Abstract
Skeletal muscle is a well-known source of glial cell line-derived neurotrophic factor (GDNF), which can produce mechanical hyperalgesia. Since some neuromuscular diseases are associated with both increased release of GDNF and intense muscle pain, we explored the role of GDNF as an endogenous mediator in muscle pain. Intramuscularly injected GDNF induced a dose-dependent (0.1–10 ng/20 μl) persistent (up to 3 wk) mechanical hyperalgesia in the rat. Once hyperalgesia subsided, injection of prostaglandin E2 at the site induced a prolonged mechanical hyperalgesia (>72 h) compared with naïve rats (<4 h; hyperalgesic priming). Selective neurotoxic destruction of IB4(+) nociceptors attenuated both GDNF hyperalgesia and hyperalgesic priming. Ergonomic muscular injury induced by eccentric exercise or mechanical vibration increased muscle GDNF levels at 24 h, a time point where rats also exhibited marked muscle hyperalgesia. Intrathecal antisense oligodeoxynucleotides to mRNA encoding GFRα1, the canonical binding receptor for GDNF, reversibly inhibited eccentric exercise- and mechanical vibration-induced muscle hyperalgesia. Finally, electrophysiological recordings from nociceptors innervating the gastrocnemius muscle in anesthetized rats, revealed significant increase in response to sustained mechanical stimulation after local GDNF injection. In conclusion, these data indicate that GDNF plays a role as an endogenous mediator in acute and induction of chronic muscle pain, an effect likely to be produced by GDNF action at GFRα1 receptors located in IB4(+) nociceptors.
Keywords: isolectin B4, ergonomic injury, chronic pain, rat
muscle pain is an extremely common symptom that considerably impacts on quality of life, generating huge direct and indirect economic loses. Muscle pain is different in many aspects from cutaneous pain (Mense 2003); one of its most problematic characteristics is that, regardless of etiology, it often persists for a much longer period of time (Sluka 2002). Furthermore, after inflammatory injury both skin (Aley et al. 2000) and muscle (Dina et al. 2008; Alvarez et al. 2012) exhibit a long lasting mechanical hyperalgesia to prostaglandin E2 but such a response persists for much longer time in muscle (Dina et al. 2008; Alvarez et al. 2012). As many muscle pain syndromes are in fact chronic, elucidating the mechanisms by which they persist could be important to the development of appropriate mechanism-based analgesic therapies.
Small diameter nociceptors have been divided into two major classes according to their dependence on nerve growth factor (NGF) or glial cell line-derived neurotrophic factor (GDNF; Molliver et al. 1997; Snider and McMahon 1998). The trophic effects of GDNF depend on receptor complexes formed by one subunit involved in ligand binding and one subunit specialized in transmembrane signaling, the receptor tyrosine kinase rearranged during transfection (Ret; Bespalov and Saarma 2007). Almost 50% of all Ret-expressing nociceptors also express the GDNF family receptor α1 (GFRα1), the canonical GDNF receptor. As distinctive features, this subpopulation of nociceptors also binds the plant lectin isolectin B4 (IB4; Molliver et al. 1997; Silverman and Kruger 1990; Streit et al. 1986) and their central projections reach the inner part of lamina II in the dorsal horn (Kitchener et al. 1993; Snider and McMahon 1998). The IB4(+) GFRα1/Ret-expressing nociceptors are not only dependent on the neurotrophic effects of GDNF (Matheson et al. 1997; Moore et al. 1996) but also display increased responsiveness to thermal (Malin et al. 2006) and mechanical (Bogen et al. 2008; Ferrari et al. 2010; Joseph and Levine 2010) stimuli after acute exposure to this neurotrophic factor. In contrast to reports indicating that GDNF enhances mechanical and thermal nociception, it has also been observed that intrathecal administration of GDNF (Boucher et al. 2000) or intraspinal delivery of viral vectors expressing GDNF (Pezet et al. 2006) attenuate cutaneous allodynia/hyperalgesia in models of neuropathic pain. Furthermore, intrathecal GDNF did not affect cutaneous mechanical or thermal nociceptive thresholds in normal rats (Boucher et al. 2000). Altogether, these reports underline the complexity of the role played by GDNF in nociception and may reflect site-specific actions exerted by this neurotrophin.
Skeletal muscle is an important source of GDNF, which provides essential trophic support for normal development, differentiation, and function of sensory neurons, motoneurons, skeletal muscle cells, and neuromuscular synapses (Cacalano et al. 1998; Henderson et al. 1994; Sakuma and Yamaguchi 2011; Yang and Nelson 2004). Highly expressed in the vicinity of the plasma membrane of skeletal muscle cells (Suzuki et al. 1998a, 1998b; Wehrwein et al. 2002), GDNF content is increased as an adaptive consequence of muscle training (Wehrwein et al. 2002).
Although GDNF is indispensable for skeletal muscle function, patients affected by painful neuromuscular diseases such as polymyositis and Duchenne type neuromuscular dystrophy exhibit increased levels of GDNF in regenerating muscle fibers (Suzuki et al. 1998a) and complain of persistent pain in affected muscles (Engel et al. 2009; Zebracki and Drotar 2008), suggesting a possible role for GDNF as an endogenous mediator of their pain. Consistent with this are reports of cutaneous mechanical hyperalgesia observed after local injection of GDNF in rats (Bogen et al. 2008; Ferrari et al. 2010; Joseph and Levine 2010) or in mice overexpressing GDNF (Albers et al. 2006). Finally, recent evidence indicates that IB4(+) nociceptors are involved in mechanical hyperalgesia induced by the inflammogen carrageenan or muscle damage induced by ergonomic interventions (Alvarez et al. 2012). While the contribution of GDNF as an endogenous mediator in persistent cutaneous pain seems to be well established, its contribution in the induction of muscle pain remains unknown. Therefore, in the present study we explored the role of GDNF and its putative targets, the IB4(+)-GFRα1/Ret-expressing nociceptors, in persistent muscle pain.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (250–330 g; Charles River, Hollister, CA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco under environmentally controlled conditions (lights on 0700–1900; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were killed by 150 mg/kg pentobarbital followed by cervical dislocation, in accordance with University of California, San Francisco, Institutional Animal Care and Use Committee standard euthanasia guidelines. The University of California, San Francisco, Committee on Animal Research approved all experimental protocols, which adhered to the ethical guidelines published by the International Association for the Study of Pain (Zimmermann 1983). Every effort was made to minimize number and suffering of animals used in the experiments.
Drugs.
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Measurement of muscle mechanical hyperalgesia.
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a digital force transducer (Chatillon DFI2; Amtek, Largo, FL) with a custom-made 7-mm-diameter probe (Alvarez et al. 2010). Rats were lightly restrained in a cylindrical acrylic holder with lateral slats that allow for easy access to the hindlimb and application of the transducer probe to the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in milliNewtons (mN), required to produce a flexion reflex in the hind leg. Baseline limb-withdrawal threshold was defined as the mean of three readings taken at 5-min intervals. Each hindlimb was treated as an independent measure, and each experiment was performed on a separate group of rats.
Eccentric exercise-induced muscle pain.
The method used to eccentrically exercise the rat hindlimb (Alvarez et al. 2010) was similar to that described by Kano et al. (2004) and Mizumura and colleagues (Taguchi et al. 2005). Briefly, isoflurane-anesthetized rats were placed in the supine position on a heating pad (to maintain body temperature at 37°C), and the right hind paw was affixed to the foot bracket of the exercise apparatus (model RU-72; NEC Medical Systems, Tokyo, Japan) with 3M Micropore surgical paper tape, such that the angle of the knee and ankle joints was ∼90° (the paw 30° from vertical). The gastrocnemius muscle was stimulated via subcutaneous needle-type electrodes attached to a model DPS-07 stimulator (Dia Medical System, Tokyo, Japan) that delivered trains of rectangular pulses (100 Hz, 700 ms, 3 V) every 3 s to give a total of 300 contractions. During these stimulus-induced contractions of the gastrocnemius muscle, the electromotor system rotated the foot to produce extension of the gastrocnemius muscle.
Mechanical vibration-induced muscle pain.
The hindlimbs of rats were vibrated with a laboratory vortex mixer (Digital Vortex Genie II; Fisher Scientific, Waltham, MA), which has a variable-speed motor with a real-time digital readout of the vibration speed. Rats were anesthetized with 3% isoflurane in oxygen, and a hind leg was affixed to the platform with 3M Micropore surgical tape so that the knee and ankle joint angles were both 90° without longitudinal rotational torque on the leg. As previously described (Dina et al. 2010), the leg was submitted to an oscillatory vibration for 15 min at a frequency of 60 to 80 Hz with a 5-mm peak-to-peak displacement amplitude.
Intramuscular injections.
Rats were briefly anesthetized with 2.5% isoflurane to facilitate the injection of GDNF or prostaglandin E2 (PGE2) into the belly of the gastrocnemius muscle (20 μl). The injection site was previously shaved and scrubbed with alcohol. Immediately after injections the skin puncture site was marked with a fine-tip indelible ink pen, so that the mechanical nociceptive threshold of the underlying injection site in the muscle could be repeatedly tested. Stock solutions of GDNF (1 μg/μl dissolved in 0.9% NaCl containing 0.5% BSA and stored at −20°C) and PGE2 (1 μg/μl dissolved in 100% ethanol and stored at −80°C) were diluted in 0.9% NaCl immediately before injection.
Intrathecal injections.
Rats were briefly anaesthetized with 2.5% isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO) in 97.5% O2. Then, a 30-gauge hypodermic needle was inserted into the subarachnoid space, on the midline, between the L4 and L5 vertebrae, and the injection was performed (20 μl). Animals regained consciousness ∼1 min after the injection. Proper intrathecal injections were systematically confirmed by checking for a sudden flicking of the tail, a reflex that is evoked by subarachnoid space access and bolus injection (Mestre et al. 1994).
The destruction of the IB4(+) population of dorsal root ganglion neurons by intrathecal injection of a cytotoxin, saporin, conjugated to IB4 (IB4-saporin) has been established previously by us (Bogen et al. 2009; Bogen et al. 2008; Joseph et al. 2008; Joseph and Levine 2010) and others (Nishiguchi et al. 2004; Vulchanova et al. 2001). IB4-saporin (Advanced Targeting Systems, San Diego, CA) was diluted with saline and a dose of 3.2 μg in 20 μl administered intrathecally 10 days before experiments (Joseph et al. 2008; Joseph and Levine 2010).
An oligodeoxynucleotide (ODNs) antisense (AS) to GFRα1, shown previously to knockdown GFRα1 protein in the rat glioma cell line C6 (Wiesenhofer et al. 2000) and to decrease GFRα1 protein, mRNA, and immunostaining in rat DRG cells (Dong et al. 2006), was synthesized by Invitrogen (San Francisco, CA). The AS ODN (5′-TAG GAA CAT GGT GCC-3′) was directed against a unique sequence of GFRα1. The mismatch (MM) ODN (5′-TAG AGA CAT GGT GCC-3′) is the AS sequence with two bases, denoted in bold face, switched. A search of the EMBL and NCBI GenBank Rattus norvegicus databases identified no homologous sequences. Before use, the ODNs were lyophilized and reconstituted in 0.9% NaCl to a concentration of 2 μg/μl. A dose of 40 μg of AS or MM ODN was administered intrathecally in a volume of 20 μl once daily for 3 consecutive days.
Measurement of GDNF levels in gastrocnemius muscle.
Twenty-four hours after either eccentric exercise or mechanical vibration, animals were deeply anesthetized with isofluorane and the ipsilateral gastrocnemius muscle was removed and flash frozen on dry ice. Each frozen muscle was cut into serial cross sections (<0.5 g) and stored at −80°C until further processing. All muscle samples were pulverized in a porcelain mortar in the presence of liquid nitrogen and transferred into 0.1 M PBS (0.225 M NaCl, 0.02 M NaH2PO43−, and 0.08 M Na2HPO43−) containing 0.1% Tween 20 and 0.05% BSA and supplemented with a 2× complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and homogenized with a tissue tearor (Biospec Products, Bartlesville, OK). Solubilized proteins were extracted by a 15 min centrifugation at 4°C and 20,000 g. The total protein concentration of each sample was determined by using the micro Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL), whereas GDNF concentration was determined using an ELISA performed with the GDNF Emax ImmunoAssay System (Promega, Madison, WI) according to the manufacturer's instructions. Absorbencies of protein extracts were measured at 450 nm for full-strength solutions and 1:10 or 1:100 dilutions by using a microplate reader (Wallac 1420 multilabel counter; Perkin Elmer, Vernon Hills, IL). The concentration of GDNF in each sample was adjusted for the dilution factor, and the results from triplicates were averaged to obtain the final concentration of GDNF per milligram of protein extracted from each muscle sample.
Single fiber in vivo electrophysiology.
The in vivo single fiber electrophysiology technique for studying muscle afferents has been described in detail previously (Chen et al. 2010). In brief, rats were anesthetized with pentobarbital sodium (initially 50 mg/kg ip with additional doses given throughout the experiment to maintain areflexia), their trachea was cannulated, and their heart rate was monitored. Anesthetized animals were positioned on their right side and an incision was made on the dorsal skin of the left leg between the mid-thigh and calf. Then, the biceps femoris muscle was partially removed to expose the sciatic nerve and gastrocnemius muscle. The edges of the incised skin were fixed to a metal loop to provide a pool that was filled with warm mineral oil that bathed the sciatic nerve and gastrocnemius muscle.
The sciatic nerve was transected proximal to the recording site to avoid the effects of reflex arc activation. While stimulation of muscular receptive field led to muscle contraction, it did not impact the recording, i.e., neural activity was still recorded. Fine fascicles of axons were then dissected from the distal stump and placed on a recording electrode. Single units were first detected by mechanical stimulation of the gastrocnemius muscle with a small blunt-tipped glass bar. Bipolar stimulating electrodes were then placed and held on the center of the receptive field of the muscle afferent by a micromanipulator (model MM-3; Narishige, Tokyo, Japan). Subsequently, electrical stimulation allowed confirmation of the mechanical receptive field according to the amplitude and duration of action potentials. Finally, the unit discharge was discriminated by Spike 2 software (CED, Cambridge, UK). Most neural activity was recorded from one or two units, no more than three units, which were significantly different from the amplitude and/or duration. The conduction velocity of each fiber was calculated by dividing the distance between the stimulating and recording electrodes by the latency of the electrically evoked action potential. Mechanical threshold, determined with calibrated von Frey filaments (VFH Ainsworth, London, UK), was defined as the lowest force that elicited at least two spikes within 1 s in at least 50% of trials. Sustained suprathreshold (10 g) mechanical stimulation was accomplished by use of a mechanical stimulator that consisted of a force-measuring transducer (Entran, Fairfield, NJ) with a blunt plastic tip that was applied by a micromanipulator (BC-3 and BE-8; Narishige) on the center of the afferent's receptive field for 60 s. Neural activity and timing of stimulus onset and termination were monitored and stored on a computer with a Micro 1401 interface (CED) and analyzed offline with Spike2 software (CED).
Statistical analysis.
Group data are expressed as means ± SE of n independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software, La Jolla, CA). The Student's t-test was used to compare one or two independent samples, whereas ANOVA followed by Dunnett's or Tukey's multiple comparison tests was used for comparing three or more samples. Data were tested for normality using the D'Agostino and Pearson omnibus normality test; if data did not pass the normality test for Gaussian distribution, Welch correction for the Student's t-test was used. Nonparametric data were analyzed using Wilcoxon's signed rank test. P < 0.05 was considered statistically significant.
RESULTS
GDNF produces acute and chronic hyperalgesia.
The unilateral injection of GDNF into the gastrocnemius muscle significantly reduced mechanical nociceptive threshold. Compared with their baselines, readings obtained 1 h after intramuscular GDNF (doses of 0.1 ng, 1 ng or 10 ng) in the injected (ipsilateral) gastrocnemius muscle (Fig. 1A), but not the untreated (contralateral) gastrocnemius muscle, exhibited a marked decrease in mechanical nociceptive threshold (hyperalgesia). The amplitude and duration of this mechanical hyperalgesia were dose dependent. The lowest dose assayed (0.1 ng/20 μl) produced a significant mechanical hyperalgesia lasting >1 wk (Fig. 1B), whereas the highest dose (10 ng/20 μl) induced hyperalgesia that lasted >3 wk (Fig. 1B). Of note, GDNF vehicle has no effect on mechanical nociceptive threshold (Fig. 2A).
Fig. 1.
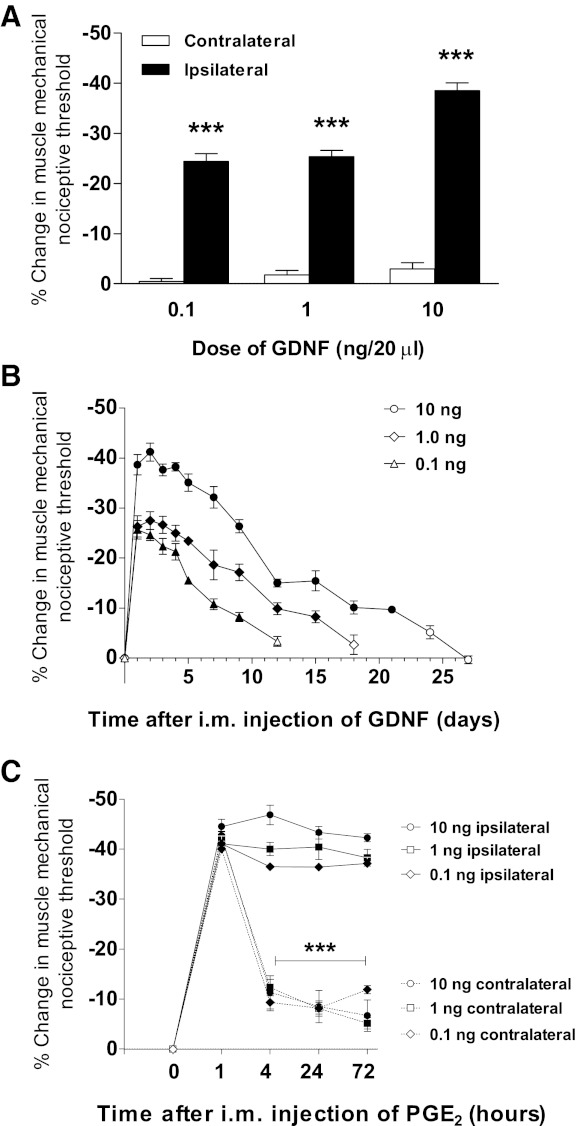
Intramuscular injection of glial cell line-derived neurotrophic factor (GDNF) produces acute and chronic pain. A: 1 h after unilateral injection of GDNF (0.1–10 ng/20 μl im; n = 6/dose), a marked mechanical hyperalgesia was observed in the injected (ipsilateral) gastrocnemius muscle (0.1 ng: 2696.2 ± 6.2 vs. 2038.3 ± 38.9 mN, P < 0.001; 1 ng: 2,671.7 ± 4 vs. 1,994 ± 11.5 mN, P < 0.001; 10 ng: 2,677.2 ± 7.9 vs. 1,647.2 ± 43.6 mN, P < 0.001, ANOVA followed by Dunnett's test) but not the contralateral muscle (0.1 ng: 2,697.2 ± 7.8 vs. 2,686.3 ± 16.6 mN, P > 0.05; 1 ng: 2,680.8 ± 2.9 vs. 2,634.3 ± 18.8 mN, P > 0.05; 10 ng: 2,677.5 ± 4.4 vs. 2,600.8 ± 17.2 mN, P > 0.05, ANOVA followed by Dunnett's test, respectively). Data are plotted as %change in mechanical nociceptive threshold of GDNF-injected (ipsilateral) and noninjected (contralateral) hindlimbs compared with baseline and differences are represented by ***P < 0.001. B: GDNF-induced muscle hyperalgesia exhibited dose-dependent amplitude and duration, persisting for >1 wk at 0.1 ng (9th day 2,474.8 ± 19.5 mN, P < 0.001, ANOVA followed by Dunnett's test) and up to 3 wk at 10 ng (21st day 2,419.3 ± 15.5 mN, P < 0.001, ANOVA followed by Dunnett's test) after injection. Data are plotted as %change in mechanical nociceptive threshold compared with baseline (n = 6/group). Solid symbols represent P < 0.05 respect to baseline. C: when nociceptive thresholds returned to pre-GDNF baseline, groups were tested for the presence of hyperalgesic priming. After bilateral PGE2 administration (1 μg/20 μl im), nociceptive thresholds were tested at 1, 4, 24, and 72 h. One hour after PGE2 injection, bilateral mechanical hyperalgesia was observed. Regardless the dose of GDNF, such hyperalgesia remained unchanged in hindlimbs ipsilateral to GDNF injection after 72 h (0.1 ng: 1,534.8 ± 28.9 vs. 1,638.8 ± 39.3 mN, P > 0.05; 1 ng: 1,528.3 ± 25.9 vs. 1,603 ± 38.9 mN, P > 0.05; 10 ng: 1,490.3 ± 14.3 vs. 1,600.3 ± 20 mN, P > 0.05, ANOVA followed by Tukey's test, respectively), indicating hyperalgesic priming. However, in hindlimbs contralateral to GDNF injection, mechanical hyperalgesia was significantly decreased 4 h after PGE2 injection (0.1 ng: 1,635 ± 2.9 vs. 2,470.8 ± 15.4 mN, P < 0.001; 1 ng: 1,569 ± 40.9 vs. 2,384.3 ± 39.6 mN, P < 0.001; 10 ng: 1,560.5 ± 19.5 vs. 2,409.2 ± 77.5 mN, P < 0.001, ANOVA followed by Tukey's test, respectively). Data are plotted as %change in mechanical nociceptive threshold compared with baseline (n = 6/group). Differences in nociceptive responses between readings taken at 1, and 4, 24, and 72 h in hindlimbs ipsi or contralateral to previous GDNF injection are represented by ***P < 0.001.
Fig. 2.
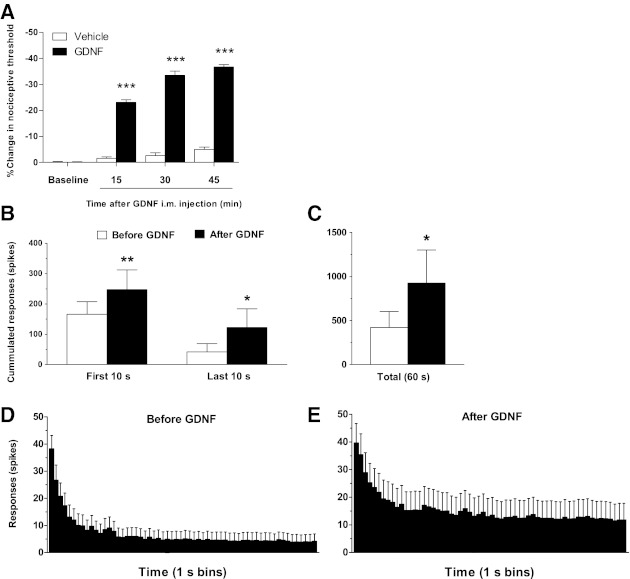
GDNF produces mechanical hyperalgesia and sensitizes muscle nociceptors. A: compared with baseline (2,649.2 ± 2.8 mN) GDNF (n = 6), but not vehicle (n = 6), produced a significant decrease in mechanical muscle threshold at 15 (2,037.3 ± 10.4 mN, P < 0.001, ANOVA followed by Dunnett's test), 30 (1,762.8 ± 18.6 mN, P < 0.001, ANOVA followed by Dunnett's test), and 45 (1,676.3 ± 8.3 mN, P < 0.001, ANOVA followed by Dunnett's test) min. after intramuscular injection. Also, 30 min after injection, GDNF significantly enhanced the nociceptor response to sustained mechanical stimulation, as revealed by increased cumulated number of spikes in recordings obtained during early (first 10 s) and late (last 10 s) parts of the stimulation period (B), as well as total response (60 s) compared with pre-GDNF control values (C). This was also evident in time-course histograms of the nociceptor response, representing recordings obtained before (D) and after (E) GDNF injection (10 ng/5 μl im; n = 16). Comparisons between pre- and post-GDNF treatment were made using one-tail Student's t-test for paired samples; *P < 0.05; **P < 0.01; ***P < 0.001.
Ten days after the nociceptive thresholds returned to baseline, rats were assessed for hyperalgesic priming. The inflammatory cytokine PGE2 (1 μg/20 μl) was injected bilaterally into the gastrocnemius muscle, and nociceptive threshold was measured after 1, 4, 24, and 72 h. PGE2 (1 μg) injected into the gastrocnemius muscle contralateral to the previous injection of GDNF (doses of 0.1, 1, or 10 ng) produced a marked diminution of mechanical threshold (1 h after PGE2 injection) that was significantly reversed by 4 h (Fig. 1C). In the GDNF-treated side, however, PGE2 induced a prolonged diminution in mechanical threshold that was unattenuated at 72 h (Fig. 1C), indicating the induction of hyperalgesic priming (Aley et al. 2000; Alvarez et al. 2012; Dina et al. 2008) by GDNF.
Local injection of GDNF sensitizes muscle nociceptors.
The set of analyzed fibers was composed for 44% Aδ (type III) fibers and 56% C (type IV) fibers. The ranges of Aδ fiber conduction velocity were between 2.57 and 5.00 m/s. The mean value of Aδ fibers was 3.83 m/s. The ranges of C-fiber conduction velocity were between 0.36 and 2.46 m/s. The mean value of C-fibers was 1.72 m/s. Before and 30 min after injection of GDNF (10 ng/5 μl; n = 16), a time at which muscle mechanical hyperalgesia is observed (Fig. 2A) (Hendrich et al. 2012), the peripheral receptive field of sensory neurons innervating the gastrocnemius muscle was tested using von Frey monofilaments. The mechanical threshold of muscle afferents recorded before GDNF treatment (1.15 ± 0.17 mN) was not significantly modified by the injection of GDNF (1.01 ± 0.16 mN; n = 16; Wilcoxon signed rank test). The conduction velocity of muscle afferents recorded before treatment (2.64 ± 0.33 m/s) was also not significantly modified by the injection of GDNF (2.63 ± 0.33 m/s; Student's t-test, with Welch's correction; n = 16).
To examine excitability in muscle nociceptors from GDNF-injected rats, we evaluated their response to a sustained (60 s) suprathreshold (10 g) von Frey filament stimulus. A baseline response of muscle afferents to this sustained mechanical stimulation was recorded before GDNF injection (421.4 ± 180.5 action potentials/60-s stimulus). After GDNF injection, this response was significantly increased (Fig. 2, B-D, 924.3 ± 375.9 action potentials/60-s stimulus; n = 16; P = 0.025, Student's t-test with Welch's correction). The analysis of the time course of the response to sustained mechanical stimulation shows a significant increase in the number of spikes during the first 10 s after application of the stimulus (Fig. 2, B–D; 247.6 ± 108.5 action potentials/10-s stimulus; n = 16; Student's t-test with Welch's correction) compared with pretreatment control (166.4 ± 40.9 action potentials/10-s stimulus; n = 16). The number of spikes recorded during the last 10 s of the stimulus application was also significantly increased (Fig. 2, B-D; 122.38 ± 61.74 action potentials/50 to 60-s stimulus; n = 16; Student's t-test with Welch's correction) compared with pretreatment control (42 ± 27.09 action potentials/50 to 60-s stimulus; n = 16).
Ergonomic insults increase GDNF in muscle.
Since the level of GDNF is upregulated in an activity-dependent manner in rat skeletal muscle (Wehrwein et al. 2002), and patients afflicted by painful neuromuscular conditions exhibit increased levels of GDNF in regenerating muscle fibers (Suzuki et al. 1998a), we hypothesized that nociceptive ergonomic stimuli might cause increased levels of GDNF in muscle, which could act to produce acute hyperalgesia and/or chronic hyperalgesic priming. Therefore, we measured GDNF levels in rats submitted to two ergonomic pain models at a time point characterized by frank muscle mechanical hyperalgesia (Alvarez et al. 2010; Dina et al. 2010). Both nociceptive ergonomic stimuli, mechanical vibration and eccentric exercise, caused increased levels of GDNF (measured as pg/mg protein) in gastrocnemius muscle compared with control animals (Fig. 3).
Fig. 3.
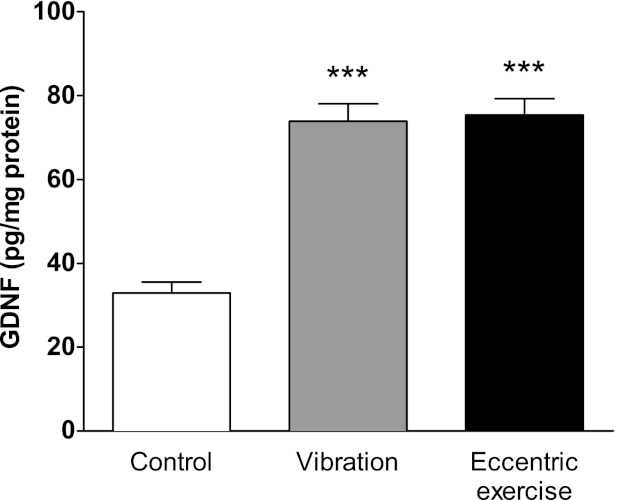
Ergonomic insults increase GDNF content in affected skeletal muscle. Rats were submitted to a hindlimb vibration or eccentric exercise. Twenty four hours later, a time point where both interventions produce muscle hyperalgesia (Alvarez et al. 2010; Alvarez et al. 2012; Dina et al. 2010), the gastrocnemius muscle was excised and GDNF content (pg/mg protein) determined by means of an ELISA procedure and compared with those exhibited by naïve rats. Both ergonomic interventions produced a significant increase in muscular content of GDNF. Comparisons between GDNF values obtained from naïve rats (white bar, n = 12) and rats submitted to vibration (grey bar, n = 7) or eccentric exercise (black bar, n = 5) were made by using a one-way ANOVA followed by a Dunnett's multiple comparisons test. ***P < 0.001.
Hyperalgesic effects of endogenously released GDNF depend on GFRα1.
Next we assessed whether the nociceptive effects of GDNF released after ergonomic insults depend on its canonical receptor, the GFRα1 receptor, to produce muscle mechanical hyperalgesia. Rats received daily intrathecal injections of ODN AS or MM to GFRα1 for 3 days, and then their hindlimbs were either vibrated or eccentrically exercised to produce long-lasting muscle mechanical hyperalgesia (Alvarez et al. 2010; Dina et al. 2010). Compared with MM ODN, AS ODN treatment significantly reduced the mechanical muscle hyperalgesia measured on days 1, 2, and 3 after vibration (Fig. 4A) or eccentric exercise (Fig. 4B).
Fig. 4.
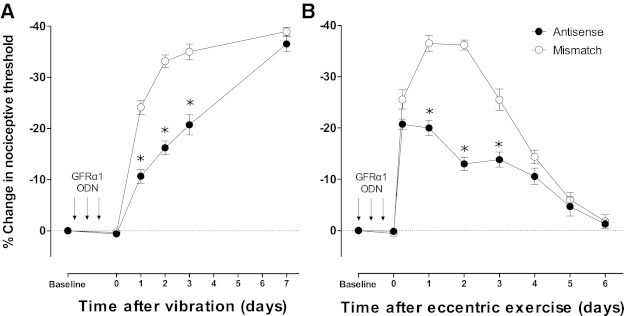
Intrathecal antisense to GFRα1 inhibits ergonomic insult-induced mechanical hyperalgesia. Rats received a daily intrathecal treatment of antisense (●) or mismatch (○) oligodeoxynucleotide (ODNs) to GFRα1 [40 μg, antisense (AS) or mismatch (MM)] during three consecutive days. They were then submitted to a hindlimb vibration (n = 9/ODN treatment) or eccentric exercise (n = 6/ODN treatment). A: preventive AS, but not MM, treatment inhibited mechanical hyperalgesia after hindlimb vibration on days 1 (AS: 2,376.7 ± 36.3 mN vs. MM: 2,025.1 ± 35.3 mN, P < 0.05), 2 (AS: 2,228.8 ± 37 mN vs. MM: 1,784.9 ± 33.1 mN, P < 0.05), and 3 (AS: 2,108.9 ± 55.7 mN vs. MM: 1,735.3 ± 41.3 mN, P < 0.05) after ergonomic insult. B: preventive AS, but not MM, treatment inhibited mechanical hyperalgesia after hid limb eccentric exercise on days 1 (AS: 2,132.7 ± 0.16 mN vs. MM: 1,680.8 ± 41.8 mN vs. P < 0.05), 2 (AS: 2,319.5 ± 337.5 mN vs. MM: 1,689.7 ± 0.16 mN, P < 0.05), and 3 (AS: 2,296.7 ± 39.2 mN vs. MM: 1,970.8 ± 53.8 mN, P < 0.05) after ergonomic insult. Comparisons to baseline were made using two-way repeated measures ANOVA (time and ODN treatment) followed by Tukey's multiple comparisons test. *P < 0.05.
Hyperalgesic effects of GDNF depend on IB4(+) nociceptors.
To determine whether IB4(+) nociceptors, the target of GDNF, play a role in GDNF-induced muscle hyperalgesia, rats were pretreated intrathecally with IB4-saporin (n = 6) to reduce the population of IB4(+) neurons and naïve rats were used as a control (n = 5). Ten days later, all rats received a unilateral GDNF intramuscular injection (10 ng/20 μl). Before the administration of GDNF, rats were tested to determine if IB4-saporin treatment produced a change in muscle mechanical nociceptive thresholds. Consistent with our previous reports (Alvarez et al. 2012), no significant effect of IB4-saporin with respect to baseline nociceptive threshold was observed.
One hour after unilateral injection of GDNF a significant reduction in mechanical nociceptive threshold was observed in naïve rats, which persisted unchanged in amplitude for at least 3 days (Fig. 5A). In contrast, only a modest hyperalgesia was observed in IB4-saporin-treated rats the first hour after GDNF injection (Fig. 5A), which then declined to baseline values in the next readings (Fig. 5A). The contralateral side did not show statistically significant changes in either IB4-saporin-treated or naïve rats (Fig. 5A).
Fig. 5.
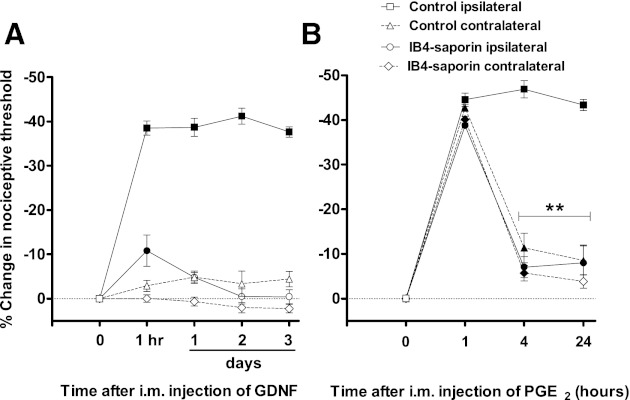
IB4-saporin abrogates acute and chronic mechanical hyperalgesia induced by GDNF intramuscularly. Rats were injected intrathecally with IB4-saporin (n = 6) or kept naïve (control group, n = 5) 10 days before unilateral GDNF injection. A: GDNF injection (10 ng/20 μl im) produced a mechanical hyperalgesia that lasted for at least 3 days in control rats (3rd day: 1,670.7 ± 30.7 mN vs. baseline: 2,674.8 ± 9.2, P < 0.001, ANOVA followed by Tukey's test). In contrast, IB4-saporin-treated rats only exhibited a modest, albeit significant, mechanical hyperalgesia that lasted ∼1 day (2,382.7 ± 11.4 mN vs. baseline: 2,669.8 ± 11.4 mN, P < 0.05, ANOVA followed by Tukey's test). B: 10 days after return to pre-GDNF baseline levels, groups were assessed for hyperalgesic priming by injecting PGE2 (1 μg/20 μl im) bilaterally. One hour after PGE2 injection, bilateral mechanical hyperalgesia was observed in naïve (1,497.4 ± 18.8 mN vs. baseline: 2,698.6 ± 7, P < 0.001, ANOVA followed by Tukey's test) and IB4(+)-saporin-treated rats (1,629.7 ± 32.9 mN vs. baseline: 2,658 ± 38.1, P < 0.001, ANOVA followed by Tukey's test). Such hyperalgesia remained unchanged after 24 h in naïve rats (1 h: 1,497.4 ± 18.8 mN vs. 24 h: 1,529.6 ± 16.6 mN, P > 0.05, ANOVA followed by Tukey's test). In contrast, PGE2-induced mechanical hyperalgesia was significantly decreased in IB4-saporin-treated rats 4 h after PGE2 injection (1 h: 1,629.7 ± 32.9 mN vs. 4 h: 2,474.2 ± 88.7 mN, P < 0.01, ANOVA followed by Tukey's test), indicating that IB4(+) nociceptors mediate hyperalgesic priming. Data are plotted as %change in mechanical nociceptive threshold compared with baseline. Solid symbols represent P < 0.05 respect to baseline. Differences in nociceptive responses comparing readings taken at 1, 4, and 24 h are represented by **P < 0.01.
Three weeks after GDNF administration, when nociceptive thresholds returned to baseline, the inflammatory cytokine PGE2 (1 μg/20 μl) was injected into the gastrocnemius muscles bilaterally. In control rats, PGE2 injected into the gastrocnemius muscle contralateral to the injection of GDNF produced a marked acute hyperalgesia (1 h after PGE2 injection) that was significantly decreased by 4 h (Fig. 5B) However, in the GDNF-treated muscle PGE2 induced a prolonged hyperalgesia that lasted at least 24 h, indicating the induction of hyperalgesic priming (Fig. 5B). In IB4-saporin-treated rats PGE2 also induced a mechanical hyperalgesia that was significantly attenuated within 4 h on both the nontreated and the GDNF-treated (Fig. 5B) hindlimbs, indicating that GDNF-induced hyperalgesic priming in muscle depends on IB4(+) nociceptors.
DISCUSSION
While cutaneous GDNF-induced hyperalgesia is a well-established phenomenon (Albers et al. 2006; Bogen et al. 2008, 2009; Ferrari et al. 2010; Joseph and Levine 2010) and high levels of GDNF have been reported in painful neuromuscular diseases, studies of the role of GDNF in skeletal muscle nociception are lacking. The results of the present study indicate that GDNF produces a long-lasting muscle hyperalgesia mediated by action at IB4(+) nociceptors.
GDNF-induced mechanical hyperalgesia and hyperalgesic priming.
Consistent with reports of GDNF-induced cutaneous hyperalgesia (Bogen et al. 2009; Bogen et al. 2008; Ferrari et al. 2010; Joseph and Levine 2010), a single intramuscular injection of GDNF produced a robust mechanical hyperalgesia, which lasted for at least 21 days. This hyperalgesia is mediated, at least in part, by nociceptor sensitization, since intramuscular injection of GDNF into the mechanical receptive field of C-nociceptors in the gastrocnemius muscle induced a large increase in their response to sustained mechanical stimulation. However, in contrast to its effects on cutaneous nociceptors, intramuscular GDNF did not affect mechanical threshold (Albers et al. 2006; Bogen et al. 2008) or conduction velocity (Albers et al. 2006) in muscle nociceptors. While we do not have a complete explanation for this difference, it might reflect a particular sensitivity of muscle nociceptors to GDNF. Indeed, while GDNF potentiates the activation of the mechanosensitive transient receptor potential channel subfamily A, member 1 (Kwan et al. 2006, 2009; Vilceanu and Stucky 2010) in skeletal muscle nociceptors, such a potentiation is absent in nociceptors innervating the skin (Malin et al. 2011).
We (Hendrich et al. 2012) have recently reported that GDNF produces sensitization of cultured IB4(+) neurons innervating muscle by reducing large-conductance voltage and calcium-activated potassium (BK) channel currents, whereas local injection of the BK inhibitor iberiotoxin increased nociceptor firing to muscle mechanical stimulation and mechanical hyperalgesia. In addition, the pretreatment with the BK activator NS1619 inhibited the mechanical hyperalgesia induced by GDNF, which in whole indicates that GDNF induces mechanical hyperalgesia by inhibiting BK currents in IB4(+) muscle nociceptors (Hendrich et al. 2012). This is consistent with the view that BK channels selectively control the excitability of IB4(+) nociceptors, especially in persistent nociceptive responses (Zhang et al. 2012).
In spite of the fact that most histological studies indicate that there is a smaller proportion of IB4(+) neurons innervating muscle compared with skin (Ambalavanar et al. 2003; Molander et al. 1987; O'Brien et al. 1989; Pierce et al. 2006; Plenderleith and Snow 1993) ranging between 5 and 40% of DRG neurons, we (Alvarez et al. 2012) have recently shown that IB4(+) nociceptors play a role in inflammation- and ergonomic insult-induced muscle hyperalgesia. In addition, we have observed a large proportion of IB4(+) nociceptors innervating the gastrocnemius muscle after injection of the tracer DiI (unpublished observations). Since most IB4(+) nociceptors also express the canonical GDNF receptor complex for GDNF, GFRα1/Ret, and ∼60% of muscle primary afferent neurons express GFRα1 (Malin et al. 2011), we tested the hypothesis that the selective destruction of IB4(+) nociceptors would abrogate the hyperalgesic effect of GDNF. Consistent with this finding, we observed a marked, albeit not complete, inhibition of GDNF-induced muscle hyperalgesia by the pretreatment with the neurotoxin saporin conjugated to IB4. This is consistent with previous studies showing that the destruction of IB4(+) nociceptors by IB4-saporin abrogates the cutaneous hyperalgesia induced by GDNF (Bogen et al. 2008, 2009; Joseph and Levine 2010). Since GDNF can also act as a low affinity agonist on the GFRα2/Ret complex receptor (Bespalov and Saarma 2007), the remaining hyperalgesic response might be due to the effect of GDNF on GFRα2/Ret expressing nociceptors unaffected by the IB4-saporin treatment. In agreement with this, a recent report indicates that ∼25% of muscle sensory neurons express GFRα2 (Malin et al. 2011).
In addition to acute hyperalgesia at the GDNF injection site, intramuscular GDNF also induced a long-lasting nociceptive response to PGE2 at that site. We have previously shown that such a change, referred to as hyperalgesic priming, is induced in skeletal muscle after intramuscular injection of the inflammogen carrageenan (Dina et al. 2008) or ergonomic interventions such as eccentric exercise (Alvarez et al. 2010) or vibration (Dina et al. 2010). There is evidence that hyperalgesic priming depends on the recruitment of PKCε signaling in addition to the normal prepriming signaling via cAMP/PKA, to produce prolonged mechanical hyperalgesia to an otherwise short-lasting proalgesic effect of PGE2 (Aley et al. 2000; Alvarez et al. 2010; Dina et al. 2008, 2010; Parada et al. 2003). On the other hand, GDNF-induced hyperalgesic priming was prevented by IB4-saporin pretreatment, which is consistent with the role of IB4(+) nociceptors in hyperalgesic priming induced by the intradermal injection of GDNF (Ferrari et al. 2010; Joseph and Levine 2010).
Endogenous GDNF mediates ergonomically induced pain.
Ergonomically induced muscle pain is a well-recognized common clinical entity, related to activities such as inappropriate exposure of extremities to vibration (Heaver et al. 2011; Necking et al. 2004) or eccentric muscle activity (Sesto et al. 2005). Notably, vibration (Okada et al. 1985; Okada 1986) and eccentric muscle activity (McNeil and Khakee 1992) can induce extensive damage of muscle fibers, especially to the sarcolemma. Indeed, sarcolemma disruption observed after exposure to these insults induces the release of cytoplasmic elements from muscle fibers (Okada et al. 1985; Okada 1986; Schwane et al. 1983). Given that GDNF is highly expressed in the vicinity of the sarcolemma (Suzuki et al. 1998a, 1998b; Wehrwein et al. 2002) and that muscle content of GDNF is increased by physical activity (Wehrwein et al. 2002), we hypothesized that ergonomic interventions should induce an acute increase in muscle content of GDNF at a time point where muscle pain is observed. Measurements of GDNF in muscle of rats exposed to either vibration or eccentric exercise confirmed this hypothesis. Importantly, pretreatment with an AS ODN to GFRα1 markedly inhibited the muscle hyperalgesia induced by these ergonomic insults.
Although some mechanisms contributing to muscle pain have been identified, those underlying eccentric exercise-induced pain have remained elusive. For instance, the interplay between lactic acid and ATP (depending on P2X5/ASIC3 channels interaction) observed in muscle ischemia/acidosis seems to play a role in ischemic muscle pain induced by acute oxygen deprivation and the associated increase of local anaerobic metabolism (Birdsong et al. 2010). However, in our experimental protocol the nociceptive responses to eccentric exercise were measured at time points where local high levels of lactic acid due to muscular anaerobic metabolism are no longer present (Mense 1993). Indeed, it has been shown that basal microvascular oxygen supply to skeletal muscle is similar between control rats and 1 or 3 days after eccentric exercise (Kano et al. 2005). Furthermore, in contrast to our observations, putative nociceptive responses induced by P2X5-ASIC3 interaction were evoked in absence of any mechanical stimuli (Birdsong et al. 2010). Also, the dorsal root ganglion neuronal subset exhibiting P2X5 and ASIC3 coexpression is mostly composed of large-diameter cells (≥50 μm) (Birdsong et al. 2010), which contrasts with the main target of GDNF: the IB4(+) subset, essentially composed by medium to small-diameter cells (∼25 μm) (Price and Flores, 2007). Thus, while local low pH is present in many conditions related to muscle injury and pain (e.g., ischemia and inflammation), the interaction between lactic acid and ATP seems not to play a role in eccentric exercise-induced mechanical hyperalgesia. In contrast, it has been recently reported that the main target for IB4 binding, the extracellular proteoglycan versican, plays a role in the sensitization of mechanosensitive currents at physiological low pH (Kubo et al. 2012a). This is in line with our previous observations regarding the involvement of IB4(+) nociceptors in eccentric exercise-induced mechanical hyperalgesia (Alvarez et al. 2012). Finally, since a large proportion of IB4(+) nociceptors also express the capsaicin receptor TRPV1 in rats (Price and Flores 2007), it is not surprising that neonatal treatment with capsaicin, which irreversibly destroys TRPV1-expressing neurons, is able to abolish eccentric exercise-induced mechanical hyperalgesia (Kubo et al. 2012b).
Taken together, these observations strongly suggest that GDNF released by muscle damage induces sensitization of IB4(+)-GFRα1/Ret nociceptors, contributing to the induction of acute persistent mechanical hyperalgesia and to hyperalgesic priming.
Clinical implications.
In spite of the fact that historically muscle pain has been not well recognized as a major symptom in neuromuscular disease (Engel et al. 2009), a growing number of studies indicate that chronic muscle pain is a common symptom in patients affected by this group of diseases (Carter et al. 2006; Engel et al. 2009; Zebracki and Drotar 2008). Findings in humans and in animal models point to disruption of the sarcolemma as a main cause of neuromuscular disease (Mendell et al. 1995; Straub et al. 1997). Consistent with this, patients affected by Duchenne type neuromuscular dystrophy exhibit increased plasma levels of some muscle fiber content, including enzymes (McMillan et al. 2011) and growth factors (D'Amore et al. 1994). Of note with respect to our findings, it has been reported that patients affected by painful neuromuscular diseases such as polymyositis and Duchenne type neuromuscular dystrophy exhibit increased expression of GDNF in regenerating muscle fibers (Suzuki et al. 1998a). It is thus tempting to speculate that in these conditions an abnormal leakage of GDNF from affected fibers might sensitize muscle nociceptors, leading to a persistent pain (Engel et al. 2009; Zebracki and Drotar 2008). Finally, it is interesting to note that muscle pain was a common complaint in patients submitted to a long-term therapy with human recombinant GDNF in a clinical trial for Parkinson disease (Nutt et al. 2003).
In summary, our data support the idea that GDNF induces acute and chronic muscle pain. Increased levels of GDNF in muscle concomitant with mechanical hyperalgesia suggest that GDNF plays a role as an endogenous mediator in muscle pain. The nociceptive effects of GDNF in skeletal muscle are likely to be produced by action at GFRα1 receptors located in IB4(+) nociceptors. These findings contribute to our understanding of chronic musculoskeletal pain syndromes.
GRANTS
This work was supported by the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.A., P.G.G., and J.D.L. conception and design of research; P.A., X.C., O.B., and P.G.G. performed experiments; P.A., X.C., and P.G.G. analyzed data; P.A. and P.G.G. interpreted results of experiments; P.A., X.C., and P.G.G. prepared figures; P.A. and J.D.L. drafted manuscript; P.A. and J.D.L. edited and revised manuscript; P.A., X.C., O.B., P.G.G., and J.D.L. approved final version of manuscript.
REFERENCES
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci 26: 2981–2990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 20: 4680–4685, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol 233: 859–865, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci 32: 819–825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol 460: 167–179, 2003 [DOI] [PubMed] [Google Scholar]
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci 28: 68–74, 2007 [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience 159: 780–786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci 28: 12–19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science 290: 124–127, 2000 [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21: 53–62, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GT, Han JJ, Abresch RT, Jensen MP. The importance of assessing quality of life in patients with neuromuscular disorders. Am J Hosp Palliat Care 23: 493–497, 2006 [DOI] [PubMed] [Google Scholar]
- Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain 151: 460–466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore PA, Brown RH, Jr, Ku PT, Hoffman EP, Watanabe H, Arahata K, Ishihara T, Folkman J. Elevated basic fibroblast growth factor in the serum of patients with Duchenne muscular dystrophy. Ann Neurol 35: 362–365, 1994 [DOI] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain 11: 369–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain 9: 457–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZQ, Ma F, Xie H, Wang YQ, Wu GC. Down-regulation of GFRalpha-1 expression by antisense oligodeoxynucleotide attenuates electroacupuncture analgesia on heat hyperalgesia in a rat model of neuropathic pain. Brain Res Bull 69: 30–36, 2006 [DOI] [PubMed] [Google Scholar]
- Engel JM, Kartin D, Carter GT, Jensen MP, Jaffe KM. Pain in youths with neuromuscular disease. Am J Hosp Palliat Care 26: 405–412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience 165: 896–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaver C, Goonetilleke KS, Ferguson H, Shiralkar S. Hand-arm vibration syndrome: a common occupational hazard in industrialized countries. J Hand Surg Eur Vol 36: 354–363, 2011 [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 266: 1062–1064, 1994 [DOI] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Chen X, Levine JD. GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience 219: 204–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain 9: 463–472, 2008 [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience 169: 431–435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Sampei K, Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand 180: 291–299, 2004 [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience 54: 545–551, 1993 [DOI] [PubMed] [Google Scholar]
- Kubo A, Katanosaka K, Mizumura K. Extracellular matrix proteoglycan plays a pivotal role in sensitization by low pH of mechanosensitive currents in nociceptive sensory neurones. J Physiol 590: 2995–3007, 2012a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Koyama M, Tamura R, Takagishi Y, Murase S, Mizumura K. Absence of mechanical hyperalgesia after exercise (delayed onset muscle soreness) in neonatally capsaicin-treated rats. Neurosci Res 73: 56–60, 2012b [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29: 4808–4819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 31: 10516–10528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson CR, Carnahan J, Urich JL, Bocangel D, Zhang TJ, Yan Q. Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor for sensory neurons: comparison with the effects of the neurotrophins. J Neurobiol 32: 22–32, 1997 [PubMed] [Google Scholar]
- McMillan HJ, Gregas M, Darras BT, Kang PB. Serum transaminase levels in boys with Duchenne and Becker muscular dystrophy. Pediatrics 127: e132–136, 2011 [DOI] [PubMed] [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140: 1097–1109, 1992 [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Sahenk Z, Prior TW. The childhood muscular dystrophies: diseases sharing a common pathogenesis of membrane instability. J Child Neurol 10: 150–159, 1995 [DOI] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54: 241–289, 1993 [DOI] [PubMed] [Google Scholar]
- Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep 7: 419–425, 2003 [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32: 197–200, 1994 [DOI] [PubMed] [Google Scholar]
- Molander C, Ygge J, Dalsgaard CJ. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett 74: 37–42, 1987 [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861, 1997 [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79, 1996 [DOI] [PubMed] [Google Scholar]
- Necking LE, Lundborg G, Lundstrom R, Thornell LE, Friden J. Hand muscle pathology after long-term vibration exposure. J Hand Surg Br 29: 431–437, 2004 [DOI] [PubMed] [Google Scholar]
- Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci 20: 474–482, 2004 [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60: 69–73, 2003 [DOI] [PubMed] [Google Scholar]
- O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 32: 493–502, 1989 [DOI] [PubMed] [Google Scholar]
- Okada A. Physiological response of the rat to different vibration frequencies. Scand J Work Environ Health 12: 362–364, 1986 [DOI] [PubMed] [Google Scholar]
- Okada A, Okuda H, Inaba R, Ariizumi M. Influence of local vibration on plasma creatine phosphokinase (CPK) activity. Br J Ind Med 42: 678–681, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience 120: 219–226, 2003 [DOI] [PubMed] [Google Scholar]
- Pezet S, Krzyzanowska A, Wong LF, Grist J, Mazarakis ND, Georgievska B, McMahon SB. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther 13: 1101–1109, 2006 [DOI] [PubMed] [Google Scholar]
- Pierce LM, Rankin MR, Foster RT, Dolber PC, Coates KW, Kuehl TJ, Thor KB. Distribution and immunohistochemical characterization of primary afferent neurons innervating the levator ani muscle of the female squirrel monkey. Am J Obstet Gynecol 195: 987–996, 2006 [DOI] [PubMed] [Google Scholar]
- Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett 159: 17–20, 1993 [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 8: 263–272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin's role in skeletal muscle adaptation. J Biomed Biotechnol 2011: 201696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc 15: 51–56, 1983 [PubMed] [Google Scholar]
- Sesto ME, Radwin RG, Block WF, Best TM. Anatomical and mechanical changes following repetitive eccentric exertions. Clin Biomech (Bristol, Avon) 20: 41–49, 2005 [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol 19: 789–801, 1990 [DOI] [PubMed] [Google Scholar]
- Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci 22: 5687–5693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron 20: 629–632, 1998 [DOI] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol 139: 375–385, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Schulte BA, Balentine JD, Spicer SS. Evidence for glycoconjugate in nociceptive primary sensory neurons and its origin from the Golgi complex. Brain Res 377: 1–17, 1986 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Kim BY, Miyata Y, Nonaka I, Arahata K, Akazawa C. Up-regulation of glial cell line-derived neurotrophic factor (GDNF) expression in regenerating muscle fibers in neuromuscular diseases. Neurosci Lett 257: 165–167, 1998a [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Miyata Y, Arahata K, Akazawa C. Prominent expression of glial cell line-derived neurotrophic factor in human skeletal muscle. J Comp Neurol 402: 303–312, 1998b [PubMed] [Google Scholar]
- Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol 564: 259–268, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLos One 5: e12177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience 108: 143–155, 2001 [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve 26: 206–211, 2002 [DOI] [PubMed] [Google Scholar]
- Wiesenhofer B, Weis C, Humpel C. Glial cell line-derived neurotrophic factor (GDNF) is a proliferation factor for rat C6 glioma cells: evidence from antisense experiments. Antisense Nucleic Acid Drug Dev 10: 311–321, 2000 [DOI] [PubMed] [Google Scholar]
- Yang LX, Nelson PG. Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience 128: 497–509, 2004 [DOI] [PubMed] [Google Scholar]
- Zebracki K, Drotar D. Pain and activity limitations in children with Duchenne or Becker muscular dystrophy. Dev Med Child Neurol 50: 546–552, 2008 [DOI] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Lee KY, Charbonnet M, Gold MS. Inflammation-induced changes in BKCa currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol Pain 8: 37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110, 1983 [DOI] [PubMed] [Google Scholar]


