Abstract
An important observation in motor physiology is that even the fastest feedback responses can be modified in a task-dependent manner. However, whether or not such responses in one limb can be modulated based on online sensory feedback from other limbs is still unknown. We tested this using a bimanual postural control task, in which the two hands either controlled two separate cursors (double-cursor task) or a single cursor displayed at the spatial average between the hands (single-cursor task). In the first experiment, the two hands were symmetrically perturbed outwards. In the double-cursor task, the participants therefore had to return their hands to the targets, whereas in the single-cursor task no correction was necessary. Within 50 ms, the electromyographic activity showed significantly smaller responses in the single- compared with the double-cursor task. In the second experiment, the perturbation direction of the left hand (inward/outward) was randomized, such that participants could not preplan their response before perturbation onset. Results show that the behavior of the right arm in the one-cursor task depended on online feedback coming from the left arm: the muscular response was modulated within 75 ms based on directionally specific information of the left arm. These results suggest that sensory feedback from one limb can quickly modify the perturbation response of another limb in a task-dependent manner.
Keywords: feedback control, task dependency, long loop reflex, task difficulty, EMG
the motor system reacts to perturbations with fast feedback responses which have been assumed to be highly automatic (i.e., unconscious and not voluntarily controlled). During postural control tasks, mechanical perturbations to the limb elicit multiple peaks in the electromyographic (EMG) response (Hammond et al. 1956). The earliest of these occurs around 20–45 ms (response 1 or R1) and is usually ascribed to spinal mechanisms. This early response is followed by two responses with longer latencies (responses 2 and 3 or R2 and R3, ∼45–100 ms) and finally a voluntary response (>100 ms). One key observation in motor physiology is that even fast feedback mechanisms are flexible and can be modified in a task-dependent manner. For example, instructing the participant to “intervene” or “not to intervene” results in changes in the EMG response as early as 50 ms after the perturbation, with larger responses for the intervene instruction. Studies over the years have demonstrated that rapid motor responses (RMR) can be modified by different factors like verbal instructions (Shemmell et al. 2009; Weerdesteyn et al. 2008; Crammond et al. 1986; Rothwell et al. 1980; Crago et al. 1976; Evarts and Granit 1976; Hammond 1956), the spatial location and shape of a target (Nashed et al. 2012; Pruszynski et al. 2008), predictability of the perturbation features (Bastiaanse et al. 2006; Goodin and Aminoff 1992; Rothwell et al. 1986), the relevance of the perturbation to task accomplishment (Kimura and Gomi 2009; Bonnet 1983), and the dynamics and stability of the environment (Kimura et al. 2006; Akazawa et al. 1983). All these studies highlight that fast corrective motor actions can be flexibly modulated depending on the behavioral context. Such flexibility is predicted by recent theories of motor control that emphasize the importance of feedback control in the production of voluntary movements (Todorov and Jordan 2002), and the common contribution of primary motor cortex for both the production of voluntary action and long-latency responses (Pruszynski et al. 2011; Scott 2004; Cheney and Fetz 1984; Evartz and Tanji 1976; also see Matthews 1991 for a review).
A dramatic example of the flexibility of feedback control comes from studies showing that perturbations to one part of the body can lead to fast and task-dependent reactions in muscles not directly affected by the stimuli to fulfill the goal of the task (Ito et al. 2005; Gielen et al. 1988; Cole et al. 1984; Marsden et al. 1976). In a seminal study, Marsden et al. (1981) showed that an unexpected pull of the left arm caused a contraction (at a latency of 85 ms) in the right triceps muscle that helped to prevent a postural perturbation by pushing the arm against a supporting table. However, if participants held an unstable object in the right hand (such as a cup of tea), the same pull of the left arm now evoked contraction of the right biceps that helped stabilize the object.
Coordinated feedback responses have been recently studied during a bimanual task (Diedrichsen 2007). In this experiment, participants controlled a cursor using both hands (single-cursor task). The cursor was displayed at the spatial average position of the two hands and had to be brought to a single target. In this condition, a mechanical perturbation to one hand led to corrective responses in both the perturbed and the unperturbed arm within 190 ms. In contrast, when each hand controlled its own cursor (double-cursor task), no feedback response was measured on the unperturbed side. With the use of task instructions that emphasized the importance of keeping the distance between the two hands stable, the direction of the coordinative feedback response could even be reversed (Diedrichsen and Gush 2009). These cases of flexible feedback control are especially interesting, as it is not merely an up- or downregulation of an existing feedback response. Rather, the motor system needs to be able to dynamically use sensory information from one limb to induce a directionally specific feedback response in the other.
The exact timing of these flexible coordinative (cross limb) feedback responses is not known as perturbations in the previous studies were applied gradually (i.e., velocity-dependent force-field, Diedrichsen 2007). Therefore, it is difficult to determine the onset time of these interlimb responses. A recent study used the bimanual single/double-cursor task to investigate the timing of interlimb feedback (Mutha and Sainburg 2009). By applying abrupt perturbations to one of the limbs against the direction of movement, these authors found that the response in the nonperturbed limb can be elicited during the long-latency time period. This experiment illustrates that corrective responses could be elicited quickly during a bimanual task. However, as the direction of perturbation was constant in the task, the appropriate response could be prearranged before movement (Kimura and Gomi 2009; Matthews 1991) and then released upon arrival of the perturbation (see Shemmell et al. 2010 for a review).
Our interest is to identify how quickly directional specific information of the perturbation can be used to modify the response in the other limb in a condition in which the appropriate response could not be preplanned. We used a postural task to determine the timing of a bilateral feedback response. Compared with voluntary reaching tasks, postural tasks have the advantage of permitting less variable quantification of the temporal aspects of rapid motor responses. For example, it has been shown that feedback gains change during the course of the reaching movement (Kimura and Gomi 2009; Kurtzer et al. 2009), whereas they are relatively stable in the postural condition.
In the first experiment, we demonstrate that participants modulate early feedback responses (>50 ms) during a postural task, based on whether the two limbs control separate cursors or a shared cursor. In this case, a preplanning of the appropriate response is possible. We also show that the size of the feedback responses (along with the voluntary responses) is scaled proportionally to the temporal constraint of the task. In the second experiment, we randomized perturbations, such that no response could be preplanned. In this experiment, we also withdrew visual information about the cursor at perturbation onset. The results show that the behavior of the right arm changes in a task-dependent manner within 75 ms based on online proprioceptive feedback coming from the left arm.
METHODS
Participants
A total of 24 individuals (23 males and 1 female, aged 17–38 yr, 21 right-handed) participated in two experiments (8 participants for experiment 1 and 16 participants for experiment 2). All participants were neurologically unimpaired, had normal or corrected-to-normal vision, and gave informed consent according to a protocol approved by the Queen's University Research Ethics Board.
Apparatus and Experimental Paradigm
Participants performed the experiments with a robotic device (KINARM Exoskeleton, BKIN Technologies, Kingston, ON, Canada) that permitted combined flexion and extension movements of the shoulder and elbow to move the hand in the horizontal plane. The robot could also apply joint or hand-based loads (Pruszynski et al. 2008; Scott 1999). Targets and hand feedback (1-cm white circle) were presented to the participant in the horizontal plane via a display composed of an overhead projector and semitransparent mirror (Fig. 1A). A metal barrier under the mirror occluded direct vision of the arm.
Fig. 1.
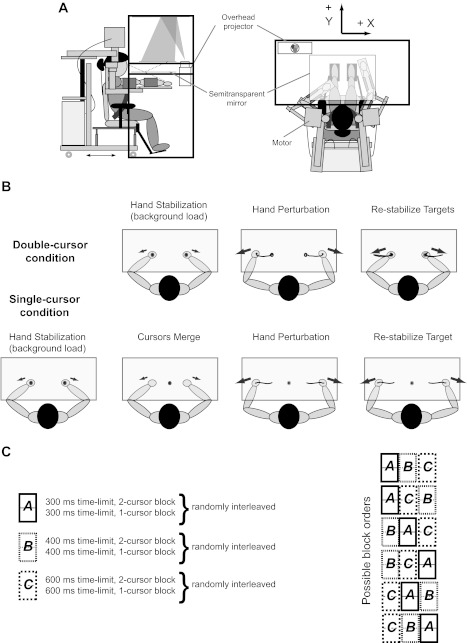
Task apparatus and experimental paradigm. A: participant sitting in the exoskeleton robot (KINARM) with their arms at the level of their shoulders. Targets and the cursors representing the index finger location are projected on a screen that is viewed through a mirror (left: side view; right: top view). B: participant is required to hold the hands in the targets against some background load (hand stabilization). In the double-cursor condition (top row), the hand is perturbed (hand perturbation) and the participant is instructed to bring the cursors back to the targets (restabilize targets). In the single-cursor condition (bottom row), following hand stabilization, the two individual cursors and target circles are replaced with a single cursor and a target circle (cursors merge). The hand is then perturbed and the participant is instructed to bring the cursor to the initial target. C: blocks of trials completed in experiment 1 (left) and possible order of blocks across participant (right).
Experiment 1: Modification of Rapid Motor Responses Based on Bimanual Task
All trials followed the same basic pattern:
In the double-cursor task, the participant was first asked to bring the hand cursors into two targets (1.2-cm diameter, located based on shoulder angle = ∼40° and elbow angle = ∼90°) against background loads (7.3 N), which pushed the hands away from the midline. These background loads were applied to preactivate the shoulder and elbow flexor muscles (Fig. 1B, small arrows show the direction of background loads). The single-cursor task started the same as the double-cursor task. However, at the beginning of the hold phase the two individual cursors and target circles were replaced with a single cursor and a single target circle (Fig. 1B).
Following a variable hold time (1,000–3,000 ms), the two arms were perturbed laterally (1,500-ms step loads with a 10-ms ramp onset, equal and opposite in direction), displacing the participants' hands laterally. The size of the perturbation and its orientation were selected for each participant such that it generated 4–5 cm of hand motion along the x-axis and minimal motion in the y-axis (7.5–13 N).
The participants were instructed to return the cursor(s) to the target(s) within 300 ms and remain there for the rest of the trial (double-cursor, 2-cm acceptance window, single-cursor, 1 cm). Target color after the perturbation signified if the cursor was in or out of the spatial target(s) (IN: solid green; OUT: solid red), and trial success was signified by displaying a 3-cm target color at the end of the trial (green: correct; red: failed). Participants were asked to avoid co-contracting the muscles before the perturbations.
Participants had to perform 30 successful trials in separate blocks (order of blocks was random). Failed trials were repeated in the end of each block. Both failed and successful trials were recorded and included in the data analysis.
We were interested to know whether changing the time limit to return the hand influenced the feedback response for identical perturbations. Thus, in separate pairs of blocks, we repeated the single- and double-cursor tasks with time limits of 400 and 600 ms (random order for pairs of blocks, see Fig. 1C for possible block orders).
Experiment 2: Modification of Rapid Motor Responses by Proprioceptive Feedback from Contralateral Limb
Experiment 2 was designed to identify how quickly online feedback from one limb could influence motor responses in the other limb when the perturbation direction was not predictable. Importantly, no visual information was available instantly following the perturbation to inform the subject of perturbation direction either. Similar to the first experiment, trials were separated into single- or double-cursor blocks. The trial flow always followed the following basic pattern:
As in experiment 1, the participant was first asked to bring the hands into two targets against background loads (6 N). The background load preactivated the flexor muscles of the right arm and the extensor muscles of the left arm. In the single-cursor task, the two individual cursors and target circles were then replaced with a single cursor and a single target circle, respectively, at the beginning of the hold phase.
In mirror-direction perturbation trials, following a variable hold time (1,000–3,000 ms), the two arms were perturbed laterally (1,500-ms step loads with a 10-ms ramp onset) (see Fig. 9, A and B; red lines). In matching-direction perturbation trials, both limbs were perturbed to the right. The same perturbation magnitude (8 N) was used for most subjects (i.e., 14 of 16). The magnitude was selected in a way that generated 4–5 cm of hand motion along the x-axis in an average subject. Slight individual adjustments of perturbation orientation were made to cause the least y-axis motion. In two subjects, the perturbation magnitude was reduced (7 and 6 N, respectively) as they were not comfortable with the original (8 N) perturbation magnitude.
As in experiment 1, the participants were instructed to return the cursor(s) to the target(s) within a fixed time (300 ms) and remain there for the rest of the trial (in this case, 3-cm acceptance window). To make sure the initial perturbation response was generated by using proprioceptive and not visual information, the cursor(s) feedback was turned off for 200 ms following the perturbation. Target color also did not change for the first 200 ms following the perturbation. After this period, the cursor was shown again, and the target color signified if the cursor was in or out of the spatial target (IN: solid green; OUT: solid red). Trial success was signified by displaying a 3-cm target color at the end of the trial (green: correct; red: failed). Participants were asked to avoid co-contracting the muscles before the perturbations.
The perturbation conditions were randomly interleaved so the participants could not predict the direction of the upcoming perturbation. In each block, participants performed 30 trials of each perturbation type (mirror vs. matching). To make sure subjects considered the temporal constraint of the task, they were told that unsuccessful trials would be repeated at the end of the block. However, missed trials were not actually repeated. As in experiment 1, both failed and successful trials were included in the data analysis.
Each participant completed four blocks (two single- and two double-cursor blocks) in an experimental session. The two blocks for each task (single- vs. double-cursor) were done consecutively, but the order was randomized across different subjects.
Fig. 9.
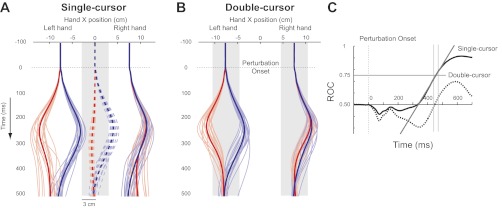
Experiment 2: kinematic results. A: mean horizontal hand paths and cursor positions across all participants in the single-cursor task (red: mirror-perturbation; blue: matching-perturbation. Thin line: single participant; thick line: Mean of all participants). Shaded boxes demonstrate the acceptable target area for each task. B: mean horizontal hand paths across all participants in the double-cursor task. C: mean ROC curves across different conditions (solid: single-cursor; dashed: double-cursor). In the single-cursor task, a line (solid gray) is fitted to a 30 ms period of ROC curve around the time it passes the threshold (0.75, thin vertical grey lines depict the regression margins), and its interception with baseline (0.5) is determined as the time when behavior deviates across tasks. Double-cursor ROC fails to pass the threshold.
Muscle Activity
Electromyographic (EMG) activity of proximal limb muscles was recorded from both limbs in experiment 1 and from the right limb in experiment 2. Specifically, we recorded activity from the brachioradialis (Br, monoarticular elbow flexor), biceps (Bi, biarticular flexor), pectoralis major (PM, monoarticular shoulder flexor), triceps lateralis (TLat, monoarticular elbow extensor), triceps longus (TLo, biarticular extensor), and the posterior deltoid (DP, monoarticular shoulder extensor). Surface EMG electrodes (DE-2.1; Delsys, Boston, MA) were attached on the muscle belly using a standard protocol (see Pruszynski et al. 2008), and the reference electrode (Dermatrode; American Imex, Irvine, CA) was attached to the ankle. We performed a set of maneuvers that elicit high levels of activation for each muscle in the plane of the task to ensure of the quality of the acquired EMG signal. EMG signals were amplified (gain = 10 K) and band-pass filtered (20–450 Hz) by a commercially available system (Bagnoli, Delsys) and then digitally sampled at 1,000 Hz.
Data Analysis
Filtering and normalization.
Perturbation onset time was adjusted by 10 ms to compensate for the time difference from commanded torque to limb movement as calculated using an accelerometer attached to the limb (see Pruszynski et al. 2008). Joint and hand position were obtained directly from the KINARM at a 1,000-Hz sample rate and then low-pass filtered (25-Hz, two-pass, sixth-order Butterworth). EMG signals were band-pass filtered (10- to 150-Hz, two-pass, sixth-order Butterworth) and full-wave rectified. In experiment 1, the EMG of the agonist (loaded) muscles was normalized to the mean preperturbation activity in single-cursor trials with a 400-ms time limit. These trials displayed minimal co-contraction between antagonist muscles (see results). For the antagonist (unloaded) muscles, we show the raw EMG signal (see Figs. 3 and 4), as they were preinhibited in the baseline period. In experiment 2, EMG was normalized to the mean baseline activity of the muscle in the mirror-direction perturbation in single-cursor trials.
Fig. 3.
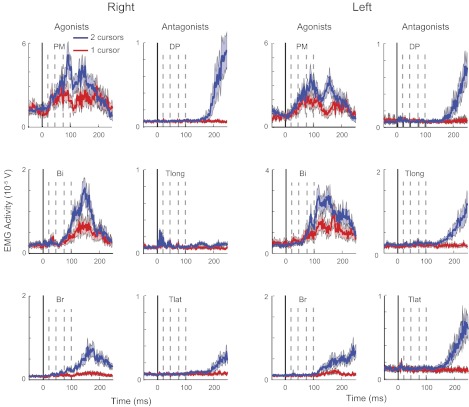
Experiment 1: EMG activity. Muscular activity of the shoulder and elbow muscles of an individual participant in the single-cursor (red) and double-cursor (blue) tasks. As the baseline activity is biased in different directions in the agonists (preexcited) and antagonists (preinhibited), the EMG is not normalized to the baseline activity to keep different muscle groups comparable. Dashed lines show each activity epoch used for EMG analysis. Shaded region denotes SE of EMG activity in each muscle. Br, brachioradialis; Bi, biceps; PM, pectoralis major; TLat, triceps lateralis; TLo, triceps longus; DP, posterior deltoid.
Fig. 4.
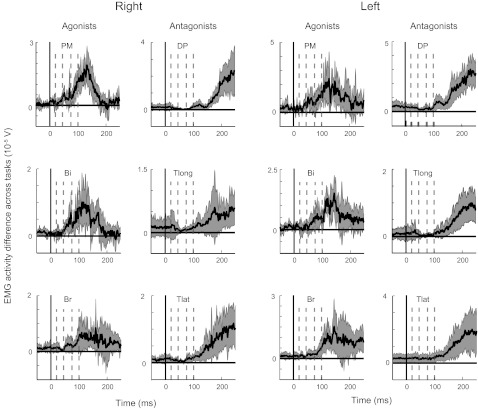
Experiment 1: differential EMG activity across tasks. Differential EMG activity of each muscle between the two tasks averaged across all participants. Dashed lines show each activity epoch used for EMG analysis. Shaded region denotes SE of EMG activity in each muscle.
Behavior and kinematics.
We were interested to know at what point in time the hand motion deviated across different conditions (i.e., single- vs. double-cursor, or 300- vs. 400-ms time limit). We therefore performed a receiver-operator characteristic (ROC) analysis at each time point. For each condition and at any time point, we determined the proportion of trials that exceeded a range of thresholds spanning the two distributions. The number of hits and false alarms was then plotted against each other for every threshold setting. The area under the curve provides a measure of how well the two distributions could be distinguished from each other (Metz 1978). Values of 0 and 1 indicate perfect discrimination (and thus complete separation between the two distributions), whereas a value of 0.5 indicates chance discrimination. Across all time points, we then found the time point (Tcriterion) where the ROC passes a criterion level (set at 0.25 or 0.75). We calculated the time at which the ROC starts deviating from baseline levels by fitting a line (Fig. 2E, the thick solid black line; using linear regression) to a 30-ms period of ROC curve, centered on the Tcriterion (Fig. 2E, the two solid vertical gray lines). In our results, we report the interception point of the regression line with the baseline ROC value (Pruszynski et al. 2008). Note that changing the ROC criterion level did not qualitatively change the result of this analysis. To find the mean deviation time across participants, we performed this analysis on the mean ROC curve across participants (Fig. 2E, thick solid red line). As an alternate approach, we identified the first point in time the hand paths were significantly different across conditions for five consecutive time points using sequential t-tests.
Fig. 2.
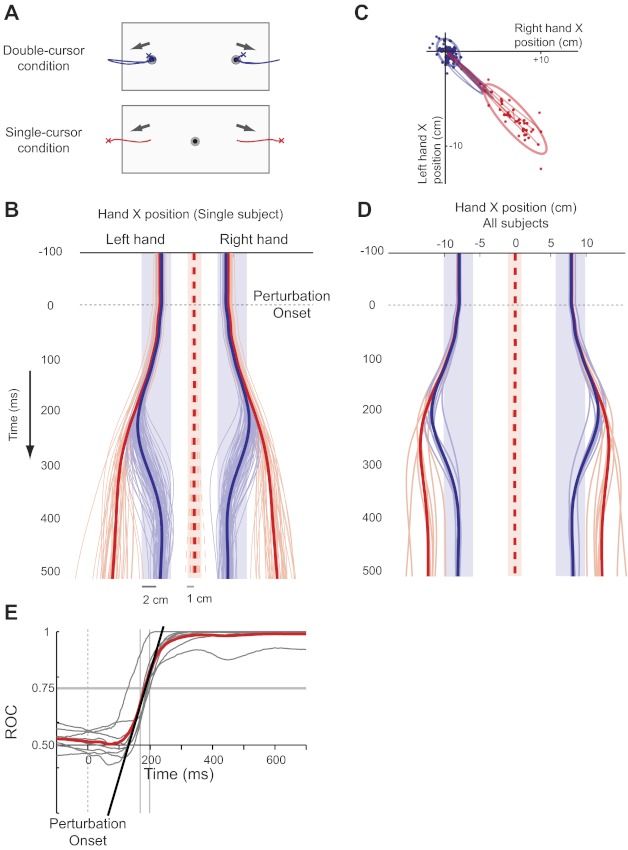
Experiment 1: kinematic results. A: hand motion in the workspace for single trials (crosses mark the hand position at the end of each trial). B: horizontal hand position (solid) and cursor position (dashed) of an individual participant through time (red: single-cursor, blue: double-cursor. Thin line: single trial; thick line: mean position). Shaded boxes demonstrate the acceptable target area for each condition. C: right and left hand positions 450 ms following the perturbation (same participant in B). Thin lines depict five random hand paths in each condition, and the thick line shows 95% confidence ellipse. Note the spread of hand path in the single-cursor task and tight distribution in the double-cursor task. D: mean horizontal hand paths and cursor position across all participants (thin line: single participant; thick line: mean of all participants). E: individual participant (thin gray) and group mean (solid red) receiver-operator characteristic (ROC) curves between single- vs. double-cursor tasks. A line (solid black) is fitted to a 30-ms period of ROC curve around the time it passes the threshold (0.75, thin vertical grey lines depict the regression margins), and its interception with baseline (0.5) is determined as the time when behavior deviates across tasks.
To evaluate interactions between the two limbs, at given time points following the perturbation, we calculated a Pearson correlation coefficient between the right and left hand positions across trials. To conform to the normality assumption of parametric tests, coefficients calculated for each subject was Fisher z-transformed before further statistical analysis. We then compared the z-values, in either tasks with 0 using a one-sample t-test, or compared the two tasks against each other using a paired t-test. Group-average correlations were calculated by averaging the z-values and then applying the inverse Fisher transformation to the mean.
Muscle activity.
Three distinct temporal epochs of time were used to quantify the rapid motor responses to the perturbations up to 100 ms: response 1 (R1, 20–45 ms), response 2 (R2, 46–74 ms), and response 3 (R3, 75–100 ms). The rationale for choosing these specific time epochs is detailed elsewhere (Pruszynski et al. 2008), but generally these timelines closely match M1, M2, and M3 responses as proposed by Lee and Tatton (1975). The R1 epoch is classically referred to as the spinal reflex period, and the R2 and R3 epochs are often referred to as long-latency responses. For statistical comparisons, we also defined two other activity epochs: baseline activity (Base, −200–0 ms) and “voluntary” activity (Vol, 120–180 ms). In our analysis, we use average muscle activity in each epoch to represent that epoch. For statistical comparison of EMGs, we use a nonparametric paired test (one-tailed Wilcoxon signed-rank test), which does not make any assumption about normality of the EMG signal. As each test is based on data from eight participants in experiment 1 and 16 subjects in experiment 2, the degrees of freedom (df) are 7 and 15 for each comparison, respectively. For all the comparisons on the task effect, for each muscle we are doing five statistical comparisons (1 for each epoch). Bonferroni correction for multiple comparisons imposes reducing the significant level to 0.05 over the number of comparisons (i.e., 0.01 in our case). Therefore in every figure, we show the P values <0.05 by one star and any values <0.01 by two asterisks. Therefore, any epoch marked with two asterisks is significant after a Bonferroni correction. We also use ANOVA (performed using SPSS software, IBM) to explore interaction of different variables (e.g., EMG activity and time limit) through our task.
It is known that increases in preperturbation activity of muscles increases the magnitude of perturbation-related activity particularly for the R1 epoch (a phenomenon termed auto-gain scaling, Pruszynski et al. 2009). In the 300-ms time-limit task in experiment 1, the baseline activity sometimes changed across the single- and double-cursor trials (see results). Therefore, to be able to compare the perturbation-related activity across the two tasks, we performed an extra analysis in which we only compared those trials with matched baseline activity (i.e., the average activity 200-ms before the perturbation). We sorted the baseline activity of different trials across the two perturbation types and selected pairs of trials with similar activity. We repeated all our EMG analysis with these subsets of trials (15–25 pairs for each participant) to make sure the effects we observe in our results is not due to co-contraction of muscles before the perturbation. To compensate for loss of trials due to resampling, we collapse similar muscles across limbs to increase statistical power.
RESULTS
Experiment 1: Modification of Rapid Motor Responses Based on Task
The first experiment examined whether and when rapid motor responses of the two limbs are modified by task constraints when the perturbation was predictable. In the double-cursor task, where the hands worked independently, the two hands were perturbed laterally and the participants rapidly returned their hands to the targets in 300 ms (blue lines in Fig. 2). The perturbation caused an ∼4-cm hand movement in the x-axis and minimal movement in the y-axis. In particular, the motion of the two hands was tightly coupled so that their motion back to the initial hand positions occurred simultaneously (Fig. 2, B and C). Similar results were observed across all of our participants (Fig. 2D). There was a relatively low success rate (51%) for this double-cursor task, reflecting the difficulty of returning both hands to the targets within 300 ms.
In the single-cursor task, the hands were perturbed laterally, but the cursor remained within the target (dashed lines in Fig. 2, x-range = 1 mm, SD = 0.6 mm, y-range = 5 mm, SD = 2 mm). This was due to the fact that the perturbations on the two limbs were symmetrically oriented (i.e., equal, opposite and oriented along the x-axis). The size of the corrective movements of the hand (red lines in Fig. 2) varied from trial to trial but was always smaller than observed for the double-cursor task (Fig. 2, B and C). There was also considerable variability in corrective responses across participants, with some participants tending to correct only minimally and others displaying substantial corrections with both hands (Fig. 2D). We then correlated the left and right hand positions 450 ms after perturbation onset across trials within each participant separately and found a strong negative correlation between hand positions [average r = −0.70, t(7)= −9.27, P < 0.0001], which was significantly bigger than that of the double-cursor task [mean r = −0.37, t(7)= −5.37, P = 0.001, paired t-test, t(7)= −3.67, P = 0.006]. This indicates that the feedback responses were coordinated more tightly across the two hands in the single-cursor compared with the double-cursor task. The success rate was also high in the single-cursor task (89%).
ROC analysis across participants demonstrated that the hands moved identically in both the single- and double-cursor tasks immediately following the perturbation, but hand positions diverged at ∼140 ms after perturbation onset (Fig. 2E; 132 ms using ROC analysis, 158 ms based on sequential t-tests). The timing of the separation of hand trajectories was similar for both the right and left hands (right hand: mean = 131 ms, median = 141 ms, SD = 28 ms; left hand: mean = 127 ms, median = 139 ms, SD = 35 ms).
Shoulder and elbow flexor muscles displayed perturbation related activity in both tasks (Fig. 3, same participant as in Fig. 2B). As the shoulder and elbow extensors responded little to the perturbation, we focus our analysis on the mono-articular shoulder and elbow flexors (i.e., PM and Br, respectively). Shoulder flexor muscles (PM) tended to show larger responses than elbow flexor muscles (Br) as the hand-based loads elicited larger torques at the shoulder compared with the elbow. For the participant in Fig. 3, preperturbation and R1 activity in right PM were similar for the single- and double-cursor tasks (P = 0.26 and 0.55, respectively). However, muscle activities were significantly smaller in the single-cursor task in the R2, R3, and Vol epochs (P = 0.001, <0.0001 and <0.0001, respectively).
Figure 4 shows the average difference in the EMG activity in each muscle across tasks, and Fig. 5 shows average activity in each epoch of different participants in each tasks. In general, EMG activity in the single-cursor task was lower compared with the double-cursor task (three-way repeated-measures ANOVA; EMG epoch * muscle * Cursor condition with Participant as a random factor, P < 0.001, df = 1, F = 76). Further analysis showed that all EMG epochs were significantly different across the two tasks (Figs. 4 and 5; R1: P = 0.02 for right PM and P = 0.07 for right Br; P < 0.01 in all other epochs across muscles and limbs). The task effect also showed a significant interaction with EMG epoch meaning that the effect was not the same across different epochs (P < 0.001, df = 4, F = 19.6). Figure 5B shows that difference in response across tasks became more pronounced in later EMG epochs (e.g., ∼20, ∼30, ∼40, and 60% response change, respectively, for R1, R2, R3, and Vol epochs or right PM).
Fig. 5.
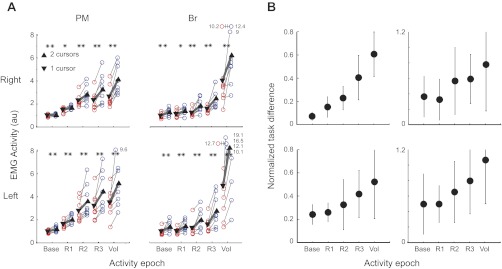
Experiment 1. EMG activity across tasks. A: group average EMG activity in different time epochs [i.e., baseline: 200 ms before perturbation, response 1 (R1): 20–45 ms, response 2 (R2): 46–74 ms, response 3 (R3): 75–100 ms, and voluntary (Vol): 120–180 ms postperturbation]. Each thin line connects the EMG measurements of one participant across tasks (*P < 0.05, **P < 0.01). Triangles show mean epoch activity across all participants, and the thick line shows the mean change across tasks. B: normalized EMG difference across tasks [(double-cursors − single-cursor)/single-cursor]. The difference gets bigger in later EMG epochs. Error bars indicate between-participant SE; au, arbitrary units.
In some cases, the muscle activity in the preperturbation baseline period was larger in the double-cursor task (Fig. 5A; P < 0.05 in four participants for right PM, one-tailed Wilcoxon signed-rank test). This difference in baseline activity suggests that these participants co-contracted their limb muscles in the more difficult, double-cursor task (Fig. 4 shows the differential activity of the antagonist muscles across tasks). This might suggest that the participants chose to increase the stiffness of the arm to oppose the large perturbations in the double-cursor task. However, the antagonist activity is rapidly inhibited in response to the perturbation (note the dip in EMG response starting around R2 in all antagonist muscles). Therefore, it is unlikely that subjects used a generalized stiffening strategy through co-contraction to accomplish the task in the double-cursor condition.
Nevertheless, because preperturbation activity can modulate the size of the initial reflexive response (Pruszynski et al. 2009), it is essential to correct for this factor. As described in methods, we resampled the data by matching pairs of trials (one for each task) with similar baseline activity. ANOVA showed a significant effect for both task condition and task condition combined with EMG epoch (P < 0.001, df = 1, F = 18.8 and P < 0.001, df = 4, F = 15.5, respectively). Further analysis showed that, in the corrected results, the baseline activities were not significantly different (P > 0.3 in both muscles; see Fig. 6). For the Br muscle, only the last three epochs showed significant task-related modulation (P = 0.07 for R1 and P < 0.05 for the other epochs). For PM, however, we still found significant changes in the activity for R1, R2, R3, and Vol epochs (all P < 0.05). Thus our results indicate some modulation of even the earliest phase of the fast feedback response for the bimanual task.
Fig. 6.
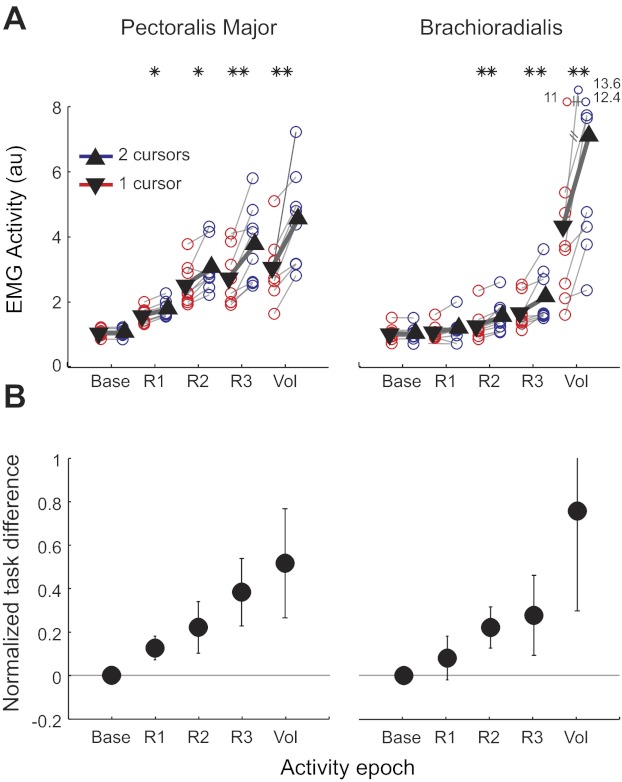
Experiment 1: baseline resampled. Muscular activity after resampling, such that the baseline EMG activity is matched across tasks. A: mean EMG activity in different time epochs (*P < 0.05, **P < 0.01). B: normalized EMG difference across tasks [(double-cursor − single-cursor)/single-cursor] in matched baseline trials. Right and left PM and Br muscle activities are pooled for this analysis. Error bars indicate SE across participants.
As the perturbation is applied at the end point, given the arm geometry of each subject, not all the muscles are equally loaded. Therefore, in some muscles (like Br) it is hard to see whether any muscle activity is evoked in response to the perturbation. We looked into each muscle's activity in response to the perturbation in the double-cursor task and compared it to its baseline. The R1 epoch was significantly larger than baseline in all subjects in all agonist (i.e., PM, Bi, and Br) muscles (paired t-test for each subject; P < 0.01 for all comparisons) except for four subjects in right Br and five subjects in left Br (P > 0.1 in these subjects). The R2 epoch was significantly larger than baseline in all subjects in all agonist muscles (P < 0.01 for all comparisons) except for two subjects in the left Br. At the population level, a one-tailed Wilcoxon signed-rank test showed a significant difference between baseline activity and R1 and R2 epochs in all muscles (P < 0.05 for all muscles) except R1 epoch for left Br (P = 0.6). The main goal of this experiment is to determine when (at the earliest) the EMG response is modulated by task knowledge or sensory information from the other limb, which is addressed by modulation of responses in the PM. Given the nature of perturbation, we have no control over the amount of motion of each individual joint in each individual subject. Therefore, it is not surprising that some muscles do not show the fastest spinal response that depends on joint motion. In fact, observing the task-dependent effects in the long-latency response of the muscles that did not show the R1 response only strengthens our discussion.
Influence of Time Constraints on Motor Responses
We also tested whether a variation of the timing constraints would influence feedback corrections. For the double-cursor task, we predicted that an increase in the time available to correct for the perturbation would diminish the magnitude of the rapid motor response. As expected, increasing the time limit to return the hands to the spatial targets made the task substantially easier, with success rates increasing from 51% for the 300 ms to 83 and 96% for the 400- and 600-ms time limits, respectively. Participants took more time to return the hands to the two targets as the time criteria increased from 300 to 600 ms (Fig. 7A). This effect can be seen by comparing the distribution of hand positions 300 ms after perturbation onset [Fig. 7A, distance from target across all participants (means ± SD): 1.5 ± 1.4 cm for 300 ms, 2.4 ± 1.5 cm for 400 ms, and 3.5 ± 1.7 cm for 600 ms]. ROC analysis demonstrated that the hands moved identically in all three conditions immediately following the perturbation, but hand positions diverged ∼135 ms postperturbation (134 ms for 300 vs. 600 ms and 136 ms for 300 vs. 400 ms).
Fig. 7.
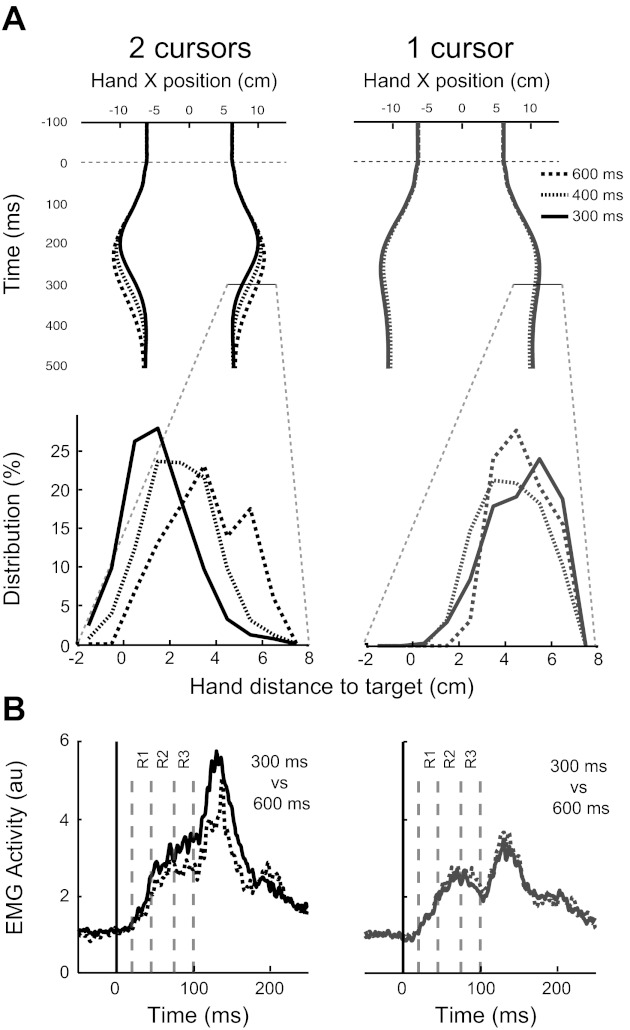
Experiment 1: effect of time limit. A, top row: mean horizontal hand position for each time limit and for all participants (black: double-cursor condition; grey: single-cursor condition). A, bottom row: Hand-position distributions 300 ms following perturbation onset for each time limit. While the distributions show a shift toward the target for the shortest time limit in the double-cursor task, the distributions do not shift for different time limits in the single-cursor task. B: PM EMG for the 300- and 600-ms time limits [or double (right)- and single-cursor (left) tasks].
Time constraint manipulation also altered the perturbation-related muscle activity in the double-cursor task. Figures 7B and 8, A and C, show decreases in EMG activity of the right PM when the time limit was increased from 300 to 600 ms (two-way repeated-measures ANOVA; EMG epoch * Time limit with Participants as a random factor, P < 0.001). The effect of changing time constraints was not the same across different EMG epochs as captured by significant interaction between EMG epochs and time limits (P = 0.001). Figure 8C shows normalized EMG modulation across time limits for different subjects (each epoch was normalized to its value in the 300-ms time limit). This figure shows that later EMG epochs were modulated more by the time limit. When comparing the EMG response in 300- and 600-ms time limits, the baseline period decreased by 13%, R1 and R2 decreased 20%, R3 by 30%, and Vol by 40% (P = 0.07, 0.01, and 0.02 comparing response modulation across R2, R3, and Vol epochs with R1 response modulation, one-tailed Wilcoxon signed-rank test). Similar results were observed for the other muscles (see Table 1 for the P values for each muscle).
Fig. 8.
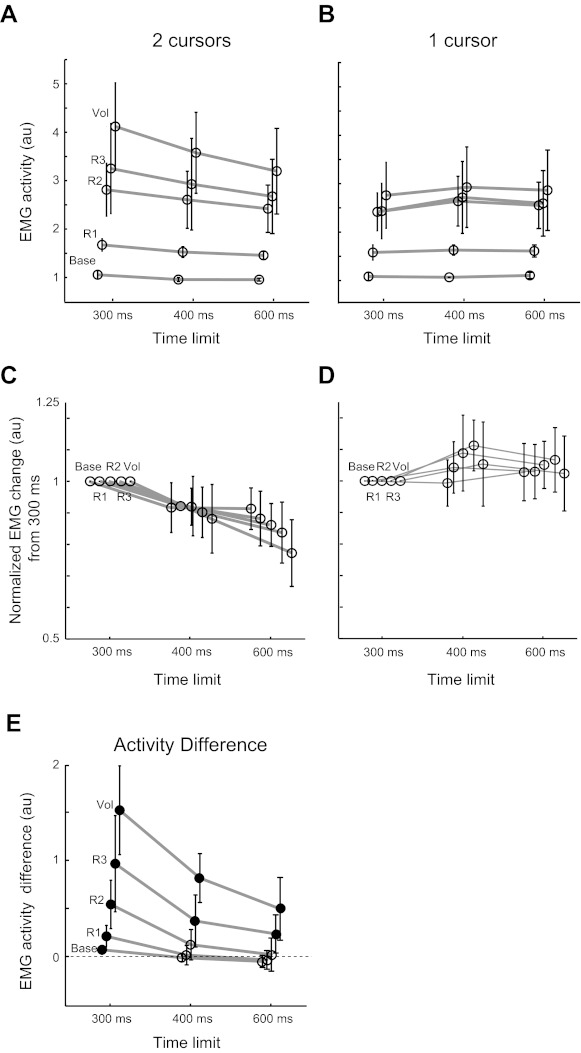
Effect of time limit on EMG modulation; EMG epoch activity across different time limits in the double-cursor task (A) and single-cursor task (B). While in the double-cursor task, EMG activity in response to the perturbation decreases as the time limit increases, the activity remains comparable in the single-cursor condition. C and D: normalized EMG modulation across time limits for different subjects (each epoch was normalized to its value in the 300-ms time limit). E: across the two tasks (single- vs. double-cursor), the difference in the EMG activity also scales with the time limit. Note that this scaling happens across all epochs (in voluntary activity as well as rapid motor responses) with a change in time limit (different activity epochs are minimally shifted horizontally to facilitate visualization of between-participant SE). Filled circles denote significant difference across the two conditions.
Table 1.
P values for the effect of different variables (time, EMG epoch, and task) on muscle response to the perturbation
| Time |
Time * EMG Epoch |
||||
|---|---|---|---|---|---|
| Single-cursor task | Double-cursor task | Single-cursor task | Double-cursor task | Cursor Condition * Time * EMG Epoch | |
| Right PM | 0.13 | <0.001 | 0.67 | 0.001 | <0.001 |
| Left PM | 0.5 | 0.01 | 0.71 | 0.1 | 0.11 |
| Right Br | 0.93 | <0.001 | 0.9 | <0.001 | <0.001 |
| Left Br | 0.56 | 0.002 | 0.99 | <0.001 | <0.001 |
PM, pectoralis major; Br, brachioradialis. P values are by three-way repeated-measures ANOVA.
In contrast, given the high success rate of the single-cursor task with a 300-ms time limit, we predicted little or no change in the feedback corrections when the time limit is increased. Task success did improve slightly (from 89% success rate for 300-ms time limit to 90 and 97% for 400 and 600 ms, respectively), but hand trajectories did not change substantially [Fig. 7A; hand position 300 ms following perturbation onset across all participants (means ± SD): 4.8 ± 1.5 cm for 300 ms, 4.5 ± 1.6 cm for 400 ms, and 5 ± 1.3 cm for 600 ms]. ROC curves (300 vs. 600 ms and 300 vs. 400 ms) did not pass threshold (0.75), and small, late differences in hand position could only be identified using a t-test (divergence time = 263 ms for 300 vs. 600 ms and 255 ms for 300 vs. 400 ms, P < 0.05). Correspondingly, EMG activity for right PM did not show significant differences across different time limits or in interaction with different EMG epochs (Figs. 7B and 8, B and D; two-way repeated-measures ANOVA, P = 0.13 and P = 0.67, respectively). Neither of the other muscles showed a significant change when altering the time limit (see Table 1 for the P values for each different muscle). As expected, there was a significant effect of cursor condition (single- vs. double-cursor) on EMG response across time (three-way repeated-measures ANOVA; EMG epoch * time limit * cursor condition with Participants as a random factor, P < 0.001 for right PM, see Table 1 for the P values of other muscles).
Since increasing the time limit decreased the feedback response in the double-cursor task but not in the single-cursor task, it also impacted the differential perturbation response across tasks. As reported, there were significant differences in all EMG epochs in the 300-ms time limit. However, when the time limit was increased to 600 ms, differences in the right PM activity across tasks was reduced and only significant in the R3 and Vol epochs (Fig. 8E; P = 0.027 and 0.003, respectively). For the right Br, even the slight difference in the Vol epoch was not present (P value in all epochs > 0.1).
Experiment 2: Modification of Rapid Motor Responses by Proprioceptive Feedback from Contralateral Limb
In this experiment, we tested whether corrective responses in one limb are modulated by the direction of the perturbation applied to the other limb. The outward perturbation of the right limb was combined with either an inward (matching-direction condition) or an outward perturbation (mirror-direction condition) of the left limb. The two conditions were intermixed randomly within the same block of trials, such that the upcoming perturbation was not predictable for the participants. Importantly, we withdrew visual information of the cursor at perturbation onset, such that we could ascertain that the feedback responses were driven by proprioceptive feedback.
In the single-cursor task, corrective responses with the right hand were only required for the matching-direction condition to return the (transiently unseen cursor) back to the central target. In this condition, both hands deviated ∼3.5 cm to the right and corrections included simultaneous motion of both hands back to their preperturbation positions (Fig. 9A). In the mirror-direction condition, corrective responses were not required and subjects tended to make smaller corrective responses (Fig. 9A). ROC analysis found that corrective responses with the right hand were different (i.e., smaller) across the two types of perturbations (mirror vs. matching) just after 300 ms [ROC, 335 ± 80 ms (means ± SD); Fig. 9C; sequential t-test, 317 ms]. There was also a clear difference in the difficulty to make corrective responses for the two load conditions as the success rates for the mirror- and matching-perturbations were 98% and 39%, respectively. In the double-cursor task, subjects had to return both hands back to the start positions for both types of perturbations (Fig. 9B). The success rate was lower for the matching- direction compared with the mirror-direction perturbation (25% and 47%, respectively). The corrections with the right hand were found to be similar for the two types of perturbations as the ROC analysis did not pass threshold (Fig. 9C). The kinematic data therefore shows that in the single-cursor task, the corrective response of the right limb is influenced by the proprioceptive information from the left limb. In the double-cursor task, we did not find such a dependence.
To determine when at the earliest task-dependent coordinative feedback occur, we examined the time course of the perturbation-related EMG of the right flexor muscles (PM, Br, and Bi). Figure 10A displays the perturbation-related EMG for the matching and mirror perturbations in the single-cursor task, and Fig. 10B displays the direct paired analysis across load conditions for each epoch. Importantly, some of the differences between the matching and mirror perturbations may be due to mechanical interactions between the arms through the trunk, or they maybe due to hardwired bilateral reflex loops. In this case, we would expect the same difference between perturbation directions to be present in the double-cursor condition (shown in Fig. 10, C and D). To establish the existence of the task-dependent coordinative feedback responses, it is therefore important to investigate the perturbation × task interaction, displayed for each muscle in Fig. 10E.
Fig. 10.
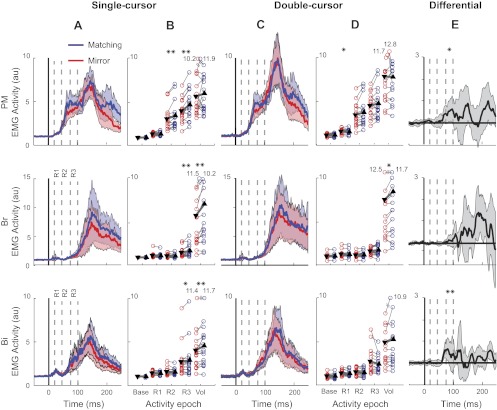
Experiment 2: EMG activity. A: muscular activity of the shoulder and elbow flexors of the right arm in the mirror (red) vs. matching (blue) perturbation conditions across all participants in the single-cursor task. Dashed lines show each activity epoch used for EMG analysis. Shades depict SE of EMG activity in each muscle. B: mean EMG activity in different time epochs. Each thin line connects one participant's EMG activity across tasks (*P < 0.05, **P < 0.01). Triangles show mean epoch activity across all participants, and the thick line shows the mean change across tasks. C and D: average muscle activity and mean epoch activity across conditions in the double-cursor task. E: differential muscle activity across conditions and across tasks [(Matching single-cursor − Mirror single-cursor) − (Matching double-cursor − Mirror double-cursor)] depicting perturbation × task interaction. Just for visualization purposes, each subject's differential signal is smoothed using a 10-ms running average.
The EMG of the right arm muscles in the single-cursor task were not significantly different between the matching and mirror-perturbations for the baseline (P = 0.09, =0.78, and =0.29 for PM, Br, and Bi, respectively) and R1 period (P = 0.39, P = 0.1, and P = 0.23 for PM, Br, and Bi, respectively). For the PM muscle, significant differences emerged in the single-cursor task in the R2 (P = 0.007) and R3 (P < 0.001) periods. Importantly, for the double-cursor task, none of these differences were significant (P = 0.5, P = 0.058, P = 0.23, and P = 0.91 for Base, R2, R3, and Vol, respectively), except for R1, which was significant in the opposite direction (i.e., the mirror was bigger than matching, P = 0.02). The perturbation × task interaction was significant for the R3 period (P = 0.02). The EMG of the right Br muscle was influenced in the single-cursor task by the direction of the perturbation of the left arm in the R3 and voluntary periods (P = 0.004). However, the perturbation × task interaction failed to reach significance for these (P > 0.09 in all epochs). Finally, the right Bi muscle was significantly influenced by the left perturbation in the R3 period in the single-cursor (P = 0.015) but not in the double-cursor task (P = 0.068). The perturbation × task interaction was also significant for the R3 period (P = 0.03). Table 2 summarizes the P values for perturbation × task interactions across all time epochs in all three muscles.
Table 2.
P values for the interaction effect of Task * Perturbation direction
| Base | R1 | R2 | R3 | Vol | |
|---|---|---|---|---|---|
| PM | 0.56 | 0.27 | 0.45 | 0.02 | 0.29 |
| Br | 0.85 | 0.83 | 0.67 | 0.16 | 0.09 |
| Bi | 0.8 | 0.77 | 0.51 | 0.03 | 0.44 |
Base, baseline; R1, R2, R3, responses 1, 2, 3; Vol, voluntary; Bi, biceps. P values are by two * two repeated-measures ANOVA.
DISCUSSION
The results of our experiments demonstrate that in a bimanual task, rapid motor responses within a limb could be modulated by the state of the other limb. More importantly, these bimanual motor responses are not limited to situations in which participants can up- or downregulate existing responses before the onset of the perturbation. Rather feedback about the behavior of one limb (i.e., perturbation direction) could be utilized (or not) to modify the other limb's response to a perturbation within 75 ms in a task-dependent manner.
Our first experiment replicated previous observations that bimanual tasks influence perturbation-related motor responses (Mutha and Sainburg 2009). As the perturbation was always the same in that experiment, participants could simply prepare the appropriate reaction (i.e., respond less in the single-cursor task, and respond more in the two-cursor task) and then release the preplanned response upon arrival of the perturbation (Shemmell et al. 2010). This preplanned response is generally known as “change of functional set” (Klous et al. 2011; Matthews 1991). In essence, therefore, the first experiment resembles the classical intervene or not to intervene manipulation studied extensively in the single limb (Evarts and Granit 1976; Phillips 1969; Hammond et al. 1956). Nevertheless, unlike these studies in which the participants are instructed to resist or yield the perturbation in our experiment the participants were not explicitly instructed to behave differently across the two tasks. Rather, they exploited the inherent redundancy in the single-cursor task by reducing their response to the perturbation. Furthermore, the high correlation between hand positions in the single-cursor task shows that the two hands responded to the perturbation in a coordinated fashion rather than launching a constant preplanned response.
Our second experiment was designed to explicitly test whether afferent information from one limb could be used dynamically and in a task-dependent manner to modify motor responses in the other limb. In this experiment, the perturbation direction of the left limb was not predictable to the participants and no visual feedback was provided for 200 ms following the perturbation. Therefore, the perturbation direction for the right limb and the visual input was identical across perturbation and task conditions. Despite this, our results show that the right response to the perturbation depended on proprioceptive information from the left hand in the single- but not in the double-cursor task. Our results clearly show that responses in the right limb can be modified flexibly and appropriately for the task based on direction-related proprioceptive input from the opposite limb within 75 ms.
Our study extends the results of a recent study in which subjects had to stabilize a simulated tray with two hands (Dimitriou et al. 2012). As in our study, participants showed task-dependent modulation of the responses to unimanual or bimanual perturbations. In this study, the tray was simulated through the forces produced by the robots grasped in each hand, as well as through a visual simulation. In our situation, the physical situation was exactly identical across tasks and the two task only differed in the behavioral goal, which was signaled through the visual scene. Yet, the task-dependent dependence between feedback responses of the two hands was still present, even for mechanical perturbations without vision. Thus our results illustrate that once the behavioral control law is established using only vision, goal-directed corrections can be generated to mechanical perturbations that do not rely on visual feedback on that trial.
The conditional and task-dependent nature of this interlimb feedback response suggests that the motor system is equipped with the ability to flexibly “utilize” sensory feedback from one limb to support rapid motor responses in another limb. The neural pathways through which these flexible responses are implemented are still unknown. Most likely, the flexible feedback process occurs at the cortical level (Ivry et al. 2004; Oliveira and Ivry 2008). Transcallosal connections between the two hemispheres clearly modulate the response to each motor cortex (Duque et al. 2008). For example, a recent study by Yedimenko and Perez (2010) demonstrates that while generating isometric forces with the right index finger, motor evoked potentials in the left index finger (evoked by TMS stimulation of the right primary motor cortex) were suppressed. Furthermore, the suppression was less strong when the direction of the force produced by both fingers was mirror symmetric. Interestingly, by testing the right hand in different postures, the authors showed that it was the direction of force in external space rather than in muscle space that determined the amount of suppression. This finding suggests that the influence does not depend on hard-wired transcallosal pathways between homologous muscles but rather that the feedback from the opposite limb is used in a flexible fashion and varies with the spatial and functional parameters of the task. Whether these modulatory influences are caused by direct connections between the two primary motor cortices, or whether they involve secondary motor regions that may represent high-order aspects of the task (Diedrichsen et al. 2010) is an important question to be addressed in future studies.
Flexible dependency of single limb responses on bimanual information has also been demonstrated for the learning of feedforward control (Jackson and Miall 2008). These studies have used velocity-dependent force fields that perturb an arm movement perpendicular to the movement direction. Many studies have shown that it is very difficult to learn alternating opposing force fields within the same limb (Krakauer et al. 2006; Caithness et al. 2004; Krouchev and Kalaska 2003). However, the state of the other arm can strongly influence the motor system to learn two conflicting force fields (Nozaki and Scott 2009; Nozaki et al. 2006). For example, the movement direction of the other hand (Howard et al. 2010) can modulate within-limb force field learning. Similarly, whether the two hands hold a common object or whether they move alone can also serve as a cue for learning opposing force fields (Howard et al. 2008). Thus, like rapid motor responses, the learning of predictive feedforward control appears to be endowed with the flexibility to utilize the movement state of the other hand to modulate control. This similarity may reflect the intimate relationship between rapid motor responses and voluntary control (Scott 2004).
One novel aspect of our results is that the temporal constraint of the task influenced the size of the rapid motor response (and early voluntary response) to the mechanical perturbation. Participants exhibited large initial motor responses when they had to return to the spatial target within 300 ms, and smaller responses if they were given 600 ms. These results suggest that if the behavioral goal is easy to achieve (as reflected by a change in the success rate), rapid motor responses are reduced overall. On the other hand, when the behavioral goal is hard to achieve (i.e., 300-ms time limit), even the R1 response could show task-dependent changes. Previous work has shown task-dependent modulation of long-latency responses based on spatial location of the target (Pruszynski et al. 2008) or limb mechanics (Kurtzer et al. 2008, 20009), but the short latency responses were not modulated in either of these conditions. The low success rate in the 300-ms condition (∼50%) might have encouraged the participant to increase the gain of spinal reflexes to raise the participant's success rate. It has been shown previously that R1 responses can change based on the behavioral goal of a task independently from the background activity of the muscle (Cheng and Loeb 2008). Yet, it is not clear why such a strategy is not used even in the conditions with less strict temporal constraints. We speculate that delaying the response to later epochs may provide more time for the brain to appropriately assess the nature of the perturbation before launching a motor response. We think the mechanism through which the R1 epoch response is modulated is different than its later counterparts; with the former being affected at the spinal level (Granit 1975) and the latter predominantly at supraspinal levels (Scott, 2004; Pruszynski and Scott 2012, Matthews 1991).
In summary, several studies have shown flexible task-dependent coupling of the two limbs in bimanual tasks. This flexibility can be observed in the feedforward control of the movement (Mechsner et al. 2001; Diedrichsen et al. 2001) and in the reactions to perturbations of each limb (Diedrichsen and Gush 2009; Ohki and Johansson 1999; Lum et al. 1993). Such similarity is congruent with the idea that rapid motor responses utilize the same neural circuitry as voluntary control (Scott 2004, 2008). In a broader sense, our results suggest that the CNS is capable of coupling any arbitrary sensory feedback with a motor output to fulfill the goal of a task (Radhakrishnan et al. 2008; Jeka and Lackner 1994). On the other hand, our finding has important implications for designing experiments to study task dependency of rapid motor responses. Changes in the magnitude of rapid motor responses with regard to temporal constraints of the task imply that if the timing of the task is not appropriately set, it is less likely to observe changes in the earlier components of the response with regard to task constraints.
GRANTS
This work was supported by the National Science and Engineering Research Council of Canada. M. Omrani received salary awards from Vanier Canada Graduate Scholarship and Ontario Graduate Scholarship programs. J. Diedrichsen was supported by a Grant of the Biotechnology and Biological Sciences Research Council.
DISCLOSURES
S. H. Scott is associated with BKIN Technologies, which commercializes the KINARM robot used in this study.
AUTHOR CONTRIBUTIONS
Author contributions: M.O., J.D., and S.H.S. conception and design of research; M.O. performed experiments; M.O. analyzed data; M.O., J.D., and S.H.S. interpreted results of experiments; M.O. prepared figures; M.O. drafted manuscript; M.O., J.D., and S.H.S. edited and revised manuscript; M.O., J.D., and S.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the laboratory of S. H. Scott for intellectual and logistic contributions. Also, we thank Dr. J. A. Pruszynski, Dr. A. Z. Khan, and Dr. G. Blohm for constructive comments.
REFERENCES
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol 49: 16–27, 1983 [DOI] [PubMed] [Google Scholar]
- Bastiaanse CM, Degen S, Baken BC, Dietz V, Duysens J. Suppression of cutaneous reflexes by a conditioning pulse during human walking. Exp Brain Res 172: 67–76, 2006 [DOI] [PubMed] [Google Scholar]
- Bonnet M. Anticipatory changes of long-latency stretch responses during preparation for directional hand movements. Brain Res 280: 51–62, 1983 [DOI] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EJ, Loeb GE. On the use of musculoskeletal models to interpret motor control strategies from performance data. J Neural Eng 5: 232–253, 2008 [DOI] [PubMed] [Google Scholar]
- Cole KJ, Gracco VL, Abbs JH. Autogenic, and nonautogenic sensorimotor actions in the control of multiarticulate hand movements. Exp Brain Res 56: 582–585, 1984 [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976 [DOI] [PubMed] [Google Scholar]
- Crammond DJ, MacKay WA, Murphy JT. Evoked potentials from passive elbow movements. II. Modification by motor intent. Electroencephalogr Clin Neurophysiol 64: 144–158, 1986 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Gush S. Reversal of bimanual feedback responses with changes in task goal. J Neurophysiol 101: 283–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hazeltine E, Kennerley S, Ivry RB. Moving to directly cued locations abolishes spatial interference during bimanual actions. Psychol Sci 12: 493–498, 2001 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci 14: 31–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J. Optimal task-dependent changes of bimanual feedback control, and adaptation. Curr Biol 17: 1675–1679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Franklin DW, Wolpert DM. Task-dependent coordination of rapid bimanual motor responses. J Neurophysiol 107: 890–901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Stefan K, Hummel F, Olivier E, Cohen LG. Memory formation in the motor cortex ipsilateral to a training hand. Cereb Cortex 18: 1395–1406, 2008 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Granit R. Relations of reflexes, and intended movements. Prog Brain Res 44: 1–14, 1976 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex, and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976 [DOI] [PubMed] [Google Scholar]
- Gielen CC, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407: 275–292, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS, Aminoff MJ. The basis, and functional role of the late EMG activity in human forearm muscles following wrist displacement. Brain Res 589: 39–47, 1992 [DOI] [PubMed] [Google Scholar]
- Granit R. The functional role of the muscle spindles–facts, and hypotheses. Brain 98: 531–556, 1975 [DOI] [PubMed] [Google Scholar]
- Hammond PH, Merton PA, Sutton GG. Nervous gradation of muscular contraction. Br Med Bull 12: 214–218, 1956 [DOI] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol 132: 17–18P, 1956 [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. Composition and decomposition in bimanual dynamic learning. J Neurosci 28: 10531–10540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. Context-dependent partitioning of motor learning in bimanual movements. J Neurophysiol 104: 2082–2091, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kimura T, Gomi H. The motor cortex is involved in reflexive compensatory adjustment of speech articulation. Neuroreport 16: 1791–1794, 2005 [DOI] [PubMed] [Google Scholar]
- Ivry RB, Diedrichsen J, Spence RM, Hazeltine E, Semjen A. A cognitive neuroscience perspective on bimanual coordination and interference. In: Interlimb Coordination, edited by Swinnen S, Duysens J. Boston, MA: Kluwer Academic, 2004, p. 259–295 [Google Scholar]
- Jackson CP, Miall RC. Contralateral manual compensation for velocity-dependent force perturbations. Exp Brain Res 184: 261–267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Exp Brain Res 100: 495–502, 1994 [DOI] [PubMed] [Google Scholar]
- Kimura T, Gomi H. Temporal development of anticipatory reflex modulation to dynamical interactions during arm movement. J Neurophysiol 102: 2220–2231, 2009 [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci 26: 9272–9281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klous M, Mikulic P, Latash ML. Two aspects of feedforward postural control: anticipatory postural adjustments, and anticipatory synergy adjustments. J Neurophysiol 105: 2275–2288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: e316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev NI, Kalaska JF. Context-dependent anticipation of different task dynamics: rapid recall of appropriate motor skills using visual cues. J Neurophysiol 89: 1165–1175, 2003 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency responses during reaching account for the mechanical interaction between the shoulder and elbow joints. J Neurophysiol 102: 3004–3015, 2009 [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008 [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions, and in human patients with motor system disorders. Can J Neurol Sci 2: 285–293, 1975 [DOI] [PubMed] [Google Scholar]
- Lum PS, Reinkensmeyer DJ, Lehman SL, A bimanual reflex during two hand grasp. Proceedings of the 15th Annual International Conference of the IEEE San Diego, CA: Med Biol Soc, 1993, pp. 1163–1164 [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain 104: 513–534, 1981 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Servo action in human posture. J Physiol 263: 187–188P, 1976 [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Servo action in the human thumb. J Physiol 257: 1–44, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex, and the motor cortex. Trends Neurosci 14: 87–91, 1991 [DOI] [PubMed] [Google Scholar]
- Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature 414: 69–73, 2001 [DOI] [PubMed] [Google Scholar]
- Metz CE. Basic principles of ROC analysis. Semin Nucl Med 8: 283–298, 1978 [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL. Shared bimanual tasks elicit bimanual reflexes during movement. J Neurophysiol 102: 3142–3155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Influence of the behavioral goal and environmental obstacles on rapid feedback responses. J Neurophysiol 108: 999–1009, 2012 [DOI] [PubMed] [Google Scholar]
- Nozaki D, Scott SH. Multi-compartment model can explain partial transfer of learning within the same limb between unimanual, and bimanual reaching. Exp Brain Res 194: 451–463, 2009 [DOI] [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci 9: 1364–1366, 2006 [DOI] [PubMed] [Google Scholar]
- Ohki Y, Johansson RS. Sensorimotor interactions between pairs of fingers in bimanual, and unimanual manipulative tasks. Exp Brain Res 127: 43–53, 1999 [DOI] [PubMed] [Google Scholar]
- Oliveira FT, Ivry RB. The representation of action: insights from bimanual coordination. Curr Dir Psychol Sci 17: 130–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. The Ferrier Lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci 173: 141–174, 1969 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Scott SH. Optimal feedback control and the long-latency stretch response. Exp Brain Res 218: 341–359, 2012 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling”. J Neurophysiol 102: 992–1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SM, Baker SN, Jackson A. Learning a novel myoelectric-controlled interface task. J Neurophysiol 100: 2397–2408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation, and conditioning of the human long latency stretch reflex. Exp Brain Res 63: 197–204, 1986 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980 [DOI] [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol 586: 1217–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Optimal feedback control, and the neural basis of volitional motor control. Nat Rev Neurosci 5: 532–546, 2004 [DOI] [PubMed] [Google Scholar]
- Scott SH. Apparatus for measuring, and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods 89: 119–127, 1999 [DOI] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol 121: 1680–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics, and verbal instruction. J Neurosci 29: 13255–13263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcheang L, Bays PM, Ingram JN, Wolpert DM. Simultaneous bimanual dynamics are learned without interference. Exp Brain Res 183: 17–25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Laing AC, Robinovitch SN. Automated postural responses are modified in a functional manner by instruction. Exp Brain Res 186: 571–580, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedimenko JA, Perez MA. The effect of bilateral isometric forces in different directions on motor cortical function in humans. J Neurophysiol 104: 2922–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


