Abstract
Excessive synchronous neuronal activity is a defining feature of epileptic activity. We previously characterized the properties of distinct glutamatergic and GABAergic transmission-dependent synchronous epileptiform discharges in mouse hippocampal slices using the 4-aminopyridine model of epilepsy. In the present study, we sought to identify the specific hippocampal neuronal populations that initiate and underlie these local field potentials (LFPs). A perforated multielectrode array was used to simultaneously record multiunit action potential firing and LFPs during spontaneous epileptiform activity. LFPs had distinct components based on the initiation site, extent of propagation, and pharmacological sensitivity. Individual units, located in different hippocampal subregions, fired action potentials during these LFPs. A specific neuron subgroup generated sustained action potential firing throughout the various components of the LFPs. The activity of this subgroup preceded the LFPs observed in the presence of antagonists of ionotropic glutamatergic synaptic transmission. In the absence of ionotropic glutamatergic and GABAergic transmission, LFPs disappeared, but units with shorter spike duration and high basal firing rates were still active. These spontaneously active units had an increased level of activity during LFPs and consistently preceded all LFPs recorded before blockade of synaptic transmission. Our findings reveal that neuronal subpopulations with interneuron properties are likely responsible for initiating synchronous activity in an in vitro model of epileptiform discharges.
Keywords: multielectrode array, seizures, brain slice
excessive or abnormally synchronous neuronal activity in the brain is a defining feature of epileptic activity (Fisher et al. 2005). The in vitro 4-aminopyridine (4-AP) model has been widely used during the last three decades to identify the mechanisms underlying the synchronization of hippocampal and parahippocampal neuronal networks (Voskyul and Albus 1985; Perreault and Avoli 1992; Avoli et al. 1996a; Ziburkus et al. 2006; Uva et al. 2009; Hill et al. 2010; Hazra et al. 2012). However, until recently, it has been difficult to simultaneously study the activity of large populations of neurons from multiple sites in vitro. With the advent of the multielectrode array (MEA), it is now possible to investigate network-level dynamics arising from the single unit activity of distinct neuronal populations in response to pharmacological or electric stimulation (Egert et al. 2002; Geracitano et al. 2005; Giustizieri et al. 2007; Jia et al. 2008; Kent and Meredith 2008; Beretta et al. 2010, 2012).
We recently reported on the pharmacology and electrophysiology of local field potential (LFP) discharges induced by a bath application of 4-AP in mouse hippocampal slices recorded with a perforated MEA (pMEA) (Gonzalez-Sulser et al. 2011). In that study, we identified the presence of two types of spontaneous activity: 1) a frequently occurring, short-duration, glutamatergic transmission-dependent LFP observed only in CA3 and 2) a less-frequent LFP occurring in both the CA3 and dentate gyrus (DG) that displayed multiple components (see materials and methods). The CA3-DG-occurring LFP is largely generated by the activation of GABAA receptors and is resistant to ionotropic glutamatergic receptor (iGluR) antagonists. The exact mechanisms that underlie its occurrence are unknown, but it is likely that the activity of particular subclasses of GABA-releasing interneurons leads to network synchronization through depolarizing GABAA receptor-mediated conductances (Avoli and de Curtis 2011). Many interneuron subtypes have been documented and classified across the hippocampus (Freund and Buzsaki 1996; Maccaferri and Lacaille 2003). Individual interneurons are capable of generating inhibitory field potentials (Bazelot et al. 2010) and can lead to synchronous bursting of CA3 pyramidal neurons in high extracellular K+ concentration (Aradi and Maccaferri 2004). However, the precise contribution of these cells to epileptiform synchronization remains unclear.
The specific aim of the present study was to identify neuronal hippocampal populations that mediate specific LFPs in the 4-AP in vitro model of epileptiform synchronization. Specifically, we found individual units in distinct hippocampal subregions that fired action potentials (APs) differentially during the various components of the LFPs. We also located a group of units that was able to generate spontaneous activity in the absence of both ionotropic excitatory and inhibitory synaptic transmission. By analyzing the relationship between the activity of these groups of units and the distinct types of LFPs during the bath application of 4-AP, we identified subclasses of cells that are likely GABAergic interneurons and presumably underlie the generation of synchronous events recorded during 4-AP application.
MATERIALS AND METHODS
Slice preparation.
C57BL/6J mice (age: 13–18 postnatal days) were killed by decapitation in line with the Animal Care and Use Committee of Georgetown University and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 80-23, Revised 1978). All efforts were made to minimize animal suffering and the number of animals used. Brains were rapidly removed and placed in an ice-cold slicing solution consisting of (in mM) 86 NaCl, 3 KCl, 4 MgCl2, 1 NaH2PO4, 75 sucrose, 25 glucose, 1 CaCl2, and 25 NaHCO3. Horizontal slices (350 μm) containing the hippocampus and the adjacent entorhinal cortex were prepared using a Vibratome 3000 Plus Sectioning System (Vibratome, St. Louis, MO). Slices were cut to 6.5 mm-sided squares to fit the pMEA chamber (Multi Channel Systems, Reutlingen, Germany). These slices contained the ventral section of the hippocampus proper, the adjacent subiculum, and part of the entorhinal cortex. These slices were placed in artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 4.5 KCl, 1 MgCl2, 10 glucose, 1 CaCl2, and 26 NaHCO3 at 32°C for 30 min to recover; afterward, they remained in aCSF at room temperature until used in experiments. All solutions were maintained at pH 7.4 by continuous bubbling with 95% O2-5% CO2.
Chemicals.
We studied the effects of specific drug treatments with the goal of assessing the presence of single unit activity in hippocampal slices during synaptic transmission blockade. We abolished glutamatergic transmission with a concurrent application of the N-methyl-d-aspartic acid (NMDA) receptor antagonist 3,3-(2-carboxypiperazine-4-yl)propyl-1-phosphonate (CPP) and the 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX). We blocked GABAA receptor signaling with an antagonist, bicuculline methobromide (BMR). Drugs were perfused over the slice by gravity at a rate of 3 ml/min. Chemicals were acquired from Sigma-Aldrich Canada (Oakville, ON, Canada), Tocris Bioscience (Ellisville, MO), and Ascent Scientific (Princeton, NJ).
pMEA recordings.
The pMEA system consists of 60 platinum electrodes, each with a 30-μm electrode diameter and a 200-μm inter-electrode spacing. The pMEA substrate is perforated to allow suctioning of the brain slice by activation of a constant vacuum pump with pressure control (Multi Channel Systems). The resulting continuous negative pressure onto the slice facilitates close contact with the electrodes, thus improving multiunit activity (MUA) acquisition. Recordings were sampled at 10 kHz, and data were acquired with either a MEA-1060 or MEA-2100 preamplifier controlled by MC Rack (4.0, Multi Channel Systems). All recording solutions were continuously preheated to 32°C before they reached the MEA chamber with a heated perfusion cannula (ALA Instruments, Farmingdale, NY). Temperature in the MEA tissue chamber was verified with an analog YS1 Telethermometer (Yellow Springs Instrument, Yellow Springs, OH).
Induction of epileptiform activity and recording sequence.
We induced spontaneous epileptiform discharges by superfusing slices with aCSF-containing 4-AP (100 μM) for a minimum of 20 min before recordings were started to allow for the establishment of spontaneous LFP activity. Data were derived from 11 brain slices from 10 mice that were selected for recording stability on the pMEA. All experiments began with a 6-min recording in aCSF + 4-AP followed by a 6-min session in aCSF + 4-AP + glutamatergic antagonists (CPP/NBQX). Experiments finished with a 6-min session in aCSF + 4-AP + glutamatergic antagonists (CPP/NBQX) + the GABAA receptor antagonist BMR.
Analysis of MUA and LFPs.
We analyzed the final 3 min of data recorded under each pharmacological condition, at which point network activity displayed stable dynamics. Voltage traces from each electrode were imported into Spike2 (version 7.05, Cambridge Electronic Design, Cambridge, UK). LFPs were extracted via a second-order low-pass Butterworth filter with a cutoff frequency of 5 Hz. MUA was extracted via a second-order high-pass Butterworth filter with a cutoff frequency of 500 Hz.
Single unit activity was extracted for 195 cells in the CA3, DG, and hilus in a subset of 7 hippocampal slices. On average, 34 ± 2 channels/slice were located over the somatic and dendritic layers of CA3 pyramidal and DG granule cells as well as the hilus; 43 ± 4% of these channels displayed unit activity, resulting in an average of 28 ± 4 cells recorded per slice from the area of interest.
Single unit activity was first extracted in the presence of CPP/NBQX. The occurrence of low-frequency LFPs recorded in this condition facilitated the detection of clean, single unit waveforms that were not distorted by the concomitant field potential activity. A positive or negative threshold was selected for spike sorting based on which polarity created the largest number of waveform crossings. Spike detection was performed on MUA with a ±5SD threshold from the total average amplitude of the recording. Once determined, this threshold was used to detect the same units in the 4-AP and 4-AP/CPP/NBQX/BMR conditions.
Spike sorting.
Unit heterogeneity produced spikes with different shapes and amplitudes that allowed us to discriminate and sort APs presumably generated by individual units. Custom-made scripts written in Spike2 were created for an automated initial pass of spike sorting of all channels of interest. Specifically, spikes were first mapped into an n-dimensional space with a mixed strategy of principal component analysis and amplitude measurements. These components were sorted using a K-means clustering algorithm to obtain independent clusters (see Fig. 2D). The K-means algorithm chooses the solution that maximizes the variance of the distance between all clustered points and the center of its cluster. Cluster separation was quantified as J3, which is given as J2/J1, where J2 is the sum of the squares of the distances from the centroid of all the points and J1 is the sum of the squares of the distances of each point from the centroid of the cluster it belongs to. Higher J3 values indicate better cluster separation.
Fig. 2.
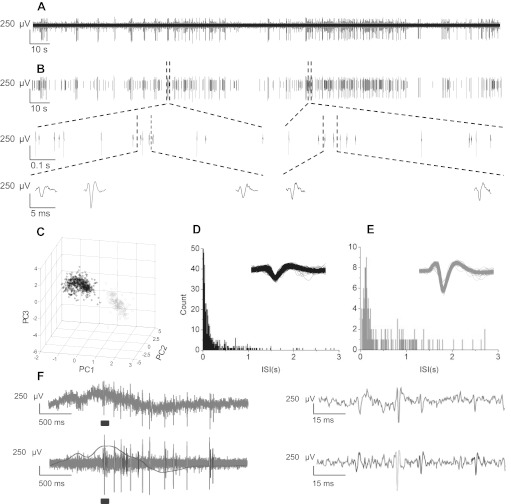
Resolution of single unit activity via spike sorting. A: example of high pass-filtered multiunit activity recorded in CA3. B: single unit activity resolved via spike sorting with principal component analysis. Two distinct cells are indicated (in black and gray). Two portions of the trace are expanded to show waveforms on 1-s and 50-ms timescales. C: waveforms from the two cells plotted according to the first three principal components of the waveform (PC1–PC3). D and E: frequency distributions of interspike intervals (ISIs) from example sorted cells shown in B. Inset, overlay of all detected waveforms. F, left: raw data trace (top) and high pass-filtered spike-sorted action potentials (APs) with low pass-filtered field potential overlayed (bottom). Right, expanded view of times over bars displaying single APs and how they are sorted. The example shows AP traces during LFP containing no refractory period infractions or collisions between spikes from cells 1 and 2.
The absolute refractory period for AP firing should cause the AP frequency to drop to zero below a minimum interspike interval, which, in similar studies, has been assessed to be ∼4 ms (Hill et al. 2011). To avoid cell refractory period violations, Spike2 cluster analysis software was used to identify pairs of spikes with intervals of <4 ms in duration (see Fig. 2F). Channels with a ratio of >1–10 refractory period violations to spike number were eliminated from the analysis. The remaining channels were manually corrected to establish clusters free of intervals of <4 ms in duration. Peak spike amplitude was monitored for stationarity and remained constant. Comparisons of the waveforms and clusters were made across the three experimental conditions to improve accuracy between drug treatments.
Analysis of LFP initation and propagation.
As in our previous study (Gonzalez-Sulser et al. 2011; see also Egert et al. 2002), we used Matlab (MathWorks, Natick, MA) to modify the analysis program MEA-Tools to calculate the frequency, amplitude, initiation, and propagation of LFPs. The initiation and propagation of events in different areas were determined by constructing contour plots from raw data files at 1 frame/sampling point (10 kHz) from each of the 60 channels plotted simultaneously using Analysis Rack within MC Rack software. This procedure was followed by an interpolation analysis using Movie Tool in MEATools with a five-point Savitzky-Golay filter before export to JPEG image format. Individual JPEGs were then concatenated and converted in AVI animations using ImageJ (Abramoff et al. 2004). We determined LFP start times by superimposing voltage traces obtained from three electrodes in the DG molecular and granule layers and by comparing them with a superposition of traces obtained from three electrodes in the CA3 pyramidal and dendritic layers. Start times, which never exceeded a 50-ms difference between channels, were derived from the average interval between the times at which each of the superimposed traces changed by >20% during the LFP. LFPs were classified depending on the site of origin as well as the degree of propagation across the slice (see Fig. 3A). However, as CA3-originating LFPs always propagated to CA1/CA2 areas, we did not consider this propagation in our LFP nomenclature. LFPs that were more frequent and initiated in the CA3 subfield were classified as CA3-restricted LFPs if they were predominantly present in CA3 and not in DG. They are referred to as LFPR-CA3 throughout this report. LFPs that propagated from CA3 to DG were classified as LFPP-CA3/DG (see Fig. 3A). LFPP-CA3/DG typically consisted of an initial CA3-only-like component (LFPP-CA3) followed by a long-duration, high-amplitude LFP afterdischarge present in the DG and, to a lesser extent, in CA3 (LFPP-DG), as shown in the examples in Fig. 3, A and B. Consequently, the start times of both LFPP-CA3 and LFPP-DG were determined and used for analysis. In most slices, the frequency of events was constant and no well-defined pauses were observed between these sequences, as reviewed by Avoli and de Curtis (2011). However, in some experiments, we recorded long pauses between sequences of LFPs, which may account for our lower frequency of LFPs compared with previous work.
Fig. 3.
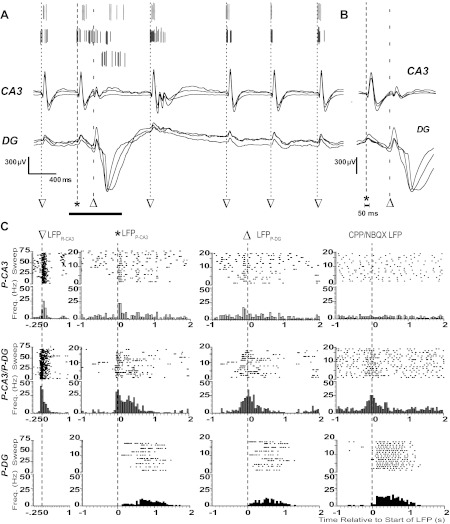
Neuronal spiking dynamics during epileptiform LFPs. A: spike-sorted APs from three example units (top) and superimposed local field potentials from three channels in CA3 (middle) and DG (bottom). Start times of LFPR-CA3 (▽), LFPP-CA3 (*), and LFPP-DG (△) are indicated with dashed lines. B: superimposed LFPs from the expanded time over the horizontal bar in A. Note that LFPP-CA3/DG and LFPR-CA3 were characterized by their presence and absence in DG, respectively. The start time of synchronous field potentials did not differ between adjacent electrodes by >50 ms, as indicated. C: raster plots for consecutive sweeps and peristimulus time histograms (PSTHs) of spike frequencies within bins centered around the start time of the indicated LFP for the three single units in A. The bin size was 50 ms for all PSTHs. Start times of LFPR-CA3, LFPP-CA3, LFPP-DG, and the CPP/NBQX LFP are indicated with dashed lines and symbols. Note that histograms show representative P-CA3 (top), P-CA3/P-DG (middle), and P-DG (bottom) units in A.
Construction of peristimulus time histograms.
Peristimulus time histograms (PSTHs) with a 50-ms bin size were generated in Spike2 for single units using the various LFP start times as a reference. This bin size was selected since the difference in LFP start times between the channels was never >50 ms and therefore provided an accurate measurement of when the field actually began across many channels (see Fig. 3B). The peak AP frequency of the PSTH was determined from the highest frequency calculated using 50-ms binning in 1.25-s windows for LFPR-CA3, which occurred more frequently, with an offset of 250 ms from the start time of the LFP. A 3-s window for LFPCPP/NBQX with a 1-s offset was used. We used variable-length windows with a first limit of 1 s before or after LFPP-CA3 and LFPP-DG, respectively, and the other limit set at half of the time interval between the two LFPs. These windows were also used to determine the other parameters characterizing PSTHs.
Patch-clamp recordings.
We performed dual loose-patch recordings with two Axopatch 1D amplifiers (Molecular Devices, Sunnyvale, CA) from visually identified pyramidal and granule neurons to identify their firing patterns during LFPs. Whole cell voltage-clamp recordings were performed from individual granule neurons at a holding voltage of −60 mV. Electrodes (3–5 MΩ) for patch recordings were pulled (PP-83, Narishige, Tokyo, Japan) from borosilicate glass capillaries (Drummond, Broomall, PA). No fire polishing or Sylgard coating was used. Loose cell-attached electrodes were filled with aCSF, whereas whole cell recording electrodes were filled with either (in mM) 145 K-gluconate, 1.1 EGTA, 5 MgATP, 0.2 NaGTP, and 10 HEPES at pH 7.2 with KOH or (in mM) 120 Cs-methanesulfonate, 5 NaCl, 10 tetraethylammonium (TEA)-Cl, 10 HEPES, 4 lidocaine N-ethyl bromide (QX-314), 1.1 EGTA, 5 MgATP, and 0.2 NaGTP at pH 7.2 with CsOH. Access resistance (<20 MΩ) was monitored but not compensated for. Correction for liquid junction potential (16 mV) was performed when indicated. Current and voltage signals at the head stage of the patch-clamp amplifier were filtered at 2 kHz with a low-pass Bessel filter and digitized at 5–10 kHz using a personal computer equipped with a Digidata 1322A data-acquisition board and pCLAMP 10 software (Molecular Devices). These signals were also acquired with two analog channels of the MCS amplifier MEA-2100, which allows for positioning of multiple external recording electrodes.
Statistics.
Normality was assessed for each group using the Kolmogorov-Smirnov test. Two-way ANOVA was used to compare LFP frequency, duration, initiation, and propagation differences between LFPs before and after pharmacological manipulation followed by Tukey post hoc testing. Paired t-tests were used to compare frequency and amplitude differences with iGluR blockade within each area. To assess between-group differences in parametric PSTH parameters, such as frequency and duration, we used one-way ANOVA with a post hoc Bonferroni correction. For nonparametric PSTH parameters, such as start time, baseline firing rate, and AP half-width and peak-to-valley measurements, we used Kruskal-Wallis ANOVA to determine significance followed by a Mann-Whitney U-test to assess the differences between specific sample pairs.
RESULTS
Using a pMEA, we recorded 4-AP-induced epileptiform activity in acute hippocampal slices (Fig. 1, A–C). In the present study, we expanded our experiments from the isolated hippocampus [coronal slice preparation (Gonzalez-Sulser et al. 2011)] to a horizontal hippocampal slice preparation. This preserves cortical inputs and is therefore a more frequently used in vitro model of temporal lobe epilepsy (Kaila et al. 1997; Barbarosie and Avoli 1997). The MEA grid of 60 electrodes (Fig. 1D) allowed us to simultaneously record LFPs and MUA from CA1, CA3, and DG regions of the hippocampus. Consistent with our previous results, we observed two distinct populations of LFPs (Fig. 1A), which initiated predominantly in CA3 (Fig. 1E) and were distinguished by their propagation patterns into DG (Gonzalez-Sulser et al. 2011). The first, a frequent, short-duration LFP (LFPR-CA3; triangles in Fig. 1A) originated predominantly in CA3 (Fig. 1E) and was restricted to the hippocampus proper (CA3/CA2/CA1). The second, a less frequently occurring LFP (LFPP-CA3/DG; diamonds in Fig. 1A) originated in CA3 and propagated to both DG and CA2/CA1. As previously observed, LFPR-CA3 was significantly more frequent than LFPP-CA3/DG (P < 0.05; Fig. 1F). These results extend our previous findings to the horizontal slice (Gonzalez-Sulser et al. 2011).
Fig. 1.
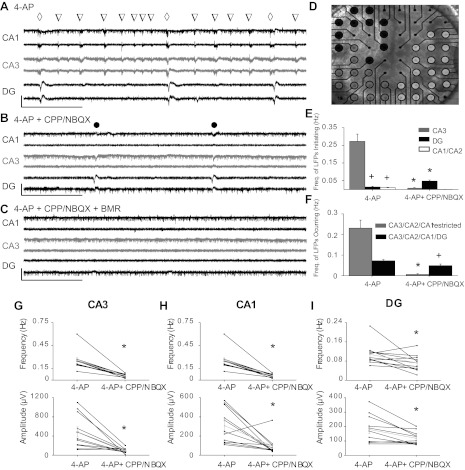
Effect of synaptic transmission blockade on 4-aminopyridine (4-AP)-induced local field potentials (LFPs) and single unit activity. Representative traces obtained from three electrodes in the CA1 (black, top) CA3 (gray, middle), and dentate gyrus (DG; black, bottom) are shown under the three pharmacological conditions: 4-AP (A), 4-AP + 3,3-(2-carboxypiperazine-4-yl)propyl-1-phosphonate (CPP)/2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) (B), and 4-AP + CPP/NBQX + bicuculline methobromide (BMR) (C). The open inverted triangles indicate LFPs detected only in CA3 (LFPR-CA3); the open diamonds indicate LFPs detected simultaneously in CA3 and DG (LFPP-CA3/DG); the solid circles indicate CPP/NBQX LFPs. Scale bars indicate a 500-μV y-axis and a 9-s x-axis. D: photograph of a corticohippocampal slice over a perforated multielectrode array (pMEA) with an indication of the assigned hippocampal subregion for each electrode overlaid in distinct colors: CA3 (gray), DG (black), and CA1/CA2 (white). The hilar region was lumped with DG for this analysis and is not labeled. E: quantification of the frequency of events initiating in the CA3 or DG in the absence or presence of CPP/NBQX. +P < 0.05 vs. DG LFPs; *P < 0.05 vs. 4-AP LFPs. F: frequency of CA3/CA2/CA1-restricted and CA3/DG-occurring LFPs in the absence or presence of CPP/NBQX. LFPs were analyzed from all electrodes in each area (at least 5 electrodes/area, including both pyramidal or granule cell layers and dendritic layers) and averaged across 11 slices. +P < 0.05 vs. CA3/DG LFPs; *P < 0.05 vs. 4-AP LFPs. G–I: average LFP frequency (top) and amplitude (bottom) recorded in CA3 (G), CA1 (H), and DG (I) in the absence and presence of CPP/NBQX. Each solid line indicates an average of 3 electrodes/subfield; shaded lines indicate overall averages across the 11 slices. *P < 0.05.
To examine the synaptic events underlying these LFPs, we blocked iGluR signaling using the NMDA- and AMPA-type iGluR antagonists CPP (20 μM) and NBQX (20 μM), respectively (Fig. 1B). iGluR blockade significantly decreased the frequency and amplitude of LFPs in all hippocampal regions (Fig. 1, G–I), preserving only a longer-duration LFP (CPP/NBQX LFP; solid circles in Fig. 1B). The addition of the GABAA receptor antagonist BMR (25 μM) to glutamatergic blockade abolished all LFPs, although MUA persisted (Fig. 1C). Interestingly, iGluR blockade altered the origination patterns of the LFPs by significantly decreasing the frequency of CA3-initating LFPs while significantly increasing the frequency of DG-originating LFPs (Fig. 1E). The occurrence of LFPR-CA3 was significantly reduced by iGluR blockade, whereas the frequency of LFPs propagating to DG was not significantly different (Fig. 1F).
Taken together, our results confirm the existence of two distinct LFPs that tend to initiate in CA3: 1) LFPR-CA3, which requires ionotropic glutamatergic transmission, and 2) LFPP-CA3/DG, which typically initiates in CA3 in the presence of ionotropic glutamatergic transmission but which originates in DG and persists at a lower amplitude after iGluR blockade (CPP/NBQX LFP). LFPP-CA3/DG had two components: LFPP-CA3 and LFPP-DG (see Fig. 3B), with an average time interval between the components of 178 ± 15 ms (n = 7 slices).
Single unit activity and LFPs.
To elucidate the mechanisms underlying ionotropic glutamatergic transmission-dependent and -independent LFPs, we examined MUA in detail to study the AP firing of individual neurons. We high- and low-pass-filtered recordings from individual channels to extract distinct single units and LFPs, respectively (Fig. 2F, bottom left). MUA (Fig. 2A) was resolved into single unit activity (Fig. 2B) via offline spike sorting (Fig. 2, C–E). Single unit activity was extracted for 195 cells in the CA3 (n = 101), DG (n = 67), and hilus (n = 27). To examine the single unit firing behavior of the neuron with respect to the various fields (Fig. 3A), we constructed PSTHs from raster plots of single unit spiking centered on the start time of the field of interest. For each single unit, we examined firing patterns with respect to LFPR-CA3, the two LFP components of propagating LFPP-CA3/DG (LFPP-CA3 and LFPP-DG; Fig. 3B), and CPP/NBQX LFP.
By examining the firing patterns of individual neurons with respect to the various fields, we grouped the units into three classes (Fig. 3C). Certain units that fired robustly during LFPR-CA3 also had large increases in AP frequency with LFPP-CA3 but displayed either a small increase (<60%) or no increase in firing with LFPP-DG (Fig. 3C, top). These units were accordingly classified as “P-CA3 units” (n = 69; Fig. 3C, top). LFPR-CA3-responding neurons that had similar increases in AP frequency with both LFPP-CA3 and LFPP-DG were termed “P-CA3/P-DG units” (n = 46; Fig. 3C, middle). Neurons that displayed a large increase in AP frequency with LFPP-DG but not with LFPP-CA3 or LFPR-CA3 were separated as “P-DG units” (n = 42; Fig. 3C, bottom). Cells that had no increase in AP frequency with any of the LFPs or that did not spike during iGluR blockade were not included in this analysis.
We also observed significant differences in baseline firing rates and cellular locations between the three groups of neurons. In recording periods without LFPs, P-DG units had a significantly lower baseline firing rate compared with P-CA3 and P-CA3/P-DG units (0.42 ± 0.11 vs 1.07 ± 0.17 and 1.01 ± 0.19 Hz, respectively, P < 0.05). P-DG units were more abundant in DG (60%; Fig. 4A), whereas P-CA3 and P-CA3/P-DG units were more prevalent in CA3 (54% and 69%, respectively). These differences were not due to recording bias: there were no significant differences in numbers of electrodes located in CA3 or DG per slice (7.1 ± 0.9 and 5.7 ± 0.8, respectively). This is consistent with LFPR-CA3 and LFPP-CA3 initiation in CA3 and LFPP-DG occurring in DG (Fig. 1F), which suggests that neurons in a particular hippocampal location have a higher probability of firing APs with LFPs occurring in those structures.
Fig. 4.
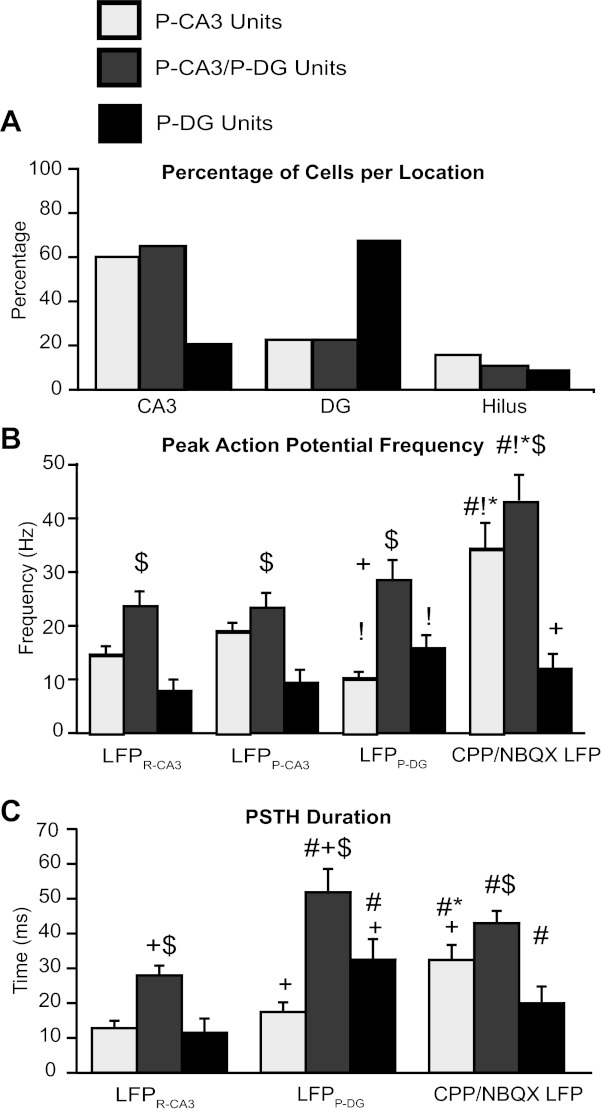
Spiking dynamics of P-CA3, P-CA3/P-DG, and P-DG units during epileptiform LFPs. A–C: quantification of P-CA3 units (light shaded bars), P-CA3/P-DG units (dark shaded bars), and P-DG units (solid bars) firing patterns with respect to the different LFPs. Single unit activity was described in terms of the percentage of cells per location (A), peak AP frequency (B), and duration (C). Note that units were not included in the statistical analysis when there was no PSTH response to that particular field. +P < 0.05 vs. CA3-P units; $P < 0.05 vs. DG-P units; #P < 0.05 vs. LFPR-CA3, !P < 0.05 vs. LFPP-CA3; *P < 0.05 vs. LFPP-DG.
To determine the activity of these three groups of neurons during LFPs, we quantified the magnitude (Fig. 4B) and duration (Fig. 4C) of the changes in AP frequency of these groups with respect to the various LFPs (Fig. 4). Most P-CA3/P-DG units displayed robust increases in AP frequency with each of the four distinct LFPs (Fig. 4B, dark shaded bars), unveiling their synchrony with these LFPs. The increased firing of P-CA3/P-DG units around the fields persisted for longer than any other cell type (Fig. 4C). In contrast, <55% of P-DG units displayed changes in AP frequency with LFPs. Those that did had significantly lower firing frequencies (Fig. 4B, solid bars) in response to the four distinct LFPs, with the most robust and longest-lasting responses occurring in response to LFPP-DG (Fig. 4, B and C). P-CA3 units increased AP frequency with LFPR-CA3 and LFPP-CA3 and had large and long-lasting increases of AP frequencies with the CPP/NBQX LFP, similar to P-CA3/P-DG units. However, in contrast to P-CA3/P-DG units, <60% of P-CA3 cells displayed changes in AP frequency with CPP/NBQX LFP. The highly variable interpeak frequency of AP firing during this interval prevented a reliable measurement of the duration of LFPP-CA3 PSTHs for the three groups of cells. In summary, peak PSTH frequencies of P-CA3 units were significantly higher for LFPP-CA3 than for LFPP-DG and vice versa for P-DG units. Peak frequencies of P-CA3/P-DG units did not differ between the two components of LFPP-CA3/DG (Fig. 4B). Most importantly, as shown in the example P-CA3/P-DG unit in Fig. 3C, middle, AP firing persisted in the time interval between LFPP-CA3 and LFPP-DG. In the time interval between LFPP-CA3 or LFPP-DG, the AP frequency of P-CA3/P-DG units did not drop below 76 ± 5% of the peak frequency with LFPP-CA3 and 67 ± 3% of the peak frequency with LFPP-DG. In contrast, the AP firing of P-CA3 and P-DG units dropped to 14 ± 2% and 5 ± 2% of peak frequency with LFPP-CA3 or LFPP-DG, respectively. These results suggest that P-CA3/P-DG units, once activated in concomitance with LFPP-CA3, maintain their firing, leading to the generation of LFPP-DG.
Recordings of activity from visually identified pyramidal and granule cells during distinct LFPs.
Based on the locations and firing patterns of the single units shown in Figs. 3–4, we hypothesized that P-CA3 and P-DG units were CA3 pyramidal cells and DG granule cells, respectively. However, MEA recordings do not allow for the precise identification of active neurons in hippocampal slices. Therefore, we performed loose-patch recordings from visually identified CA3 pyramidal and DG granule neurons within hippocampal slices while recording with pMEA (Fig. 5A). Consistent with our hypothesis, we observed that CA3 pyramidal and DG granule cells had similar activities to P-CA3 and P-DG units, respectively. The CA3 pyramidal neuron shown in Fig. 6A responded to all LFPs; however, as with P-CA3 units, they had a larger increase in AP frequency with LFPP-CA3 compared with LFPP-DG. In our sample of 18 CA3 pyramidal neurons from 10 slices derived from 7 mice, 14 neurons had similar PSTH distributions, as in the example shown in Fig. 5B. Three neurons had P-CA3/P-DG unit properties with similar peak frequencies to both LFPP-CA3 and LFPP-DG, and one neuron had an increase in AP frequency only with LFPP-DG.
Fig. 5.
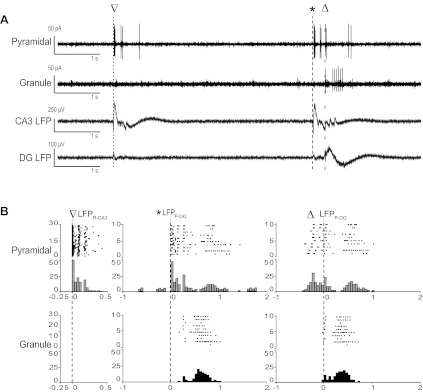
Firing pattern of pyramidal and granule neurons during distinct LFPs. A: examples of currents reflecting AP firing recorded in the loose cell-attached mode from visually idenfied granule and pyramidal neurons. LFPs recorded from the DG and CA3 are also shown to illustrate the timing of the distinct events while LFPs were recorded with a pMEA. B: raster plots for consecutive sweeps and PSTHs of spike frequencies within bins centered around the onset time of the indicated LFP for the activity of the neurons shown in A. The bin size was 50 ms for all PSTHs. The inverted triangles indicate LFPR-CA3; asterisks indicate LFPP-CA3, and the open triangles indicate LFPP-DG.
Fig. 6.
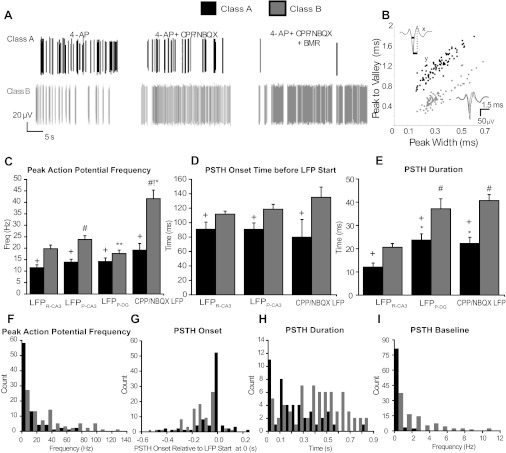
Spiking dynamics of class A and class B units during epileptiform LFPs. A: representative examples of class A (top) and class B (bottom) AP firing in 4-AP before (left) and after the successive application of CPP/NBQX (middle) or CPP/NBQX/BMR (right) B: scatterplot of mean spike waveform parameters of class A (solid) and B (shaded) units. Cells were classified based on the width at half-height (x) and peak-to-valley time (y) (an example is shown in the top left inset). The bottom right inset shows examples of extracellular waveforms recorded from a class A unit (solid) and a class B unit (shaded). C–E: quantification of class A and class B firing patterns with respect to the different LFPs. Single unit activity is shown in terms of peak AP frequency (C), onset time before the LFP start (D), and duration (E). F–I: distribution of values of peak AP frequency (F), onset time (G), duration (H), and baseline (I) for individual class A and B units derived from their PSTH responses to the CPP/NBQX LFP. Note that onset times are reported as negative values in G when they preceded the start time of the LFP. +P < 0.05 vs. class B units; #P < 0.05 vs. LFPR-CA3; !P < 0.05 vs. LFPP-CA3; *P < 0.05 vs. LFPP-DG.
In contrast, 10 of 12 DG granule cells (10 slices, 7 mice) fired APs preferentially with LFPP-DG and significantly less so with LFPP-CA3 (Fig. 5B). In addition, all CA3 pyramidal neurons (compared with only 38% of granule cells) fired APs during LFPR-CA3. CPP/NBQX perfusion blocked the synchronous firing of six of seven granule neurons and three of six pyramidal neurons. P-CA3-like pyramidal and P-DG-like granule cells had spike durations and basal frequencies as well as peak and interpeak PSTH frequencies consistent with their matching groups deriving from spike sorting (not shown). We therefore concluded that our spike sorting was able to identify distinct classes of neurons based on their firing behavior during various LFPs.
To determine whether single unit activity preceded the generation of LFPs, we examined the onset time of the AP frequency increase (taken as time of 5% of the PSTH peak) with respect to the time of LFPs. The onset time did not differ significantly between the three groups with respect to either LFPR-CA3 (101 ± 5 ms for P-CA3/DG units, 110 ± 6 ms for P-CA3 units, and 86 ± 17 ms for P-DG units) or LFPP-CA3/DG (116 ± 12 ms for P-CA3/DG units, 107 ± 19 ms for P-CA3 units, and 106 ± 14 ms for P-DG units). However, the onset time for P-CA3/P-DG units was significantly earlier than that of other neuronal classes during the CPP/NBQX LFP (159 ± 16 ms for P-CA3/DG units vs. 88 ± 20 ms for P-CA3 units and 108 ± 28 ms for P-DG units, P < 0.05). This suggested a major role for P-CA3/P-DG units in the generation of the CPP/NBQX LFP. We also observed that P-CA3/P-DG units significantly increased their baseline firing rate in the presence of ionotropic glutamatergic transmission antagonists (1.01 ± 0.19 vs. 1.71 ± 0.30 Hz, P < 0.05). This suggests that in the P-CA3/P-DG group, there are more neurons capable of spontaneous firing in the absence of ionotropic excitatory drive.
Spontaneously active neurons in the absence of synaptic transmission.
We hypothesized that the ionotropic glutamatergic transmission-independent LFPs identified in CA3 and DG (Fig. 1B) were mediated by GABAA receptors. We therefore blocked GABAergic transmission after iGluR blockade (Fig. 1C). Ionotropic glutamatergic and GABAergic blockade eliminated all LFPs but revealed a population of spontaneously active units (Fig. 1C). Neurons that did not generate APs or fired only occasionally during blockade of ionotropic glutamatergic and GABAergic synaptic transmission (CPP/NBQX/BMR) were considered class A neurons (n = 94; Fig. 6A, top). Neurons that fired APs at a frequency of >0.5 Hz (range: 0.6–150 Hz) when ionotropic glutamatergic and GABAergic synaptic transmission was blocked were considered class B neurons (n = 90; Fig. 6A, bottom). Cells (n = 11) that were active in CPP/NBQX/BMR but did not display unit activity in the presence of 4-AP were not analyzed further.
Spontaneously active class B cells were significantly more abundant in CA3 than in DG per slice (8 ± 1.4 and 3 ± 1.2, respectively, n = 7 slices, P < 0.05), whereas class A cells were evenly distributed between CA3 and DG (4.5 ± 1.6 and 5.7 ± 1.4, n = 7 slices). The average AP duration of class B cells was significantly shorter than for class A cells for both the half-width (0.30 ± 0.01 vs. 0.33 ± 0.01 ms, P < 0.05; Fig. 5B) and peak-to-valley duration (0.62 ± 0.02 and 1.19 ± 0.03 ms, P < 0.001), suggesting that at least some of the class B units were interneurons (Ranck et al.1973, Ylinen et al. 1995, Takahashi et al. 2007).
Interestingly, 73% of P-CA3/P-DG units were class B cells, whereas only 51% and 25% of P-CA3 and P-DG units, respectively, were class B cells. Although there was no difference in the AP half-width, P-CA3/P-DG units also had a significantly shorter AP peak-to-valley measurement compared with P-CA3 and P-DG units (0.76 ± 0.33 ms for P-CA3/P-DG units vs. 0.96 ± 0.38 ms for P-CA3 units and 0.95 ± 0.33 ms for P-DG units, P < 0.01). This suggests that there is an overlap between the class B and P-CA3/P-DG unit classifications and that both populations are most likely interneurons.
We hypothesized that cells that were spontaneously active in the absence of ionotropic glutamatergic and GABAergic synaptic transmission differed significantly in their firing relationship with LFPs. As shown in Fig. 6C, class B neurons had significantly higher increases in AP frequency with all LFPs compared with class A cells and had higher increases in AP frequency with LFPP-CA3 than with LFPR-CA3 and LFPP-DG. Importantly, this group of cells also had larger peak PSTHs after iGluR blockade, suggesting they were major contributors to the CPP/NBQX LFP.
The onset of class B cell firing preceded all LFPs more than the onset of class A cell firing, including during blockade of ionotropic glutamatergic transmission (Fig. 6D), further supporting the notion that class B cells underlie the ionotropic glutamatergic-independent LFP. Class B unit PSTHs had longer durations than class A PSTHs in all LFPs (Fig. 6E), implying that they have greater excitability. Both class A and B cells had significantly longer durations during LFPP-DG and the CPP/NBQX LFP than LFPR-CA3 (Fig. 6D), suggesting that the LFPP-DG component of LFPP-CA3/DG and the CPP/NBQX LFP share similar mechanisms leading to their generation. Furthermore, class B cells had significantly higher basal frequencies than class A cells (1.34 ± 0.15 vs. 0.41 ± 0.08 Hz, P < 0.05). Finally, upon ionotropic glutamatergic transmission blockade, class B units increased their baseline firing rate (1.34 ± 0.15 vs. 2.18 ± 0.25 Hz, P < 0.05), whereas class A units decreased their baseline firing rate (0.41 ± 0.08 vs. 0.22 ± 0.05 Hz, P < 0.05). The pivotal role of class B neurons in the CPP/NBQX LFP was further supported by the distribution of class A and class B units (Fig. 6, F–I) obtained considering four distinct parameters characterizing the PSTHs triggered by the CPP/NQBX LFP. From these results, it is evident that during blockade of ionotropic excitatory synaptic transmission, many class B units precede the start times of the LFPs earlier and have higher peak frequencies and baseline firing rates with a longer duration of the response to LPFs than class A units (Fig. 6, F–I). This suggests that class B neurons are characterized by high excitability even during iGluR blockade and that their activity underlies the occurrence of the CPP/NBQX LPF. Interestingly, these cells were located predominantly in the broader CA3 area (22 of 34 cells).
Our findings therefore suggest that spontaneously active class B cells are likely interneurons and that this population heavily overlaps with P-CA3/P-DG units. Furthermore, P-CA3/P-DG units precede and therefore likely drive the ionotropic glutamatergic transmission-independent CPP/NBQX LFP. The CPP/NBQX LFP predominantly originates in DG, unlike the CA3-originating fields recorded in the presence of ionotropic glutamatergic transmission. This suggests that spontaneously active interneurons generate the CPP/NBQX LFP observed in DG after iGluR blockade.
We therefore performed whole cell recordings from DG granule cells in the presence and absence of blockers of iGluR and GABAergic receptors. Under control conditions, DG cells experienced both inwardly directed excitatory postsynaptic current (EPSC) sequences and very small outwardly directed inhibitory postsynaptic currents (IPSCs) in correspondence to the LFPs (Fig. 7A). iGluR blockers abolished EPSCs (Fig. 7B) and dramatically enhanced IPSCs (Fig. 7B). These IPSCs were subsequently abolished by BMR (Fig. 7C). Similar results were observed in four granule cells, where the average increase of the outward currents was 593 ± 256%. As this large increase could have been due to the small size of outward currents at −60 mV (range: 30–90 pA), we repeated the experiments at 0 mV (range: 0.3–1 nA) and observed a 253 ± 141% increase. To further gain insight into the ionic nature of the currents measured in granule cells, we performed recordings with intracellular solution containing QX-314, CsMeSO4, and TEA-Cl to block voltage-gated Na+ and K+ currents. Under this condition, we calculated a Cl− equilibrium potential of −68 mV. In recordings from four granule neurons from four distinct slices (Fig. 7D), the reversal potential of the current measured in correspondence with the P-CA3/DG LFP was −56 ± 4 mV and with the CPP/NBQX LFP was −53 ± 5 mV (Fig. 7E). The difference between the calculated and measured Cl− equilibrium potential may relate to permeability to HCO3 and/or the difficulty of obtaining proper voltage clamping of dendrites of DG granule neurons in the presence of 4-AP. Taken together with the BMR abolishment of all currents and LFPs, these results show that Cl− currents are major contributors to the LFPs recorded in DG. These findings support our data from pMEA recordings, suggesting that in the presence of CPP/NBQX, GABAergic interneurons continue to be synchronously active, and demonstrate that synaptic transmission is completely blocked by ionotropic glutamatergic and GABAergic transmission antagonists.
Fig. 7.
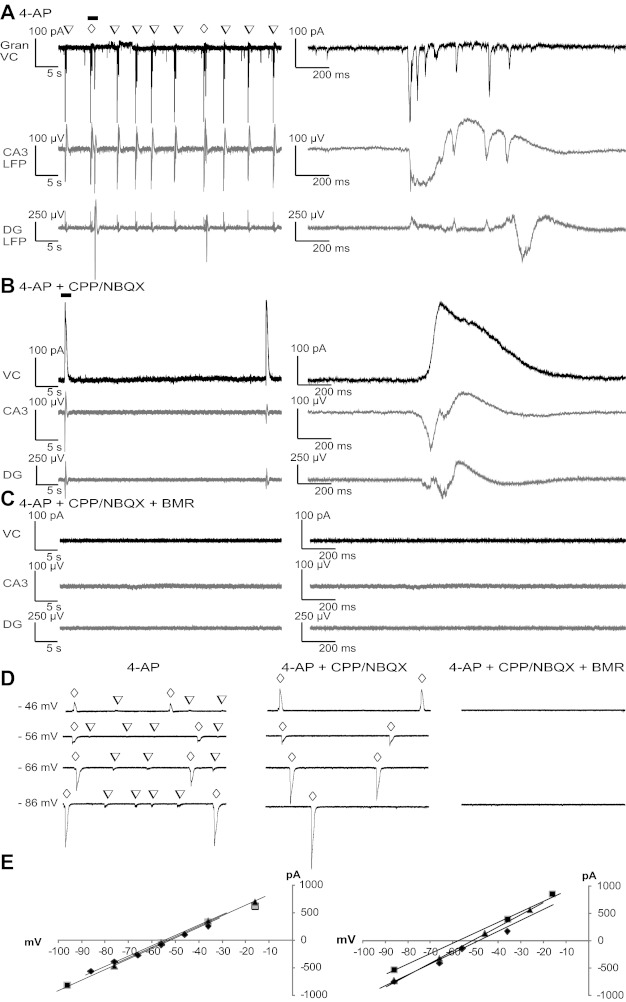
Synaptic input to DG granule neurons during distinct LFPs. A–C: example currents from whole cell voltage-clamp recordings at −60 mV with a K-gluconate-based pipette solution from a granule neuron are shown together with LFPs recorded from CA3- and DG-located pMEA electrodes. Excitatory postsynaptic current (EPSC) sequences occurring during the LFPs (A) were blocked by perfusion with ionotropic glutamate receptor (iGluR) antagonists (B), revealing large outward currents that disappeared with perfusion with BMR along with the CPP/NBQX LFP (C). The open inverted triangles indicate LFPR-CA3; the open diamonds indicate LFPP-CA3/DG. D: whole cell currents recorded from granule neurons using a pipette solution with blockers of voltage-gated Na+ and K+ channels and a higher intracellular Cl− concentration. Occurrences of LFPR-CA3 and LFPP-CA3/DG are marked by open inverted triangles and open diamonds, respectively. Whole cell currents were compared at different holding potentials in the presence and absence of blockers of iGluR and GABAA receptors. Voltages on the left of the traces are holding potentials corrected for the liquid junction potential. E: quantification of the voltage dependence of the peak current in correspondence to LFPP-CA3/DG measured from at least three events at each voltage in four distinct neurons in the absence (left) and presence (right) of CPP/NBQX.
DISCUSSION
In this study, we correlated LFPs with the activity of single units recorded from electrodes that were located in the hippocampal regions of the CA3, DG, and hilus that are known to be critical for seizure initiation (Gloor 1997). Here, we present evidence for classes of neurons that are likely to be pivotal in the generation of 4-AP-induced slow field potentials, which are mediated by GABAA receptor activation and continue to occur in the presence of iGluR blockers. LFPs recorded in horizontal hippocampal slices during 4-AP application initiate in the CA3 subfield, as previously reported by us in coronal slices analyzed with pMEA (Gonzalez-Sulser et al. 2011) and in agreement with earlier studies (Ziburkus et al. 2012, Hill et al. 2010; for a review, see Avoli and de Curtis 2011). Moreover, we found that blockade of ionotropic glutamatergic transmission can elucidate two major types of synchronous activity: 1) LFPR-CA3 discharges, which depend on iGluRs, and 2) LFPP-CA3/DG, which had both ionotropic glutamatergic (LFPP-CA3) and GABAergic (LFPP-DG) dependence (Perreault and Avoli 1992; Perkins and Wong 1996; Avoli and de Curtis 2011; Lamsa and Kaila 1997). Accordingly, all LFPs were blocked using GABAA and iGluR antagonists, and, under these conditions, we were able to record activity from many neurons, suggesting that nonsynaptic mechanisms controlling the intrinsic firing of single units do operate in the presence of 4-AP. It has been previously shown by Salah and Perkins (2008) that metabotropic glutamate receptors (mGluRs) are activated in the 4-AP in vitro model, although coapplication of mGlu1 and mGlu5 antagonists did not prevent the emergence of epileptiform activity. Furthermore, when iGluRs antagonists were applied in that study, mGluR blockade did not change the rate of the GABA-mediated LFP events.
We found three populations of neurons that displayed increases in AP frequency distinctly with the ionotropic glutamatergic and GABAergic components of LFPP-CA3/DG: P-CA3, P-CA3/DG, and P-DG units. P-CA3 units responded preferentially to LFPP-CA3, P-CA3/P-DG units had similar responses to both LFPP-CA3 and LFPP-DG, and P-DG units had a large response to LFPP-DG. P-CA3 units were primarily located in CA3, whereas P-DG units were mainly located in DG. These results align well with our findings showing that ionotropic glutamatergic-dependent field potentials originate in CA3, whereas GABAergic LFPs propagate to DG or initiate in that structure in the absence of glutamatergic transmission (Fig. 1) (Gonzalez-Sulser et al. 2011). Interestingly, P-CA3/DG units had very high peak frequencies that did not differ between LFPP-CA3 and LFPP-DG. In addition, these units had longer PSTH durations and had a higher level of sustained AP firing between LFPP-CA3 and LFPP-DG peaks, suggesting that they fired APs synchronously with LFPP-CA3 and that their sustained activity was involved in generating LFPP-DG. Moreover, activation of P-CA3/DG units preceded the LFP start time of the CPP/NBQX LFP compared with the other two unit groups, highlighting their importance in generating LFPs after iGluR blockade and suggesting their involvement in the propagation of activity into DG after it initiates in CA3 during LFPP-CA3/DG. These units were mainly located in CA3 and had the highest percent representation in the hilus, ideal locations from which to signal DG and cause the sporadic activation of that structure after the start of the LFP in CA3.
We also analyzed our pool of single units based on their activity in the presence of iGluR and GABAA receptor antagonists. Cohen and Miles (2000) reported that these antagonists as well as mGluR and muscarinic ACh receptor antagonists do not block MUA in hippocampal slices in high extracellular K+ concentration. These results suggest that under our experimental conditions, we may also have higher extracellular K+ concentration and that the intrinsic properties of a subset of hippocampal neurons allow them to generate AP initiation. In our study, active units (class B) had shorter AP duration than inactive units (class A). In addition, class B units had longer PSTHs than class A units, higher basal and peak firing frequencies, and an earlier onset of PSTHs. Although it is necessary to take into account that uncertainty will exist when deriving information about cell types from extracellular recordings (Cohen and Miles 2000), the short AP duration suggests that some class B units are likely GABAergic interneurons (Ranck et al. 1973; Ylinen et al. 1995; Cohen and Miles 2000; Takahashi et al. 2007). Some interneuron subclasses (including parvalbumin-positive fast spikers, somatostatin-expressing, oriens-lacunosum moleculare, and basket cells) have been shown to fire at higher frequencies during epileptiform activity in vitro as well as under more physiologically relevant conditions (Spampanato et al. 2007; Ziburkus et al. 2006; Freund and Buzsaki 1996). The majority of P-CA3/DG units had sustained activity in relation to LFPPCA3. These cells overlapped with class B units and had short spike durations, suggesting that they are GABAergic interneurons with higher excitability. Furthermore, this class of units significantly preceded the LFP in CPP/NBQX, supporting their role in the initiation of these LFPs. Simultaneous loose-patch and pMEA recordings from slices in which CA3 pyramidal and DG granule neurons were visually identified showed that the activity of these neurons during LFPs is similar to the firing patterns of P-CA3, P-DG, and class A units, further supporting our hypothesis that P-CA3/DG and class B cells are likely to be GABAergic interneurons.
These findings also lend support to the hypothesis that both LFPDG and LFPs recorded in the absence of ionotropic excitatory synaptic transmission reflect the synchronous activation of a subpopulation of GABAergic interneurons that become overexcitable by 4-AP; these cells are expected to release GABA, which, by activating GABAA receptors, leads to transient increases in extracellular K+ concentration, which is likely to support further neuronal synchronization and the spread of epileptiform activity in this model (Avoli et al. 1996a; Perkins and Wong 1996; Kaila et al. 1994; Kaila et al. 1997; for a review, see Avoli and de Curtis 2011). Our results from whole cell recordings from DG granule neurons show that iGluR antagonists induce large synchronized IPSCs during the CPP/NBQX LFP. This supports the findings that the activity of GABAergic interneurons underlies the CPP/NBQX LFP. However, further studies will be required to investigate the underlying mechanisms of synchronization. We speculate that blockade of ionotropic excitatory control of critical interneurons that inhibit large populations of GABAergic cells, or a gap junction-mediated mechanism (Traub et al. 2001a, 2001b), or both may play a role in the generation of LFPs.
Conclusions.
Our study further characterized network activity within an in vitro experimental model of epileptiform synchronization. These data suggest different roles for neuronal populations with characteristics of GABAergic interneurons in the initiation and propagation of LFPs. Further study will be needed to identify the anatomic and functional correlates of these neuronal subgroups.
GRANTS
This work was supported in part by the Luce Foundation (to R. Dzakpasu and J. Wang), Consejo Nacional de Ciencia y Tecnología (Mexico) Scholarship 209104 (to A. Gonzalez-Sulser), National Science Foundation Partnerships for International Research and Education Grant 0730255 (to A. Gonzalez-Sulser and J. Wang), National Institutes of Health Grants T32-NS-041218-10 and FNS-080462A (to B. N. Queenan), Canadian Institutes of Health Research Grants 8109 and 74609 (to M. Avoli), and the Savoy Foundation (to M. Avoli). J. Wang is a lecturer in the Department of Instrument Science and Technology (Xi'an Jiaotong University, Shaanxi, China) and is also supported by the China Scholarship Council-Georgetown University Postdoc Fellowship Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.G.-S., M.A., S.V., and R.D. conception and design of research; A.G.-S. performed experiments; A.G.-S., J.W., B.N.Q., S.V., and R.D. analyzed data; A.G.-S., B.N.Q., M.A., S.V., and R.D. interpreted results of experiments; A.G.-S. and J.W. prepared figures; A.G.-S., S.V., and R.D. drafted manuscript; A.G.-S., B.N.Q., M.A., S.V., and R.D. edited and revised manuscript; A.G.-S., J.W., B.N.Q., M.A., S.V., and R.D. approved final version of manuscript.
REFERENCES
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with imageJ. Biophotonics 11: 36–42, 2004 [Google Scholar]
- Aradi I, Maccaferri G. Cell type-specific synaptic dynamics of synchronized bursting in the juvenile CA3 rat hippocampus. J Neurosci 24: 9681–9692, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol 95: 104–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Kurcewicz I, Pumain R, Barbarosie M. Extracellular free potassium and calcium during synchronous activity induced by 4-aminopyridine in the immature rat hippocampus. J Physiol 493: 707–717, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci 17: 9308–14, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazelot M, Dinocourt C, Cohen I, Miles R. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J Physiol 588: 2077–2090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear J, Lothman EW. An in vitro study of focal epileptogenesis in combined hippocampal-parahippocampal slices. Epilepsy Res 14: 183–193, 1993 [DOI] [PubMed] [Google Scholar]
- Berretta N, Bernardi G, Mercuri NB. Firing properties and functional connectivity of substantia nigra pars compacta neurones recorded with a multi-electrode array in vitro. J Physiol 588: 1719–1735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Ledonne A, Mango D, Bernardi G, Mercuri NB. Hippocampus versus entorhinal cortex decoupling by an NR2 subunit-specific block of NMDA receptor in a rat in vitro model of temporal lobe epilepsy. Epilepsia 53: 80–84, 2012 [DOI] [PubMed] [Google Scholar]
- Cattani AA, Bonfardin VD, Represa A, Ben-Ari Y, Aniksztejn L. Generation of slow network oscillations in the developing rat hippocampus after blockade of glutamate uptake. J Neurophysiol 98: 2324–2336, 2007 [DOI] [PubMed] [Google Scholar]
- Cohen I, Miles R. Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. J Physiol 524: 485–502, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Prida LM, Huberfeld G, Cohen I, Miles R. Threshold behavior in the initiation of hippocampal population bursts. Neuron 49: 131–142, 2006 [DOI] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time-dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res 87: 581–596, 1991 [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci 23: 7873–7880, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert U, Heck D, Aertsen A. Two-dimensional monitoring of spiking networks in acute brain slices. Exp Brain Res 142: 268–274, 2002 [DOI] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46: 470–2, 2005 [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. New York: Oxford Univ. Press, 1997 [Google Scholar]
- Gonzalez-Sulser A, Wang J, Motamedi GK, Avoli M, Vicini S, Dzakpasu R. The 4-aminopyridine in vitro epilepsy model analyzed with a perforated multi-electrode array. Neuropharmacology 60: 1142–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra A, Rosenbaum R, Bodmann B, Cao S, Josić K, Žiburkus J. β-Adrenergic modulation of spontaneous spatiotemporal activity patterns and synchrony in hyperexcitable hippocampal circuits. J Neurophysiol 108: 658–671, 2012 [DOI] [PubMed] [Google Scholar]
- Hill AJ, Jones NA, Williams CM, Stephens GJ, Whalley BJ. Development of multi-electrode array screening for anticonvulsants in acute rat brain slices. J Neurosci Methods 185: 246–256, 2010 [DOI] [PubMed] [Google Scholar]
- Hill DN, Mehta SB, Kleinfeld D. Quality metrics to accompany spike sorting of extracellular signals. J Neurosci 31: 8699–705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci 17: 7662–7672, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J, Meredith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLos One 3: e3884, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Kaila K. Ionic mechanisms of spontaneous GABAergic events in rat hippocampal slices exposed to 4-aminopyridine. J Neurophysiol 78: 2582–91, 1997 [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictal-like discharges in the rat entorhinal cortex. J Neurophysiol 79: 352–360 [DOI] [PubMed] [Google Scholar]
- Perkins KL, Wong RK. Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J Neurophysiol 76: 3886–3894, 1996 [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci 12: 104–115, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah A, Perkins KL. Effects of subtype-selective group I mGluR antagonists on synchronous activity induced by 4-aminopyridine/CGP 55845 in adult guinea pig hippocampal slices. Neuropharmacology 55: 47–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Mody I. Spike timing of lacunosom-moleculare targeting interneurons and CA3 pyramidal cells during high-frequency network oscillations in vitro. J Neurophysiol 1: 96–104, 2007 [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in temporal lobe epilepsy. Epilepsia 35: 721–727, 1994 [DOI] [PubMed] [Google Scholar]
- Ranck JB. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Behavioral correlates and firing repertoires. Exp Neurol 41: 462–531, 1973 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sakurai Y. Coding of spatial information by soma and dendrite of pyramidal cells in the hippocampal CA1 of behaving rats. Eur J Neurosci 26: 2033–2045, 2007 [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R, Wong RK. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science 243: 1319–1325, 1989 [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci 21: 9478–86, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig R, Piechotta A, Draguhn R, Schmitz D. Synaptic and nonsynaptic contributions to giant ipsps and ectopic spikes induced by 4-aminopyridine in the hippocampus in vitro. J Neurophysiol 85: 1246–1256, 2001b [DOI] [PubMed] [Google Scholar]
- Uva L, Avoli M, de Curtis M. Synchronous GABA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur J Neurosci 29: 911–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Brain A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15: 30–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol 95: 3948–3954, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


