Abstract
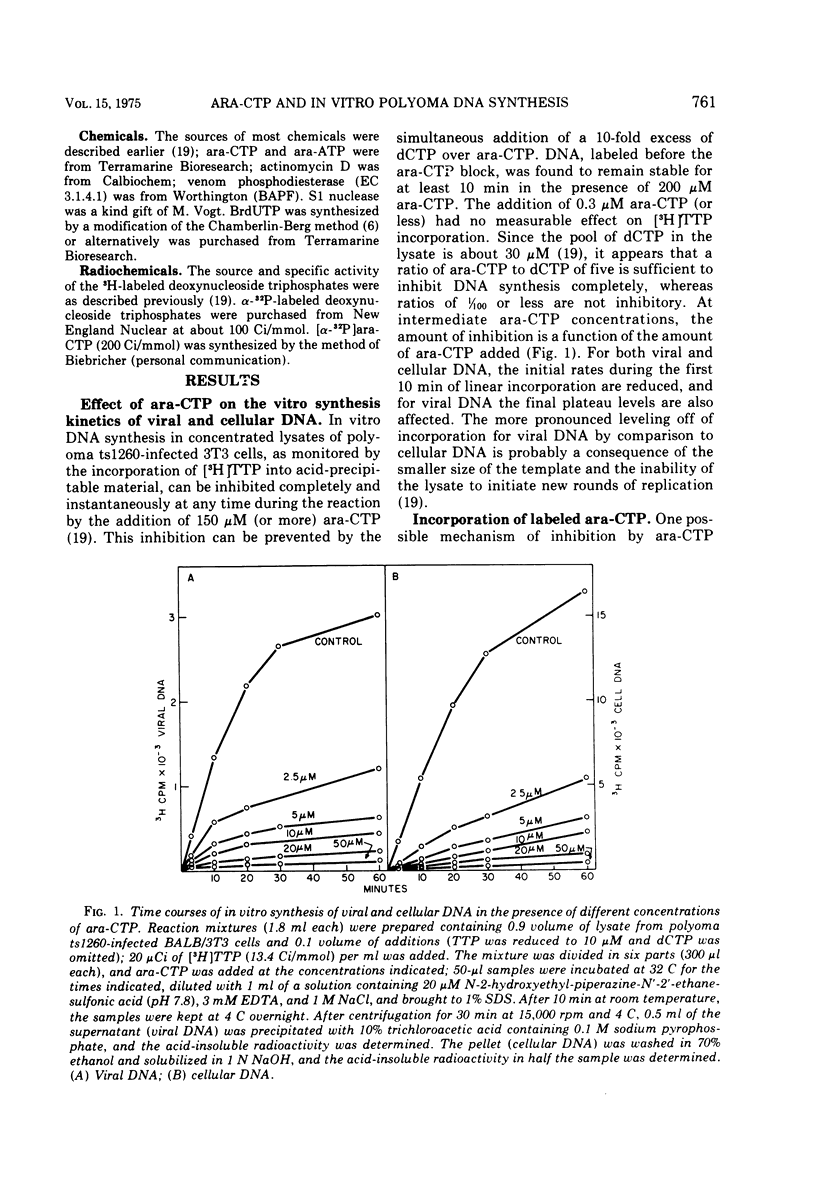
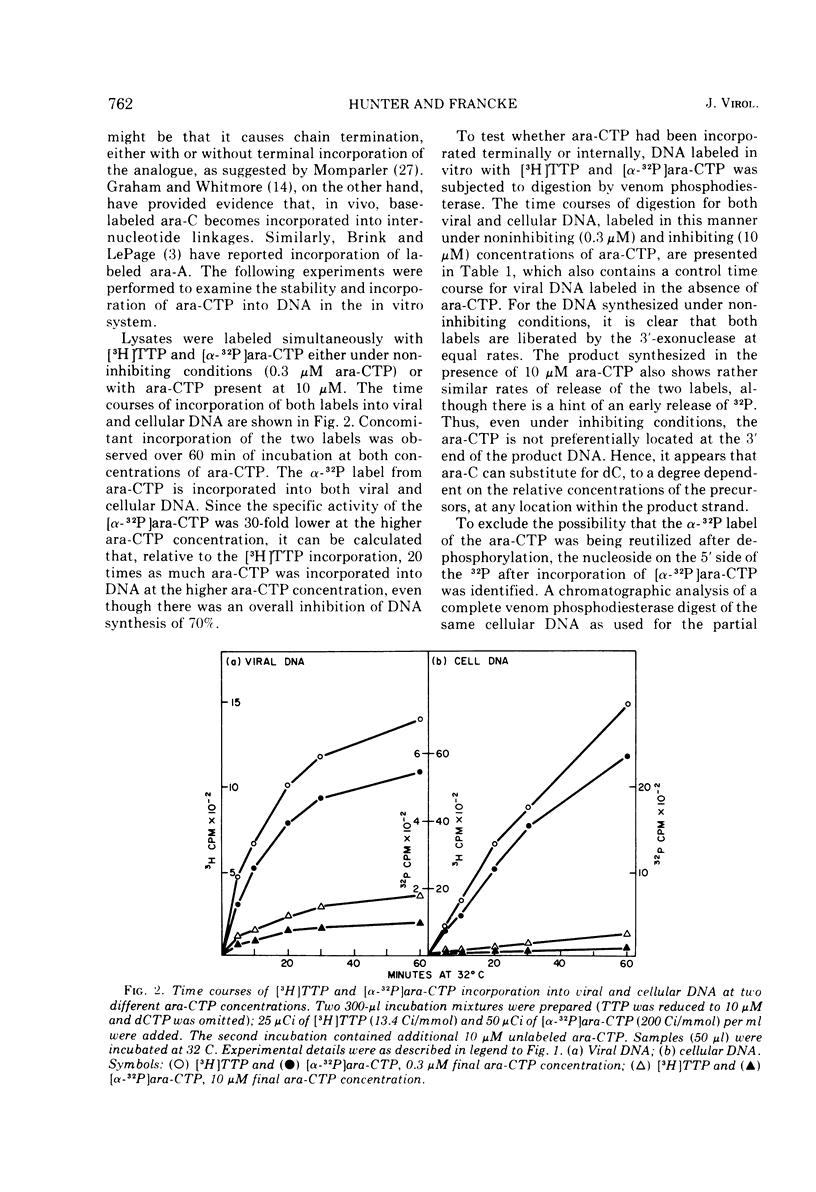
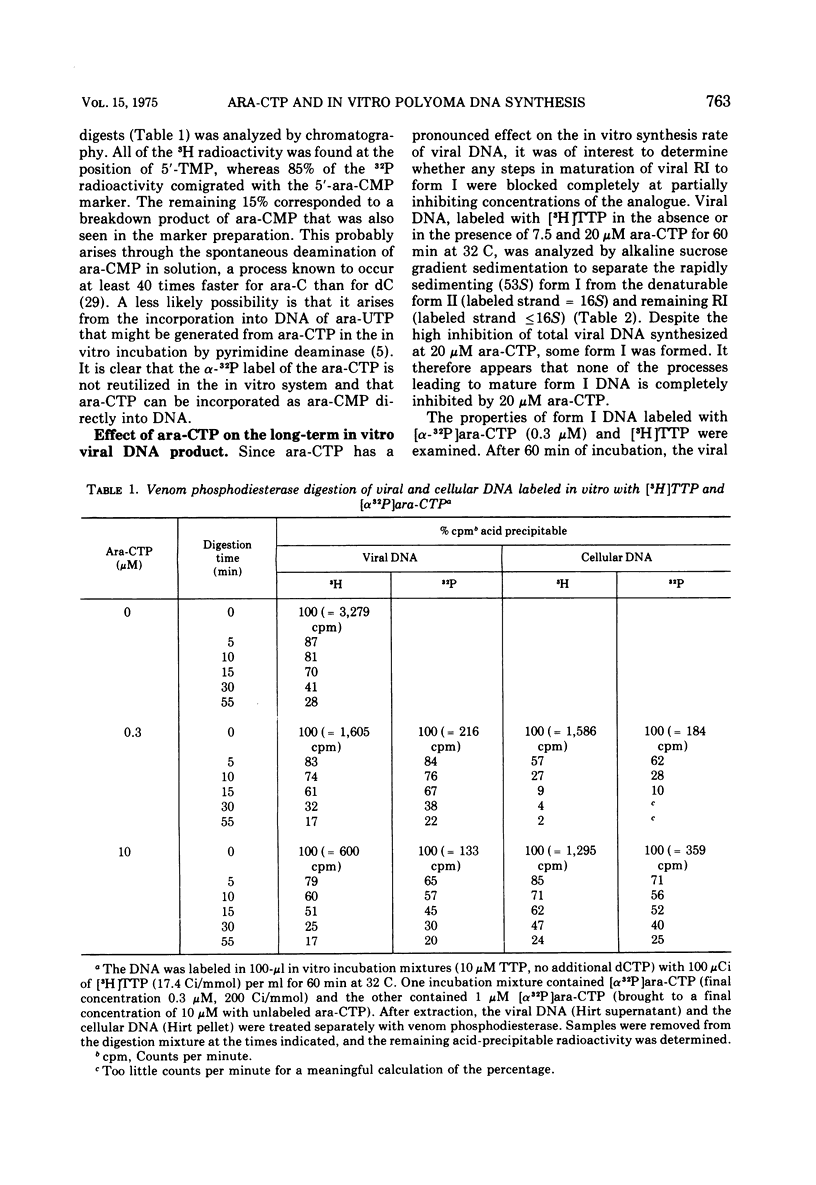
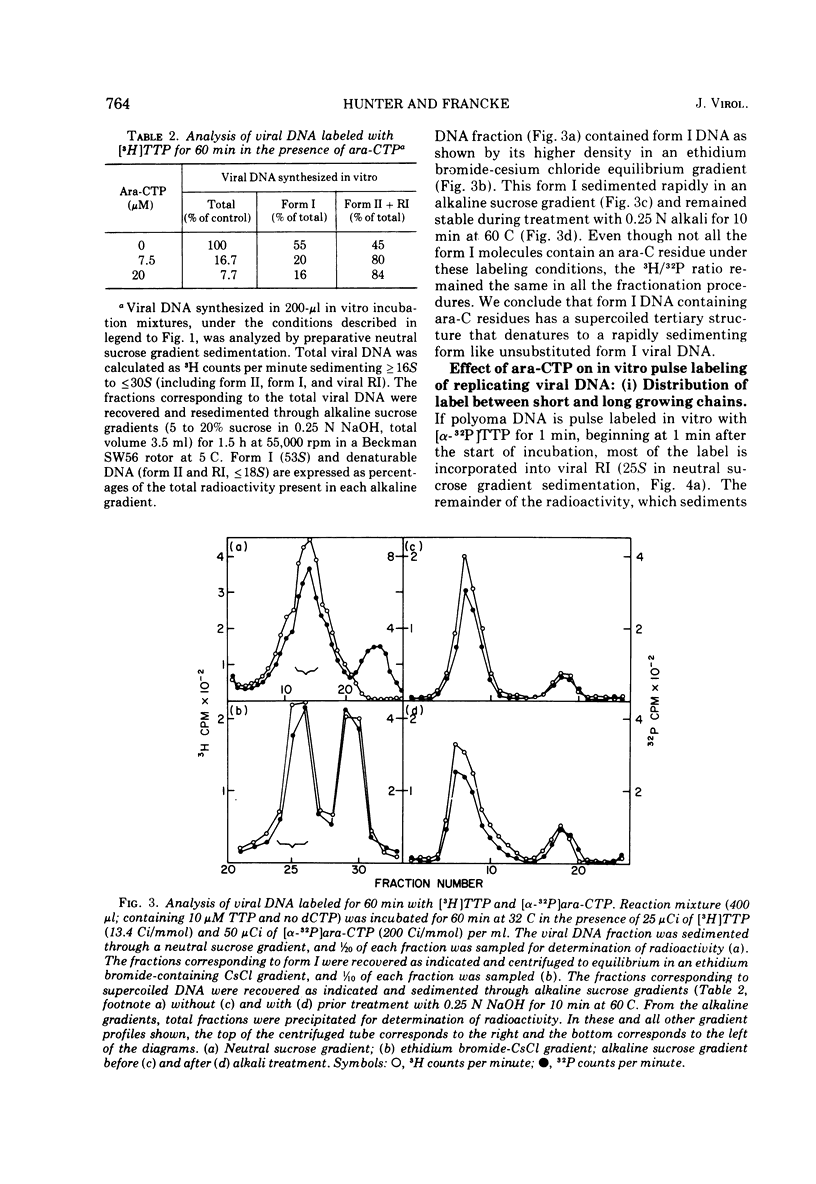
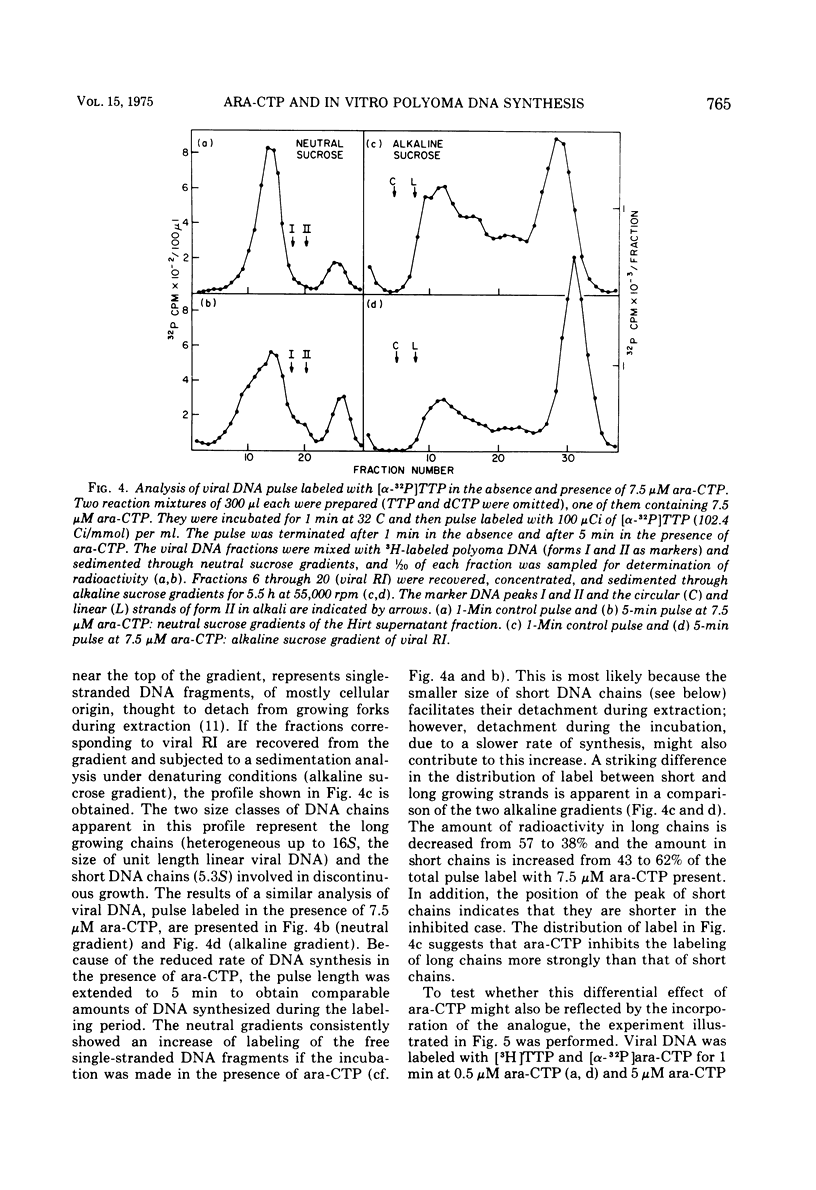
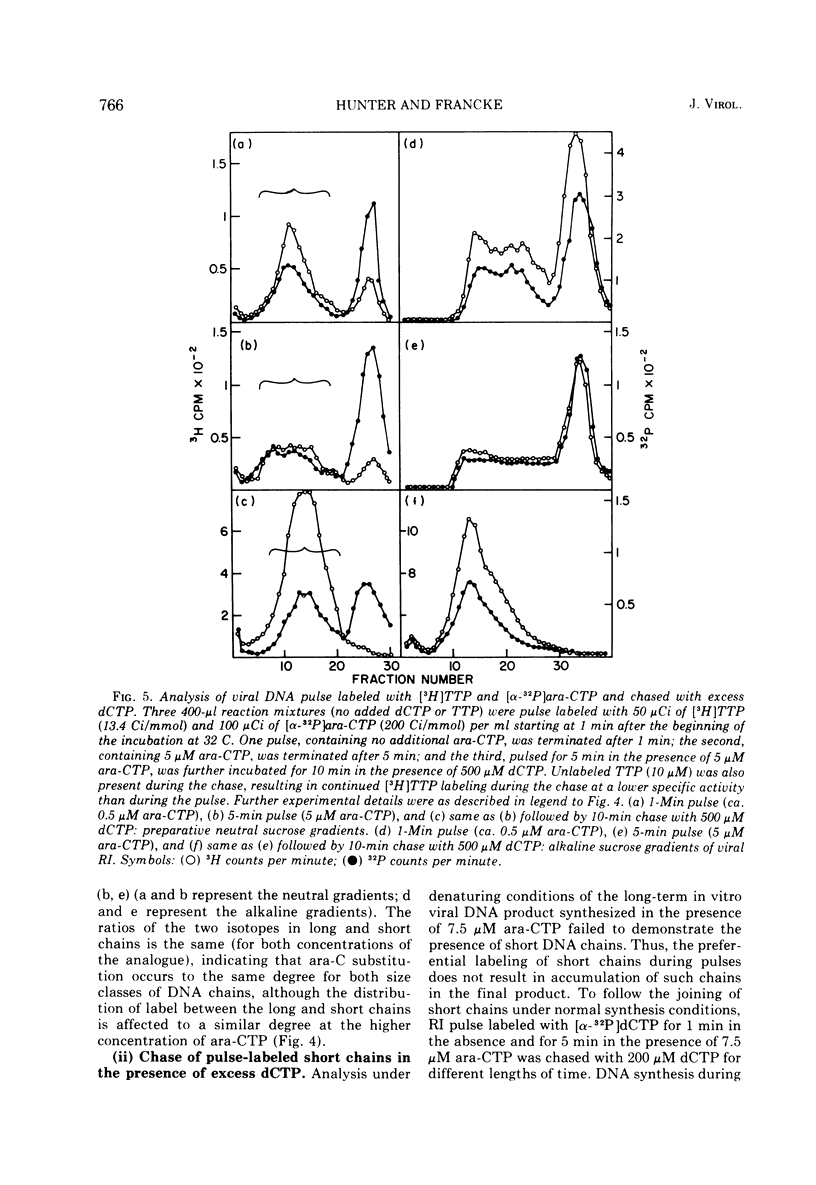
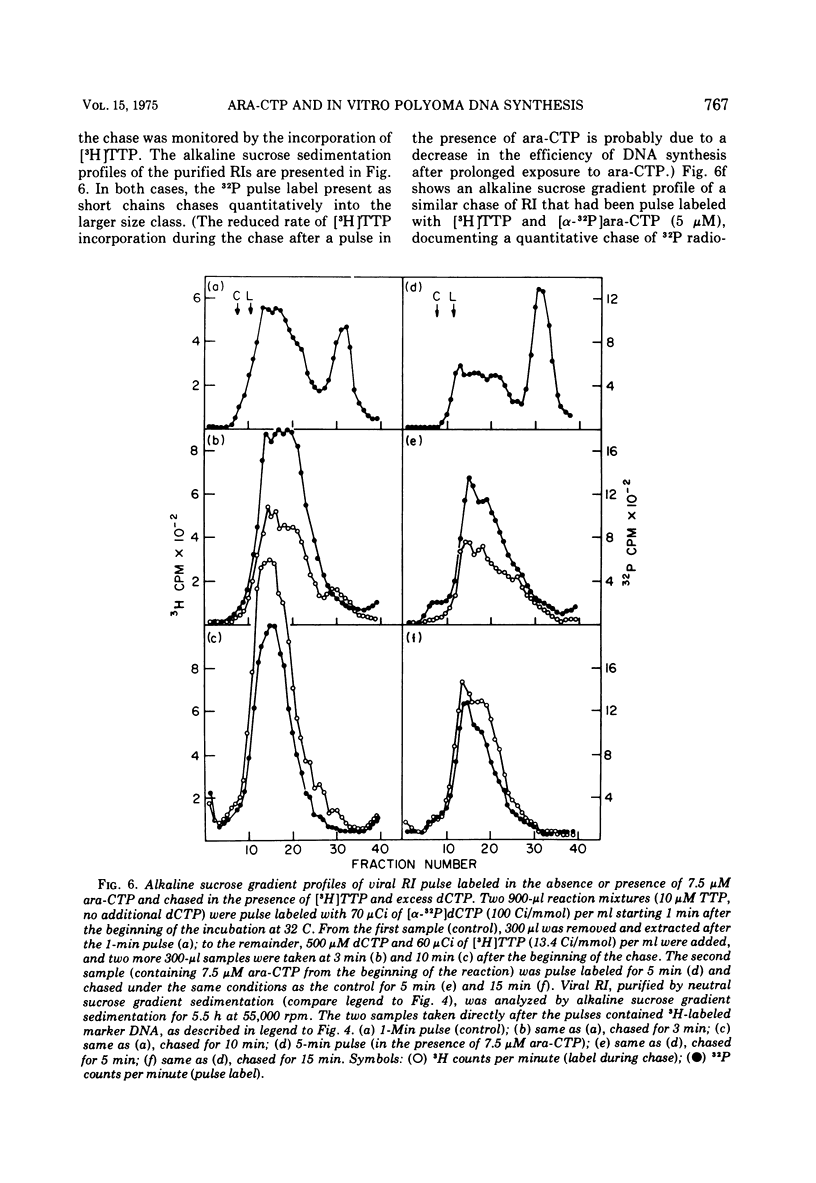
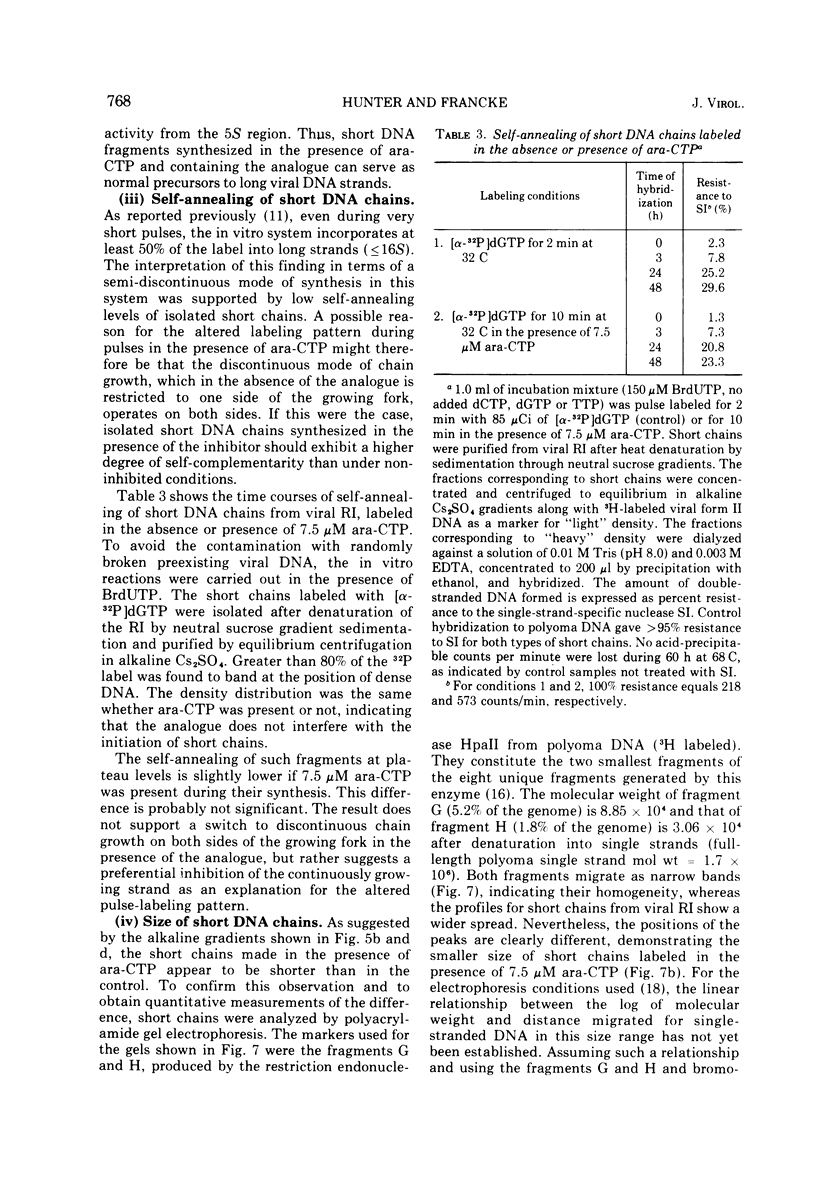
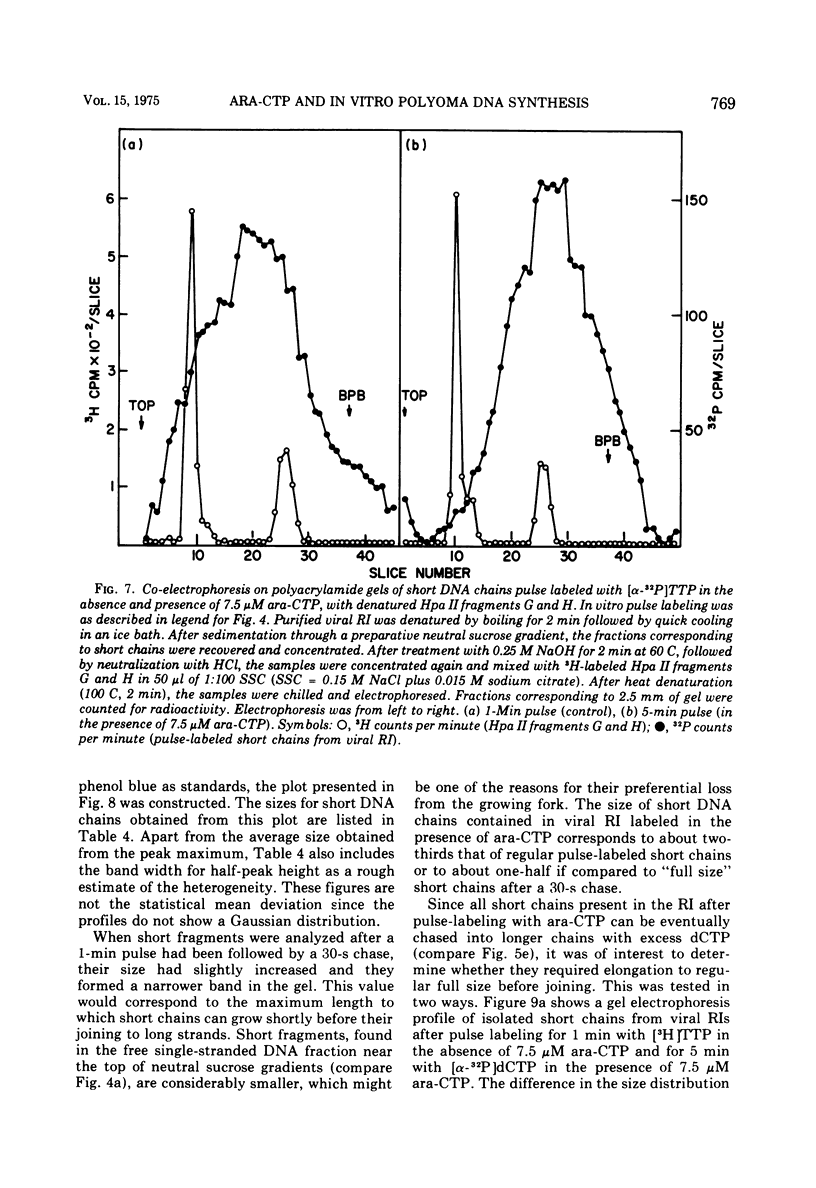
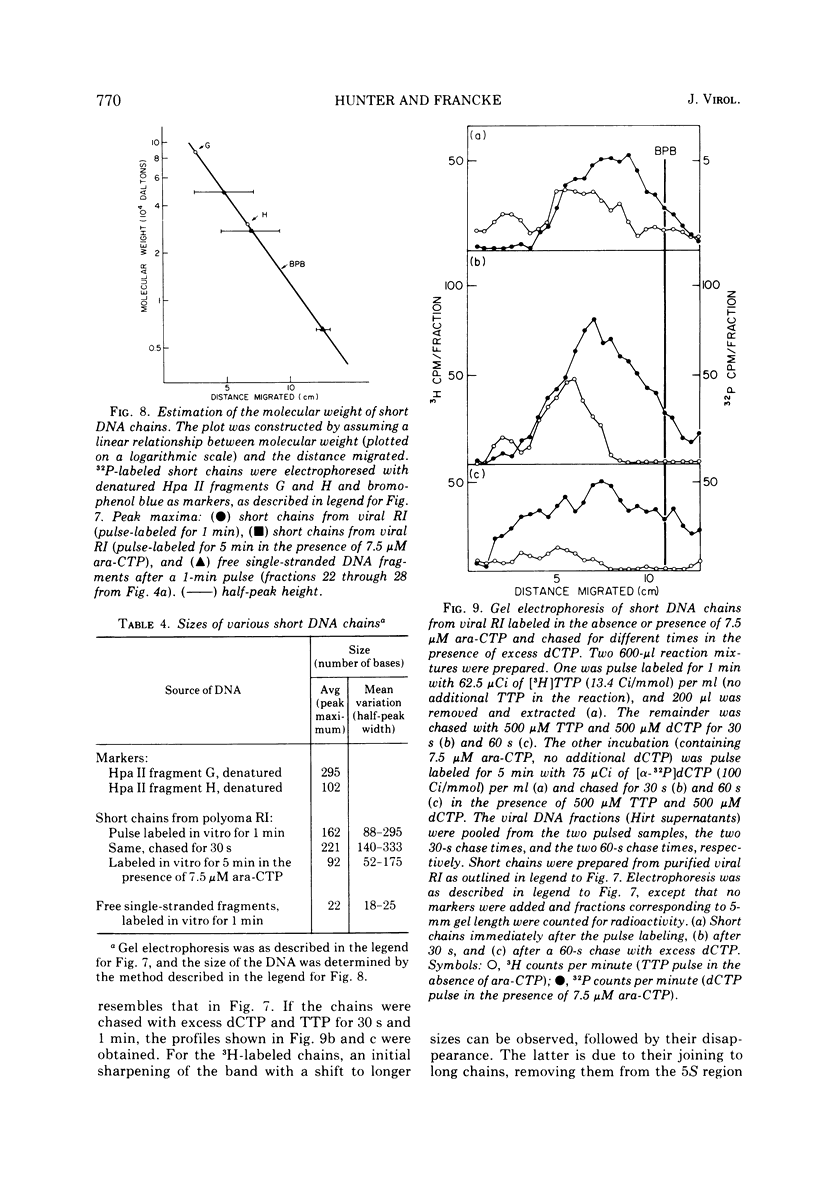
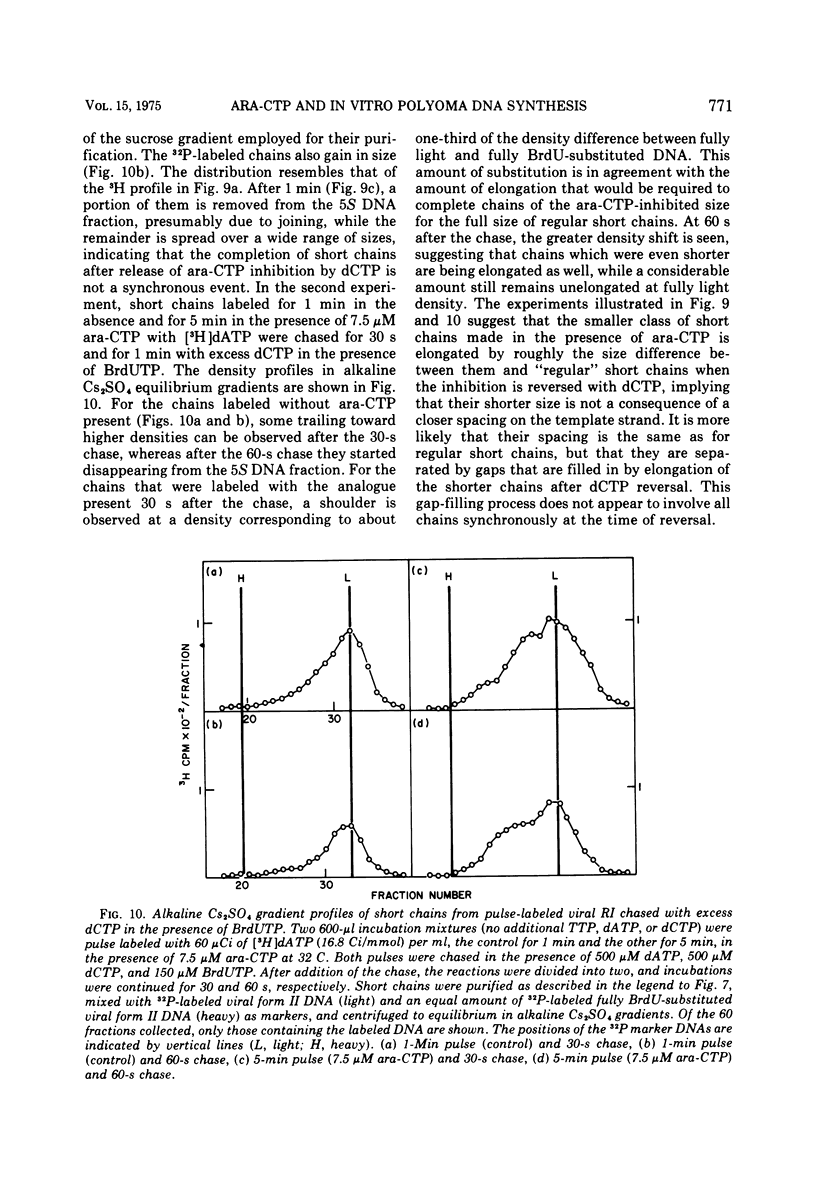
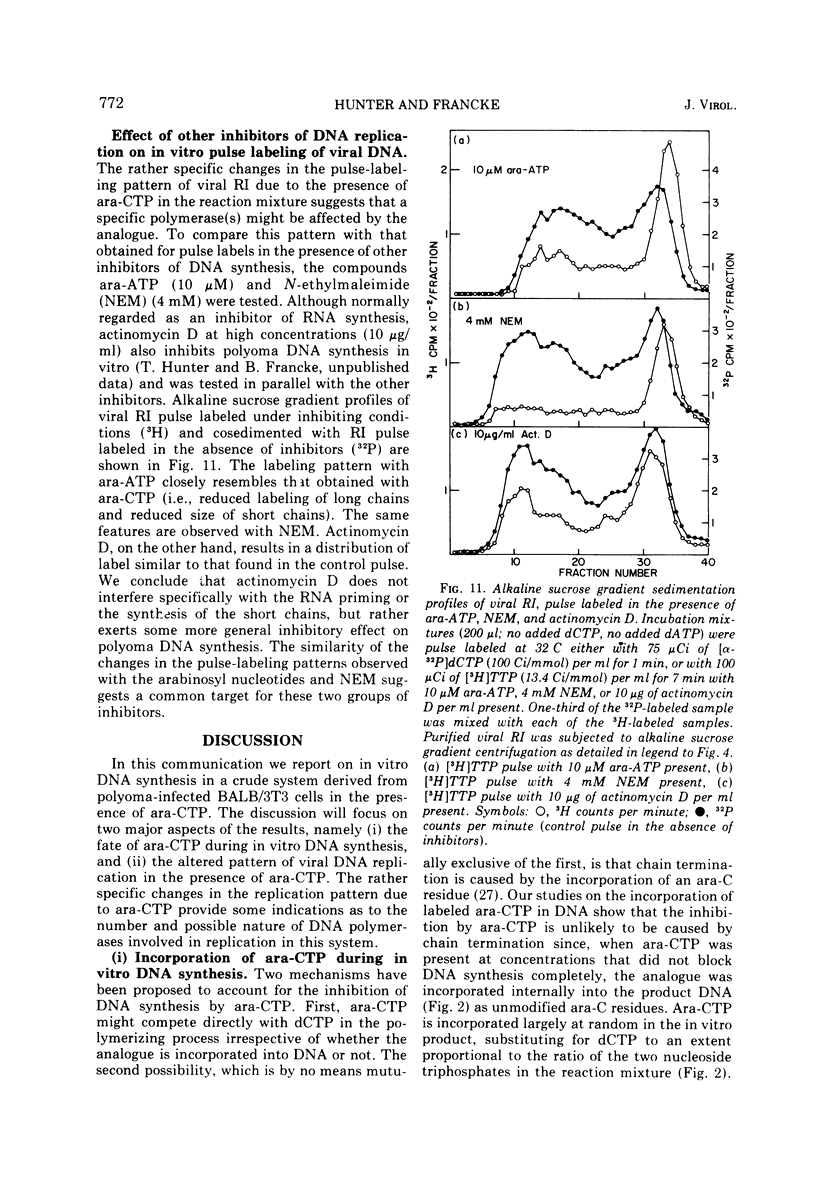
The effects of 1-beta-D-arabinofuranosyl CTP (ara-CTP) on DNA replication were studied in an in vitro system from polyoma-infected BALB/3T3 cells. Ara-CTP concentrations of larger than or equal to 150 muM were found to block in vitro DNA synthesis completely, and concentrations of smaller than or equal to 0.3 muM had no inhibitory effect. Intermediate concentrations resulted in a concentration-dependent reduction of the in vitro synthesis rate. Long-term labeling with [alpha-32-P]ara-CTP demonstrated the incorporation of the analogue into cellular and viral DNA concomitantly with [3-H]TTP. In pulse-labeling experiments, at noninhibitory concentrations of the analogue, ara-CTP was incorporated into short DNA fragments and long growing strands to relatively the same extent as TTP. Partial venom phosphodiesterase digestion liberated the incoporated are-CTP at essentially the same rate as incorporated TTP, excluding a predominantly terminal incorporation, and after total venom phosphodiesterase digestion greater than 80% of the incorporated ara-CTP was recovered as 5'-ara-CMP. Analysis of the long-term in vitro viral DNA product made in the presence of partially inhibiting ara-CTP concentrations demonstrated that none of the steps leading to mature viral DNA were totally inhibited at the ara-CTP concentrations used. Pulse labeling of replicating viral DNA in the presence of ara-CTP revealed two consistent differences in the pattern found in control pulses: (i) predominant labeling of short chains (5S) with reduced amounts of radioactivity in the longer growing viral DNA strands (smaller than or equal to 16S), and (ii) a one-third to one-half reduction in size for short DNA chains labeled in the presence of ara-CTP. Release of the ara-CTP inhibition with excess dCTP resulted in covalent extension of these smaller short chans to approximately the size of regular short chains labeled in the absence of the inhibitor. Isolated short chains synthesized in the presence of ara-CTP exhibited a slightly lower degree of self-complementarity than regular short chains. The predominant labeling of short chains during pulses is, therefore, not a consequence of discontinuous growth on both sides of the replication fork. Similar results were obtained with ara-ATP and N-ethylmaleimide. The experiments indicate that ara-CTP acts primarily on DNA-polymerizing activities, affecting different DNA polymerases to varying degrees. The results are discussed in terms of the possible number and identity of polymerases involved in viral (and cellular) DNA replication.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Henderson M. A., Wood W., Lindsay J. G. Multiple forms of nuclear deoxyribonucleic acid polymerases and their relationship with the soluble enzyme. Biochem J. 1973 Feb;131(2):237–246. doi: 10.1042/bj1310237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK J. J., LEPAGE G. A. 9-BETA-D-ARABINOFURANOSYLADENINE AS AN INHIBITOR OF METABOLISM IN NORMAL AND NEOPLASTIC CELLS. Can J Biochem. 1965 Jan;43:1–15. doi: 10.1139/o65-001. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. The effect of arabinofuranosylcytosine on the growth cycle of simian virus 40. Virology. 1965 Dec;27(4):490–495. doi: 10.1016/0042-6822(65)90174-1. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- Camiener G. W., Smith C. G. Studies of the enzymatic deamination of cytosine arabinoside. I. Enzyme distribution and species specificity. Biochem Pharmacol. 1965 Oct;14(10):1405–1416. doi: 10.1016/0006-2952(65)90175-9. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A chemical model for transcriptional initiation of DNA replication. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1354–1360. doi: 10.1016/s0006-291x(72)80124-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase in mammalian cells. J Biol Chem. 1971 Sep 25;246(18):5835–5837. [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sclair M. Specific endonuclease R fragments of bacteriophage phiX174 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):574–582. doi: 10.1128/jvi.9.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):99–121. doi: 10.1016/0022-2836(74)90426-4. [DOI] [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 1-beta-d-arabinofuranosylcytosine and the 5'-triphosphate of 9-beta-d-arabinofuranoxyladenine. Cancer Res. 1968 Oct;28(10):2061–2067. [PubMed] [Google Scholar]
- Graham F. L., Whitmore G. F. Studies in mouse L-cells on the incorporation of 1-beta-D-arabinofuranosylcytosine into DNA and on inhibition of DNA polymerase by 1-beta-D-arabinofuranosylcytosine 5'-triphosphate. Cancer Res. 1970 Nov;30(11):2636–2644. [PubMed] [Google Scholar]
- Graham F. L., Whitmore G. F. The effect of-beta-D-arabinofuranosylcytosine on growth, viability, and DNA synthesis of mouse L-cells. Cancer Res. 1970 Nov;30(11):2627–2635. [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Newbold J. E., Pagano J. S. Analysis of simian virus 40 DNA with the restriction enzyme of Haemophilus aegyptius, endonuclease Z. J Virol. 1973 Apr;11(4):508–514. doi: 10.1128/jvi.11.4.508-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Francke B. In vitro polyoma DNA synthesis: characterization of a system from infected 3T3 cells. J Virol. 1974 Jan;13(1):125–139. doi: 10.1128/jvi.13.1.125-139.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Francke B. Letter: In vitro polyoma DNA synthesis: involvement of RNA in discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):123–130. doi: 10.1016/0022-2836(74)90427-6. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Levine A. J. DNA replication in SV40-infected cells. IX. The inhibition of a gap-filling step during discontinuous synthesis of SV40 DNA. Virology. 1973 Dec;56(2):580–594. doi: 10.1016/0042-6822(73)90059-7. [DOI] [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. I. Characterization of fragments. J Virol. 1973 Sep;12(3):600–608. doi: 10.1128/jvi.12.3.600-608.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. II. Behavior during incubation of isolated nuclei. J Virol. 1973 Sep;12(3):609–615. doi: 10.1128/jvi.12.3.609-615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momparler R. L. Effect of cytosine arabinoside 5'-triphosphate on mammalian DNA polymerase. Biochem Biophys Res Commun. 1969 Feb 21;34(4):464–471. doi: 10.1016/0006-291x(69)90405-7. [DOI] [PubMed] [Google Scholar]
- Momparler R. L., Rossi M., Labitan A. Partial purification and properties of two forms of deoxyribonucleic acid polymerase from calf thymus. J Biol Chem. 1973 Jan 10;248(1):285–293. [PubMed] [Google Scholar]
- Notari R. E., Chin M. L., Cardoni A. Intermolecular and intramolecular catalysis in deamination of cytosine nucleosides. J Pharm Sci. 1970 Jan;59(1):27–32. doi: 10.1002/jps.2600590103. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Discontinuous DNA replication in vitro. I. Two distinct size classes of intermediates. Nat New Biol. 1972 Dec 20;240(103):233–235. doi: 10.1038/newbio240233a0. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Winnacker E. L., Eliasson R., Reichard P. Discontinuous elongation of both strands at the replication forks in polyoma DNA replication. Nat New Biol. 1973 Oct 17;245(146):203–205. doi: 10.1038/newbio245203a0. [DOI] [PubMed] [Google Scholar]
- Rama Reddy G. V., Goulian M., Hendler S. S. Inhibition of E. coli DNA polymerase II by ara-CTP. Nat New Biol. 1971 Dec 29;234(52):286–288. doi: 10.1038/newbio234286a0. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Thoren M. M. Inhibition in the joining of DNA intermediates to growing simian virus 40 chains. J Virol. 1973 May;11(5):721–729. doi: 10.1128/jvi.11.5.721-729.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Gallo R. C. DNA-dependent DNA polymerases I and II from normal human-blood lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2879–2884. doi: 10.1073/pnas.69.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]



