCK E. F., TAMM I. Biochemical basis for alterations in structure and function of HeLa cells infected with Newcastle disease virus. J Exp Med. 1961 Nov 1;114:617–632. doi: 10.1084/jem.114.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. E. Inhibition of host-cell protein and ribonucleic acid synthesis by Newcastle disease virus. J Virol. 1968 Jan;2(1):1–6. doi: 10.1128/jvi.2.1.1-6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



