Abstract
Respiratory depression is a therapy-limiting side effect of opioid analgesics, yet our understanding of the brain circuits mediating this potentially lethal outcome remains incomplete. Here we studied the contribution of the rostral ventromedial medulla (RVM), a region long implicated in pain modulation and homeostatic regulation, to opioid-induced respiratory depression. Microinjection of the μ-opioid agonist DAMGO in the RVM of lightly anesthetized rats produced both analgesia and respiratory depression, showing that neurons in this region can modulate breathing. Blocking opioid action in the RVM by microinjecting the opioid antagonist naltrexone reversed the analgesic and respiratory effects of systemically administered morphine, showing that this region plays a role in both the analgesic and respiratory-depressant properties of systemically administered morphine. The distribution of neurons directly inhibited by RVM opioid microinjection was determined with a fluorescent opioid peptide, dermorphin-Alexa 594, and found to be concentrated in and around the RVM. The non-opioid analgesic improgan, like DAMGO, produced antinociception but, unlike DAMGO, stimulated breathing when microinjected into the RVM. Concurrent recording of RVM neurons during improgan microinjection showed that this agent activated RVM ON-cells, OFF-cells, and NEUTRAL-cells. Since opioids are known to activate OFF-cells but suppress ON-cell firing, the differential respiratory response to these two analgesic drugs is best explained by their opposing effects on the activity of RVM ON-cells. These findings show that pain relief can be separated pharmacologically from respiratory depression and identify RVM OFF-cells as important central targets for continued development of potent analgesics with fewer side effects.
Keywords: rostral ventromedial medulla, analgesia, improgan, pain modulation, rat
while opioids remain the most powerful tool available for treating moderate to severe pain, their utility is limited by side effects, especially potentially lethal respiratory depression. Given this risk and the low therapeutic index for many opioids, clinicians often undertreat pain (Nickerson and Attaran 2012; Webster et al. 2011). Despite the clinical and social significance of opioid-induced respiratory depression, the underlying neural mechanisms and circuits are still not fully understood.
In contrast to respiratory depression, the analgesic actions of opioids have been studied intensely, and we now know that these agents produce pain relief by engaging an endogenous brain stem pain modulatory system. This system is the driving force behind the natural suppression or enhancement of pain in different behavioral states (Fields 2004). Its output influences pain behavior via projections from the rostral ventromedial medulla (RVM) to dorsal horn nociceptive circuits. Inactivation or lesion of the RVM can interfere with the analgesic effects of systemically administered opioids, and μ-opioid agonists applied directly in the RVM produce a potent analgesia (Fields et al. 2006; Proudfit 1980, 1981).
Two classes of RVM neurons, the “ON-cells” and “OFF-cells,” respond to opioids (Fields et al. 2006; Heinricher et al. 2009). ON-cells facilitate nociception, and these neurons are defined by activation during nociceptive withdrawal behaviors. Conversely, OFF-cells suppress nociception, and this cell class is defined by a withdrawal-related pause in activity. Drugs that prevent the OFF-cell pause produce behavioral antinociception, independent of whether ON-cell activity is changed (Heinricher and Ingram 2008; Heinricher et al. 2010b; Neubert et al. 2004). μ-Opioids, for instance, given systemically or locally in the RVM, produce continuous OFF-cell firing while inhibiting ON-cell activity. Whether these changes in OFF-cell and ON-cell activity collectively or separately relate to other effects of opioids, including respiratory depression, is not yet known.
The constituent regions of the RVM, including portions of raphe magnus, raphe pallidus, and raphe obscurus at the level of the facial nucleus, have also been tied to other regulatory functions, including thermogenesis and cardiovascular control (Cao et al. 2004; Lovick 1997; Nakamura and Morrison 2007). Although these areas have not been strongly implicated in opioid-mediated respiratory depression, they have been linked to respiratory modulation (Dias et al. 2007, 2012; Hellman et al. 2007, 2009; Madden and Morrison 2005; Menuet et al. 2011; Rice et al. 2009; Taylor et al. 2006; Verner et al. 2004). Nevertheless, the neuronal and physiological overlap of these homeostatic functions with pain modulation is not well understood, in part because of the lack of mechanistic studies that include both parameters.
Here we show that the RVM contributes to opioid-induced respiratory depression at doses that simultaneously produce behavioral analgesia. In this same brain region, the non-opioid analgesic improgan also relieves pain yet stimulates respiration. This functional separation reflects independent actions of the two distinct populations of opioid-sensitive RVM neurons, the ON-cells and the OFF-cells. Thus, while these results demonstrate an overlap of opioid-induced respiratory depression and analgesia within a common brain stem region, they also show promise for dissociating these two effects pharmacologically, at the level of functionally distinct neuronal populations.
MATERIALS AND METHODS
Animals.
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University and followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
Surgical preparation and anesthesia.
Deep surgical anesthesia was induced in male Sprague-Dawley rats (250–350 g, Charles River) with 4% isoflurane in humidified O2 at 1.25 l/min, and the animals were placed in a stereotaxic apparatus. For surgical preparation (≤20 min), the isoflurane concentration was reduced to 3% and a small craniotomy performed to allow placement of a recording electrode and/or glass microinjection pipette in the RVM. Animals were placed on a circulating warm-water pad to support body temperature.
After surgical preparation the isoflurane concentration was reduced from 3% to 1.5% over 30 min (−0.5% every 10 min) and then further adjusted in increments of 0.25% until a tail flick (TF) reflex was evoked (see Nociceptive testing) without other signs of discomfort. This concentration was maintained for at least 30 min prior to the initiation of the experimental protocol. Isoflurane concentration and gas flow rate were fixed for the duration of the protocol.
Nociceptive testing.
Nociceptive thresholds were measured by TF latency. With a feedback-controlled radiant heat source, the ventral side of the animal's tail was maintained at 34°C between trials and then heated at a constant rate of 1.7°C/s until a tail movement was detected or the cutoff temperature of 53°C was reached. A motion transducer detected movement of the tail. Three locations, at 2, 4, and 6 cm from the tip of the tail, were tested in rotation to avoid sensitization and tissue damage. The holding temperature allowed us to rule out the possibility that any changes in latency could be attributed to changes in skin temperature. TF latency was defined as the difference in time between the point at which the tail surface temperature reached 36°C and the occurrence of the reflex. This protocol, with trials at 5-min intervals, produces a stable measure of nociceptive responsiveness over several hours (Martenson et al. 2005). The baseline threshold was the average withdrawal latency of three trials taken immediately prior to the drug injection. To aid in comparisons of drug effect among groups, TF latency is sometimes expressed as percentage of maximum possible effect: %MPE = (postdrug latency − baseline latency)/(cutoff latency − baseline latency).
Respiration, heart rate, and rectal temperature.
Breathing was monitored by using two different noninvasive methods, both of which provide accurate measurements of breathing rate and relative tidal volume compared with whole body plethysmography (Cleary et al. 2012). Initial experiments used accelerometry-based plethysmography (Devonshire et al. 2009), where an accelerometer was attached to the chest wall of the animal to detect movements associated with breathing. In later experiments, respiration was monitored by ventilation pressure transduction (Cleary et al. 2012), which measures small changes in pressure just outside the animal's nose resulting from inhalation and exhalation. The respiratory signals were amplified, filtered, and recorded for off-line analysis (Spike2; CED, Cambridge, UK). Respiratory rate was determined by averaging the interbreath interval over the 60-s period before each TF. Relative respiratory amplitude was determined by expressing peak-to-peak amplitude, a correlate of tidal volume (Cleary et al. 2012), as a percentage of the predrug baseline. Heart rate was derived from the electrocardiogram. Body temperature was measured with a rectal thermometer (TH-5; Physitemp, Clifton, NJ).
RVM recording.
For the improgan microinjection experiments (see Experimental protocols), a gold- and platinum-plated stainless steel recording microelectrode (Microprobe, Gaithersburg, MD) was attached to the microinjection pipette so that the tips were separated by no more than several hundred micrometers (Heinricher et al. 1994, 2010a, 2010b; Neubert et al. 2004). The assembly was lowered to the RVM with the use of anatomical landmarks. Cell recordings made before, during, and after the improgan microinjection were stored for later off-line analysis to ensure accurate discrimination throughout the recording. Prior to the start of the recording protocol, each neuron was unambiguously classified as an ON-, OFF, or NEUTRAL-cell with standard criteria (Fields et al. 1983). ON- and OFF-cells are defined by a sudden activation or cessation in firing rate, respectively, beginning just prior to a nociceptive reflex such as the TF response. NEUTRAL-cells show no change in firing rate correlated with the occurrence of a nociceptive reflex. Spontaneous firing was determined by measuring the firing rate in a 30-s period immediately prior to each TF trial (5-min intervals). Reflex-related changes in firing were determined in a 3-s period beginning 0.5 s prior to the TF response.
Experimental protocols.
In the first set of experiments, the contribution of neurons in the RVM to the antinociceptive and respiratory-depressant actions of systemically administered morphine was determined by microinjection of the opioid antagonist naltrexone in the RVM. TF trials were initiated at 5-min intervals throughout the protocol. After a 15-min baseline period, morphine (0.66 mg/kg iv) was given. Ten minutes later, naltrexone (3 μg/200 nl) or artificial cerebrospinal fluid (aCSF, 200 nl) was microinjected into the RVM over a period of ∼5 min or into surrounding regions as off-site controls. Naloxone (0.27 mg/kg iv) was given systemically at the end of the experiment to show that morphine effects were receptor mediated and reversible.
In the second set of experiments, we examined the effects of direct RVM microinjection of [d-Ala2,N-Me-Phe4,Gly-ol]-enkephalin (DAMGO, 200 pmol/200 nl) or the non-opioid analgesic improgan (15 or 30 nmol in 200 nl; Hough et al. 2000) on nociception, respiratory parameters, heart rate, and body temperature. In the improgan experiments, activity of an RVM neuron was also recorded as described above. A 15-min baseline was established, followed by microinjection of drug or vehicle (injected over 5–10 min, beginning immediately after the last baseline TF). TF, respiratory measurements, heart rate, rectal temperature, and cell activity (in the improgan experiments) were recorded for the next hour.
In the third set of experiments, we microinjected either the GABAA receptor antagonist bicuculline (22 pmol/200 nl) to disinhibit RVM neurons or the GABAA receptor agonist muscimol (18 pmol/200 nl) to suppress activity of RVM neurons (Heinricher and Tortorici 1994; Martenson et al. 2009). The protocol in this third set of experiments was identical to that for DAMGO and improgan, with TF, respiration, heart rate, and rectal temperature recorded before and after microinjection of bicuculline or muscimol. However, cell data were not recorded in this set of experiments.
Verification of microinjection and recording sites.
Microinjection locations and recording sites were marked either by fluorescent beads (FluoSpheres, Invitrogen, Eugene, OR) injected with the drug or by an electrolytic lesion created after the experimental protocol. Animals were overdosed with isoflurane and then transcardially perfused with physiological saline followed by 10% formalin. Brains were removed and stored overnight in 10% formalin. The brain stem was sectioned at 60 μm on a cryostat and mounted for microscopic examination.
Identification of opioid-sensitive brain stem neurons.
To identify neurons in the region of the RVM that contain postsynaptic μ-opioid receptors and that could thus drive RVM opioid-induced changes in nociception and respiration, we microinjected a peptide μ-opioid agonist, dermorphin, that was fluorescently labeled with Alexa Fluor 594 (Arttamangkul et al. 2000, 2006). Dermorphin-A594 was dissolved in either 3% DMSO in saline (6 pmol/200 nl injections) or 30% DMSO in saline (66 pmol/200 nl).
For injection of dermorphin-A594 into the RVM, animals were initially anesthetized with 5% isoflurane for placement of a jugular catheter, and the anesthetic was then switched from inhaled isoflurane to intravenous methohexital (30–60 mg·kg·h). After a stable baseline was achieved for at least 25 min, dermorphin-A594 was injected into the RVM. In some experiments, 45 min prior to the injection of dermorphin-A594 an injection of the irreversible μ-opioid antagonist β-funaltrexamine (β-FNA, 300 nl, 6 nmol; Tocris Bioscience), was injected into the RVM. Heart rate, respiratory rate, and rectal temperature were measured as described above. Nociceptive threshold was measured by placing a Peltier device on the left hind paw, slowly increasing temperature from 35 to 53°C, and noting the temperature at which a withdrawal was initiated. EMG recordings from the left calf were used to determine the beginning of the withdrawal. Antinociception is expressed as %MPE. These experiments allowed a comparison of the analgesic efficacy and respiratory and autonomic depressive effects of dermorphin-A594 with those of DAMGO and improgan.
Physiological and nociceptive parameters were monitored before and after injection of dermorphin-A594. Sixty minutes after injection, animals were overdosed with methohexital and perfused transcardially with solutions of physiological saline and of 10% formalin. The brains were removed, fixed overnight in 10% formalin, and sectioned at 60 μm with a cryostat. Sections were mounted on glass slides with Permount, visualized on an Olympus BX51 fluorescent microscope (Olympus, Center Valley, PA), and photographed with a Microfire A/R camera attachment (Optronics, Goleta, CA). For each brain, an experimenter blinded to the treatment conditions photographed eight representative brain stem sections between −1.08 and −3.96 mm (relative to the interaural line), with the same intensity and exposure for each photograph.
Mean fluorescence for each section was quantified with the open-source image processing package Fiji (http://www.fiji.sc). Fluorescence was measured in the RVM, a midline area roughly 2 mm in width and 1 mm in height directly dorsal to the pyramidal tracts at the level of the facial nucleus. Background intensity for each section was also measured and then subtracted from the overall fluorescence.
Statistical analysis.
All data are represented as means + SE. Drug effects on TF latency, hind paw withdrawal threshold, respiratory rate, heart rate, and rectal temperature were determined by one- or two-way ANOVA, with post hoc comparisons used where indicated. Differences in mean RVM fluorescence between treatment groups and the effects of dermorphin-A594 relative to baseline were analyzed by unpaired and paired t-tests, respectively. Respiratory amplitude was analyzed with a Friedman's analysis of variance by rank. RVM neurons exhibit a wide range of basal firing rates. A within-cell analysis approach was therefore used in which cell firing data after treatment were compared to baseline with a Wilcoxon's signed-rank test for matched samples. Analyses were performed with GraphPad Prism or StatView. P < 0.05 was considered statistically significant.
RESULTS
RVM contributes to antinociceptive and respiratory-depressant actions of systemically administered morphine.
The RVM is defined functionally as the area where low-current electrical stimulation produces behavioral antinociception and includes the nucleus raphe magnus and adjacent reticular formation at the level of the facial nucleus (Fields and Heinricher 1985). We first determined whether this region is required for respiratory-depressant actions of systemically administered morphine, as well as for analgesia. Respiratory parameters (rate and amplitude) were measured in parallel with the TF response evoked by noxious radiant heat. The latter is an index of nociception widely employed in awake behaving animals that can also be used in lightly anesthetized subjects (Fields and Heinricher 1985).
As shown in Fig. 1, systemically administered morphine produced both potent analgesia and a significant decrease in respiratory rate (ANOVA, P < 0.05 compared with baseline for all groups). Both effects were reversed by focal application of the opioid antagonist naltrexone in the RVM but not by aCSF vehicle. Naltrexone microinjections in areas immediately surrounding the RVM (dorsal, rostral, and caudal) were ineffective (Fig. 1, naltrexone placement control group). Subsequent systemic administration of naloxone, a highly lipophilic, short-acting opioid antagonist, reversed antinociception and respiratory depression in RVM-vehicle and placement control groups, showing that both effects were opioid receptor mediated and reversible. These data demonstrate that opioid receptors in the RVM contribute to respiratory depression as well as to antinociception produced by systemically administered morphine.
Fig. 1.
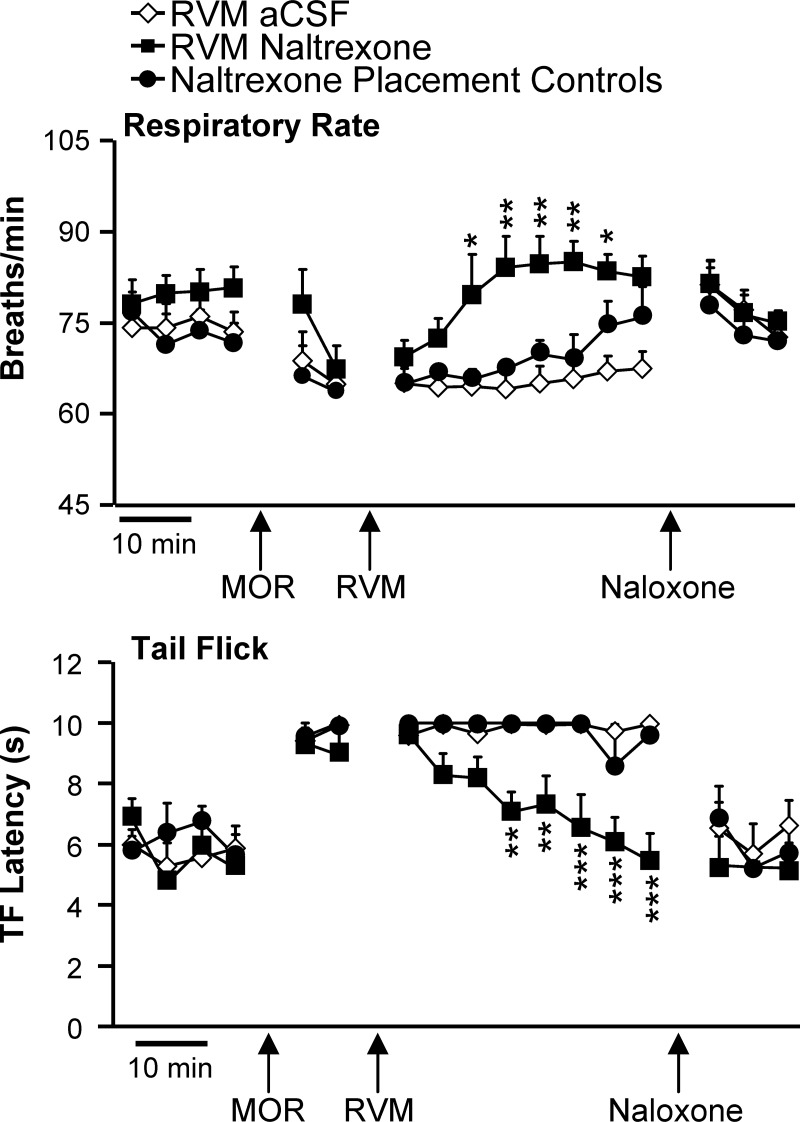
Respiratory depression (top) and antinociception (bottom) produced by systemically administered morphine are blocked by an opioid receptor antagonist in the rostral ventromedial medulla (RVM). Animals underwent baseline testing and were given morphine systemically (MOR). At the point labeled RVM, naltrexone or artificial cerebrospinal fluid (aCSF) was microinjected into the RVM. Injections that missed the RVM are shown as placement controls. All animals then received naloxone systemically, to verify the reversibility of any effect. Respiration was quantified, and tail flick (TF) trials were initiated at 5-min intervals throughout the protocol (6–9 animals/group, no difference among groups in baseline). *P < 0.05, **P < 0.01, ***P < 0.001 compared with aCSF by ANOVA followed by Bonferroni post hoc test (significant effect of MOR on breathing and TF relative to baseline not marked for clarity; repeated-measures ANOVA, P < 0.05 compared with baseline for all groups).
Distribution of neurons in RVM driving opioid-induced changes in respiration, heart rate, and pain threshold.
We next determined the distribution of neurons in the RVM and surrounding areas that could be the direct target of μ-opioid agents. By microinjecting the μ-opioid agonist dermorphin labeled with an Alexa Fluor 594 fluorophore (dermorphin-A594), we could identify individual cells in the RVM and surrounding regions that bind the agonist and internalize the μ-opioid receptor. These labeled neurons are potential drivers for the physiological and behavioral effects produced by opioid microinjections into the RVM.
We first determined that dermorphin-A594, like DAMGO, could produce antinociception and alter breathing when microinjected in the RVM. The higher dose of dermorphin-A594 (66 pmol/200 nl) produced significant effects on heat-evoked withdrawal (%MPE: 64.6 ± 18.4, n = 5, P < 0.05), respiratory rate (−16.2 ± 3.6 breaths/min, P < 0.05), heart rate (−23.0 ± 7.9 beats/min, P < 0.05), and body temperature (−0.28 ± 0.10°C, P < 0.05), consistent with results from microinjections of DAMGO into the RVM (see next section).
To identify the minimal distribution of neurons that could produce behavioral effects, we used the lowest dose of dermorphin-A594 (6 pmol/200 nl) that consistently produced measurable, albeit small, antinociception (%MPE: 9.5 ± 3.5, n = 4, P < 0.05) and then mapped the distribution of fluorescently labeled neurons. With this lower dose, respiratory rate was significantly decreased (−10.8 ± 3.0 breaths/min, P < 0.05), although there were no changes in heart rate (−25.0 ± 11.0 beats/min, P > 0.05) or body temperature (−0.21 ± 0.09°C, P > 0.05). Many neurons with strong A594 fluorescence were visible in the area immediately surrounding the injection site (Fig. 2), including numerous cells in the nucleus raphe magnus, nucleus raphe pallidus, raphe obscurus, and reticularis gigantocellularis pars alpha. Distinctly fluorescent single neurons were visible as far as 1 mm rostral and caudal to the injection site. Some larger neurons were also visible in the area dorsal to the injection site (nucleus reticularis gigantocellularis), predominantly in the sections containing the injection site or the track of the injector.
Fig. 2.
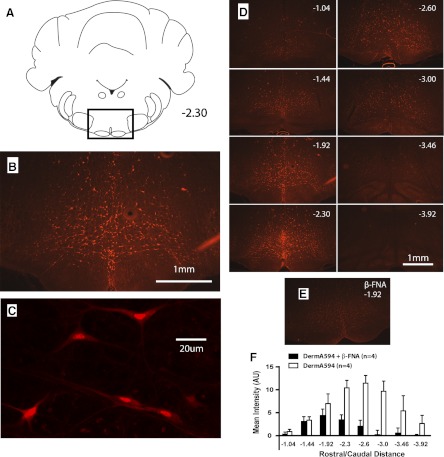
Dermorphin-A594 labeling of single neurons in and around the RVM. A: images were taken from a ventral area of sections representing the RVM and rostrally and caudally adjacent brain stem. B: representative image showing the distribution of fluorescent cells at 1.92 caudal to the interaural line after a 200-nl microinjection of dermorphin-A594. C: view of individual RVM neurons with dermorphin-A594 labeling. D: representative sections from same animal as in B showing the distribution of fluorescent neurons at different rostral/caudal levels. Distance from interaural line is given. E: labeling for dermorphin-A594 from representative animal pretreated with β-funaltrexamine (β-FNA). F: pretreatment with β-FNA significantly attenuated fluorescence from dermorphin-A594 microinjection (4 animals/group). AU, arbitrary unit. Schematics showing the extent of the RVM, including raphe pallidus, can be found in Fig. 3.
In control experiments with this lower dose of dermorphin, injecting the irreversible μ-opioid antagonist β-FNA 45 min prior to dermorphin-A594 injection significantly attenuated mean fluorescent labeling in the RVM (dermorphin-A594: 6.4 ± 1.1 arbitrary units averaged across all rostro-caudal levels, n = 4; β-FNA pretreatment: 1.8 ± 0.63, n = 4; P < 0.05 by unpaired t-test, Fig. 2F).
RVM supports opioid-induced respiratory depression.
To compare the analgesic and respiratory effects of direct local RVM administration of the μ-opioid agonist DAMGO with those of the non-opioid analgesic improgan (Hough et al. 2000), we recorded nociception, respiration, heart rate, and body temperature simultaneously before and after microinjection of the two agents. We also recorded RVM neuronal activity in the improgan experiments, but not in the DAMGO experiments, since the effect of local DAMGO injection on activity of RVM neurons has been defined previously (Heinricher et al. 1994).
Microinjections of DAMGO or improgan in the RVM at sites shown in Fig. 3 produced potent antinociception. TF latency was increased significantly by both agents, but not by vehicle (Fig. 4). The peak antinociceptive effects of improgan and DAMGO were seen at 10–20 and 35–45 min after injection, respectively, consistent with the known time courses of these agents. Injections of improgan in areas surrounding the RVM, mostly dorsal and rostral (see Fig. 3), resulted in a small, but statistically significant, increase in TF latency (1.8 ± 0.5 s, n = 23, P < 0.01).
Fig. 3.
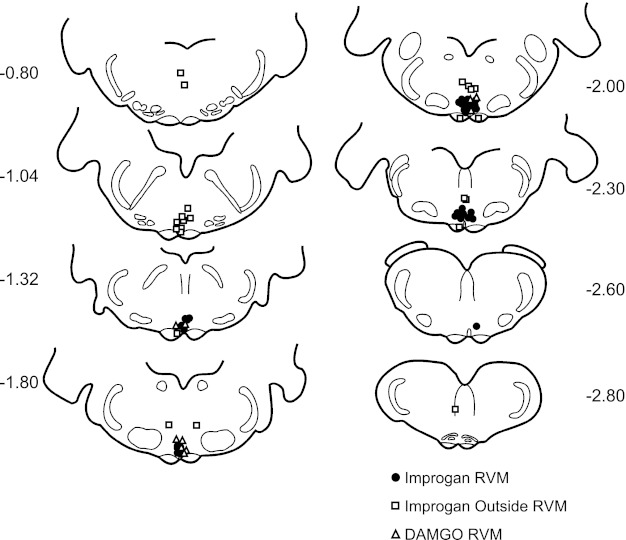
Locations of improgan and DAMGO microinjection sites in and around the RVM. There were 36 microinjections of improgan inside, and 23 outside, the RVM. Ten DAMGO microinjections were inside the RVM. Distances from lambda are indicated adjacent to each section. The RVM encompasses the ventromedial medulla at the level of the facial nucleus, ventral to a line drawn across the dorsal aspect of the facial nucleus and medial to the lateral edges of the pyramidal tracts.
Fig. 4.
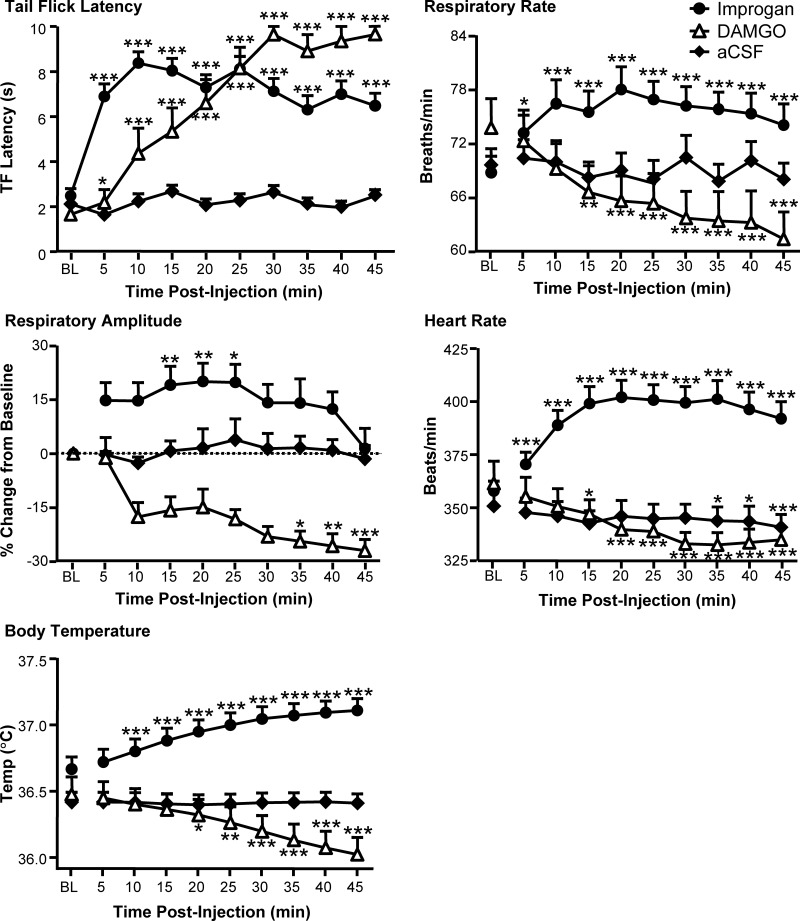
Effects of improgan and DAMGO microinjections into the RVM on TF latency, respiratory rate, respiratory amplitude, heart rate, and body temperature. Improgan and DAMGO were injected over a period of 5–10 min immediately following a 15-min baseline (BL, average of 3 trials). There were no differences among groups in any of these parameters in baseline (1-way between-groups ANOVA, 10–36 animals/group). *P < 0.05, **P < 0.01, ***P < 0.001 compared with preinjection baseline by repeated-measures ANOVA followed by Dunnett's test (respiratory rate, heart rate, body temperature) or Friedman's analysis of variance by ranks followed by Dunn's test (respiratory amplitude).
Although both DAMGO and improgan produced antinociception when microinjected into the RVM, only DAMGO produced a significant respiratory depression, decreasing both respiratory rate and amplitude (Fig. 4). In marked contrast, improgan in the RVM stimulated both respiratory rate and amplitude. The peak effects of improgan and DAMGO were seen at 10–20 and 35–45 min after injection, respectively, for both rate and amplitude. Vehicle injection had no effect on respiration, and injections of improgan in areas surrounding the RVM produced only a modest increase in respiration (4.8 ± 0.18 breaths/min, P < 0.05). These data demonstrate that the analgesic actions of drugs in the RVM are not inextricably linked to respiratory depression.
Effects of DAMGO and improgan on heart rate and body temperature are also distinct.
DAMGO microinjection resulted in a decrease in heart rate, while improgan induced a substantial increase (Fig. 4). Peak effects on heart rate were evident at 10–20 min and 35–45 min after injection with improgan and DAMGO, respectively. A small but statistically significant decrease in heart rate (6.0 ± 2.0 beats/min) was seen in vehicle-treated controls over the course of the experiment. Injections of improgan in areas surrounding the RVM produced a statistically significant increase in heart rate (25 ± 6.6 beats/min, P < 0.01).
Like heart rate, body temperature was also differentially affected by DAMGO and improgan. DAMGO microinjection decreased, whereas improgan increased, body temperature (Fig. 4). The peak effects of improgan and DAMGO on temperature were seen at 35–45 min after injection. The delayed time course for improgan in this case presumably reflects the kinetics of whole body temperature change. Injections of improgan in areas surrounding the RVM produced a small but statistically significant increase in body temperature (0.1 ± 0.03°C, P < 0.01).
Thus, like respiratory depression, reduced autonomic output following manipulations of the RVM can also be dissociated from analgesia.
Changes in RVM neuronal activity from DAMGO and improgan administration.
From a pain-modulating perspective, all neurons recorded in the RVM can be assigned to one of three mutually exclusive classes: OFF-cells (defined by nociceptive reflex-related inhibition of activity), ON-cells (characterized by nociceptive reflex-related activation), and NEUTRAL-cells (unresponsive to noxious stimuli; Fields et al. 2006). Both OFF-cells and ON-cells function as pain-modulating neurons, respectively suppressing and facilitating spinal nociceptive processing. The effects of μ-opioids on the firing of these RVM cell classes have been well documented. Local or systemically administered μ-opioid receptor agonists, including morphine and DAMGO, indirectly activate OFF-cells through presynaptic disinhibition, suppress ON-cell firing through direct inhibition, and do not alter NEUTRAL-cell firing (Fields et al. 2006; Heinricher and Ingram 2008). The effects of locally administered improgan on the different RVM cell classes have not been studied. We therefore recorded the activity of physiologically identified neurons within the RVM during the improgan injections described above (Fig. 4). As expected from previous work, these neurons exhibited a wide range of basal firing rates.
Improgan activated the pain-inhibiting OFF-cells in the RVM. Ongoing firing of these neurons was increased substantially (Fig. 5). Furthermore, improgan prevented the characteristic inhibition of OFF-cell firing during noxious stimulation (P = 0.03, n = 7, Wilcoxon's signed-rank test compared with baseline; data not shown). Improgan activation of OFF-cells thus mimics the net opioid effect of increasing the firing of these neurons (Heinricher et al. 1994). However, unlike opioids, improgan also strongly activated both ON-cells and NEUTRAL-cells (Fig. 5).
Fig. 5.
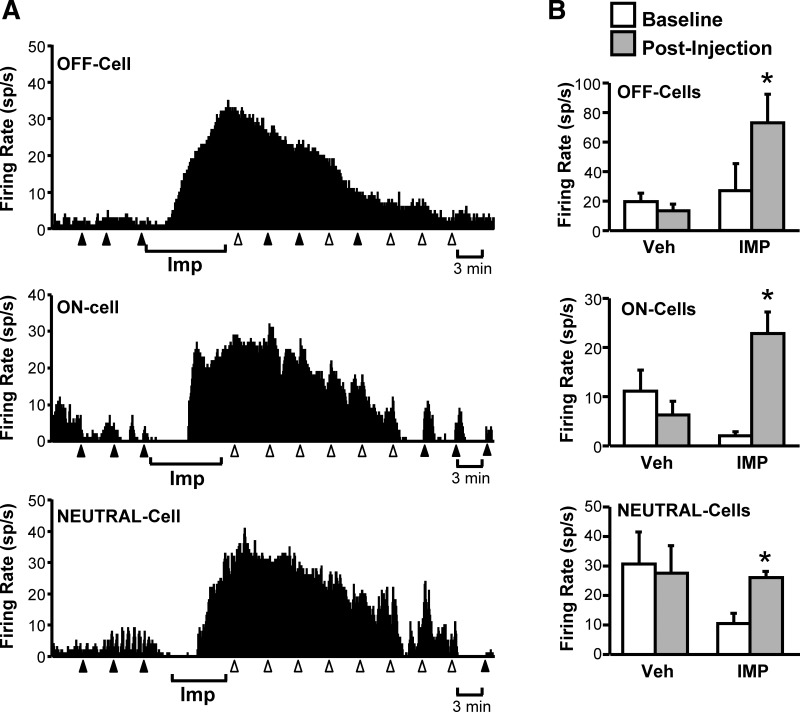
All RVM neuronal classes are activated after local application of the non-opioid analgesic improgan. A: ratemeter records showing firing rate (in spikes/s) of a typical OFF-cell, ON-cell, and NEUTRAL-cell before and after local microinjection of improgan during the period indicated below the trace. Triangles indicate TF trials, with filled triangles indicating that the animal responded to the heat and open triangles that there was no response prior to the cutoff time. B: group data confirm that all three RVM cell classes exhibit an increase in firing rate after improgan (IMP), but not vehicle (Veh), microinjection (6–8 cells/group). *P < 0.05 compared with preinjection baseline, Wilcoxon signed-rank test.
The differential effects of RVM DAMGO and improgan on respiration and autonomic parameters is therefore best explained by changes in the firing of the ON-cells, since only this cell class responds differentially to the two drugs.
Functional effects of stimulating or blocking all RVM neurons.
To corroborate the behavioral and physiological effects of RVM DAMGO and improgan, we examined the effects of nonselective excitation or inhibition of all RVM neurons on nociception, respiration, and autonomic parameters. The goal of these experiments was to contrast effects of selective manipulations of ON- and OFF-cells by using opioids with nonselective activation or inhibition to confirm the contributing role of these two cell classes in analgesia, heart rate, thermogenesis, and respiratory control.
To nonselectively excite RVM neurons, we microinjected the GABAA receptor antagonist bicuculline into the RVM. Like improgan, bicuculline activates both ON- and OFF-cell classes (Heinricher and Tortorici 1994). The physiological response to bicuculline generally mimicked the response to improgan rather than DAMGO, with antinociception accompanied by increases in respiratory rate, heart rate, and body temperature (Fig. 6). These data verify the above finding with improgan that concurrent activation of ON- and OFF-cells in RVM stimulates respiration at the same time that it produces analgesia.
Fig. 6.
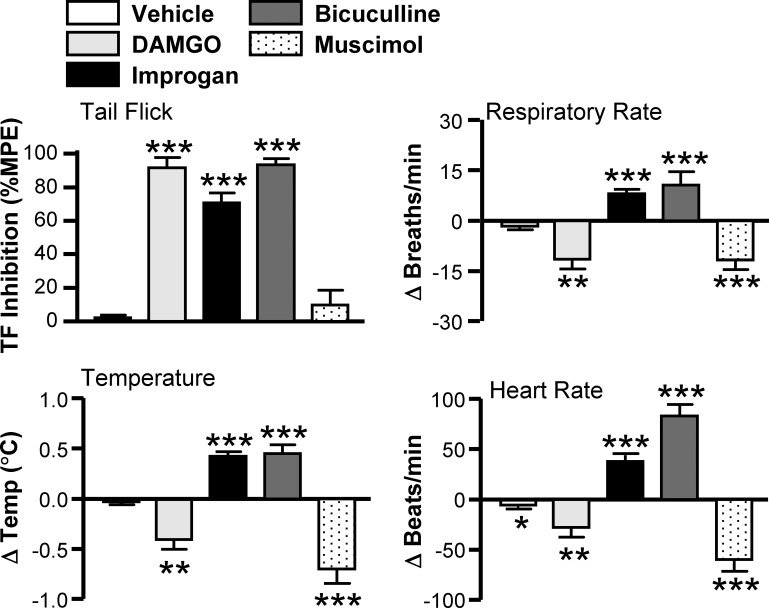
Effects of RVM improgan and DAMGO compared with bicuculline and muscimol: TF latency (expressed as % maximum possible effect, %MPE), change in respiratory rate, change in heart rate, and change in body temperature. Each data set was analyzed with an ANOVA followed by a Dunnett's test for comparison to aCSF vehicle control (9–36 animals/group). *P < 0.05 compared with preinjection baseline; **P < 0.01 and ***P < 0.001 compared with aCSF group.
To confirm that suppression of activity of a subset of RVM neurons was relevant to opioid-induced respiratory depression, we blocked activity of all RVM neurons by microinjecting the GABAA receptor agonist muscimol (Martenson et al. 2009). Breathing, heart rate, and body temperature were all significantly reduced after RVM blockade, although nociceptive threshold was not altered (Fig. 6). Inhibiting all RVM neurons thus reproduces the respiratory depressant actions of DAMGO and, furthermore, points to a role for this region in maintenance of basal respiratory function.
DISCUSSION
These experiments show that the RVM, a region long implicated in pain modulation and homeostatic regulation, contributes to both the analgesic and respiratory-depressant properties of μ-opioids. To determine whether RVM mechanisms of antinociception can be separated from those mediating respiratory depression, we compared the behavioral, physiological, and neuronal effects of DAMGO with those of improgan, a non-opioid analgesic (Table 1). While both drugs produced analgesia when microinjected into the RVM, DAMGO produced respiratory depression, whereas improgan stimulated breathing. Locally applied DAMGO, like systemically administered morphine, is known to activate OFF-cells and suppress ON-cell firing (Heinricher et al. 1994). Here, local improgan activated both ON- and OFF-cells. Thus, while OFF-cells show the same response to both DAMGO and improgan, the two drugs have opposing effects on ON-cells. The differential respiratory response to these two analgesic drugs in the RVM is therefore most readily explained by their opposing effects on the activity of ON-cells. By contrast, the common analgesic response to the agents is accounted for by their ability to activate OFF-cells.
Table 1.
Responses of RVM neurons and associated changes in tail flick latency, respiratory rate, heart rate, and temperature to local application of vehicle, DAMGO, improgan, bicuculline, and muscimol in RVM
| Change in Firing |
Behavioral or Physiological Effect |
|||||
|---|---|---|---|---|---|---|
| Treatment | ON-cell | OFF-cell | Tail flick | Resp rate | Heart rate | Temperature |
| Vehicle | No effect | No effect | No effect | No effect | Mild effect | No effect |
| DAMGO | ↓ | ↑ | Hypoalgesia | ↓ | ↓ | ↓ |
| Improgan | ↑ | ↑ | Hypoalgesia | ↑ | ↑ | ↑ |
| Bicuculline | ↑ | ↑ | Hypoalgesia | ↑ | ↑ | ↑ |
| Muscimol | ↓ | ↓ | No effect | ↓ | ↓ | ↓ |
Summary of the responses of rostral ventromedial medulla (RVM) neurons and the associated changes in tail flick latency, respiratory rate, heart rate, and temperature to local application of vehicle, DAMGO, improgan, bicuculline, and muscimol in the RVM. Activation of OFF-cells is coupled to hypoalgesia, whereas changes in respiratory rate and autonomic parameters are linked to drug effects on the ON-cells.
Neural basis for analgesia and respiratory depression mediated by RVM.
While histochemical and anatomical approaches to the study of RVM neurons are as yet incomplete, their physiological classification is comprehensive. That is, by definition, every RVM neuron recorded can be identified as an ON-, OFF-, or NEUTRAL-cell. These three cell classes have been identified in barbiturate-, ketamine-, and isoflurane-anesthetized rats as well as in decerebrate-unanesthetized and awake animals (Clarke et al. 1994; Heinricher et al. 2010b; Leung and Mason 1995, 1999; McGaraughty et al. 1993a, 1993b). ON-cells facilitate nociception, and local or systemically administered μ-opioids suppress ON-cell activity. OFF-cells suppress nociception, and opioids increase OFF-cell firing through disinhibition. Sustained OFF-cell activity mediates the analgesic action of morphine and other μ-opioids. The NEUTRAL-cells do not respond to μ-opioid agonists, whether given systemically or locally (Fields et al. 2006; Heinricher and Ingram 2008). Therefore, one or both of the two opioid-sensitive cell classes, the ON-cells and OFF-cells, must mediate the physiological and behavioral effects of μ-opioids in the RVM, including respiratory depression and analgesia.
To better understand how μ-opioids act in the RVM to depress respiration, we compared the effects of opioids with those of improgan, a non-opioid analgesic. This compound does not cross the blood-brain barrier, but when administered intracerebroventricularly it acts at an unknown receptor site to stimulate descending antinociception through RVM OFF-cell activation (Heinricher et al. 2010b; Nalwalk et al. 2004), a finding consistent with the present results. The surprising observation in the present study was that improgan, applied directly in the RVM, produced a powerful respiratory stimulation in parallel with analgesia, allowing us to investigate the cellular basis for the differential influence on respiratory control and nociception. Locally administered improgan activated not only OFF-cells, mimicking the effect of μ-opioids on these neurons, but also ON-cells, an effect opposite to that of μ-opioids. Although NEUTRAL-cell firing was also increased by local improgan, these neurons do not respond to opioids (Barbaro et al. 1989), which argues against a role for this cell class in opioid-induced respiratory modulation via the RVM. These data therefore confirm the already substantial evidence that the OFF-cells are the analgesic output from the RVM (Fields et al. 2006; Heinricher and Ingram 2008) but, more important, suggest that RVM effects on respiration are mediated by ON-cells. A role for ON-cells in opioid-induced respiratory depression was unexpected but fits well with established interactions between pain and respiration. For instance, acute noxious stimuli, which activate ON-cells, have long been recognized to attenuate opioid-induced respiratory depression (Borgbjerg et al. 1996; Kamei et al. 2011; McQuay 1988). Should OFF-cells play any role in modulating respiration or autonomic parameters, that influence is masked by the overriding effect of the ON-cells.
Dissociation of analgesia from respiratory depression at the level of RVM.
Since OFF-cells appear to mediate analgesia but not respiratory depression, our data imply that further separation of respiratory depression from analgesia is possible, based on both neural substrate and pharmacology. μ-Opioid activation of OFF-cells is indirect, through a presynaptic mechanism, whereas inhibition of ON-cells is a direct postsynaptic effect (Heinricher and Ingram 2008; Heinricher et al. 1992; Pan et al. 1990). Because the pre- and postsynaptic actions of μ-opioids invoke distinct second messengers and channels (Heinricher and Ingram 2008), presynaptic mechanisms could be critical targets for “pure” opioid-like analgesia. Focusing on OFF-cell-selective pathways, including the presynaptic μ-opioid receptors and downstream molecules, therefore has the potential to provide potent pain relief without the risk of respiratory depression. Indeed, cannabinoids, like opioids, act in the RVM to produce analgesia but do not produce significant respiratory depression. This disparity between opioid and cannabinoid actions could be explained by the fact that cannabinoids do not have direct postsynaptic inhibitory actions on RVM ON-cells (Meng et al. 1998; Vaughan et al. 1999).
The RVM has the potential to modulate respiration through several pathways. Raphe magnus and raphe obscurus both send direct projections to the phrenic motor nucleus (Holtman et al. 1984, 1986; Hosogai et al. 1998), and stimulation of either raphe magnus or pallidus influences activity of phrenic motoneurons (Lalley 1986; Millhorn 1986). Alternatively, the RVM has numerous afferent and efferent connections within the brain stem and could modulate relays at various stages of the chemosensory pathways or contribute to chemosensory-evoked activations (Guyenet et al. 2010; Huckstepp and Dale 2011; Nattie 2011; Pattinson et al. 2009). For example, medullary raphe regions are recognized to modulate chemosensory function of the retrotrapezoid nucleus (Depuy et al. 2011; Dias et al. 2008; Hilaire et al. 2010; Mulkey et al. 2007; Viemari and Tryba 2009).
Distribution of opioid-inhibited neurons in RVM and surrounding brain stem.
Because of technical challenges with the use of MOR1 antibodies in the medullary core, the distribution of neurons with postsynaptic μ-opioid receptors in the RVM and surrounding brain stem regions has not been defined precisely, and attempts to quantify or visually identify RVM neurons that express the μ-opioid receptor have met with limited success. We found that the fluorescent μ-opioid dermorphin-A594 microinjected into the RVM labeled somata of neurons that bound and internalized this ligand. This approach holds significant promise for labeling functional receptors where immunohistochemical techniques are not optimal. In addition, it gives a more direct measure of the spread of the injected drug than traditional dye approaches or calculations of injectate volumes.
Labeled neurons were found primarily in raphe magnus and nucleus reticularis gigantocellularis pars alpha but were also concentrated in raphe pallidus. Neurons in the area of raphe magnus and reticularis gigantocellularis pars alpha that exhibit inhibitory responses to μ-opioid agonists have been found without exception to be ON-cells (Barbaro et al. 1989). Whether opioid-sensitive neurons in raphe pallidus also exhibit the physiological properties of ON-cells has not been investigated systematically. Raphe pallidus is strongly implicated in homeostatic regulation, especially control of body temperature (Cao and Morrison 2003; Madden and Morrison 2005; Morrison 2011). However, raphe pallidus has significant anatomical and functional overlap with more dorsal aspects of the RVM, and neurons from throughout the RVM project to the intermediolateral cell column (IML; Berner et al. 1999; Henry and Calaresu 1974; Loewy 1981; Morrison 2011). Functional projections to the IML from the medullary raphe raise core temperature by engaging multiple mechanisms of thermogenesis, including brown adipose tissue activation, vasoconstriction, and fusimotor activity (Blessing and Nalivaiko 2001; McAllen et al. 2010; Nakamura et al. 2004). Control of thermogenesis by opioid-sensitive ON-cells fits with previous observations that DAMGO injected into the RVM attenuates stimulus-evoked increases in activity of brown adipose tissue (Nason and Mason 2006).
Some labeled neurons were also found immediately rostral and caudal to the RVM, at the level of the superior olive and in the area of raphe obscurus dorsal to the inferior olive. Opioid-sensitive cell populations rostral and caudal to the RVM have also not been characterized, but neurons with respiration-related activity have been identified in the medial medulla immediately caudal to the RVM (Lindsey et al. 1994; Pilowsky et al. 1995) The observation that an opioid microinjected in the RVM can directly influence neurons beyond the conventional boundaries of this region raises the possibility that opioid-induced analgesia and respiratory depression are mediated not by RVM OFF- and ON-cells but by opioid-responsive neurons in surrounding regions (Depuy et al. 2011; Zhang et al. 2007). However, it seems unlikely that these areas were the primary target of the injected analgesic drugs, since local application of an opioid antagonist in areas surrounding the RVM did not prevent the analgesic or respiratory-depressant effects of systemically administered morphine. Furthermore, it has been shown that microinjections of DAMGO caudal and lateral to the RVM, at the level of raphe obscurus, do not activate OFF-cells or produce behavioral antinociception (Heinricher et al. 1994). Nevertheless, it is doubtful that a clear functional boundary can be drawn between the RVM and adjacent reticular areas, and there is likely to be significant anatomical overlap in the distributions of neurons important in pain modulation, respiration, and autonomic function (Kerman 2008; Lovick 1997; Rathner et al. 2001; Strack et al. 1989).
Integration of pain modulation and homeostatic regulation in RVM.
Control of respiration occurs through the cooperative actions of a network of brain regions, with contributions from the cerebral cortex, hypothalamus, and multiple sites in the brain stem (Dean and Nattie 2010; Feldman et al. 2003; Guyenet 2008; Guyenet et al. 2010; Horn and Waldrop 1998; Nattie and Li 2009). While the outputs of these areas may converge before reaching respiratory motor neurons, no single brain site is responsible for all aspects of breathing. Thus, just as systemically administered opioids modulate nociception through synergistic spinal and supraspinal actions (Bodnar 2000; Budai and Fields 1998; Hirakawa et al. 1999; Yaksh and Rudy 1978), these agents likely depress respiration through concurrent actions in multiple brain areas, including rostral ventrolateral medulla, pre-Bötzinger complex, nucleus ambiguus, and cerebral cortex (Gray et al. 1999; Hassen et al. 1983; Lalley 2006; Miyawaki et al. 2002; Montandon et al. 2011; Pattinson et al. 2009; Stucke et al. 2008; Zhang et al. 2007). The present findings reinforce the idea of a distributed opioid influence on respiration by showing that activation of opioid receptors in the RVM, a well-known pain-modulating region, can also significantly depress breathing. While these data show that the RVM contributes to decreases in respiration at clinically relevant analgesic doses, higher, potentially lethal doses almost certainly have multiple targets, including direct effects on respiratory premotor neurons (Lalley 2003; Mustapic et al. 2010; Stucke et al. 2008).
While the contribution of RVM ON-cells to opioid-induced respiratory depression is novel, the finding is not out of line with a long-standing view of this region as important for coordinating physiological and behavioral aspects of defense in response to both internal and external challenges to homeostasis (Bandler and Shipley 1994; Lovick 1997). The neuronal basis of this coordination of function deserves further study. Whether a single neuron can modulate nociception, respiration, and autonomic parameters in parallel or if defined cell populations or subpopulations separately regulate each of these functions is a long-standing question that is yet to be resolved (Brazier and Hobson 1980).
Conclusions.
Given the multiple functions integrated within the RVM, it has been argued that separating opioid-mediated analgesia from side effects would be impossible (Mason 2011). While the present data show that respiration, body temperature, and heart rate can be modulated by altering the activity of opioid-sensitive neurons in the RVM, the effects on all three homeostatic parameters are separable from pain inhibition.
An important clinical and scientific goal is to develop drugs that effectively relieve pain without producing respiratory depression. Our findings demonstrate a common central site of opioid action for respiratory depression and analgesia, but also show promise for further dissociation of these effects pharmacologically at the level of functionally distinct neuronal populations within the RVM.
GRANTS
This work was supported by NIH Grants DA-022492, DA-027835, and NS-070374.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S.P., D.R.C., and S.A. performed experiments; R.S.P., D.R.C., and M.M.H. analyzed data; R.S.P., D.R.C., and M.M.H. prepared figures; D.R.C., J.W.N., L.B.H., and M.M.H. conception and design of research; D.R.C. and M.M.H. interpreted results of experiments; D.R.C., L.B.H., and M.M.H. edited and revised manuscript; D.R.C. and M.M.H. approved final version of manuscript; M.M.H. drafted manuscript.
ACKNOWLEDGMENTS
Confocal microscopy and analysis were carried out at the Advanced Light Microscope Core at The Jungers Center at the Oregon Health and Science University, supported by National Institutes of Health (NIH) Grant P30-NS-061800. D. R. Cleary was supported by the Neurobiology of Disease Fellowship from the Oregon Health and Science University Brain Institute for part of this work.
Present address of R. S. Phillips: Dept. of Physics, University of New Hampshire, Durham, NH 03824.
REFERENCES
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharmacol 58: 1570–1580, 2000 [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci 26: 4118–4125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17: 379–389, 1994 [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Motor Res 6: 413–425, 1989 [DOI] [PubMed] [Google Scholar]
- Berner NJ, Grahn DA, Heller HC. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res 831: 155–164, 1999 [DOI] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neuroscience 105: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci 7: 181–194, 2000 [DOI] [PubMed] [Google Scholar]
- Borgbjerg FM, Nielsen K, Franks J. Experimental pain stimulates respiration and attenuates morphine-induced respiratory depression: a controlled study in human volunteers. Pain 64: 123–128, 1996 [DOI] [PubMed] [Google Scholar]
- Brazier MA, Hobson JA. (Editors). The Reticular Formation Revisited. New York: Raven, 1980, p. 552 [Google Scholar]
- Budai D, Fields HL. Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol 79: 677–687, 1998 [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004 [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res 980: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- Clarke RW, Morgan MM, Heinricher MM. Identification of nocifensor reflex-related neurons in the rostroventromedial medulla of decerebrated rats. Brain Res 636: 169–174, 1994 [DOI] [PubMed] [Google Scholar]
- Cleary DR, Phillips RS, Wallisch M, Heinricher MM. A novel, non-invasive method of respiratory monitoring for use with stereotactic procedures. J Neurosci Methods 209: 337–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB, Nattie EE. Central CO2 chemoreception in cardiorespiratory control. J Appl Physiol 108: 976–978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Preston MJ, Dommett EJ, Murphy KL, Greenfield SA. Design and evaluation of a low-cost respiratory monitoring device for use with anaesthetized animals. Lab Anim 43: 382–389, 2009 [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol 105: 83–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Branco LG, Gargaglioni LH. Opioid μ-receptors in the rostral medullary raphe modulate hypoxia-induced hyperpnea in unanesthetized rats. Acta Physiol (Oxf) 204: 435–442, 2012 [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol 103: 1780–1788, 2007 [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 5: 565–575, 2004 [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: Wall and Melzack's Textbook of Pain (5th ed.), edited by McMahon S, Koltzenburg M. London: Elsevier, 2006, p. 125–142 [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci 3: 2545–2552, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci 308: 361–374, 1985 [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286: 1566–1568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol 518: 3883–3906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen AH, Feuerstein G, Faden AI. Differential cardiovascular effects mediated by mu and kappa opiate receptors in hindbrain nuclei. Peptides 4: 621–625, 1983 [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: The Senses, A Comprehensive Reference, Vol. 5, Pain, edited by Bushnell MC, Basbaum AI. San Diego, CA: Academic, 2008, p. 593–626 [Google Scholar]
- Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol 104: 3222–3230, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Nalwalk JW, Hough LB. Neural basis for improgan antinociception. Neuroscience 169: 1414–1420, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48: 533–543, 1992 [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 63: 279–288, 1994 [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60: 214–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience 63: 533–546, 1994 [DOI] [PubMed] [Google Scholar]
- Hellman KM, Brink TS, Mason P. Activity of murine raphe magnus cells predicts tachypnea and on-going nociceptive responsiveness. J Neurophysiol 98: 3121–3133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman KM, Mendelson SJ, Mendez-Duarte MA, Russell JL, Mason P. Opioid microinjection into raphe magnus modulates cardiorespiratory function in mice and rats. Am J Physiol Regul Integr Comp Physiol 297: R1400–R1408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JL, Calaresu FR. Excitatory and inhibitory inputs from medullary nuclei projecting to spinal cardioacceleratory neurons in the cat. Exp Brain Res 20: 485–504, 1974 [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174: 76–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa N, Tershner SA, Fields HL. Highly delta selective antagonists in the RVM attenuate the antinociceptive effect of PAG DAMGO. Neuroreport 10: 3125–3129, 1999 [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Anastasi NC, Norman WP, Dretchen KL. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res 362: 214–220, 1986 [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Gillis RA. Projections from the raphe nuclei to the phrenic motor nucleus in the cat. Neurosci Lett 44: 105–111, 1984 [DOI] [PubMed] [Google Scholar]
- Horn EM, Waldrop TG. Suprapontine control of respiration. Respir Physiol 114: 201–211, 1998 [DOI] [PubMed] [Google Scholar]
- Hosogai M, Matsuo S, Sibahara T, Kawai Y. Projection of respiratory neurons in rat medullary raphe nuclei to the phrenic nucleus. Respir Physiol 112: 37–50, 1998 [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WM, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res 880: 102–108, 2000 [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity—towards a better understanding of mechanism. J Physiol 589: 5561–5579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M, Hayashi SS, Nakanishi Y. Effect of chronic pain on morphine-induced respiratory depression in mice. Neuroscience 174: 224–233, 2011 [DOI] [PubMed] [Google Scholar]
- Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res 187: 1–16, 2008 [DOI] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol 380: 349–371, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003 [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regul Integr Comp Physiol 290: R1387–R1396, 2006 [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Effects of isoflurane concentration on the activity of pontomedullary raphe and medial reticular neurons in the rat. Brain Res 699: 71–82, 1995 [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological properties of raphe magnus neurons during sleep and waking. J Neurophysiol 81: 584–595, 1999 [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J Neurophysiol 72: 1830–1851, 1994 [DOI] [PubMed] [Google Scholar]
- Loewy AD. Raphe pallidus and raphe obscurus projections to the intermediolateral cell column in the rat. Brain Res 222: 129–133, 1981 [DOI] [PubMed] [Google Scholar]
- Lovick TA. The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol 82: 31–41, 1997 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martenson M, Houts J, Heinricher M, Ogden B. A simple device for humidification of inspired gases during volatile anesthesia in rats. Contemp Top Lab Anim Sci 44: 46–48, 2005 [PubMed] [Google Scholar]
- Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain 142: 236–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. From descending pain modulation to obesity via the medullary raphe. Pain 152: S20–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R, Tanaka M, Ootsuka Y, McKinley M. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol 109: 27–33, 2010 [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Reinis S, Tsoukatos J. Investigating the role of anaesthetics on the rostral ventromedial medulla: implications for a GABAergic link between ON and OFF cells. Neurosci Lett 149: 119–122, 1993a [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Reinis S, Tsoukatos J. Two distinct unit activity responses to morphine in the rostral ventromedial medulla of awake rats. Brain Res 604: 331–333, 1993b [DOI] [PubMed] [Google Scholar]
- McQuay HJ. Potential problems of using both opioids and local anaesthetic. Br J Anaesth 61: 121, 1988 [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature 395: 381–383, 1998 [DOI] [PubMed] [Google Scholar]
- Menuet C, Borghgraef P, Matarazzo V, Gielis L, Lajard AM, Voituron N, Gestreau C, Dutschmann M, Van Leuven F, Hilaire G. Raphe tauopathy alters serotonin metabolism and breathing activity in terminal tau.P301l mice: possible implications for tauopathies and Alzheimer's disease. Respir Physiol Neurobiol 178: 290–303, 2011 [DOI] [PubMed] [Google Scholar]
- Millhorn DE. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol 381: 169–179, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience 109: 133–144, 2002 [DOI] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBötzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31: 1292–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. Central neural pathways for thermoregulatory cold defense. J Appl Physiol 110: 1137–1149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol 103: 409–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24: 5370–5380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res 1021: 248–255, 2004 [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci 26: 1190–1198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Central chemoreception: then … and now. J Appl Physiol 110: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain 110: 158–165, 2004 [DOI] [PubMed] [Google Scholar]
- Nickerson JW, Attaran A. The inadequate treatment of pain: collateral damage from the war on drugs. PLoS Med 9: e1001153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol 427: 519–532, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 29: 8177–8186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, Miyawaki T, Minson JB, Sun QJ, Arnolda LF, Llewellyn-Smith IJ, Chalmers JP. Bulbospinal sympatho-excitatory neurons in the rat caudal raphe. J Hypertens 13: 1618–1623, 1995 [PubMed] [Google Scholar]
- Proudfit HK. Effects of raphe magnus and raphe pallidus lesions on morphine-induced analgesia and spinal cord monoamines. Pharmacol Biochem Behav 13: 705–714, 1980 [DOI] [PubMed] [Google Scholar]
- Proudfit HK. Time-course of alterations in morphine-induced analgesia and nociceptive threshold following medullary raphe lesions. Neuroscience 6: 945–951, 1981 [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphe-spinal neurons in rats. J Physiol 535: 841–854, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CD, Lois JH, Kerman IA, Yates BJ. Localization of serotoninergic neurons that participate in regulating diaphragm activity in the cat. Brain Res 1279: 71–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 491: 274–296, 1989 [DOI] [PubMed] [Google Scholar]
- Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol 100: 2878–2888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol 153: 203–216, 2006 [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol 127: 935–940, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner TA, Goodchild AK, Pilowsky PM. A mapping study of cardiorespiratory responses to chemical stimulation of the midline medulla oblongata in ventilated and freely breathing rats. Am J Physiol Regul Integr Comp Physiol 287: R411–R421, 2004 [DOI] [PubMed] [Google Scholar]
- Viemari JC, Tryba AK. Bioaminergic neuromodulation of respiratory rhythm in vitro. Respir Physiol Neurobiol 168: 69–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, Grey T, Johnson EM, Lee LK, Passik SD, Peppin J, Porucznik CA, Ray A, Schnoll SH, Stieg RL, Wakeland W. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med 12, Suppl 2: S26–S35, 2011 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain 4: 299–359, 1978 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid μ receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288–297, 2007 [DOI] [PubMed] [Google Scholar]


