Abstract
Context:
Surgical outcomes of vitrectomy for idiopathic macular hole using a “heavy” Brilliant Blue G (HBBG) solution for staining and removal of the internal limiting membrane (ILM).
Settings and Design:
Prospective interventional case series conducted in a tertiary eye care hospital.
Materials and Methods:
Nineteen patients (20 eyes) with idiopathic macular hole were enrolled to undergo vitrectomy with ILM peeling using HBBG. BBG dye was made heavy by mixing with 10% dextrose normal saline (DNS) solution in 2:1 ratio. The adequacy of ILM staining was noted intraoperatively. The closure rates of macular hole and visual improvement were recorded. Patients were followed up postoperatively on day 1, week 1, and subsequently at 1, 3, and 6 months, and every 6th month thereafter.
Statistical Analysis:
Wilcoxon signed-rank test was used; P < 0.05 was considered significant.
Results:
Preoperative best-corrected visual acuity (BCVA) ranged from 20/1000 to 20/63 (median: 20/100). Intraoperatively, the ILM stained very well in all eyes, and was easily removed. All macular holes closed postoperatively. The mean follow-up was 6.15 ± 2 months (range: 4-10; median: 6 months). Final BCVA ranged from 20/20 to 20/80 (median: 20/40), amounting to a significant visual improvement (P = 0.0001). BCVA improved by 1-8 Snellen lines in 19 eyes (95%); 16 eyes (80%) improved by ≥2 lines; 13 eyes (65%) achieved a final BCVA of 20/40 or better.
Conclusions:
Addition of 10% DNS to BBG dye allowed good ILM staining with less dye during macular hole surgery, and provided excellent anatomic and visual outcomes.
Keywords: Brilliant Blue G, dextrose normal saline 10%, internal limiting membrane, macular hole
Brilliant Blue G (BBG) is fast emerging as a forerunner among the currently available dyes used for the staining of the internal limiting membrane (ILM) during vitrectomy for several macular pathologies.[1,2] At the same time, it is well known that any dye is potentially toxic to the retina, and therefore should be used in minimum amount and concentration, for the shortest possible time, and with the most favorable pH and osmolality.[3,4] Recently, attempts have been made to modify two popular dyes, Trypan Blue[5,6] and BBG,[7] using 10% dextrose and heavy water (deuterium oxide) respectively, to make the solution denser than vitreous and commonly used intraocular infusion fluids, i.e. to make the dye “heavy.” This addition serves two purposes. The macular contact time of the dye is prolonged, which directly sediments on the posterior pole rather than dispersing in the vitreous cavity. At the same time, less dye is used, in terms of both volume and concentration. We used 10% dextrose normal saline (DNS) to produce heavy BBG (HBBG) and used this heavy solution to stain ILM during macular hole surgeries.
Materials and Methods
This pilot study was conducted with the approval of the Institutional Review Board and in accordance with the Tenets of Helsinki Declaration. All patients were explained about the safety and experimental nature of the used dye, and informed consents were obtained preoperatively. Twenty eyes of 19 consecutive surgical inpatients (14 women) were included in this prospective interventional case series between March 2010 and December 2010. The inclusion criteria consisted of senile idiopathic macular holes, with visual symptoms solely attributed to macular hole, and optical coherence tomography (OCT) documentation of a full-thickness macular hole. To avoid confounding variables in outcome assessment with a new dye, we maintained consistency in case selection by excluding secondary macular holes (e.g. traumatic) and other indications of ILM peeling (e.g. vitreomacular traction, diabetic macular edema, etc). All patients underwent a detailed clinical evaluation including best-corrected visual acuity (BCVA) measurements, slit-lamp biomicroscopy, and indirect ophthalmoscopy.
All surgeries were performed by two experienced vitreoretinal surgeons (DS and KBN). Simultaneous phacoemulsification with posterior chamber intraocular lens implantation was done if visually significant cataract was present. All eyes underwent a 23-gauge three-port pars plana vitrectomy with triamcinolone-assisted removal of the posterior hyaloid interface. Visible epiretinal membranes (ERMs) were peeled with an intraocular forceps. ILM was stained with HBBG, which was prepared by mixing BBG 0.05% dye (Ocublue Plus, Aurolab, Madurai, India) with 10% DNS in the ratio 2:1. This resulted in a final concentration of dextrose 3.33% and BBG 0.033%. The final osmolality and pH of the solution were 332 mOsm/kg and 7.85, respectively. The absolute densities of HBBG, conventional BBG, and Ringer's Lactate used for infusion were 0.9944, 0.9867, and 1.0145 g/ml, respectively, at room temperature. The relative densities of HBBG and conventional BBG in Ringer's Lactate solution were 0.9801 and 0.9726, respectively. This small increase in density resulted in significantly greater gravitation of HBBG in Ringer's Lactate solution [Fig. 1]. Intraoperatively, HBBG was injected slowly so that it trickled down in a continuous stream and settled over the macula [Fig. 2a]. The volume injected was 0.1-0.15 ml. The dye was allowed to stay for 1-2 min with plugged infusion and sclerotomies, and was aspirated thereafter. The staining intensity was graded by the two surgeons on a scale of 1-4 (+ to ++++), where + indicated minimally adequate staining (similar to Trypan Blue) and ++++ indicated optimum staining comparable to indocyanine green (ICG) dye [Fig. 2b]; both surgeons had previously used Trypan Blue, conventional BBG, and ICG dyes. An edge of ILM was lifted with Tano's membrane scraper and peeled with end-gripping forceps [Fig. 2c]. This was followed by fluid-air exchange and injection of perfluoropropane (C3F8 16%; 12 patients) or sulfur hexafluoride (SF6 20%; 8 patients). All patients were asked to maintain a face-down position for 1 week after surgery.
Figure 1.
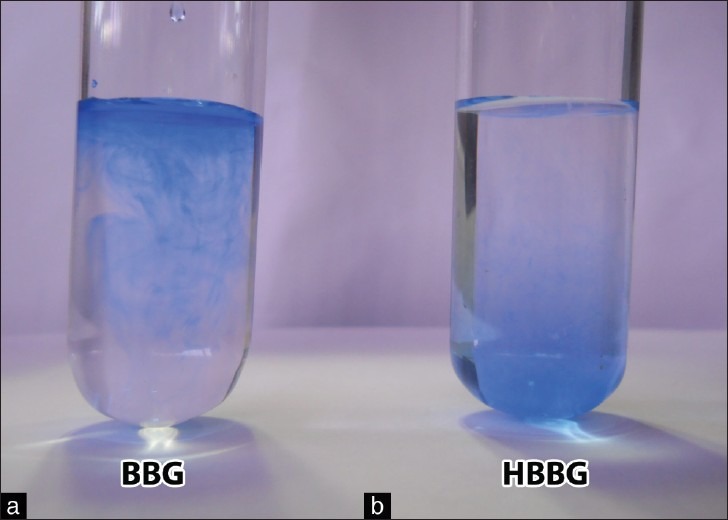
Note the diffuse spread of the conventional Brilliant Blue dye (BBG, 0.05%) immediately after introduction into a test tube containing Ringer's Lactate solution (a). Dilution of conventional BBG with 10% dextrose normal saline in 2:1 ratio makes the dye heavy (HBBG, 0.033%), which sediments to the bottom of the test tube (b)
Figure 2.
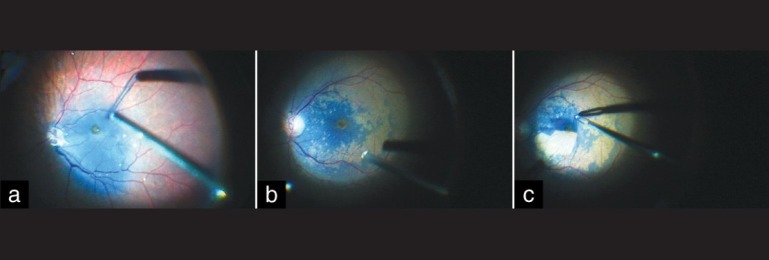
Intraoperative steps of internal limiting membrane peeling (ILM) using heavy Brilliant Blue G (HBBG). (a) HBBG flows out from the injection cannula in an uninterrupted stream, sedimenting on the posterior pole. There is triamcinolone residue on the optic nerve head, from the just concluded posterior vitreous interface removal. (b) Note the excellent staining of ILM obtained with HBBG (not the same eye as in Figure a). The temporal macula did not stain uniformly due to presence of epiretinal membrane. (c) Maculorrhexis in progress: A large flap of ILM has been fashioned with end-gripping forceps
Patients were examined postoperatively on day 1, week 1, and subsequently at 1, 3, and 6 months, and every 6th month thereafter. Data regarding intraoperative and postoperative complications, best-corrected visual acuity (BCVA), and closure of the macular hole were obtained at each visit by fundus photography and OCT. Anatomic and functional results at the final visit were used for this analysis.
Statistical methods
Statistical analysis was done with STATA software, version 8.1 (StataCorp, College Station, TX, USA). Wilcoxon signed-rank test was used for statistical analysis. P-value less than 0.05 was considered statistically significant. BCVA was converted to logarithm of minimal angle of resolution (logMAR) for analysis.
Results
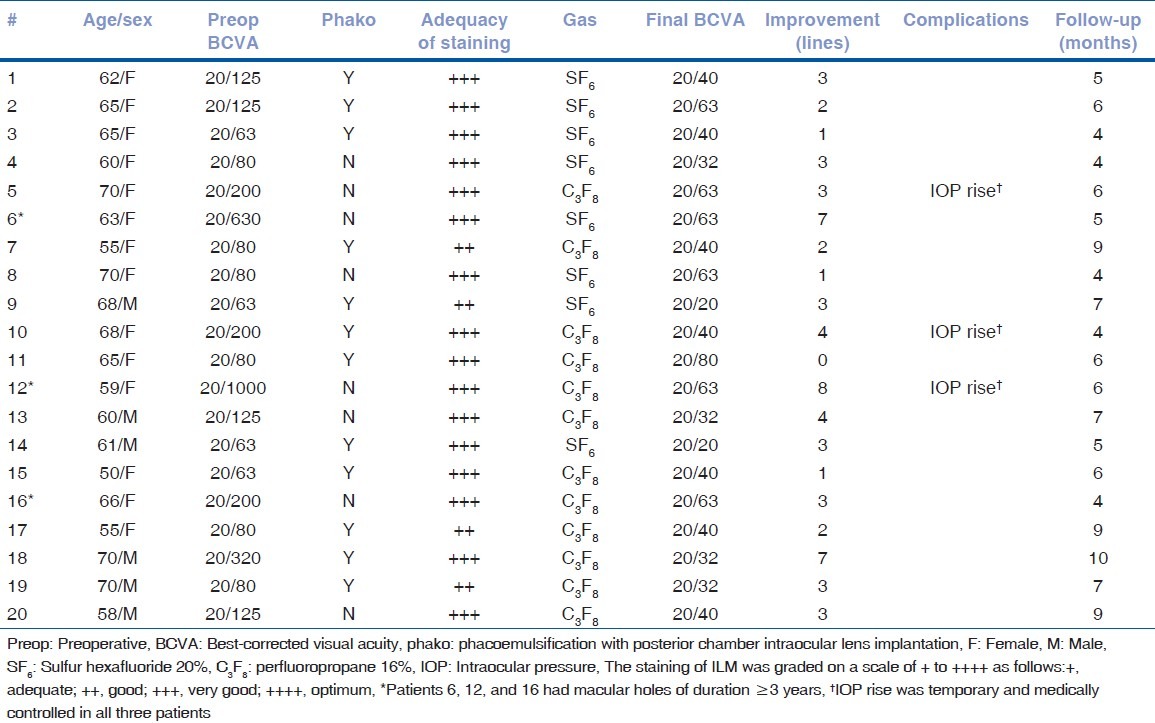
Twenty eyes of 19 patients were included in the study. The mean age was 63 ± 5.7 years (range: 50-70 years). All macular holes were of stages 3 to 4. Twelve patients underwent combined phacoemulsification with vitrectomy; three were already pseudophakic. Preoperative BCVA ranged from 20/1000 to 20/63 (median: 20/100). Mean and median logMAR BCVA values were 0.803 ± 0.35 and 0.7, respectively. Intraoperatively, HBBG was observed to flow in a continuous stream from the injection cannula. The staining intensity was graded by the two surgeons as +++ in 16 eyes and ++ in 4 eyes. Subjectively, both surgeons concurred that the staining intensity was similar or better than that obtained with conventional BBG dye, but marginally inferior to the staining with ICG. An ERM was identified in three patients by negative or patchy staining of macula; ERM was removed along with the ILM [Fig. 2b]. All patients were followed up for 4-10 months (mean follow-up: 6.15 ± 2 months; median: 6 months). During the review visits, no patient developed retinal detachment or other major complications; three patients had a transient rise in intraocular pressure, which was managed with topical medications. Macular hole closure was achieved in all the cases. Postoperative BCVA ranged from 20/20 to 20/80 at the final follow-up. Mean and median postoperative logMAR BCVA values were 0.315 ± 0.16 and 0.3, respectively (Snellen equivalent: 20/40). The improvement in the mean postoperative BCVA was statistically significant (P = 0.0001). Visual acuity improved by at least 1 line in 19 eyes (95%) (range: 1-8 lines). An improvement of 2 or more Snellen lines was achieved in 16 eyes (80%). Thirteen (65%) achieved a final BCVA of 20/40 or better. One patient whose BCVA failed to improve had posterior capsular opacification, for which laser capsulotomy was not performed till the duration of the study. The postoperative anatomic and functional outcomes are summarized in Table 1.
Table 1.
Macular hole surgery with heavy Brilliant Blue G: Demographics and outcomes
Discussion
Enaida et al.[8] in their pioneering clinical trial used 0.5 ml of BBG, 0.025% dye (0.25 mg/ml) for staining ILM, followed by immediate washout. However, similar consistent staining is not universally reported with these parameters; others observed inadequate staining in nearly a third of eyes.[9] We and other investigators have used BBG concentrations of 0.5 mg/ml and 0.83 mg/ml, with good anatomic and functional outcomes.[10–12] Specifically, we have previously reported good results with 0.5 mg/ml concentration of BBG dye allowed to stay for 2-3 min in the vitreous cavity.[10] However, subsequent reports of successful use of “heavy” Trypan Blue solutions for ERM and macular hole surgery[5,6] inspired us to minimize the volume and concentration of BBG dye in a similar fashion, without compromising on the quality of staining. We found that injection of only a much smaller volume of HBBG (about 0.1 ml: a fifth of the volume used by Enaida et al.)[8] sufficed in spite of the 33% dilution because the dye streamed down and accumulated directly on the macula. Even when the dye dispersed due to inadvertent forceful injection on occasions, it subsequently gravitated to the posterior pole. We also found that waiting for 1-2 min was enough to allow good staining of ILM. We used a 2:1 mixture of the dye with 10% dextrose, unlike previous reports which diluted Trypan Blue dye 1:1.[5,6] We arrived at this ratio to optimize osmolarity and pH, which may be as or more important than dye nature, concentration, and volume.[4] One should not be tempted to use yet higher concentrations of dextrose to make the dye still heavier. Glucose solutions only with concentration of 4.4% or less were found to be non-toxic to the retinal tissue in a recent experimental study; significant electrophysiological toxicity was observed with glucose concentration of 8% and above.[13] In spite of the reported safety in clinical experience,[5,6] others caution against Trypan Blue solution in 5-10% glucose as it is likely to cause retinal acid-base disturbance.[14] Though several experimental and clinical studies have attested to the safety of 5% glucose solution with BBG, ICG, and endogenous blood,[4,13–15] we used a safer concentration of 3.33% dextrose in the final DNS solution for intravitreal injection. Both surgeons found the staining of ILM to be marginally better with HBBG as compared to their recently reported experience with undiluted 0.5 mg/ml BBG, used for 2-3 min.[10]
Haritoglou et al.[7] have reported the use of heavy water to prepare HBBG for ILM staining in eight patients with macular hole or epimacular membrane. We endorse the results of their pilot study in a larger case series with consistent case selection and a longer follow-up, and propose a much more readily available and inexpensive solvent (10% DNS) to obtain the same results. Recently, a ready-to-use modified BBG dye has been marketed; the dye is made denser by addition of 4% polyethylene glycol and is claimed to similarly gravitate to the macula.[16] We were unable to find any published clinical experience on the safety or efficacy of this dye formulation, though its safety has been shown on retinal pigment epithelial cell lines.[17] We achieved good visual and anatomic outcomes in this study in spite of reducing the volume, concentration, and duration of application of BBG dye. Our anatomic (hole closure rates) and visual outcomes (percent improved, and number with BCVA of 20/40 or better) compared favorably with some of the recent studies using Trypan Blue (conventional and heavy), BBG, ICG, autologous blood, or triamcinolone.[6,9–11,15] We attempted to adhere to the basic tenets of optimal pH, osmolality, and lowest working concentration of dye. Applying the logic “the smaller the amount of intraocular dye, the greater the safety of application,” diluting BBG with 10% DNS may be a safer and more efficient way of using the dye. However, only a comparative trial can settle the question of whether the reported outcomes with heavy BBG in this pilot study are significantly superior to those obtained with the conventional BBG dye.
Acknowledgement
The authors acknowledge the support of TIFAC-CORE, a government of India initiative, for this project. The authors also thank Mr Ventakesh Kannan, Division Head, Aurolab pharmacy, for the biochemical analyses.
Footnotes
Source of Support: TIFAC-CORE, a government of India.
Conflict of Interest: None declared.
References
- 1.Rodrigues EB, Penha FM, de Paula Fiod Costa E, Maia M, Dib E, Moraes M, Jr, et al. Ability of new vital dyes to stain intraocular membranes and tissues in ocular surgery. Am J Ophthalmol. 2010;149:265–77. doi: 10.1016/j.ajo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: Principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332–40. doi: 10.1016/j.ajo.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Morales MC, Freire V, Asumendi A, Araiz J, Herrera I, Castiella G, et al. Comparative effects of six intraocular vital dyes on retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:6018–29. doi: 10.1167/iovs.09-4916. [DOI] [PubMed] [Google Scholar]
- 4.Penha FM, Maia M, Eid Farah M, Príncipe AH, Freymüller EH, Maia A, et al. Effects of subretinal injections of indocyanine green, trypan blue and glucose in rabbit eyes. Ophthalmology. 2007;114:899–908. doi: 10.1016/j.ophtha.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Lesnik Oberstein SY, Mura M, Tan SH, de Smet MD. Heavy trypan blue staining of epiretinal membranes: An alternative to infracyanine green. Br J Ophthalmol. 2007;91:955–7. doi: 10.1136/bjo.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesnik Oberstein SY, de Smet MD. Use of heavy Trypan blue in macular hole surgery. Eye (Lond) 2010;24:1177–81. doi: 10.1038/eye.2010.3. [DOI] [PubMed] [Google Scholar]
- 7.Haritoglou C, Schumann RG, Kampik A, Gandorfer A. Heavy brilliant blue G for internal limiting membrane staining. Retina. 2011;31:405–7. doi: 10.1097/IAE.0b013e3181feccd7. [DOI] [PubMed] [Google Scholar]
- 8.Enaida H, Hisatomi T, Hata Y, Ueno A, Goto Y, Yamada T, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26:631–6. doi: 10.1097/01.iae.0000236469.71443.aa. [DOI] [PubMed] [Google Scholar]
- 9.Henrich PB, Haritoglou C, Meyer P, Ferreira PR, Schötzau A, Katamay R, et al. Anatomical and functional outcome in brilliant blue G assisted chromovitrectomy. Acta Ophthalmol. 2010;88:588–93. doi: 10.1111/j.1755-3768.2008.01477.x. [DOI] [PubMed] [Google Scholar]
- 10.Shukla D, Kalliath J, Neelakantan N, Naresh KB, Ramasamy K. A comparison of brilliant blue G, trypan blue and indocyanine green dyes to assist internal limiting membrane peeling during macular hole surgery. Retina. 2011;31:2021–25. doi: 10.1097/IAE.0b013e318213618c. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Gogia V, Shah VM, Nag TC. Comparative evaluation of anatomical and functional outcomes using brilliant blue G versus triamcinolone assisted ILM peeling in macular hole surgery in Indian population. Graefes Arch Clin Exp Ophthalmol. 2011;249:987–95. doi: 10.1007/s00417-010-1609-1. [DOI] [PubMed] [Google Scholar]
- 12.Cervera E, Díaz-Llopis M, Salom D, Udaondo P. High dose intravitreal brilliant blue G. Arch Soc Esp Oftalmol. 2007;82:473. doi: 10.4321/s0365-66912007000800004. [DOI] [PubMed] [Google Scholar]
- 13.Januschowski K, Mueller S, Spitzer MS, Lueke M, Bartz-Schmidt KU, Szurman P. The effects of the intraocular dye brilliant blue G (BBG) mixed with varying concentrations of glucose on retinal function in an isolated perfused vertebrate retina. Graefes Arch Clin Exp Ophthalmol. 2011;249:483–9. doi: 10.1007/s00417-010-1508-5. [DOI] [PubMed] [Google Scholar]
- 14.Costa Ede P, Rodrigues EB, Farah ME, Dib E, Penha F, Magalhães O, Jr, et al. Vital dyes and light sources for chromovitrectomy: Comparative assessment of osmolarity, pH, and spectrophotometry. Invest Ophthalmol Vis Sci. 2009;50:385–91. doi: 10.1167/iovs.08-2285. [DOI] [PubMed] [Google Scholar]
- 15.Lai CC, Wang NK, Chuang LH, Wu WC, Yeung L, Hwang YS, et al. Blood clump-assisted vitrectomy and internal limiting membrane peeling for macular hole repair. Retina. 2011;31:2014–20. doi: 10.1097/IAE.0b013e31821504a5. [DOI] [PubMed] [Google Scholar]
- 16.Zuidland: Dutch Ophthalmic Research Centre International BV; [Last accessed 2012 Jan 20]. Dorc. nl[internet] [updated 2011 Dec 15]. Available from: http://www.dorc.nl/products.php?group=18143,18208,18447 . [Google Scholar]
- 17.Awad D, Schrader I, Bartok M, Mohr A, Gabel D. Comparative toxicology of trypan blue, brilliant blue G, and their combination together with polyethylene glycol on human pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:4085–90. doi: 10.1167/iovs.10-6336. [DOI] [PubMed] [Google Scholar]


