Abstract
Endogenous cannabinoid type 1 (CB1) receptors demonstrate a cell type-specific expression and are potent modulators of synaptic transmission within the central nervous system. We aimed to investigate whether two classes of multipolar interneuron in the neocortex displayed a form of short-term synaptic plasticity, depolarization-induced suppression of inhibition (DSI), and whether the DSI was mediated by a common receptor. Paired whole cell recordings combined with biocytin labeling were performed between pyramidal cells and either multipolar adapting or multipolar nonadapting interneurons in layers II–IV of male Wistar rat (postnatal day 17–22) somatosensory cortex. Inhibitory postsynaptic potentials elicited by multipolar adapting interneurons were sensitive to DSI, which was blocked by the CB1 receptor antagonist AM-251 (8 μM), indicating that the suppression of inhibition was mediated by CB1 receptors. Two subpopulations of multipolar nonadapting interneuron-to-pyramidal cell connections were discovered on the basis of their susceptibility to DSI. Whereas 50% were insensitive to DSI, the remaining half were sensitive to DSI, which could not be prevented by AM-251. DSI at these connections was also insensitive to the group I (mGluRIa) and III metabotropic glutamate receptor antagonists (RS)-1-aminoindan-1,5-dicarboxylic acid (100 μM) and (RS)-α-cyclopropyl-4-phosphonophenylglycine (100 μM) and the group III agonist l-2-amino-4-phosphonobutanoate (50 μM). However, multipolar nonadapting interneuron-to-pyramidal cell connections were sensitive to the endocannabinoid anandamide (9 μM), mimicking the effects of DSI, which also could not be prevented by AM-251, implying a CB1 receptor-independent suppression of inhibition. These results reveal an interneuron type-specific modulation of synaptic transmission via CB receptors in the neocortex.
Keywords: interneuron, depolarization-induced suppression of inhibition, inhibitory postsynaptic potential
within the central nervous system (CNS), endocannabinoids are key modulators of synaptic transmission that have been demonstrated to act as retrograde signaling molecules to suppress neurotransmitter release through the activation of presynaptic cannabinoid type 1 (CB1) receptors (Kreitzer and Regehr 2001a; Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001).
CB1 receptor mRNA is predominantly localized presynaptically in subsets of neocortical and hippocampal GABAergic interneurons with an adapting firing pattern (Irving et al. 2000), particularly CCK-expressing cells (Hájos and Freund 2002; Katona et al. 1999). These receptors are thought to be absent in nonadapting multipolar interneurons, which usually contain parvalbumin (PV), or bi-tufted adapting somatostatin (SOM)-containing interneurons (Bodor et al. 2005; Tsou et al. 1999). In the neocortex, CB1 receptors are differentially expressed across the different layers, with the highest levels in layers II/III and V (Fortin and Levine 2007), whereas layers IV–VI show equal representation of CB1 receptors at CCK- and calbindin (CBD)-containing interneurons as revealed by immunocytochemical studies (Bodor et al. 2005; Galarreta et al. 2008). Interestingly, CB1 receptor mRNA, often undetected by immunocytochemical studies, is also expressed in subsets of pyramidal cells (Hill et al. 2007; Marsicano and Lutz 1999).
A form of short-term plasticity mediated by the CB1 receptor is termed depolarization-induced suppression of inhibition (DSI). It describes depolarization of the postsynaptic cell, leading to release of endocannabinoids as a retrograde messenger, which in turn activates presynaptic CB1 receptors and, at inhibitory synapses, leads to a subsequent reduction of GABAA receptor-mediated transmission (Alger et al. 1996; Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001). DSI has been demonstrated to occur within the neocortex (Trettel and Levine 2003), in addition to other regions of the brain such as the hippocampus (Wilson and Nicoll 2001) and cerebellum (Kreitzer and Regehr 2001a). It is a phenomenon that has thus far been reported to be lacking between neocortical interneurons (Galarreta et al. 2008; Lemtiri-Chlieh and Levine 2007) but is present between CCK-immunopositive interneurons in the hippocampus (Ali 2007; Iball and Ali 2011). Previous studies have implicated endogenous cannabinoids as the retrograde messenger in DSI (Ali 2007; Ferraro et al. 2001; Kreitzer and Regehr 2001b; Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001; see also Freund 2003 for review). Endocannabinoids have been shown to have presynaptic actions since they do not affect the quantal size of miniature GABA-mediated events (Alger et al. 1996; Morishita and Alger 1997), which is consistent with a presynaptic location of cannabinoid receptors. It has been suggested that the effects of group I metabotropic receptors (mGluRs) on DSI may be a result of the activation of endocannabinoids, with glutamate acting as a trigger for endocannabinoid production rather than as a retrograde signal (Maejima et al. 2001; Vásquez et al. 2003). At neocortical connections between fast-spiking interneurons and pyramidal cells, DSI has been reported to be both absent (Galaretta et al. 2008) and present (Harkany et al. 2004), where glutamate is implicated as the retrograde transmitter.
In this study, we sought to determine whether DSI occurred at two subclasses (adapting and nonadapting) of multipolar interneuron-to-pyramidal cell connection and if the suppression of inhibition was mediated by the CB1 receptor. Paired whole cell recordings between pyramidal cells and either multipolar adapting or multipolar nonadapting interneurons in acute slices of rat somatosensory cortex (postnatal days 17–22; P17–22) were obtained, during which neurons were filled with biocytin for subsequent labeling and reconstruction. Since the DSI that occurred at a subset of connections appeared to be CB1 receptor independent, the involvement of group I and III mGluRs was thus investigated.
MATERIALS AND METHODS
Slice preparation.
These procedures were reviewed by ethical committees and comply with British Home Office regulations under the Animals (Scientific Procedures) Act 1986. Male Wistar rats (P17–22) were anesthetized by an intraperitoneal injection of sodium pentobarbitone (60 mg/kg; Euthatal, Merial, UK) and perfused with a sucrose-containing artificial cerebrospinal fluid (ACSF) solution that consisted of (in mM) 248 sucrose, 3.3 KCl, 1.4 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 15 glucose, and 25.5 NaHCO3, bubbled with 95% O2 and 5% CO2. After decapitation and removal of the brain, coronal slices of cortex 300 μm thick were cut in ice-cold ACSF using an automated vibratome (Leica, Wetzlar, Germany). This standard ACSF contained (in mM) 121 NaCl, 2.5 KCl, 1.3 NaH2PO4, 2 CaCl2, 1 MgCl2, 20 glucose, and 26 NaHCO3, equilibrated with 95% O2 and 5% CO2. Slices were incubated in ACSF for 1 h at room temperature (20–23°C) before recordings began.
Electrophysiology.
Cortical slices were placed in a submerged chamber and perfused with ACSF at a rate of 1–2 ml/min. Paired whole cell somatic recordings were obtained from layer II–IV interneurons and pyramidal cells of rat somatosensory cortex at room temperature (20–23°C). Electrodes with resistances of 8–11 MΩ were made from filamented borosilicate glass capillaries (Harvard Apparatus, Edenbridge, UK) and filled with a solution containing (in mM) 134 K+-gluconate, 10 HEPES, 10 phosphocreatine, 2 Na2ATP, 0.2 Na2GTP, and 0.2% (wt/vol) biocytin. Cells were chosen for recording on the basis of the shape of their soma and dendritic projections by using video microscopy under near-infrared differential interference contrast illumination. Neurons were further characterized by their electrophysiological properties obtained from a series of 500-ms depolarizing and hyperpolarizing current pulses and neuroanatomy revealed by the labeling of biocytin in recorded cells (see below). The inhibitory connections reported in this study were between interneuron and pyramidal cells (n = 24), of which nine connections were reciprocally connected and excitatory postsynaptic potentials (EPSPs) elicited in the interneurons were not included in the present study.
Unitary inhibitory postsynaptic potentials (IPSPs) were elicited by a depolarizing current step (+0.05 nA, 5–10 ms) repeated at 0.33 Hz. To induce DSI, a protocol similar to one described previously was used (Ali 2007). Three action potentials were induced in the postsynaptic pyramidal cell with an interspike interval of 30 ms. A single IPSP was then elicited by the interneuron 1 s following the pyramidal cell depolarization. This was repeated at 0.33 Hz for 30 sweeps and alternated with 30 sweeps of the same protocol, minus the pyramidal cell depolarization. We expected the DSI protocol to decrease IPSP amplitudes significantly from control (range of reduction between 20 and 60% of control), and these synaptic connections were categorized as “DSI sensitive.” Pairs that showed no change in IPSP amplitudes during the DSI protocol were classified as “DSI-insensitive” connections.
Recordings were carried out in the current-clamp mode of operation (SEC 05LX npi amplifiers; npi electronics, Tamm, Germany), low-pass filtered at 2 kHz, and digitized at 5 kHz using a CED 1401 interface (Cambridge Electronic Design, Cambridge, UK). Input resistance was monitored throughout experiments by means of a hyperpolarizing current step (−0.001 nA, 10 ms). Signal software (Cambridge Electronic Design) was used to acquire recordings and generate current steps.
Drugs.
AM-251 was dissolved in DMSO and applied at concentrations of 4, 6, 8, and 10 μM during preliminary studies, with 8 μM being the optimal concentration (also in accordance with previous studies: Fortin et al. 2004; Galarreta et al. 2008). The final concentration of DMSO was ≤0.1%. (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), and l-2-amino-4-phosphonobutanoate (l-AP4) were dissolved in distilled water and used at concentrations of 100, 100, and 50 μM, respectively. All drugs were acquired from Tocris (Bristol, UK), stored in vials as stock at −20°C, and delivered to the bath in the ACSF.
Data analysis.
Data were analyzed off-line using Signal software. The input resistance and membrane time constant were determined from voltage changes in response to hyperpolarizing current steps (−0.1 nA, 500 ms). A depolarizing current step (+0.2 nA, 500 ms) was used to induce trains of action potentials from which the first interspike interval was measured as the time between the first and second action potentials, and the adaption ratio by dividing the final interspike interval by the first.
IPSP amplitudes and the time from the peak of the presynaptic action potential to the onset of the IPSP (latency of onset) were measured from single sweeps. The 10–90% rise time and width at half-amplitude (HW) measurements were obtained from averages. Average IPSPs contain between 50 and 150 events. The failure of a presynaptic action potential to elicit IPSP was measured from 100 sweeps and is shown as the failure rate (%). Apparent failures of transmission were assigned a value of 0 mV and were not included in averages. The paired-pulse ratio (PPR) was calculated by dividing the second average postsynaptic amplitude by the first average postsynaptic amplitude in control and during drug application.
Values are given as means ± SD. A two-tailed paired t-test was used to compare two groups of data. For comparisons between more than two groups of data, a one-way ANOVA was used and a Bonferroni correction imposed for multiple comparisons. Significance was accepted where P < 0.05.
Histological processing and the reconstruction of recorded cells.
After the termination of recording, slices were submerged overnight at 4°C in fixative containing 4.0% paraformaldehyde, 2.5% glutaraldehyde, and 0.2% picric acid in 0.1 M phosphate buffer. Slices were then embedded in gelatin, immersed in fixative for 40 min, and sectioned at 60 μm. For cryoprotection prior to freeze-thawing, sections were incubated in a series of sucrose- and glycerol-containing 0.1 M phosphate buffer solutions (2 × 10 min in 10% sucrose, 2 × 20 min in 20% sucrose with 6% glycerol, and 2 × 30 min in 30% sucrose and 12% glycerol). Sections were freeze-thawed over liquid nitrogen (3 × 30 s) and immersed in 1% hydrogen peroxide for 30 min, followed by an overnight incubation at 4°C in a preformed avidin and biotinylated horseradish peroxidase solution (Vectastain Elite ABC kit; Vectorlabs, Burlingame, CA). Cells were visualized by incubating sections in 3,3′-diaminobenzidine and nickel chloride for 15 min, followed by the addition of 1% hydrogen peroxide. Osmium tetroxide (1%) was used to intensify staining. Sections were flattened and placed in an ascending sequence of ethanol solutions: 50% for 2 times 10 min, 70% for 2 × 20 min, 95% for 2 × 20 min, and 100% for 30 min, followed by immersion in propylene oxide. Sections were embedded in epoxy resin (Durcupan; Fluka, Buchs, Switzerland).
Cells were reconstructed using a microscope with an attached drawing tube (Olympus, London, UK) at a magnification of ×1,000, using a ×100 oil-immersion objective lens (Olympus). Three-dimensional reconstructions of the soma and dendrites of neurons were produced using Neurolucida (MBF Bioscience, Williston, VT). To compare the relative length of axon between groups of interneurons, the length of axon was measured within a 60-μm section containing the soma by means of a 100 × 100-μm square centered to the cell body. The scale of reconstructions was obtained from slides and has not been corrected for shrinkage that may have occurred during processing of the slices.
RESULTS
Multipolar adapting and a subset of multipolar nonadapting interneuron-to-pyramidal cell pairs are sensitive to DSI.
To investigate as to whether multipolar adapting and multipolar nonadapting interneuron-to-pyramidal cell connections were sensitive to DSI, pyramidal cells were depolarized before unitary IPSPs were recorded at 0.33 Hz (for DSI protocol, see materials and methods, Electrophysiology). Individual pairs were deemed sensitive to DSI by a reduction in the average amplitude, which was in the range of 30–60% reduction of control average amplitudes. However, it was evident from viewing single-sweep amplitude plots that there appeared to be no change at those classified as insensitive, whereas at those categorized as sensitive, a reduction in IPSP amplitudes could be observed from sweep to sweep, with a reduction of IPSP amplitude always by ≥18%.
All multipolar adapting to pyramidal cell pairs (n = 6) demonstrated a sensitivity to DSI. Five of 18 multipolar nonadapting interneuron-to-pyramidal cell pairs were found to be insensitive to DSI; a stronger depolarization protocol eliciting long trains of 3–6 action potentials in the postsynaptic neuron also did not induce DSI (not shown). Multipolar nonadapting interneuron-to-pyramidal cell pairs sensitive to DSI were then selected for, and a total of 13 pairs were recorded. Examples of reconstructed interneurons from the three subtypes of connection are shown in Fig. 1, Ai–Ci, and the anatomic and electrophysiological properties of the interneurons in Tables 1 and 2, respectively. Although the multipolar adapting interneurons possessed soma that were on average longer in the horizontal and vertical orientations, and a greater total length of dendrite, with fewer primary dendrites and a greater number of branch points per dendrite, compared with both groups of nonadapting interneuron (P < 0.05, Bonferroni), a significant difference was not found between the two nonadapting groups in any of the anatomic properties measured. With regard to the active and passive membrane properties, there were again no significant differences between the two nonadapting groups, whereas the adapting interneurons displayed a higher input resistance, longer membrane time constant and action potential HW, smaller afterhyperpolarization amplitude, and a greater adaption ratio and first interspike interval (P < 0.05, Bonferroni).
Fig. 1.
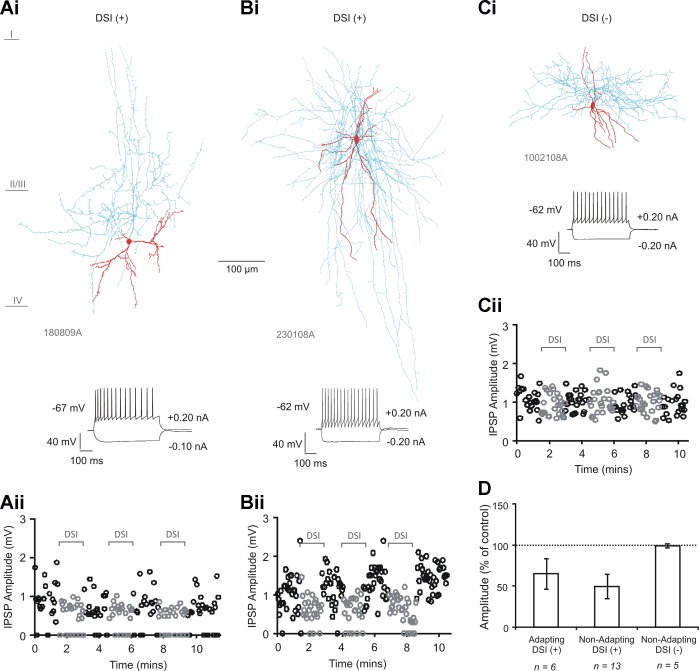
Multipolar adapting and a subset of multipolar nonadapting interneuron-to-pyramidal cell pairs are sensitive to depolarization-induced suppression of inhibition (DSI). Ai–Ci: biocytin-labeled reconstructions of a multipolar adapting interneuron (from an interneuron-to-pyramidal cell connection) sensitive to DSI (Ai), a multipolar nonadapting interneuron (from an interneuron-to-pyramidal cell connection) sensitive to DSI (Bi), and a multipolar nonadapting interneuron (from an interneuron-to-pyramidal cell connection) insensitive to DSI (Ci). Interneuron soma and dendrites are shown in red and axon in blue. Aii–Cii: single-sweep measurements of inhibitory postsynaptic potential (IPSP) amplitudes (at 0.33 Hz) over time, during the DSI protocol, for the 3 respective interneuron-to-pyramidal cell pairs. Gray circles show amplitudes during DSI. D: average IPSP amplitudes during DSI for the 3 groups of interneurons. Bars denote group average and error bars indicate SD. IPSP amplitudes were reduced at the multipolar adapting interneuron-to-pyramidal cell group (n = 6, P < 0.005, paired t-test) and at the multipolar nonadapting interneuron-to-pyramidal cell group (n = 13, P < 0.005, paired t-test). DSI+, DSI sensitive; DSI−, DSI insensitive.
Table 1.
Neuroanatomic properties of adapting and nonadapting multipolar interneurons sensitive and insensitive to DSI
| Multipolar Adapting |
Multipolar Nonadapting |
||
|---|---|---|---|
| DSI (+) | DSI (+) | DSI (−) | |
| Soma | |||
| Vertical diameter, μm | 9.5 ± 2.3* | 6.0 ± 2.5 | 6.0 ± 2.0 |
| Horizontal diameter, μm | 7.0 ± 3.0* | 5.8 ± 2.0 | 5.5 ± 4.0 |
| Dendrites | |||
| Total length, μm | 426 ± 32.0* | 210 ± 15.0 | 200 ± 16.0 |
| No. of primary dendrites | 5.5 ± 2.5* | 8.0 ± 3.5 | 8.0 ± 2.0 |
| No. of branch points per dendrite | 6.0 ± 2.0* | 2.5 ± 1.5 | 3.0 ± 1.5 |
| Axon | |||
| Vertical span, μm | 375 ± 15.0 | 230 ± 26.0 | 380 ± 16.5 |
| Horizontal span, μm | 575 ± 23.0 | 457 ± 34.8 | 589 ± 20.0 |
| Relative length, μm | 2534 ± 173 | 1549 ± 100 | 1126 ± 59 |
Values are means ± SD for depolarization-induced suppression of inhibition (DSI)-sensitive (+) multipolar adapting (n = 6) and nonadapting (n = 13) interneurons and DSI-insensitive (−) multipolar nonadapting interneurons (n = 5).
P < 0.05, significantly different from the other 2 subclasses of interneuron (Bonferroni).
Table 2.
Electrophysiological properties of multipolar adapting and nonadapting interneurons sensitive and insensitive to DSI
| Multipolar Adapting |
Multipolar Nonadapting |
||
|---|---|---|---|
| DSI (+) | DSI (+) | DSI (−) | |
| Input resistance, MΩ | 290 ± 11.0* | 223 ± 14.0 | 220 ± 12.5 |
| Time constant, ms | 19.5 ± 6.52* | 12.56 ± 8.6 | 12.0 ± 9.0 |
| 1st interspike interval | 17.0 ± 1.40* | 11.0 ± 2.0 | 10.0 ± 1.50 |
| Adaption ratio | 3.20 ± 0.80* | 1.24 ± 0.53 | 1.15 ± 1.0 |
| AP HW, ms | 2.1 ± 0.43* | 1.1 ± 0.23 | 1.25 ± 0.30 |
| AHP amplitude, mV | 4.2 ± 1.50* | 7.80 ± 2.30 | 7.5 ± 1.80 |
Values are means ± SD for properties of DSI-sensitive multipolar adapting (n = 6) and nonadapting (n = 13) interneurons and DSI-insensitive multipolar nonadapting interneurons (n = 5).
P < 0.05, significantly different from the other 2 subclasses of interneuron (Bonferroni). AP HW, action potential half-width; AHP, afterhyperpolarization.
The properties of IPSPs recorded at multipolar adapting and multipolar nonadapting interneuron-to-pyramidal cells sensitive and insensitive to DSI are shown in Table 3. Single-sweep IPSP amplitude measurements over time for examples of the three subtypes of interneuron during the DSI protocol are shown in Fig. 1, Aii–Cii. During DSI, average IPSPs were reduced in amplitude to 64.85 ± 18.69% of control at the multipolar adapting interneuron-to-pyramidal cell group (Fig. 1D, n = 6, P < 0.005, paired t-test) and to 49.64 ± 14.67% of control at the multipolar nonadapting interneuron-to-pyramidal cell group (n = 13, P < 0.005, paired t-test). There was no significant change in the nonadapting group insensitive to DSI (99.18 ± 2.22% of control, n = 5, P ≥ 0.05, paired t-test).
Table 3.
Properties of IPSPs recorded at multipolar adapting and nonadapting interneuron-to-pyramidal cell pairs sensitive and insensitive to DSI
| Multipolar Adapting |
Multipolar Nonadapting |
||
|---|---|---|---|
| DSI (+) | DSI (+) | DSI (−) | |
| Amplitude, mV | 1.70 ± 1.49 | 1.39 ± 1.05 | 1.54 ± 1.14 |
| 10–90% RT, ms | 9.50 ± 2.10 | 6.80 ± 2.10 | 6.29 ± 1.11 |
| HW, ms | 78.10 ± 27.44 | 53.90 ± 12.52 | 59.97 ± 14.84 |
| Latency of onset, ms | 1.75 ± 0.52 | 1.59 ± 0.26 | 1.51 ± 0.61 |
| Failure rate, % | 4.00 ± 5.23 | 2.08 ± 5.11 | 0.00 ± 0.00 |
Values are means ± SD for properties of inhibitory postsynaptic potentials (IPSPs) recorded at DSI-sensitive multipolar adapting (n = 6) and nonadapting (n = 13) interneuron-to-pyramidal cell pairs and at DSI-insensitive multipolar nonadapting interneuron-to-pyramidal cell paris (n = 5). RT, rise time; HW, half-width.
DSI had no effect on the latency of onset of IPSPs in either interneuron group sensitive to DSI (adapting, 101.48 ± 3.73% of control; nonadapting, 100.31 ± 2.62% of control; P ≥ 0.05, paired t-test), suggesting that while inhibition is suppressed, timing is maintained.
AM-251 prevents DSI at multipolar adapting but not multipolar nonadapting interneuron-to-pyramidal cell pairs.
To establish whether DSI was mediated by CB1 receptors at multipolar adapting and multipolar nonadapting interneuron-to-pyramidal cell connections, AM-251 (8 μM) was applied to four of each type of connection. Examples of single-IPSP amplitudes recorded from both a multipolar adapting interneuron-to-pyramidal cell connection and a multipolar nonadapting interneuron-to-pyramidal cell connection during DSI and in the presence of AM-251, with the average IPSPs, are shown in Fig. 2, A and B, respectively. AM-251 blocked DSI at all multipolar adapting interneuron-to-pyramidal cell pairs, with the group IPSP average increasing from 76.45 ± 11.80% of control during DSI to 112.74 ± 10.80% of control during DSI with AM-251 (Fig. 2C, n = 4). However, the addition of AM-251 to all multipolar nonadapting interneuron-to-pyramidal cell pairs (n = 4) did not prevent DSI (Fig. 2C, from 48.31 ± 9.21% of control during DSI to 48.15 ± 9.32% of control during DSI in the presence of AM-251), suggesting that DSI at these connections is mediated by a CB1 receptor-independent mechanism. AM-251 alone (without the DSI protocol) appeared to increase the IPSP amplitudes at two of the four multipolar adapting interneuron-to-pyramidal cell pairs, as can be observed in the example shown in Fig. 2A, although this was not statistically significant for the group (to 122.33 ± 29.03% of control, n = 4, P ≥ 0.05). At the multipolar nonadapting interneuron-to-pyramidal cell connections, there appeared to be no change (to 100.07 ± 6.21% of control, n = 4, P ≥ 0.05).
Fig. 2.
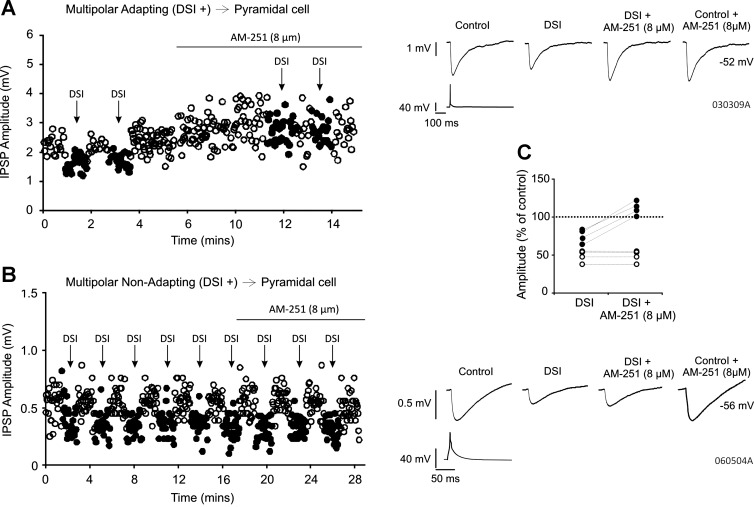
AM-251 (8 μM) blocks DSI at multipolar adapting but not multipolar nonadapting interneuron-to-pyramidal cell pairs. A and B: single-sweep IPSP amplitudes over time and average IPSPs obtained from a multipolar adapting and multipolar nonadapting interneuron-to-pyramidal cell connection sensitive to DSI, respectively, under control conditions and in the presence of AM-251 (8 μM). Filled circles show amplitudes during DSI. Holding potentials of the pyramidal cells were −52 and −56 mV, respectively. C: AM-251 blocks DSI at all multipolar adapting interneuron-to-pyramidal cell pairs but not at the multipolar nonadapting interneuron-to-pyramidal cell pairs. Each circle represents 1 pair: filled circles, multipolar adapting; open circles, multipolar nonadapting.
That DSI was mediated presynaptically at the multipolar nonadapting interneuron-to-pyramidal cell group was indicated by an increase in the apparent failures of transmission in the nonadapting group from 2.08 ± 5.11% in control to 18.46 ± 11.74% during DSI (n = 13, P <0.05, paired t-test).
Group I and group III mGluRs are not involved in mediating DSI at neocortical multipolar nonadapting interneuron-to-pyramidal cell pairs.
Previous studies within cortical regions have suggested that group I mGluRs cooperate with the endocannabinoid system (Ohno-Shosaku et al. 2001). We therefore performed experiments with the postsynaptic group I mGluR (targeting the mGluR1a) antagonist AIDA (100 μM) to investigate whether the mGluR1a was involved in mediating DSI at multipolar nonadapting interneuron-to-pyramidal cell connections insensitive to AM-251. The involvement of presynaptic group III mGluRs was also tested using the agonist l-AP4 (50 μM) and the antagonist CPPG (100 μM). Examples of average IPSPs obtained from three multipolar nonadapting interneuron-to-pyramidal cell pairs in the presence of AIDA, CPPG, or l-AP4 are shown in Fig. 3, A–C, respectively.
Fig. 3.
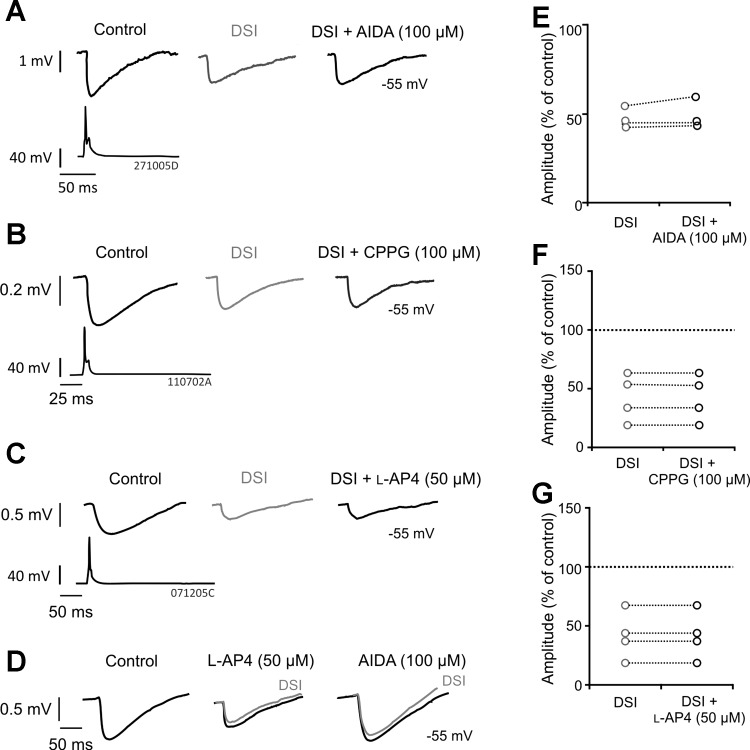
(RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), and l-2-amino-4-phosphonobutanoate (l-AP4) do not prevent DSI at multipolar nonadapting interneuron-to-pyramidal cell connections. A–C: average IPSPs obtained from paired whole cell recordings between multipolar nonadapting interneurons and pyramidal cells during DSI and in the presence of AIDA [100 μM; postsynaptic group I metabotropic glutamate receptor (mGluR1a) antagonist], CPPG (100 μM; group III mGluR antagonist), or l-AP4 (50 μM; group III mGluR agonist), respectively. D: positive control of l-AP4 and AIDA. The example shown was recorded in the hippocampus between a CA1 basket and pyramidal cell under similar conditions to the present study. The IPSPs illustrate cooperation of group I and III mGluRs, since l-AP4 reduced the IPSPs, occluding subsequent DSI, which was then prevented by subsequent addition of AIDA. E–G: individual pair average IPSP amplitude responses to DSI alone and DSI with AIDA, CPPG, or l-AP4, demonstrating that the drugs do not prevent DSI. Each circle represents 1 pair.
During DSI, IPSPs were reduced to 48.63 ± 5.66% of control (n = 3) and continued to be suppressed following application of AIDA (Fig. 3D, 50.64 ± 5.66% of control). The presence of CPPG had no effect on IPSP amplitudes during DSI (41.99 ± 14.01% of control), which during DSI without CPPG were reduced to 42.13 ± 19.95% of control (Fig. 3E, n = 4). In addition, l-AP4 (50 μM) also failed to prevent DSI (n = 4, 52.44 ± 13.94% of control during DSI), where DSI alone reduced IPSPs to 52.34 ± 14.01% of control (Fig. 3F). These results suggest that neither mGluR1a nor group III mGluRs are involved in CB1 receptor-insensitive DSI present between multipolar nonadapting interneurons and pyramidal cells. However, to illustrate the positive control of these drugs, Fig. 3D shows the action of l-AP4 and AIDA at a synaptic connection between a CA1 basket and pyramidal cell (recorded under similar conditions to the present study). These IPSPs illustrate a cooperation of group I and III mGluRs, because l-AP4 reduced the IPSPs, occluding subsequent DSI, which was then prevented by subsequent addition of AIDA.
Multipolar nonadapting interneuron sensitivity to anandamide.
To test whether cannabinoid receptor agonist had an affect at multipolar nonadapting interneuron-to-pyramidal cell connections that exhibited DSI but were insensitive to group I and III mGluR pharmacology, the effects of an endogenous cannabinoid, anandamide, were tested. Eight unidirectional (nonreciprocal) inhibitory pairs were challenged with the nonselective cannabinoid agonist anandamide (9 μM) to test whether they were susceptible to modulation by cannabinoids. AM-251 (8 μM) was also applied to five of these connections, to assess whether modulation by anandamide was mediated by the CB1 receptor.
Anandamide significantly reduced average IPSP amplitudes in five multipolar nonadapting interneuron-containing pairs (Fig. 4). The average amplitude decreased to 1.04 ± 0.35 mV from a control average of 1.9 ± 0.55 mV (n = 5, P < 0.05, paired t-test). On addition of AM-251, the average IPSP amplitude did not change significantly in the five pairs sensitive to anandamide and remained at an average amplitude of 1.02 ± 0.45 mV (for examples, see Fig. 4, C and D). The average IPSP amplitudes did not return to control levels in the presence of AM-251 following a decrease in the presence of anandamide, which is contrary to the result to be expected if the reduction in the average IPSP amplitude was mediated by the CB1 receptor (see Ali 2007). Four of five of these connections were also sensitive to DSI (Fig. 4D). Multipolar adapting (n = 3) and a single bipolar adapting connection appeared to be insensitive to anandamide (data not shown).
Fig. 4.
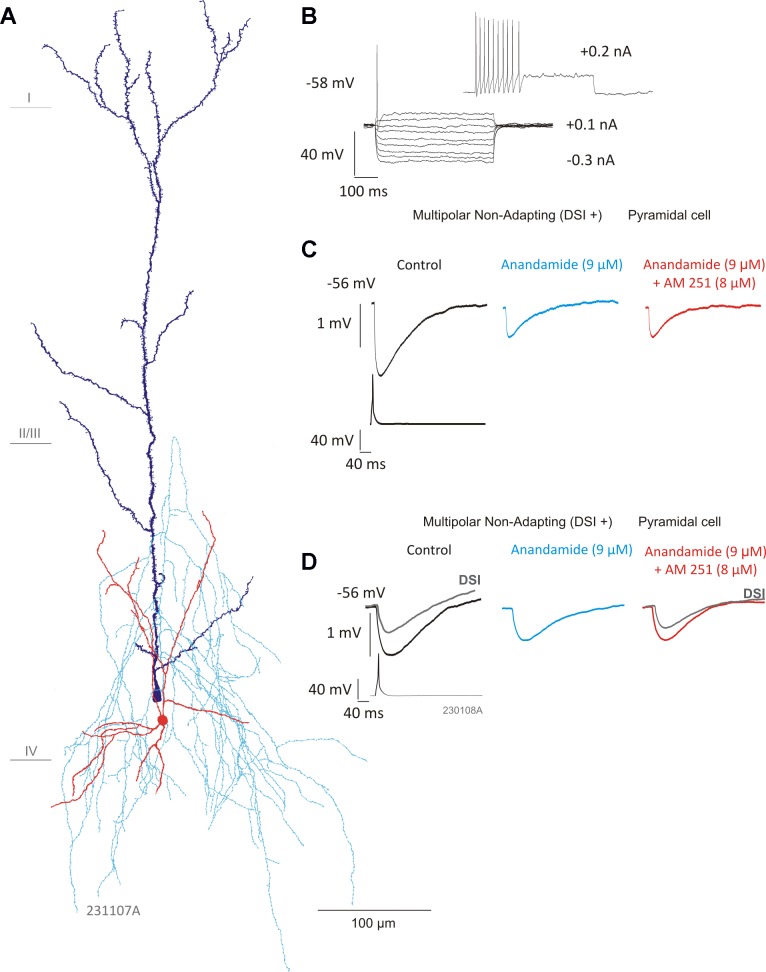
Multipolar nonadapting interneuron IPSPs are sensitive to anandamide. A: reconstruction of a multipolar nonadapting interneuron connected to a pyramidal cell. Interneuron cell body and dendrites are shown in red, axon in light blue, and pyramidal cell in dark blue. B: the firing pattern of the interneuron in response to hyperpolarizing and depolarizing current pulses. C: average IPSP amplitudes were significantly reduced in the presence of anandamide (9 μM; n = 5, P < 0.05, Student's t-test). However, AM-251 (8 μM) did not appear to prevent the effects of anandamide. D: another multipolar nonadapting interneuron-to-pyramidal cell connection (reconstruction shown in Fig. 1B) sensitive to DSI (gray traces, superimposed) and anandamide, but not AM-251, suggesting a CB1 receptor-independent mechanism.
DISCUSSION
We have shown multipolar adapting interneuron-to-pyramidal cell connections to be sensitive to DSI and that the suppression of inhibition is blocked by AM-251 (8 μM), implying it is CB1 receptor mediated. We report two populations of multipolar nonadapting interneuron-to-pyramidal cell connection based on their sensitivity to DSI and demonstrate that the suppression of inhibition at the connections exhibiting DSI is not affected by AM-251, AIDA, CPPG, or l-AP4, suggesting that it is neither CB1 receptor nor postsynaptic mGluR1a or presynaptic group III mGluR mediated. In contrast to previous studies, inhibition of elicited synaptic currents produced by 3,5-dihydroxyphenylglycine (DHPG), an agonist of mGluR1 and mGluR5, was abolished by CB1 receptor antagonism (Chevaleyre and Castillo 2003; Maejima et al. 2001; Ohno-Shosaku et al. 2001; Varma et al. 2001). We therefore cannot eliminate the involvement of mGluR5 in the present study.
DSI sensitivity and interneuron subtypes.
In this study, multipolar adapting interneuron-to-pyramidal cell connections were sensitive to DSI. We demonstrated that AM-251 blocked DSI at these connections, in accordance with results obtained in previously published work within the neocortex (Galaretta et al. 2008; Trettel and Levine 2003; Trettel et al. 2004) and other brain regions such as the hippocampus, cerebellum, and thalamus (Kreitzer and Regehr 2001a; Sun et al. 2011; Wilson and Nicoll 2001), indicating that the suppression of inhibition is mediated by CB1 receptors.
Within the neocortex, CB1 receptor-dependent DSI has previously been reported to occur between irregular spiking interneurons and pyramidal cells (Galarreta et al. 2008). However, fast-spiking interneuron-to-pyramidal cell connections have been shown to demonstrate a CB1 receptor-independent DSI where the involvement of glutamate is proposed (Harkany et al. 2004), whereas a later study has reported fast-spiking interneuron-to-pyramidal connections to be insensitive to a DSI (Galarreta et al. 2008). This dissimilarity could be explained by the different species used (rat vs. mouse). There are also differences in the methods used to induce DSI within the literature, and it may consequently be the case that a particular DSI protocol is not sufficient to induce DSI at a specific interneuron-to-pyramidal synapse; for example, the number and frequency of postsynaptic action potentials used to stimulate DSI within the neocortex affect the degree and duration of suppression (Fortin et al. 2004). However, it seems unlikely that this is the case in the study by Galarreta et al. 2008, since a suppression of inhibition was also not induced by a cannabinoid receptor agonist. Using the same protocol and species in the present study, we report two different types of multipolar nonadapting interneuron-to-pyramidal cell connection: DSI insensitive and CB1 receptor-independent DSI.
Neither the neuroanatomic or electrophysiological properties compared in this study distinguish two groups of multipolar nonadapting interneurons on the basis of their sensitivity to DSI. In the present study we attempted to define the two groups of multipolar interneurons by performing double immunocytochemistry (see Ali 2007) to detect the calcium binding proteins and neuropeptides, but we were unsuccessful. This is a common experimental limitation with long synaptic recordings in the neocortex. However, previous neuroanatomic studies correlated multipolar nonadapting interneurons as PV containing but multipolar adapting interneurons as CCK containing (Kawaguchi and Kubota 1997). It may be that the expression of neuropeptides and calcium binding proteins better defines populations of neocortical interneurons sensitive to DSI and CB1 receptor pharmacology, since the CB1 receptor has been colocalized with CCK-, VIP-, and CBD-expressing cells but not with PV-, calretinin-, or SOM-immunopositive cells (Bodor et al. 2005; Wȩdzony and Chocyk 2009). In addition, VIP and SOM mRNA-containing cells have been reported to coexpress CB1 receptor mRNA (Hill et al. 2007).
It is interesting that particular interneuron-to-pyramidal cell connections are insensitive to DSI, which would suggest a cell type-specific time window for neuromodulation. For example, under high frequency of pyramidal cell activation, the subpopulation of multipolar nonadapting interneurons that were insensitive to DSI will not be able to reduce the presynaptic GABA release via retrograde release of endocannabinoid. One of the reasons why certain interneuronal connections are insensitive to DSI may be a lack of presynaptic CB1 receptors, which could prevent a suppression of GABA release by endocannabinoids. It could also be the case that at certain synapses the ability to synthesize and release endocannabinoids is absent through a lack of “machinery,” which is suggested at neocortical interneuron-to-interneuron connections, since a CB1 receptor agonist reduces inhibition whereas DSI does not, and the intracellular [Ca2+] rise upon depolarization is close to that of pyramidal cells (Lemtiri-Chlieh and Levine 2007). Therefore, in the present study, we cannot exclude the lack of functional presynaptic cannabinoid receptors at DSI-insensitive synapses because it still remains to be determined whether this subpopulation is sensitive to CB1 receptor agonist, which would clarify whether the insensitivity was due to the lack of CB1 receptors or the lack of the endocannabinoid.
CB1-independent DSI at multipolar nonadapting interneuron-to-pyramidal cell connections.
We propose that an alternative receptor or messenger is involved at CB1-independent DSI exhibited by multipolar nonadapting interneurons, perhaps the involvement of another type of CB receptor. The evidence for this in the present study is that all the multipolar nonadapting interneurons studied were sensitive to anandamide, implying that these connections are modulated by the nonselective cannabinoid agonist. As with pairs sensitive to DSI, connections susceptible to modulation by anandamide did not respond to the CB1 receptor antagonist AM-251. Since interneurons with a nonadapting firing pattern often express PV (Kawaguchi and Kondo 2002) and colocalization of the CB1 receptor with PV-expressing neurons was not found in the neocortex (Bodor et al. 2005), the reduction in IPSP amplitude by anandamide may have been caused through means independent of CB1 receptor activation, as has been previously implied in CB1 knockout mice and pharmacological studies (Adams et al. 1998; Di Marzo et al. 2010). For example, the inhibition of voltage-gated calcium channels (VGCCs) may result from anandamide in a CB1 receptor-independent manner through which anandamide directly acts on VGCCs (Lozovaya et al. 2009). Therefore, at synapses devoid of presynaptic CB1 receptors, a CB1-independent inhibition of presynaptic VGCCs by anandamide may lead to a reduction in neurotransmitter release and, consequently, result in a smaller IPSP.
A number of additional targets within the brain have been suggested for anandamide, such as the orphan G protein-coupled receptor GPR55. GPR55 can be activated by anandamide, and interestingly, AM-251 has also been reported to act as an agonist at this receptor (Ryberg et al., 2007). Anandamide can also bind to the CB2 receptor, and with relatively recent published work suggesting the expression of CB2 receptors within the neocortex, it becomes an interesting potential modulator of GABAergic transmission (Ki of 0.27 μM; McPartland et al. 2007, see section 7.2.3). CB2 receptors have been also been reported to modulate synaptic transmission within the entorhinal cortex (Morgan et al. 2009). However, expression of the CB2 receptor in neurons of the CNS is controversial (see Atwood and Mackie 2010).
It is interesting to note that the multipolar adapting interneurons displayed average IPSP amplitudes that were not significantly reduced in the presence of anandamide (9 μM), but all were sensitive to DSI and AM-251. These results imply that modulation by the nonselective cannabinoid agonist may be cell type specific and that 2-arachidonoyl-N-gylcerol (2-AG) is probably the preferred endocannabinoid operating at these synapses. This is consistent with the observations that, unlike anandamide, 2-AG is present at relatively high levels in the CNS (Kondo et al., 1998), which also suggests that 2-AG and anandamide play specific roles.
In summary, we found multipolar adapting interneuron-to-pyramidal cell connections to be sensitive to DSI, although only a subpopulation of multipolar nonadapting interneuron-to-pyramidal cell connections were sensitive. Whereas DSI was blocked by AM-251 at multipolar adapting interneuron-to-pyramidal cell pairs, indicating involvement of the CB1 receptor, AM-251 in addition to AIDA, CPPG, and l-AP4 (50 μM) failed to affect DSI at multipolar nonadapting interneuron-to-pyramidal cell pairs, suggesting it is mediated by neither CB1 receptors nor mGluR1a or group III mGluRs.
GRANTS
This work was supported by Medical Research Council New Investigator Grant GO501263 (to A. B. Ali).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.D.-M. and A.B.A. performed experiments; C.L.D.-M. and A.B.A. analyzed data; C.L.D.-M. and A.B.A. prepared figures; C.L.D.-M. and A.B.A. drafted manuscript; C.L.D.-M. and A.B.A. edited and revised manuscript; C.L.D.-M. and A.B.A. approved final version of manuscript; A.B.A. conception and design of research; A.B.A. interpreted results of experiments.
REFERENCES
- Adams I, Compton D, Martin B. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther 284: 1209–1217, 1998 [PubMed] [Google Scholar]
- Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, Lenz RA. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol 496: 197–209, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB. Postsynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol 98: 861–869, 2007 [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160: 467–479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyíri Mackie K, Ledent C, Hájos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci 25: 6845–6856, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 8: 461–472, 2003 [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Anandamide serves two masters in the brain. Nat Neurosci 13: 1446–1448, 2010 [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Cassano Bebe T, BW, Siniscalchi A, O'Connor WT, Magee P, Tanganelli S, Cuomo V, Antonelli T. Cannabinoid receptor agonist WIN 55,212–2 inhibits rat cortical dialysate gamma-aminobutyric acid levels. J Neurosci Res 66: 298–302, 2001 [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex 17: 163–174, 2007 [DOI] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol 92: 2105–2112, 2004 [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495, 2003 [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdélyi F, Szabó G, Hestrin S. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex 18: 2296–2305, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids 31: 73–82, 2002 [DOI] [PubMed] [Google Scholar]
- Harkany T, Holmgren C, Härtig W, Quershi T, Chaudhry FA, Storm-Mathisen J, Dobaszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Jr, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: involvement of vesicular transporter 3. J Neurosci 24: 4978–4988, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Férézou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical and glutamatergic neurons. J Neurophysiol 97: 2580–2589, 2007 [DOI] [PubMed] [Google Scholar]
- Iball I, Ali AB. Endocannabinoid release modulates electrical coupling between CCK cells connected via chemical and electrical synapses in CA1. Front Neural Circuits 5: 17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Coutts AA, Harvey J, Rae MG, Mackie K, Bewick GS, Pertwee RG. Functional expression of cell surface cannabinoid CB(1) receptors on presynaptic inhibitory terminals in cultured rat hippocampal neurons. Neuroscience 98: 253–262, 2000 [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 1: 4544–4558, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31: 277–287, 2002 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486, 1997 [DOI] [PubMed] [Google Scholar]
- Kondo S, Kondo H, Nakane SS, Kodaka T, Tokumura A, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and -independent mechanisms. FEBS Lett 429: 152–156, 1998 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001a [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21: RC174, 2001b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chilieh F, Levine E. Lack of depolarization-induced suppression of inhibition (DSI) in layer 2/3 interneurons that receive cannabinoid-sensitive inhibitory inputs. J Neurophysiol 98: 2517–2524 2007 [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Min R, Tsintsadze V, Burnashev N. Dual modulation of CNS voltage-gated calcium channels by cannabinoids: focus on CB1 receptor-independent effects. Cell Calcium 46: 154–162, 2009 [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res 40: 205–210, 2001 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11: 4213–4225, 1999 [DOI] [PubMed] [Google Scholar]
- McPartland J, Glass M, Pertwee R. Meta-analysis of cannabinoid ligand binding affinity, and receptor distribution: interspecies differences. Br J Pharmacol 152: 583–593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NH, Stanforf IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 57: 356–368, 2009 [DOI] [PubMed] [Google Scholar]
- Morishita W, Alger BE. Sr2+ supports depolarization-induced suppression of inhibition and provides new evidence for a presynaptic expression mechanism in rat hippocampal slices. J Physiol 505: 307–317, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729–738, 2001 [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152: 1092–1101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Wu CS, Lu HC, Beierlein M. Target-dependent control of synaptic inhibition by endocannabinoids in the thalamus. J Neurosci 31: 9222–9230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol 556: 95–107, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Endocannabinoids mediate rapid retrograde signaling at interneuron→pyramidal neuron synapses of the neocortex. J Neurophysiol 89: 2334–2338, 2003 [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93: 969–975, 1999 [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 15: RC188, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez C, Navarro-Polanco RA, Huerta M, Trujillo X, Andrade F, Trujillo-Hernández B, Hernández L. Effects of cannabinoids on endogenous K+ and Ca2+ currents in HEK293 cells. Can J Physiol Pharmacol 81: 436–442, 2003 [DOI] [PubMed] [Google Scholar]
- Wȩdzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalised with calbindin- but not parvalbumin- and calretinin-positive GABAergic neurons. Pharmacol Rep 61: 1000–1007, 2009 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001 [DOI] [PubMed] [Google Scholar]


