Abstract
Background
Clear case definitions of malaria are an essential means of evaluating the effectiveness of present and proposed interventions in malaria. The clinical signs of malaria are nonspecific, and parasitemia accompanied by a fever may not be sufficient to define an episode of clinical malaria in endemic areas. We defined and quantified cases of malaria in people of different age groups from 2 areas with different rates of transmission of malaria.
Methods
A total of 1602 people were followed up weekly for 2 years, and all the cases of fever accompanied by parasitemia were identified. Logistic regression methods were used to derive case definitions of malaria.
Results
Two case definitions of malaria were derived: 1 for children 1–14 years old and 1 for infants (<1 year old) and older children and adults (≥15 years old). We also found a higher number of episodes of clinical malaria per person per year in people from an area of low transmission of malaria, compared with the number of episodes in those from an area of higher transmission (0.84 vs. 0.55 episodes/person/year; incidence rate ratio, 0.66 [95% confidence interval, 0.61–0.72]; P < .001).
Conclusions
Case definitions of malaria are bound to be altered by factors that affect immunity, such as age and transmission. Case definitions may, however, be affected by other immunity-altering factors, such as HIV and vaccination status, and this needs to be borne in mind during vaccine trials.
Strategies presently in place to control malaria (e.g., bed nets) and future interventions (e.g., vaccines) that are aimed at reducing morbidity and mortality need careful evaluation of their effectiveness. To measure morbidity, a clear case definition of clinical malaria is needed [1]. In areas where malaria is not endemic, clinical malaria can be defined as a history of fever accompanied by peripheral parasitemia. However, in some areas where malaria is endemic, >80% of the “asymptomatic” general population <10 years old have peripheral parasitemia [2]. In such areas, parasitemia accompanied by clinical symptoms of malaria does not necessarily imply that clinical malaria is present. An illness that includes a fever may be confused with clinical malaria because of accompanying parasitemia. All these factors contribute to the complexity involved in the diagnosis of malaria in endemic areas.
In the past, the usual approach to this problem was to base definitions of clinical disease on parasitemia above certain thresholds accompanied by fever [1]. This was done because high-level parasitemia was assumed to be more likely to cause fever than low-level parasitemia. However, such cutoff values for parasite density may lead to an unquantifiable level of misdiagnosis of malaria [3]. The fraction of fevers attributable to parasitemia can also be used to calculate the number of fevers that would be eliminated if malaria was eradicated [4]. This classic method of calculating the fraction of fevers attributable to parasitemia compares the proportion of the population that is febrile and has parasitemia with the proportion of the population that is afebrile and has parasitemia. In areas of high endemicity, this method is not useful, because most of the afebrile population will have parasitemia [1]. To overcome this problem, logistic regression has been used to model the risk of fever as a continuous function of the parasite density [5]. This method can also be used to estimate the sensitivity and specificity of various cutoff values for parasite density that are used in case definitions of clinical malaria.
In the present study, we have derived age-specific case definitions of clinical malaria in 2 populations with similar ethnic and socioeconomic characteristics in 2 areas in a single district in Kenya with different rates of transmission. We have explored the effect that differences in case definitions would have on inferences made during intervention trials.
MATERIALS AND METHODS
Study area
The study sites were located in the Kilifi District, on the coast of Kenya, within 20 km of each other. The low transmission area (Ngerenya) was located north of the Kilifi Creek, and the high transmission area (Chonyi) was located south of the Kilifi Creek (figure 1). Both areas have 2 rainy seasons: the “long” rains in May–July and the “short” rains in November. The study participants were predominantly from the Mijikenda tribe and had similar beliefs and customs.
Figure 1.
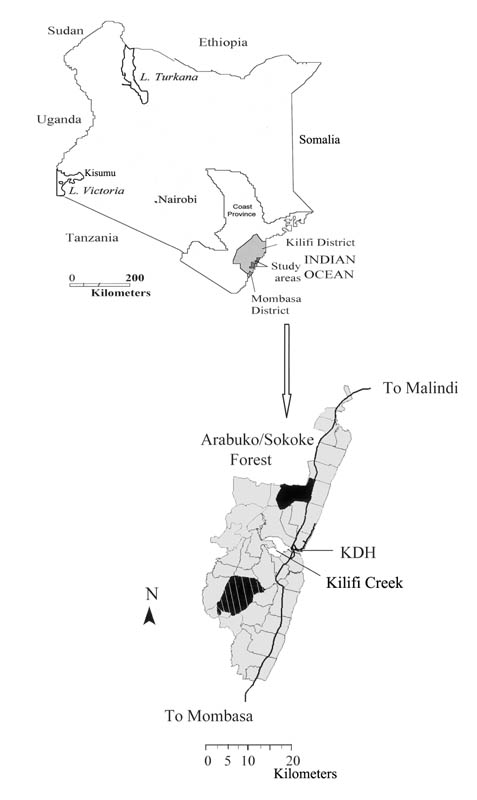
Maps of the study area showing Ngerenya (black area) and Chonyi (hatched area), which are part of a larger study area under demographic surveillance (gray area). Black solid lines denote the main tarmac roads. KDH, Kilifi District Hospital.
Residents of Ngerenya have, on average, 10 infective bites/person/year [6], whereas residents of Chonyi have an estimated 22–53 infective bites/person/year [7]. There was, however, higher bed net use in children <10 years old in Ngerenya, compared with that in children in Chonyi (69% vs. 6%), probably because a large trial of bed net use had been conducted in the mid-1990s in Ngerenya.
Consent was sought from the parents of young children (≤14 years old), from older children (≥15 years old), and from adults from randomly selected households in mapped clusters in Ngerenya and Chonyi. The local dialect was used to explain the study protocol to the participants, and a copy of the consent form was left in each household so that the family members could discuss it. A total of 52 households with 783 participants were selected in Chonyi, and 72 households with 819 participants were selected in Ngerenya.
Active surveillance
Each household was visited by a field-worker once a week, and an axillary temperature was obtained for every participant by use of an electronic digital thermometer (Becton Dickinson). Any participant with a temperature <36 °C was tested twice more, to ensure that the apparent low temperature was not the result of poor placement of the thermometer. Any participant with a fever (axillary temperature ≥37.5 °C) or a history of fever was given local bus fare to travel to the study clinic at the Kilifi District Hospital, where a blood smear was performed. The study clinic was open daily, and participants were encouraged to seek treatment whenever they were ill. All investigations and treatments provided at the study clinic were free.
Participants who could not come to the study clinic had a blood smear performed in the field; if the participants were found to be parasitemic, field-workers returned the following day to dispense malaria treatment. Sulfadoxine-pyrimethamine was used as first-line treatment throughout the study. The study was conducted between May 1999 and May 2001.
Cross-sectional parasitological surveys
A total of 6 surveys were conducted: 3 during rainy seasons, which were considered to be times of high transmission of malaria (July 1999, July 2000, and June 2001), and 3 during dry seasons, which were considered to be times of low transmission of malaria (March 2000, October 2000, and March 2001). Thick and thin blood smears were performed and axillary temperatures were recorded for all consenting participants. The blood smears performed during these cross-sectional surveys were not analyzed immediately unless the participants were clinically ill. As a result, participants with asymptomatic infections did not receive treatment.
Laboratory investigations
Thick and thin blood smears were air-dried, and the thin blood smears were fixed in 100% methanol. Slides were then stained in 2% Giemsa (diluted in a buffer with a pH of 7.2) for 30 min and were either analyzed immediately, if they were obtained from participants who attended the study clinic, or stored, if they were obtained during the cross-sectional surveys. The number of parasites/200 white blood cells (WBCs) was counted. The density of parasites per microliter of blood was calculated using either each participant’s WBC count, if it was for a participant who attended the study clinic, or an average count of 8000 WBCs/μL of blood, if it was for a participant seen during the cross-sectional surveys for whom no WBC counts were performed. During a single cross-sectional survey conducted in Ngerenya at the start of the longitudinal survey, a comparison of the parasite densities calculated using either the average of 8000 WBCs/μL of blood or the individual WBC counts in healthy study participants showed no statistically significant differences (data not shown).
Data processing and statistical methods
All information on participants was entered into a FoxPro database (version 6.0; Microsoft). All data were entered twice, and these double entries were cross-checked for errors before they were conflated. All data analyses were performed using Stata software (version 7.0; StataCorp).
Estimating attributable fractions and case definitions
The malaria attributable fraction (MAF) is defined as the fraction of fevers attributable to malaria (or parasitemia) and is the fraction of fevers that would be eliminated if malaria was eradicated. For the calculation of the MAFs, a case patient was defined as a participant with a fever at the time of the visit by a field-worker either during a weekly follow-up visit or during a cross-sectional survey; a control subject was defined as a healthy study participant for whom blood smears were performed during the cross-sectional surveys. However, if a participant had a fever on 2 consecutive weeks, only the first week’s data were included. Similarly, data from a participant who had been ill during the week before or during a cross-sectional survey were not used in the definition of asymptomatic parasitemia. The MAFs and case definitions were derived using logistic regression methods and the technique described by Smith et al. [5]. The logistic regression model used was
where p is the probability that a participant with parasites at a density x had a fever, and where τ is the power function of the parasite density. The power function was derived using maximum likelihood estimation for the different age groups and was used to model the relationship between fever and parasite density as a continuous function. MAFs were calculated for each area and for different age groups. Bootstrapping, by use of 100 repeat samplings, was used to calculate the confidence intervals (CIs) for the MAFs. The sensitivity and specificity of various age-specific cutoff values for parasite density used in case definitions of clinical malaria were estimated, and a general case definition of clinical malaria, used for both study areas, was derived.
Incidence rates
To derive annualized incidence rates of clinical malaria, passively and actively detected cases were combined and expressed per total number of person-years of risk. If malaria occurred in 2 consecutive weeks in the same participant, only the first episode was counted as a case of clinical malaria. Poisson regression was used to estimate the association between incidence of clinical malaria and study area after adjusting for age.
RESULTS
Prevalence of parasites
Differences in endemicity between the 2 study areas were evident because of the different prevalences of parasites. In children 1–9 years old, the prevalence of parasites in Ngerenya was 24.9% (95% CI, 23.1%–26.7%), whereas the prevalence of parasites in Chonyi was 40.5% (95% CI, 38.5%–42.6%). In children <1 year old, the average prevalence of parasites in Ngerenya was 6.6%, whereas the average prevalence of parasites in Chonyi was 13.8%.
MAFs
The overall MAF in Ngerenya was 50.2% (95% CI, 48.6%–51.7%), and the overall MAF in Chonyi was 47.9% (95% CI, 46.1%–49.4%). Age-specific MAFs for Chonyi and Ngerenya are presented in figure 2. In children <1 year old, there was evidence that more children had fevers due to parasitemia in Chonyi than in Ngerenya, although the difference had borderline statistical significance (P = .05). There was no evidence of a difference in the MAFs in the 2 areas in children 1–3 years old. However, the MAF was significantly higher in Ngerenya than in Chonyi, in participants 5–19 years old (P < .001), whereas there was no evidence of a difference in the MAFs in adults ≥20 years old (P = .9).
Figure 2.
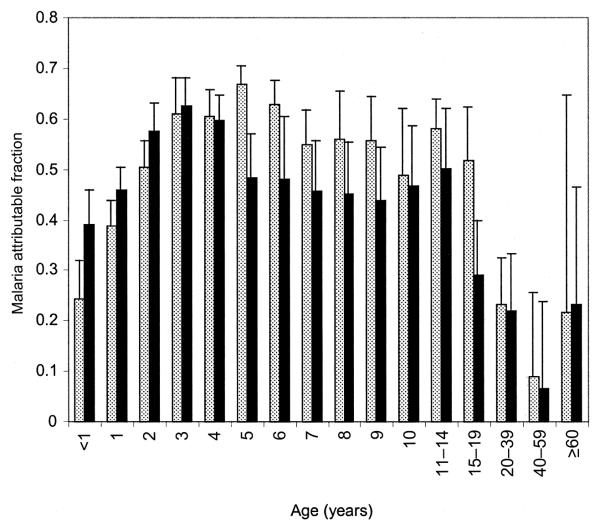
Logistic regression estimates of malaria attributable fractions by age in Ngerenya (hatched bars) and Chonyi (black bars)
Sensitivities and specificities of various cutoff values in parasite density
Figure 3 shows estimates of the sensitivities and specificities of various cutoff values in parasite density used in case definitions of clinical malaria in the different age groups. The use of fever accompanied by any parasitemia as a case definition of clinical malaria would give an estimate of the number of cases of clinical malaria that was consistent with the calculated proportion of fevers attributable to malaria in children <1 year old in both areas. As is illustrated in table 1, this definition would, however, have a low specificity in children >1 year old and would have led to an ~16% overestimate of the number of episodes of clinical malaria, compared with that expected from the calculation of the MAF. A single best definition with maximum combined sensitivity and specificity (as is shown in table 2) was derived for both study areas. A case of clinical malaria was defined as fever accompanied by any parasitemia in children <1 year old and in older children and adults (≥15 years old) but was defined as fever accompanied by a parasitemia of ≥2500 parasites/μL of blood in children 1–15 years old.
Figure 3.
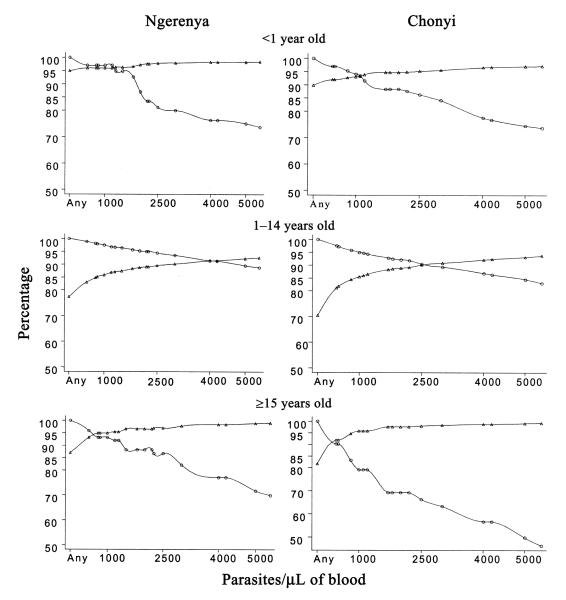
The sensitivity (circles) and specificity (triangles) of various cutoff values for parasite density used in case definitions of malaria, by age group in Ngerenya and Chonyi.
Table 1.
Fevers attributable to malaria according to different case definitions in children ≤5 years old from Ngerenya and Chonyi.
| Estimated total fevers attributable to malaria by MAF (95% CI) |
Total fevers attributable to malaria according to different case definitions |
||||||
|---|---|---|---|---|---|---|---|
| Age group, area | Age specifica |
Any parasitemia |
1000 parasites/μL of blood |
2500 parasites/μL of blood |
5000 parasites/μL of blood |
10,000 parasites/μL of blood |
|
| <1 year | |||||||
| Ngerenya | 65 (54–76) | 73 | 75 | 71 | 57 | 52 | 41 |
| Chonyi | 109 (9–127) | 113 | 126 | 114 | 102 | 86 | 76 |
| 1–5 years | |||||||
| Ngerenya | 634 (609–668) | 627 | 734 | 689 | 657 | 610 | 546 |
| Chonyi | 465 (442–482) | 458 | 526 | 500 | 465 | 435 | 371 |
NOTE. For parasitemia, the limit of detection by microscopy is 40 parasites/μL of blood, so a level >40 parasites/μL of blood implies any parasitemia. The other levels shown are cutoff values for parasite density commonly used in case definitions. CI, confidence interval; MAF, malaria attributable fraction.
In children <1 year old, clinical malaria was defined as any parasitemia and an axillary temperature of ≥37.5 °C; in children 1–5 years old, clinical malaria was defined as ≥2500 parasites/μL of blood and an axillary temperature of ≥37.5 °C.
Table 2.
Sensitivity and specificity estimates according to different cutoff values for parasite density in case definitions of clinical malaria in people of different age groups from Ngerenya and Chonyi.
| Cutoff value for parasite density |
Ngerenya |
Chonyi |
|||
|---|---|---|---|---|---|
| Age group | Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | |
| <1 year | Any parasitemia | 100 | 95 | 100 | 90 |
| 1–5 years | ≥2500 parasites/μL of blood | 95 | 89 | 91 | 89 |
| 6–9 years | ≥2500 parasites/μL of blood | 95 | 88 | 91 | 92 |
| 10–14 years | ≥2500 parasites/μL of blood | 90 | 93 | 88 | 93 |
| ≥15 years | Any parasitemia | 100 | 87 | 100 | 82 |
NOTE. In all age groups, parasitemia was accompanied by an axillary temperature of ≥37.5 °C.
Incidence
A total of 1522 person-years of risk in Ngerenya yielded 1274 episodes of clinical malaria, and a total of 1519 person-years of risk in Chonyi yielded 841 episodes of clinical malaria. The overall rate of clinical malaria in Ngerenya was 0.84 episodes/person/year (95% CI, 0.79–0.88 episodes/person/year), compared with 0.55 episodes/person/year (95% CI, 0.52–0.59 episodes/person/year) in Chonyi, with evidence of a lower incidence being observed in the area of higher transmission (incident rate ratio [IRR], 0.66 [95% CI, 0.61–0.72]; P < .001).
Figure 4 shows the age-specific incidence of clinical malaria in the 2 study areas. Only in children <1 year old was the incidence of clinical malaria higher in Chonyi than in Ngerenya (IRR, 1.56 [95% CI, 1.18–2.06]; P = .002). There was no evidence of a difference in the incidence of clinical malaria in children 1–3 years old in the 2 areas. However, in children 4–19 years old, there was evidence of a higher incidence of clinical malaria in Ngerenya, compared with that in Chonyi (IRR, 0.45 [95% CI, 0.4–0.51]; P < .001). Incidence rates of clinical malaria were similar in adults ≥20 years old in the 2 areas.
Figure 4.
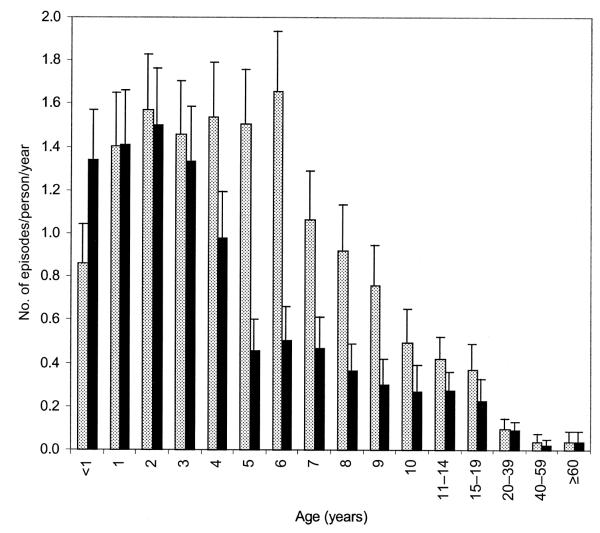
Age-specific incidence of malaria in Ngerenya (hatched bars) and Chonyi (black bars)
DISCUSSION
Intervention trials in malaria may have several potential end points, but the most obvious and most convincing is a reduction in episodes of clinical malaria. However, it is difficult to define clinical malaria, because symptoms are nonspecific and because asymptomatic parasitemia is common in endemic areas. Various approaches to the derivation of a definition have been taken—from including any febrile illness accompanied by a peripheral parasitemia (an appropriate definition from the point of view of treatment allocation), which is a deliberately oversensitive strategy, to setting arbitrary cutoff values for parasite density. Smith et al. [5] used logistic regression to model the risk of fever as a continuous function of the parasite density. This method can also estimate the sensitivity and specificity of various cutoff values for parasite density used in case definitions. During recent years, this method has been applied in different settings in Africa that have entomological inoculation rates (EIRs) of 8.5–300 infective bites/person/year [1, 5, 8–14]. Studies suggest that an optimum case definition of clinical malaria differs with both endemicity and age [5, 11, 12, 14]. However, studies in areas of different endemicity that are separated in space and time and that potentially differ widely in many other ways may be difficult to compare.
We have employed the method of Smith et al. [5] to cross-sectional and longitudinal data collected simultaneously in 2 cohorts with similar ethnic and socioeconomic profiles who live within 20 km of each other in areas that have differing rates of transmission of malaria. Both areas exhibit classic stable endemicity, in which severe disease essentially disappears in children >5 years old. Ngerenya has low transmission (EIR, 10), whereas Chonyi has low-moderate transmission (EIR, 22–53) of malaria. Although the EIRs appear to be fairly low, the 2–4-fold difference in transmission is reflected in the parasite rates in infants and in patterns of acquisition of antimalarial antibody responses(data not shown). Differences between the 2 areas are also apparent in the age-specific pattern of clinical disease, in which disease is concentrated in the younger age groups in the higher transmission area. Interestingly, the amount of clinical disease (i.e., the number of episodes during a lifetime) was significantly higher in the lower transmission area—an observation reported for mild malaria in 2 areas of Senegal [15] and for severe malaria in a number of different settings [16]. However, by the age of 20 years, adults in the 2 areas have the same incidence of clinical malaria, and this similarity may be due to the strong partial immunity that is thought to occur after continuous, uninterrupted exposure to parasites in endemic areas [17].
We observed differences by both endemicity and age in the optimum definition of clinical malaria. We compared our case definitions by age with those from a study conducted in the Ghanian village of PramPram, in which there is an EIR of 8 in children up to 2 years old [11], and with those from a study conducted in Siaya, Kenya, in which there is an EIR of 300 in people of all ages [12]. In both studies, there were differences according to age in the cutoff values for parasite density used for case definitions of clinical malaria. Along with the results of our study, these findings raise 2 main points. First, the age at which the peak cutoff value for parasite density for a case definition of clinical malaria was achieved appeared to differ with the rate of transmission. This peak occurred at the youngest age in Siaya (EIR, 300), at the oldest age in Ngerenya (EIR, 10), and at an intermediate age in Chonyi (EIR, 22–53). Second, it suggests that, in most endemic areas, 2 concurrent case definitions of clinical malaria would be useful—1 for children 1–14 years old and 1 for very young children (<1 year old) and older children and adults (≥15 years old). This is probably because tolerance for parasites is high in older children, whereas tolerance is low in young children, because they have not yet acquired it, and in adults, who may lose it as they develop immunity that more effectively limits the growth of parasites. Transmission rates may, however, alter the age at which different case definitions of clinical malaria should be applied. In areas of high transmission, such as Siaya, children <6 months old and any person >5 years old may require a certain definition, and children between 6 months old and 5 years old may require another. In the 2 study areas in Kilifi, a cutoff value for age of 1 year old was most appropriate.
In the present study, there were differences between the 2 areas not only in age but also in the most appropriate cutoff value for parasite density to use in the case definition of clinical malaria. This raises the question of how much effort should be put into deriving an exact definition of clinical malaria in any given study. In theory, it would be possible to use a different definition for each age group in each of the several subareas within an overall study area. However, the differences between the age groups are relatively small, and the best approach is likely to be a compromise—in our study, a single definition (with 2 age-specific components) that had sensitivities and specificities of >80% in both areas, which is reasonable for a diagnostic test, was appropriate.
That both age and rate of transmission affect the definition of clinical malaria is tantamount to saying that the definition varies with immune status. Interestingly, there were differences in case definitions of clinical malaria in HIV-positive and HIV-negative adults in Uganda [13]. The same definition (fever accompanied by parasitemia of >1250 parasites/μL of blood) had a sensitivity of 51% and 84% in diagnosing malaria in HIV-negative and HIV-positive adults, respectively.
That the definition of clinical malaria varies with immune status needs to be borne in mind when malaria interventions are compared, especially in vaccine trials, in which improvement in the immune status of the vaccinated group is desired. It is difficult to imagine a study in which different definitions of clinical malaria are used for an intervention group and a control group, especially because there would be no way to know whether the intervention had worked and therefore changed a subject’s immune status. One potentially useful approach would be to include a standard analysis that used a fixed case definition in addition to the age-specific and area-specific definitions. This standard analysis in which the MAF for each group is calculated makes no a priori assumption about the definition of clinical malaria.
Acknowledgments
We thank all the clinical staff of the Kenya Medical Research Institute at Kilifi who made this study possible, especially Victor Mung’ala Odera and Norbert Peshu. A special acknowledgment is made of the dedicated field-workers who conducted all the surveys, especially Johnson Masha, Salma Said, and Monica Omondi, who entered the data.
Financial support: Wellcome Trust (K.M. [grant 631342] and R.W.S. [grant 058992] are Senior Research Fellows); Kenya Medical Research Institute.
Footnotes
This article is published with the permission of the director of the Kenya Medical Research Institute.
References
- 1.Armstrong-Schellenberg JRM, Smith T, Alonso PL, Hayes RJ. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today. 1994;10:439–41. doi: 10.1016/0169-4758(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith T, Charlwood JD, Kihonda J, et al. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]
- 3.Rougemont A, Breslow N, Brenner E, et al. Epidemiological basis for clinical diagnosis of childhood malaria in endemic zone in West Africa. Lancet. 1991;338:1292–5. doi: 10.1016/0140-6736(91)92592-p. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM, Bradley AK, Greenwood AM, et al. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–86. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–58. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 6.Mbogo CN, Snow RW, Khamala CP, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am J Trop Med Hyg. 1995;52:201–6. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 7.Mbogo CM, Mwangangi JM, Nzovu J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734–42. [PubMed] [Google Scholar]
- 8.Alonso PL, Smith T, Schellenberg JR, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344:1175–81. doi: 10.1016/s0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 9.D’Alessandro U, Leach A, Drakeley CJ, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–7. doi: 10.1016/s0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- 10.Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 11.McGuinness D, Koram K, Bennett S, Warner G, Nkrumah F, Riley E. Clinical case definition for malaria: clinical malaria associated with very low parasite densities in African infants. Trans R Soc Trop Med Hyg. 1998;92:527–31. doi: 10.1016/s0035-9203(98)90902-6. [DOI] [PubMed] [Google Scholar]
- 12.Bloland PB, Boroga DA, Ruebush TK, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60:641–8. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 13.Whitworth J, Morgan D, Quigley M, et al. Effects of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–6. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 14.Vounatsou P, Smith T, Kitua AY, Alonso PL, Tanner M. Apparent tolerance of Plasmodium falciparum in infants in a highly endemic area. Parasitology. 2000;120:1–9. doi: 10.1017/s0031182099005211. [DOI] [PubMed] [Google Scholar]
- 15.Trape JF, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–40. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 16.Snow R, Omumbo JA, Lowe B, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 17.Day KP, Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol Today. 1991;12:A68–70. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]


