Abstract
GABAergic and glycinergic inhibition play key roles in the function of spinal motor pathways. However, there is little direct information on the extent to which inhibition controls the activity of spinal neurons during behavior or the relative effectiveness of GABA and glycine on cell activity under normal conditions. These issues were investigated in three macaque monkeys trained to perform voluntary ramp-and-hold wrist movements and grip. Pipettes with an extracellular recording electrode and iontophoresis barrels were used to eject GABA, glycine, and/or their respective antagonists, bicuculline and strychnine, as the activity of single neurons was recorded in the C6–T1 spinal segments during hand movements. The firing rate of the vast majority of neurons decreased when an inhibitory neurotransmitter was ejected from the electrode, suggesting that most movement-related spinal neurons are sensitive to both GABA and glycine. Most movement-related neurons exhibited increased activity during iontophoresis of an antagonist, suggesting that both GABAergic and glycinergic inhibition actively regulate the majority of spinal neurons during movement. These conclusions were supported by the responses of neurons tested with both agonists or both antagonists. Bicuculline and strychnine produced the largest increases in firing rate during dynamic movements (ramp phase), smaller increases during maintained torque/force (hold phase), and the smallest increase during the rest period. Since excitatory inputs also tend to increase progressively from rest to static to dynamic muscle contractions, this result is consistent with coupled excitatory and inhibitory inputs to spinal neurons during movement.
Keywords: interneurons, inhibition, wrist, iontophoresis
the critical roles played by GABAergic and glycinergic inhibition in regulating neuronal excitability in spinal motor pathways have been investigated under a variety of conditions. Early work demonstrated the effects of GABA and glycine on spinal neurons in anesthetized animals (Curtis 1974; Werman et al. 1968). More recently, studies in spinal slices, isolated cord preparations, and decerebrate cats have extensively characterized inhibitory control of locomotor circuits (Kiehn 2006; McCrea and Rybak 2008). However, we know of only one study that examined spinal inhibition during normal behavior. Taepavarapruk et al. (2002) characterized the state-dependent effects of GABA and glycine on the activity of spinocerebellar neurons during wakefulness and sleep in unanesthetized cats. The extent to which inhibition contributes to movement-related activity under natural conditions is unknown.
The functional differences between spinal GABAergic and glycinergic systems are also not clearly understood. Several lines of evidence suggest that the two neurotransmitters mediate different functions (e.g., Hinckley et al. 2005; Takazawa and MacDermott 2010) and that interactions between them may provide important regulation of spinal excitability (e.g., Lu et al. 2008; Raiteri et al. 1992). For example, in the isolated spinal cord of neonatal rats, application of either GABA or glycine receptor antagonists transformed the alternating pattern of left-right, flexor-extensor motor activity to a synchronous activity (Cowley and Schmidt 1995), yet it has been demonstrated that glycinergic neurons provide most of the commissural inhibition to hindlimb motoneurons (Kjaerulff and Kiehn 1997). These issues have not been studied during normal behavior.
The current experiments were undertaken to elucidate further the roles of inhibitory systems in the spinal cord. The effects on neuronal activity of exogenously applied glycine and its antagonist, strychnine, and of GABA and the GABAA receptor antagonist bicuculline, were characterized during voluntary wrist movements in behaving monkeys.
MATERIALS AND METHODS
The activity of single spinal neurons was recorded in three male macaque monkeys (Macaca nemestrina) performing trained hand movements. The experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington.
Behavioral training and animal preparation.
The monkeys sat upright in a chair and grasped the handle of an experimental apparatus with the right hand. The torques about the flexion-extension, radial-ulnar, and pronation-supination axes of the wrist and the grip force exerted by the hand were measured by transducers mounted on the device. Monkeys were trained for 4–6 mo to produce voluntary wrist torque and grip force to position a cursor into target boxes displayed on a screen in front of the animal (for details of the task, see Fig. 3 of Perlmutter 2009). Trials started when the animal positioned the cursor in a central target box by relaxing the wrist. A peripheral target then appeared on the screen. The monkey moved the cursor into the box by generating ramp-and-hold grip force or wrist torque in a direction specified by the attributes of the box (see Figs. 1 and 2, torque traces). The speed with which the monkeys acquired the targets was not controlled; the ramp phase of the task, during which dynamic torque/force was generated, typically lasted ∼0.5 s. The hold phase of the task, in which the monkey held the cursor in a target, was varied randomly between 1.1 and 1.5 s. After the hold criterion was met, the monkey moved the cursor back to a central target box by relaxing the wrist and then received an applesauce reward. Neuronal firing was not studied when the monkey was not engaged in the behavioral task (i.e., all targets were off for an extended period).
Fig. 3.
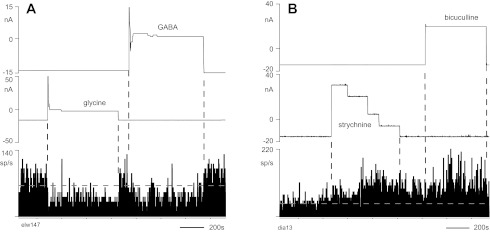
Responses of 2 neurons to both GABAergic and glycinergic drugs. Iontophoresis currents for GABA and glycine (A) and for bicuculline and strychnine (B) are shown above firing rate histograms for neurons in C6 and C7, respectively. Horizontal dashed lines indicate mean firing rate during the period before drug ejection. The high variability in firing during periods of constant ejection current is due to the directionally tuned, task-related activity of the neurons; the monkey performed hand movements in different directions throughout the ∼30-min period shown. Note that firing rate decreased after strychnine ejection but had not yet returned to the control level before bicuculline ejection was started.
Fig. 1.
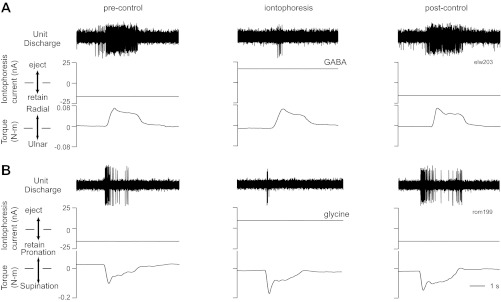
Example responses of 2 movement-related spinal neurons to iontophoresis of inhibitory neurotransmitters. A: activity of a C7 neuron for single trials of radial deviation in the pre-control period (left), during iontophoresis of GABA (center), and several seconds after iontophoresis was stopped (right). B: response of a C8 neuron to ejection of glycine during wrist supination (format as in A). Both A and B show the extracellularly recorded signals (top), iontophoresis currents (middle), and torque profiles during the trial (bottom).
Fig. 2.
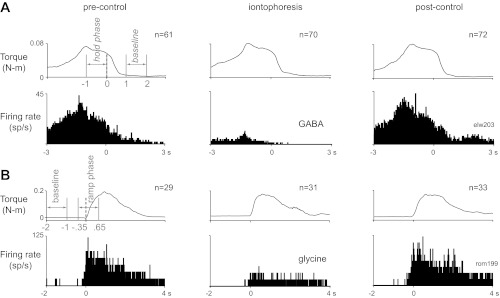
Average responses of the 2 neurons shown in Fig. 1. A: average torque traces (top) and peri-event histograms (bottom) of the discharge of the neuron shown in Fig. 1A in the pre-control period (left), during iontophoresis of GABA (center), and several seconds after iontophoresis was stopped (right); traces are aligned on the end of the hold phase. Data from movements in all directions are combined. B: average responses of the neuron shown in Fig. 1B to iontophoresis of glycine, aligned on torque onset (format as in A). The baseline and hold phases (A) and baseline and ramp phases (B) for calculation of hold- and ramp-related firing, respectively, are indicated in gray on the pre-control torque traces. n, No. of trials.
After training was completed, aseptic surgeries were performed to implant head stabilization lugs and a spinal recording chamber over the C5–C7 vertebrae. All surgeries were performed with the animal under 1–1.5% isoflurane or sevoflurane anesthesia. Antibiotics and analgesics were given postoperatively. Details of the implant procedures were reported previously (Perlmutter et al. 1998).
Iontophoresis electrodes.
Drugs were ejected near recorded neurons using custom-made, seven-barreled glass micropipettes, made according to the method of Haidarliu et al. (1995). An electrode was constructed from an etched tungsten rod (diameter 200 μm, length 10 cm; A-M Systems) and seven glass capillary tubes with filaments (outer diameter 1.0 mm, inner diameter 0.58 mm, length 10 cm; A-M Systems). The seven capillaries were glued together with six tubes surrounding the seventh. The sharpened tungsten rod was back-fed into the central tube. The pipettes were pulled together by a vertical microelectrode puller (PE-2; Narishige, Tokyo, Japan). Finally, the tip of the electrode was ground until the impedance of the tungsten electrode ranged from 0.6 to 1.3 MΩ and the tip was conical in shape. The overall diameter of the tungsten rod and the glass pipettes near the exposed tip was 10–25 μm. The tungsten provided enough strength for the electrode to penetrate the dura mater several times without damage, and the conical shape of the tip helped prevent clogging of the lumens of the pipettes.
Extracellular signals were recorded with a high signal-to-noise ratio from the tungsten electrode. The six surrounding glass barrels were filled with one or more of the following drugs (Sigma): GABA (0.5 M, pH 3.5), bicuculline methiodide (20 mM, pH 4.0), glycine (0.5 M, pH 8.0), strychnine hydrochloride (10 mM, pH 5.5), and sodium chloride (0.5 M, pH 3.5; 20 mM, pH 4.0; 0.5 M, pH 8.0; and 10 mM, pH 5.5 were used separately as controls for GABA, bicuculline, glycine, and strychnine, respectively). Chlorinated silver wires connected the barrels to an intracellular amplifier, which was used to generate and monitor iontophoresis currents.
Recording procedure.
During recording, the head and vertebral implants were secured to the primate chair with flexible attachments. A seven-barreled electrode was advanced through the intact dura into the C6–T1 spinal segments. Activity of spinal neurons was recorded through the central tungsten electrode while the monkey performed the behavioral task for 2–4 h per day. The amplified and bandpass-filtered (0.3–6 kHz) signals were fed to a template-matching discriminator (MSD; Alpha-Omega Engineering) to isolate the extracellular activity of 1–2 units. Spikes (sampled at 40 kHz), wrist torque and grip force (500 Hz), and iontophoresis current were digitized using a custom-designed data acquisition system.
Isolated neurons that exhibited changes in firing rate during performance of the task were studied. The input-output connectivity of the cells was not investigated. Activity of each neuron was recorded for several minutes while the monkey generated wrist torques and grip forces before ejection of drugs. During this period, a retaining current of −15 nA (DC) was applied to each drug barrel to prevent unwanted diffusion from the pipettes. Positive currents of 20–50 nA were then applied to one or more drug barrels for iontophoresis. Current was adjusted to produce a change in movement-related firing rate without silencing or saturating the cell and was adjusted during recording, if necessary, to maintain approximately the same change in rate. This assessment was subjective, since firing rate was continually being modulated in relation to the ongoing task.
Most neurons were studied during iontophoresis of one drug due to either loss of recording stability or prolonged recovery of firing after ejection of the antagonist. Some neurons were studied with both GABAergic and glycinergic drugs.
The response of some neurons to iontophoresis of sodium chloride was also examined to test the possibility that neurons responded to the ejection current itself, rather than the drugs. No behavioral changes and no signs that the monkey was aware of the drug ejection were observed during iontophoresis.
Data analysis.
The effects of iontophoretically applied drugs were examined separately during the baseline (relaxed wrist), ramp (dynamic torque), and hold (static torque) phases of the task. Data for movements in all directions were combined in histograms of activity across trials. For the ramp phase, neuronal firing was aligned on the onset of the change in torque/force, determined by a slope detection algorithm. Baseline firing was computed as the mean discharge rate during the period 1–2 s before movement onset. Ramp-related activity was taken as the mean rate from 0.35 s before until 0.65 s after the torque/force onset (see Fig. 2B, torque trace). For the hold phase, neuronal activity was aligned on the end of the hold period. Baseline discharge was computed as the mean rate between 1 and 2 s after the hold, when the wrist was relaxed. Hold-related activity was taken as the mean rate for the period 1 s before the end of the hold (see Fig. 2A, torque trace). Task-related neurons were identified by a significant change in firing rate during ramp or hold phases relative to baseline (Mann-Whitney, P < 0.05). Drug effects were identified by significant changes in firing rate between control and drug epochs (Mann-Whitney, P < 0.05).
The movement-related responses of the neurons were quantified with several measures. The mean changes in baseline firing and ramp- and hold-related activity during iontophoresis relative to the control period were compared. In addition, the ramp and hold response amplitudes and a dynamic index were determined for neurons studied with the antagonists. Ramp (hold) response amplitude was calculated as the mean firing rate in the ramp (hold) phase minus the mean baseline rate. The dynamic index was taken as the difference between the mean firing rates in the ramp phase and the hold phase. The significance of drug-evoked changes in firing rate was determined with a Wilcoxon signed-rank test; response amplitudes and dynamic indexes were compared with paired t-tests (the distributions of these measures were approximately normal).
RESULTS
Three hundred eighty-nine neurons in the cervical enlargement exhibited activity modulated during the wrist task. Neurons were studied during iontophoresis of one or more drugs.
Effects of GABA and glycine iontophoresis.
The activities of 127 and 105 spinal neurons were recorded during iontophoresis of GABA and glycine, respectively, including 30 neurons studied with both. Figures 1 and 2 show the responses of two movement-related neurons before, during, and after iontophoresis of one of the two drugs. The neurotransmitters inhibited the discharge of the cells, although their firing patterns during movement were qualitatively unchanged (Fig. 2). The discharge recovered to control levels after the iontophoresis was stopped (Figs. 1 and 2).
The vast majority of movement-related neurons were inhibited by iontophoresis of the neurotransmitters. GABA application reduced the firing rate of 115/127 (91%) tested neurons; glycine inhibited 96/105 (91%) neurons. Most neurons were inhibited during all task epochs (i.e., baseline, ramp, and hold phases). The remaining cells did not respond to the ejected agonists.
Although the majority of neurons were tested with only GABA or glycine, these results suggest that about 83% of movement-related neurons in the cervical enlargement are sensitive to both neurotransmitters (joint probability, assuming independence of effects). This conclusion is supported by the responses of 30 neurons tested with both drugs. Of these, 24 (80%) were inhibited by both GABA and glycine (Fig. 3A). Four cells were inhibited by only one drug, and two were unaffected by either.
The results of iontophoresis experiments can be confounded by responses to the applied current itself, independent of any effect of the ejected drug. To test this possibility, an electrode barrel was occasionally filled with sodium chloride at the same concentration as the drug used in that experimental session. Current passed through the sodium chloride barrel did not significantly change (P > 0.05) the firing rate of 18 neurons responsive to GABA and 20 different neurons responsive to glycine (Fig. 4A1). In addition, the changes in firing rate during drug and sodium ion ejection were significantly different in all cases (paired t-test, P < 0.001, Fig. 4B).
Fig. 4.
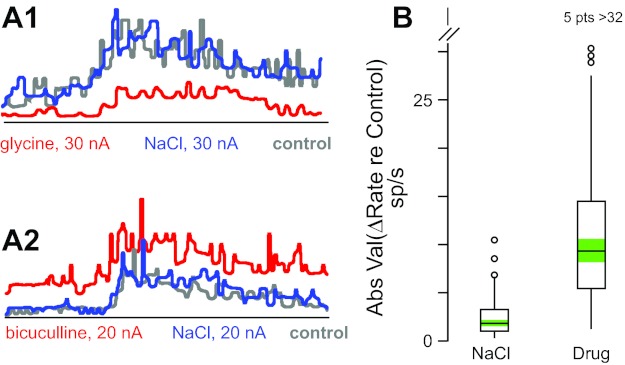
Iontophoresis current does not affect neuron firing rate. A: average peri-event histograms for 2 neurons (A1, A2) in the pre-control period (gray), during iontophoresis of sodium chloride (blue), and during iontophoresis of glycine (A1, red) or bicuculline (A2, red). Traces are aligned on torque onset; data from movements in all directions are combined. B: box plots of effects on firing rate of iontophoresis of sodium chloride (NaCl) and neurotransmitter agonists and antagonists (Drug: glycine, GABA, strychnine, and bicuculline effects combined); ordinate shows absolute value of difference between rate during iontophoresis and control rate [absolute value normalizes effects of agonists (decrease) and antagonists (increase)]; data include all responses (n = 143) during ramp and hold phases for all neurons (n = 65) that were tested with NaCl and had a significant response to at least 1 agonist or antagonist. Box plots show the interquartile range of the data (box), the median (horizontal line), 1.5 times the interquartile range (vertical lines), data points outside this range (circles; 5 data points with change in rate >32 spikes/s not shown for Drug plot), and the 95% confidence intervals around the median for each distribution (green shading). NaCl and Drug distributions are significantly different at the P < 0.001 level (paired t-test).
Effects of bicuculline and strychnine iontophoresis.
The sensitivity of neurons to exogenously applied GABA and glycine does not indicate the extent of inhibitory control during normal behavior. To investigate this issue, the antagonists bicuculline and strychnine were iontophoretically applied as the monkeys performed the wrist task. Bicuculline and strychnine reduced the inhibitory effects of GABA and glycine, respectively, for all neurons (n = 59) for which the two drugs were simultaneously applied (example for bicuculline in Fig. 5). The antagonists increased the discharge of cells in the baseline, ramp, and hold phases of the task (Figs. 6 and 7), although the overall relation of activity to torque was not qualitatively changed. The discharge of the neurons recovered to control levels after iontophoresis was stopped (Figs. 6 and 7).
Fig. 5.
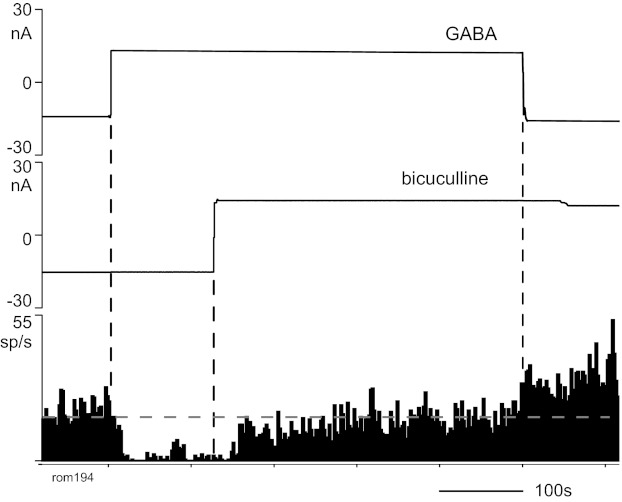
Response of a neuron to iontophoresis of GABA and bicuculline. The inhibition of cell activity by GABA was reduced by simultaneous ejection of bicuculline. The firing rate increased above baseline when the GABA ejection was then terminated. Format as in Fig. 3.
Fig. 6.
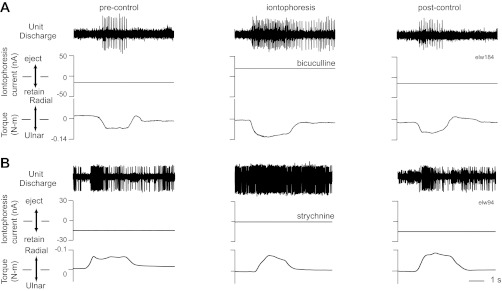
Example responses of 2 movement-related neurons to iontophoresis of antagonist drugs. A: activity of a C7 neuron for single trials of ulnar deviation before, during, and after iontophoresis of bicuculline. B: response of another C7 neuron to iontophoresis of strychnine for trials of radial deviation. Format as in Fig. 1.
Fig. 7.
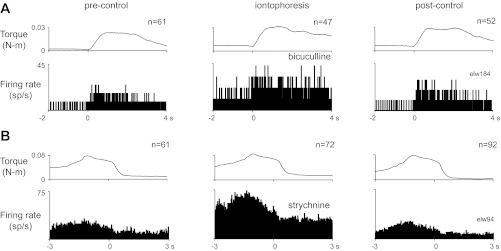
Average responses of the 2 neurons shown in Fig. 6. A: average responses for the neuron shown in Fig. 6A before, during, and after iontophoresis of bicuculline; traces are aligned on torque onset. B: average responses of the neuron shown in Fig. 6B to iontophoresis of strychnine, aligned on the end of the hold phase. Data from movements in all directions are combined. Format as in Fig. 2.
Most movement-related neurons were excited by iontophoresis of the antagonists. Bicuculline increased the firing rate of 93/111 (84%) tested neurons; strychnine excited 96/121 (79%) neurons. The antagonists decreased the activity of three neurons and had no effect on the firing rate of the other cells. These data suggest that about 66% of spinal neurons are under active inhibition from both GABA and glycine during execution of voluntary hand movements (joint probability, assuming independence of responses to each drug). This is consistent with the results from 34 neurons tested with iontophoresis of both antagonists. The discharge rate of 24 of the 34 neurons (71%) was increased by both bicuculline and strychnine (Fig. 3B). Eight cells were excited by only one antagonist, and two were unaffected by either.
The relative effect of the antagonists on firing during different task epochs was compared. The average discharge of the neurons during dynamic and static torque/force production, with and without ejection of bicuculline and strychnine, are shown in Table 1 (data averaged for all directions of movement). The antagonists tended to increase firing rate more during the ramp and hold phases of the task than during baseline (Wilcoxon signed-rank test, P < 0.05). This led to significantly larger increases in response amplitudes for individual neurons during application of bicuculline and strychnine than during control (Fig. 8; paired t-test, P < 0.05). In addition, there was a substantially larger effect on mean response amplitude during the ramp than the hold phase. This was quantified by comparing the dynamic index of the responses before and after application of the antagonists (Fig. 8). Both bicuculline and strychnine produced a significant increase in the neurons' mean dynamic index relative to control (paired t-test, P < 0.05).
Table 1.
Firing during different task epochs
| Spikes/s |
|||
|---|---|---|---|
| Baseline | Ramp phase | Hold phase | |
| Control | 13.3 ± 13.3 (389) | 24.2 ± 18.8 (389) | 18.4 ± 14.8 (389) |
| Bicuculline | +10.6 ± 9.6 (91) | +17.6 ± 13.8* (88) | +13.8 ± 9.5† (82) |
| Strychnine | +9.5 ± 8.7 (96) | +12.8 ± 11.6* (92) | +11.2 ± 8.7† (83) |
Control values are firing rates (means ± SD) with no. of cells given in parentheses. Values for bicuculline and strychnine are changes in firing rate (means ± SD) with parentheses giving no. of cells with a significant increase (+) in rate during ramp and/or hold (baseline), ramp (ramp phase), or hold (hold phase).
P < 0.05, significant difference from change in baseline rate for cells with significant effect of drug during ramp phase (Wilcoxon signed-rank test with Bonferroni correction).
P < 0.05, significant difference from change in baseline rate for cells with significant effect of drug during hold phase (Wilcoxon signed-rank test with Bonferroni correction).
Fig. 8.
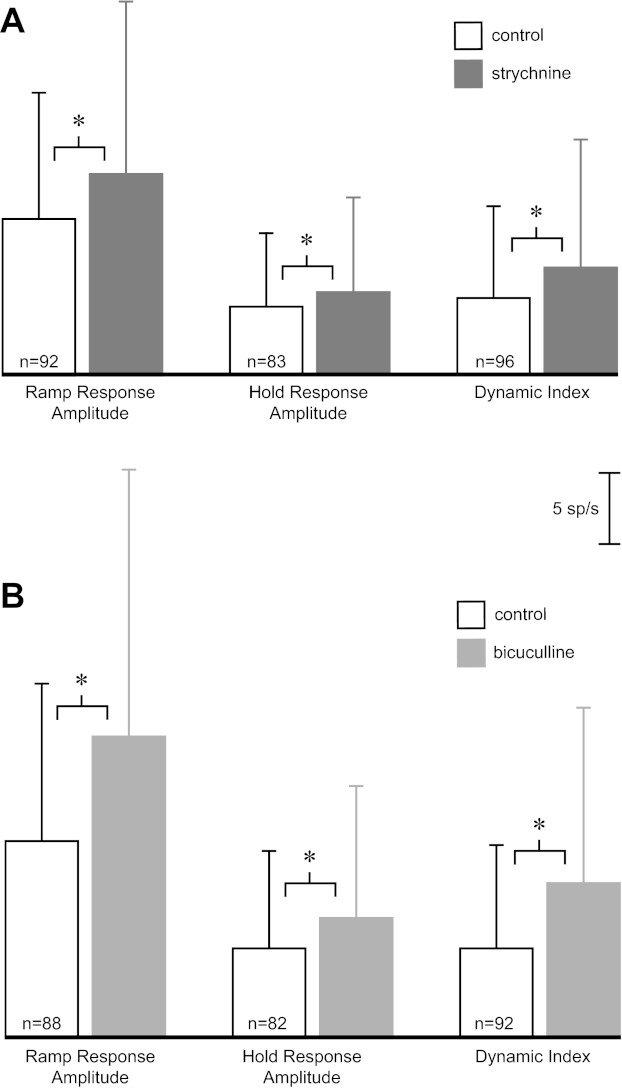
Change in response amplitudes with iontophoresis of antagonists. Bars show means ± SD of ramp response amplitude, hold response amplitude, and dynamic index for all neurons with a significant drug effect during ramp, hold, and ramp and/or hold phases, respectively. Open bars are average responses in control condition. Shaded bars are average responses with iontophoresis of strychnine (A) and bicuculline (B). The number of cells in each comparison is shown within each open bar (n). The calibration scale applies to A and B. *P < 0.05, significant differences between control and drug conditions (paired t-test with Bonferroni correction).
Sodium chloride was ejected with the same iontophoresis currents used for bicuculline or strychnine for 22 and 16 neurons, respectively. Sodium chloride did not change the firing rate of any of these neurons (P > 0.05, Fig. 4A2), and there was a significant difference between the change in discharge rate during sodium chloride and drug ejection for all neurons (paired t-test, P < 0.001, Fig. 4B).
DISCUSSION
GABA and glycine were highly effective in modulating the firing rates of a high proportion of neurons with activity related to hand movements. Furthermore, it appears that these cells are under active inhibitory control from both glycinergic and GABAergic systems during normal behavior, since most neurons were responsive to the antagonists bicuculline and strychnine during voluntary movements and at rest.
Methodological issues.
The reliability of in vivo iontophoresis as a probe of neuronal sensitivity to GABAergic and glycinergic compounds needs consideration. Five issues are relevant.
First, the concentration of the ejected drug at dendritic and somatic receptors of the recorded neurons is unknown. Factors that influence this parameter include the proximity of the electrode tip to the neuron, the size of the cell body and the extent of the dendritic tree, the presence of physical or chemical barriers preventing the drug from reaching receptor sites, the degree of dilution of the drug in the extracellular space, and the effects of the electrochemical properties of individual iontophoresis pipettes on release concentration (Aston-Jones and Siggins 1995; Salmoiraghi and Stefanis 1967). Even if GABA and/or glycine receptors were present on the recorded neurons, it is impossible to determine if the iontophoresed drugs reached enough of the membrane to affect the cell's firing rate. However, this caveat does not weaken the main finding of the study; these factors tend to increase the chances of false negative results with the use of iontophoresis and would lead to an underestimate of the proportion of neurons sensitive to the ejected drugs.
Second, the currents used to retain or eject drugs from the pipette could have themselves affected cell discharge. In these experiments, applied charge was not typically balanced using a sodium chloride reference barrel because of the frequency with which any barrel clogged on penetration of the dura mater. However, possible electrotonic effects were checked in some recording sessions by passing current through a sodium chloride-filled barrel at intensities typical for iontophoresis. In no case did the current change the firing rate of a recorded neuron. In addition, in the vast majority of cases neuronal discharge was modulated in the direction expected by application of inhibitory neurotransmitters or their antagonists. In contrast, electrotonic effects are expected to depolarize or hyperpolarize cell membranes independently of the iontophoresed drug. Consequently, it is unlikely that the applied currents themselves contributed substantially to the observed changes in neuronal discharge.
Third, the nonspecific actions of GABAergic and glycinergic antagonists may lead to misleading conclusions about responsiveness. At high concentrations, strychnine blocks GABAA, as well as glycine, receptors on rat spinal motoneurons (Jonas et al. 1998). Cross-sensitivity between the two antagonists has also been described in the lamprey spinal cord (Safronov et al. 1989). In addition, high doses of bicuculline have non-GABAergic effects, including blockade of calcium-dependent potassium channels and increases of intracellular calcium (Debarbieux et al. 1998; Johansson et al. 2001; Kurt et al. 2006; Mestdagh and Wulfert 1999). Such effects could produce increases in firing independent of GABA disinhibition. Nonspecific effects cannot be ruled out. However, most tested cells were inhibited by GABA and/or glycine, indicating the presence of the corresponding receptors. In addition, ejection currents were kept low throughout the recordings. Currents were increased slowly until a response was detected and then kept at relatively low levels just sufficient to change firing rate significantly; this was typically achieved by reducing the ejection current throughout the recording period to hold mean firing rate approximately constant.
Fourth, changes in firing rate of recorded neurons could result from indirect effects of ejected drugs. The rapid decrease in concentration of iontophoresed compounds away from the pipette tip (Herz et al. 1969) and the limited influence that the small number of neurons within the effective radius of diffusion are likely to have on the recorded cell suggest that few of the observed responses were due to effects on somatic or dendritic receptors on neurons other than the recorded cell. Iontophoresis also could have modulated transmitter release in terminals presynaptic to the cell under study, altering the synaptic drive to the recorded neuron. Unfortunately, the techniques used in this study cannot distinguish presynaptic and postsynaptic effects of the ejected drugs.
Fifth, GABA acts not only at ionotropic GABAA receptors but also at metabotropic GABAB receptors, which are not blocked by bicuculline. This may contribute to the larger percentage of neurons that were responsive to GABA compared with bicuculline.
Inhibition of individual spinal neurons by both glycine and GABA.
The present results are consistent with previous studies that showed spinal neurons receive both GABAergic and glycinergic synaptic inputs. Anatomically, the distributions of glycine and GABA receptors in the spinal cord overlap substantially (Persohn et al. 1991; van den Pol and Gorcs 1988), GABA and glycine are colocalized in single spinal neurons and axon terminals (Liu et al. 2010; Maxwell et al. 1995; Ornung et al. 1996; Todd and Sullivan 1990; Weber et al. 2007), and glycine and GABAA receptors are found on individual neurons and even within single postsynaptic densities (Bohlhalter et al. 1994; Todd 1996). Physiologically, experiments in anesthetized animals and spinal cord slices have shown that some spinal neurons are inhibited by both glycine and GABA (Curtis et al. 1968; Hirayama et al. 1990; Werman et al. 1968), that evoked inhibitory responses in dorsal horn neurons, motoneurons, Renshaw cells, and some other ventral horn neurons are blocked by strychnine and bicuculline (Curtis et al. 1976; Game and Lodge 1975; Hirayama et al. 1990; Inquimbert et al. 2007; Schneider and Fyffe 1992; Yoshimura and Nishi 1995), and that both neurotransmitters are coreleased at individual spinal synapses (Gao et al. 2001; Jonas et al. 1998).
However, we are aware of only one previous study that examined inhibition of spinal neurons under normal behavioral conditions. Taepavarapruk et al. (2002) combined recording and iontophoresis in intact, unanesthetized cats to show that single dorsal spinocerebellar neurons are inhibited by both GABA and glycine and that the extent of inhibition varies with the animal's level of wakefulness and sleep.
The present study adds to these findings with evidence that most (at least 80%) movement-related cervical spinal neurons have receptors for both glycine and GABA. In addition, the effects of iontophoresis of bicuculline and strychnine suggest that most of these cells are under the control of both inhibitory neurotransmitters during normal behavior.
Inhibitory control at rest and during active movement.
Bicuculline and strychnine increased the firing of movement-related neurons during both the rest and movement phases of the task. This implies that at rest neurons exhibit low firing due to both reduced descending excitation and active inhibition, at least when the animal is awaiting behavioral cues to initiate a trained movement. Furthermore, the data suggest that before and during movement, descending motor commands (and probably proprioceptive feedback once movement begins) drive increases in cell discharge, but active inhibition continues to regulate excitability. The antagonists increased firing for all directions of movement, even those for which the cell was maximally activated in the wrist task (not shown). It appears that inhibitory inputs modulate firing even when activity is high. Continuous, coupled excitatory and inhibitory inputs have been reported to be important for controlling the firing of neurons throughout the central nervous system, including the spinal cord (e.g., Okun and Lampl 2008; Shu et al. 2003; Stein 2010). For locomotor rhythm generation, it appears that a finely tuned balance between excitation and inhibition in lumbar networks is required to achieve appropriate motor patterns (Sherwood et al. 2011). The current data support the view that spinal motor circuits are continuously regulated by active inhibition.
Both bicuculline and strychnine had a larger effect on the firing of individual neurons during the movement phases than during the baseline phase of the task, and during dynamic movements (ramp phase) than during maintained torques/force (hold phase). This task dependence would result if the neurons received more inhibition during maintained torque/force than during baseline, and still more inhibition during dynamic movement. Since, on average, spinal neurons exhibit a larger increase in firing rate during dynamic than static hand movements (Maier et al. 1998), the effect of the antagonists parallels the average firing rate of the neurons during the task. That is, as firing rate (and presumably excitation) increases during movement, inhibition increases. Such a relationship would support the model of coupled excitatory and inhibitory inputs in spinal motor circuits (Berg et al. 2007). Alternatively, phase-dependent changes in firing rate with blockade of GABA or glycine receptors may reflect a nonlinear input-output relationship underlying cell firing rate (Silver 2010). As neurons become more depolarized during movement, similar decreases in inhibitory input could produce larger increases in firing rate.
Role of glycine and GABA in the spinal cord.
The presence of both GABA and glycine synapses on individual movement-related spinal neurons raises the question of the relative roles of the two neurotransmitters in spinal processing during movement. One possibility is that the two inhibitory systems mediate different functions, as suggested by results from other preparations. These studies found differences between the spinal distribution of glycine and GABAA receptors (Albin et al. 1993; Todd 1996), the responses of spinal neurons in different laminae to glycine and GABA (Inquimbert et al. 2007; Takazawa and MacDermott 2010), the kinetics of inhibitory currents activated by glycine and GABA (Game and Lodge 1975; Gao et al. 2001; Inquimbert et al. 2007; Jonas et al. 1998; Yoshimura and Nishi 1995), and the effects of GABAergic and glycinergic antagonists on locomotor rhythm generation (Alford et al. 2003; Butt et al. 2002; Hinckley et al. 2005; Stein 2010). A second possibility is that GABA and glycine interact postsynaptically to regulate inhibitory inputs (Lu et al. 2008), as suggested by the corelease of GABA and glycine from presynaptic terminals in the spinal cord (Gao et al. 2001; Jonas et al. 1998). The present study cannot address the relative function of GABA and glycine but indicates that both systems modulate the activity of most spinal neurons during voluntary movements.
GRANTS
This study was supported by National Institutes of Health Grants R01 NS40867, R01 NS12542, and RR00166 and Christopher and Dana Reeve Foundation Award PB-0403-2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.W. and S.I.P. conception and design of research; G.W. and S.I.P. performed experiments; G.W. and S.I.P. analyzed data; G.W. and S.I.P. interpreted results of experiments; G.W. and S.I.P. prepared figures; G.W. drafted manuscript; G.W. and S.I.P. edited and revised manuscript; G.W. and S.I.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank C. Kirby and L. Shupe for expert technical assistance.
REFERENCES
- Albin RL, Hollingsworth Z, Sakurai SY, Gilman S. Inhibitory and excitatory amino acid neurotransmitter binding sites in cynomolgus monkey (Macaca fascicularis) cervical spinal cord. Brain Res 604: 354–357, 1993 [DOI] [PubMed] [Google Scholar]
- Alford S, Schwartz E, Viana di Prisco G. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist 9: 217–228, 2003 [DOI] [PubMed] [Google Scholar]
- Aston-Jones GS, Siggins GR. Electrophysiology. In: Psychopharmacology: The Fourth Generation of Progress, edited by Bloom FE, Kupfer DJ. New York: Raven, 1995, p. 41–63 [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Mohler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res 642: 59–69, 1994 [DOI] [PubMed] [Google Scholar]
- Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res Brain Res Rev 40: 107–117, 2002 [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol 74: 1109–1117, 1995 [DOI] [PubMed] [Google Scholar]
- Curtis DR. Amino acid neurotransmitters and the brain. Med J Aust 2: 723–731, 1974 [PubMed] [Google Scholar]
- Curtis DR, Game CJ, Lodge D, McCulloch RM. A pharmacological study of Renshaw cell inhibition. J Physiol 258: 227–242, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Hosli L, Johnston GA. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res 6: 1–18, 1968 [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol 79: 2911–2918, 1998 [DOI] [PubMed] [Google Scholar]
- Game CJ, Lodge D. The pharmacology of the inhibition of dorsal horn neurones by impulses in myelinated cutaneous afferents in the cat. Exp Brain Res 23: 75–84, 1975 [DOI] [PubMed] [Google Scholar]
- Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J Neurophysiol 86: 492–502, 2001 [DOI] [PubMed] [Google Scholar]
- Haidarliu S, Shulz D, Ahissar E. A multi-electrode array for combined microiontophoresis and multiple single-unit recordings. J Neurosci Methods 56: 125–131, 1995 [DOI] [PubMed] [Google Scholar]
- Herz A, Zieglgansberger W, Farber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res 9: 221–235, 1969 [DOI] [PubMed] [Google Scholar]
- Hinckley C, Seebach B, Ziskind-Conhaim L. Distinct roles of glycinergic and GABAergic inhibition in coordinating locomotor-like rhythms in the neonatal mouse spinal cord. Neuroscience 131: 745–758, 2005 [DOI] [PubMed] [Google Scholar]
- Hirayama T, Ono H, Fukuda H. Effects of excitatory and inhibitory amino acid agonists and antagonists of ventral horn cells in slices of spinal cord isolated from adult rats. Neuropharmacology 29: 1117–1122, 1990 [DOI] [PubMed] [Google Scholar]
- Inquimbert P, Rodeau JL, Schlichter R. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III–IV of the young rat spinal cord. Eur J Neurosci 26: 2940–2949, 2007 [DOI] [PubMed] [Google Scholar]
- Johansson S, Druzin M, Haage D, Wang MD. The functional role of a bicuculline-sensitive Ca2+-activated K+ current in rat medial preoptic neurons. J Physiol 532: 625–635, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science 281: 419–424, 1998 [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006 [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci 17: 9433–9447, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABAA-antagonists bicuculline and gabazine in sensory cortex. Hear Res 212: 224–235, 2006 [DOI] [PubMed] [Google Scholar]
- Liu TT, Bannatyne BA, Maxwell DJ. Organization and neurochemical properties of intersegmental interneurons in the lumbar enlargement of the adult rat. Neuroscience 171: 461–484, 2010 [DOI] [PubMed] [Google Scholar]
- Lu T, Rubio ME, Trussell LO. Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron 57: 524–535, 2008 [DOI] [PubMed] [Google Scholar]
- Maier MA, Perlmutter SI, Fetz EE. Response patterns and force relations of monkey spinal interneurons during active wrist movement. J Neurophysiol 80: 2495–2513, 1998 [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Todd AJ, Kerr R. Colocalization of glycine and GABA in synapses on spinomedullary neurons. Brain Res 690: 127–132, 1995 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestdagh N, Wulfert E. Bicuculline increases Ca2+ transients in rat cerebellar granule cells through non-GABAA receptor associated mechanisms. Neurosci Lett 265: 95–98, 1999 [DOI] [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci 11: 535–537, 2008 [DOI] [PubMed] [Google Scholar]
- Ornung G, Shupliakov O, Linda H, Ottersen OP, Storm-Mathisen J, Ulfhake B, Cullheim S. Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: a postembedding electron microscopic study. J Comp Neurol 365: 413–426, 1996 [DOI] [PubMed] [Google Scholar]
- Perlmutter SI. Primate interneurons. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009, p. 1045–1054 [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol 80: 2475–2494, 1998 [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience 42: 497–507, 1991 [DOI] [PubMed] [Google Scholar]
- Raiteri M, Bonanno G, Pende M. gamma-Aminobutyric acid and glycine modulate each other's release through heterocarriers sited on the releasing axon terminals of rat CNS. J Neurochem 59: 1481–1489, 1992 [DOI] [PubMed] [Google Scholar]
- Safronov BV, Baev KV, Batueva IV, Rusin KI, Suderevskaya EI. Peculiarities of receptor-channel complexes for inhibitory mediators in the membranes of lamprey spinal cord neurones. Neurosci Lett 102: 82–86, 1989 [DOI] [PubMed] [Google Scholar]
- Salmoiraghi GC, Stefanis CN. A critique of iontophoretic studies of central nervous system neurons. Int Rev Neurobiol 10: 1–30, 1967 [DOI] [PubMed] [Google Scholar]
- Schneider SP, Fyffe RE. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol 68: 397–406, 1992 [DOI] [PubMed] [Google Scholar]
- Sherwood WE, Harris-Warrick R, Guckenheimer J. Synaptic patterning of left-right alternation in a computational model of the rodent hindlimb central pattern generator. J Comput Neurosci 30: 323–360, 2011 [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003 [DOI] [PubMed] [Google Scholar]
- Silver RA. Neuronal arithmetic. Nat Rev Neurosci 11: 474–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Alternation of agonists and antagonists during turtle hindlimb motor rhythms. Ann NY Acad Sci 1198: 105–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk N, McErlane SA, Soja PJ. State-related inhibition by GABA and glycine of transmission in Clarke's column. J Neurosci 22: 5777–5788, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol 588: 2571–2587, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur J Neurosci 8: 2492–2498, 1996 [DOI] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol 296: 496–505, 1990 [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci 8: 472–492, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I, Veress G, Szucs P, Antal M, Birinyi A. Neurotransmitter systems of commissural interneurons in the lumbar spinal cord of neonatal rats. Brain Res 1178: 65–72, 2007 [DOI] [PubMed] [Google Scholar]
- Werman R, Davidoff RA, Aprison MH. Inhibitory action of glycine on spinal neurons in the cat. J Neurophysiol 31: 81–95, 1968 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. J Physiol 482: 29–38, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]


