Abstract
Nuclear and cytoplasmic O-GlcNAc transferase (OGT) is a unique and universally expressed enzyme catalyzing O-GlcNAcylation of thousands of proteins. Although OGT interferes with many crucial intracellular processes, including cell cycle, only few studies have focused on elucidating the precise role of the glycosyltransferase during cell cycle entry. We first demonstrated that starved MCF7 cells reincubated with serum quickly induced a significant OGT increase concomitantly to activation of PI3K and MAPK pathways. Co-immunoprecipitation experiments performed upon serum stimulation showed a progressive interaction between OGT and β-catenin, a major factor in the regulation of cell cycle. OGT expression was also observed in starved HeLa cells reincubated with serum. In these cells, the O-GlcNAcylation status of the β-catenin-2XFLAG was increased following stimulation. Moreover, β-catenin-2XFLAG was heavily O-GlcNAcylated in exponentially proliferating HeLa cells when compared to confluent cells. Furthermore, blocking OGT activity using the potent inhibitor Ac-5SGlcNAc prevented serum-stimulated cyclin D1 synthesis and slightly delayed cell proliferation. At last, interfering with OGT expression (siOGT) blocked cyclin D1 expression and decreased PI3K and MAPK activation. Together, our data indicate that expression and catalytic activity of OGT are necessary and essential for G0/G1 transition.
Keywords: serum-stimulated cell cycle entry, O-GlcNAc transferase, cyclin D1, β-catenin
Introduction
O-GlcNAcylation (O-linked β-N-acetylglucosaminylation) is a post-translational modification that reversibly modifies proteins present in the cytosol, the nucleus and the mitochondria.1, 2, 3 Two enzymes control the O-GlcNAcylation cycle. First, the O-GlcNAc transferase (OGT, uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase or O-GlcNAc transferase) transfers the N-acetylglucosamine residue onto target proteins. Second, the O-GlcNAcase (OGA, N-acetyl β-glucosaminidase or O-GlcNAcase) removes the GlcNAc residue.4 OGT (EC. 2.4.1.255) is assigned to the GT41 family in the CAZY (carbohydrate-active enzyme) database5 and is found in all living beings (animals, plants, protists, bacteria, viruses) except in yeasts, in which its expression remains controversial. OGT participates in many fundamental cellular processes including cell cycle.2 Our group previously showed that hormonal stimulation of physiologically G2-blocked Xenopus laevis oocytes triggered a quick increase in O-GlcNAcylation levels and that inhibition of OGT impaired G2/M transition.6, 7 In parallel, Slawson et al.8 observed that OGT localized to the mitotic spindle and midbody during mitosis and that its overexpression resulted in supernumerary chromosomes. Later, the same authors showed that both OGT and OGA physically interact with Aurora B and PP1 to regulate the stability of the midbody and the phosphorylation and/or O-GlcNAcylation of vimentin at M-phase.9, 10 Finally, it was observed that OGT expression and O-GlcNAcylation reached a maximum level at the M-phase of the cell cycle.11 Taken together, these studies show that OGT and O-GlcNAcylation in general are needed for germ cells meiosis and somatic cell mitosis. However, to our knowledge, there is no report focusing on the expression and activity of OGT during cell cycle entry (G0/G1). Here, we describe that following serum stimulation, OGT is significantly overexpressed. Blockade of OGT delays serum-stimulated cyclin D1 synthesis and cell proliferation. OGT silencing also prevents cyclin D1 expression and diminishes PI3K and MAPK activation. These are the first results demonstrating that OGT is indispensable for G0/G1 transition.
Results and Discussion
OGT and OGA is a couple of non-redundant enzymes that controls O-GlcNAc cycling. Although much attention has been paid to OGT and although it has been described that this enzyme interferes with many crucial intracellular processes including cell cycle,2, 6, 7, 8, 9, 10, 11 no study has focused on its expression and function during cell cycle entry.
OGT is upregulated upon stimulation of MCF7 cells
As described previously,11 G0/G1 arrested MCF7 cells were stimulated by addition of fetal calf serum (FCS) and samples were collected at different times to assess OGT expression using western blot (Figures 1a and b). We observed a significant OGT increase (2.5-fold) 30 min after stimulation. FCS stimulation activated PI3K and MAPK pathways as attested by the use of antiphospho-Akt and antiphospho-Erk1/2 antibodies (Figure 1a). Cells treated with the protein synthesis inhibitor cycloheximide before stimulation did not show OGT increase in response to FCS (Figure 1c), indicating that protein translation is required to enhance OGT level. In parallel, OGT mRNA level, assessed by real-time PCR, remained unchanged 60 min post FCS treatment (Figure 1d). Thus, we conclude that OGT is regulated at the translational level shortly after addition of FCS. This is the first time that OGT induction is reported so rapidly after FCS treatment. Yang et al.11 recently showed that, upon FCS stimulation, OGT levels increased during all the cell cycle, suggesting that OGT might have some oncogenic properties. This hypothesis was first proposed by Caldwell et al.12 who reported higher hexosamine biosynthetic pathway activity and OGT levels in breast cancer cells, and demonstrated that interfering with the glycosyltransferase expression reduced tumor growth. Thus, OGT synthesis and activity may regulate fundamental factors involved in the control of the cell cycle.
Figure 1.

Stimulation of starved MCF7 cells with FCS increases OGT level. (a) MCF7 cells were maintained in a Dulbecco's modified Eagle's medium supplemented with 10% (v/v) FCS, 2 mℳℒ-glutamine, 5 IU/ml penicillin and 50 μg/ml streptomycin at 37 °C in a 5% (v/v) CO2-enriched humidified atmosphere. Cells were stopped at G0/G1 using the FCS-starvation method.11 Cells were FCS-starved for 48 h and then cell cycle was released by FCS addition. FCS-induced cells were collected at the indicated times after FCS addition. Cells were washed with 10 ml of cold phosphate-buffered saline and lysed directly on ice with lysis buffer (10 mℳ Tris/HCl, 150 mℳ NaCl, 1% Triton X-100 (v/v), 0.5% sodium deoxycholate (w/v), 0.1% sodium dodecyl sulfate (w/v) and proteases inhibitors, pH 7.4). Cell lysates were centrifuged (20 000 g, 10 min, 4 °C), pellets were discarded and supernatants boiled for 10 min in Laemmli buffer. Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and electroblotted on a nitrocellulose sheet (GE Healthcare, Orsay, France). Equal loading was verified using Ponceau red staining. Membranes were saturated for 45 min with 5% non-fatty acid milk in (TBS)-Tween buffer (15 mℳ Tris/HCl, 140 mℳ NaCl and 0.05% Tween20 (v/v), pH 8.0). Proteins were immunodetected using the following primary antibodies; OGT: rabbit polyclonal TI14, 1/2000 (Sigma-Aldrich, Saint-Quentin Fallavier, France); α-tubulin: mouse monoclonal B-5-1-2, 1/5000 (Santa Cruz Biotechnology, Heidelberg, Germany); Erk2: D-2, 1/5000 (Santa Cruz Biotechnology); phospho-Erk1/2: rabbit polyclonal, 1/1000 (Cell Signaling, Danvers, MA, USA); phospho-AKT: rabbit polyclonal, 1/1000 (Cell Signaling) and AKT: mouse monoclonal, 1/2000 (Cell Signaling). Membranes were incubated with primary antibodies overnight at 4 °C, washed three times (TBS–Tween, 10 min) and incubated with appropriate horseradish peroxidase-labelled secondary antibodies at a dilution of 1/10 000 for 1 h. After three more washes, detection was performed with enhanced chemiluminescence (GE Healthcare). (b) Histograms represent densitometric analyses of western blots (WBs). Results correspond to the mean value ±s.d. of three experiments (*P<0.05, **P<0.01, ***P<0.001 respectively, NS not significant). (c) Starved MCF7 cells were stimulated with FCS with or without the protein synthesis inhibitor cycloheximide (CHX) at a concentration of 15 μg/ml. OGT expression was analyzed by WB and protein loading was verified using alpha-tubulin. (d) FCS-starved and 1 h FCS-stimulated MCF7 cells OGT mRNA levels were determined by real-time PCR. Quantitative reverse transcriptase–PCR: Total RNA was reverse transcribed using random primers and MultiScribe reverse transcriptase (Applied Biosystems, Villebon sur Yvette, France). Real-time PCR analysis was performed by Power SYBR Green (Applied Biosystems) in a MX3005P fluorescence temperature cycler (Stratagene, Paris, France) according to the manufacturer's instructions. Results were normalized with respect to RPLP0 RNAs used as internal control. The primers used are as follows: OGT sense 5′-TGGCTTCAGGAAGGCTATTG-3′ and antisense 5′-CAAGTCTTTTGGATGTTCATATG-3′, and RPLP0 sense 5′-GTGATGTGCAGCTGATCAAGA-3′ and antisense 5′-GATGACCAGCCCAAAGGAGA-3′. Results correspond to the mean value ±s.d. of three experiments (NS not significant). Molecular mass markers are indicated at the left.
β-catenin and OGT interact rapidly upon G1 phase entry
We and others have previously reported that the oncoprotein β-catenin, a major cell cycle factor, can be O-GlcNAcylated by OGT.13, 14, 15, 16 However, the role of this modification remained poorly understood. Studies performed on plakoglobin,17 a member of the catenin family, showed that mutation of Thr14, an O-GlcNAcylated site close to the D-box that controls plakoglobin degradation, into alanine slightly increased plakoglobin stability. We also reported that β-catenin stability is regulated by the glucose status, the hexosamine biosynthetic pathway (which provides UDP-GlcNAc, the substrate for O-GlcNAcylation processes) flux and O-GlcNAcylation.14, 18, 19 At last, RNA interference of OGA in the colorectal cancer metastatic cell line SW620 resulted in β-catenin overexpression.20 Our hypothesis is that O-GlcNAcylation may serve as a protective signal for short half-life proteins such as β-catenin. Upon cell cycle entry, the expression of β-catenin continuously increases during G1 phase until it reaches a maximal level at G2/M transition.21, 22, 23 To establish that this increase is due to O-GlcNAcylation, we investigated to measure the OGT interaction with β-catenin after addition of FCS to quiescent cells. First, we observed a concomitant increase of OGT and β-catenin as soon as 15 min after stimulation with FCS (Figure 2a, left panel). Co-immunoprecipitations performed under the same conditions showed that OGT and β-catenin interacted within minutes (Figure 2a, right panel) as soon as G0/G1 transition was triggered, as confirmed by phosphorylation of Erk and according to the β-catenin target gene cyclin D1 profile (Figure 2a, left panel). In this way, note that β-catenin transcriptional activity doubled upon FCS stimulation (Figure 2b) as attested by reporter gene analysis using TOP/FOP Flash constructs.
Figure 2.
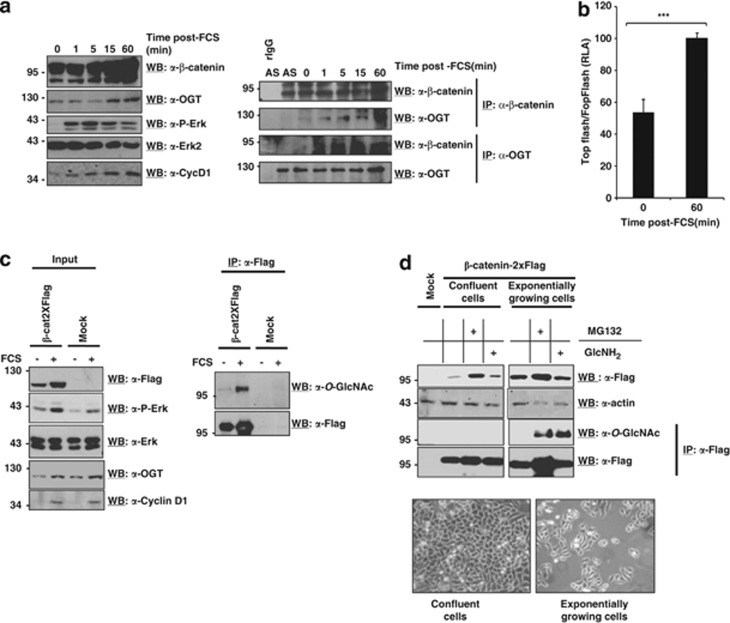
Upon cell cycle entry, as OGT content increases, the glycosyltransferase interacts with β-catenin (a). MCF7 cells were starved for 48 h and then re-supplemented with FCS for the indicated time periods. Levels of β-catenin, OGT and Erk2 were determined by western blot (WB). Activation of the MAPK pathway was probed using an antiphospho-Erk and cell cycle entry was checked using a mouse monoclonal anticyclin D1 (Santa Cruz Biotechnology) at a final dilution of 1/1000 (left panel). Co-immunoprecipitations using β-catenin or OGT-directed antibodies were performed during the time course experiment to evaluate the interaction between the two partners (right panel). MCF7 cells were washed with 10 ml of cold phosphate-buffered saline and lysed on ice in a lysis buffer containing 20 mℳ Tris/HCl, 150 mℳ NaCl, 0.5% NP-40 (v/v) and a cocktail of proteases inhibitors, pH 8.0. Whole cell extracts were centrifuged at 20 000 g for 10 min at 4 °C and supernatants were collected and first precleared with Sepharose-labelled protein A (GE Healthcare) for 1 h. Beads were then discarded and supernatants were incubated with rabbit anti-OGT (DM17 from Sigma) or rabbit anti-β-catenin (H-102 from Santa Cruz Biotechnology) antibodies overnight at 4 °C and then with Sepharose-labelled protein A for 1 h. Beads were gently centrifuged for 1 min and washed four times for 5 min each with the lysis buffer. Controls for immunoprecipitation specificities were performed with non-immune rabbit IgG (Santa Cruz Biotechnology). FCS stimulation of starved cells increases β-catenin transcriptional activity (b). Starved MCF7 cells were transfected with β-galactosidase, TOP Flash containing three optimal copies of the TCF/LEF-binding site, FOP Flash containing mutated copies of the TCF/LEF-binding site and β-catenin-2XFlag vector by the Lipofectamine2000 (Invitrogen, Saint-Aubin, France) reagent (2 μl) in six-wells plates with 0.2 μg of DNA for 24 h. Cells were stimulated by FCS for 1 h and TOP/FOP Flash reporter assays were performed. Histogram represents the relative luciferase activity. Results correspond to the mean value ±s.d. of three experiments (***P<0.001). Addition of FCS to starved cells increases OGT and β-catenin levels and β-catenin O-GlcNAcylation (c). HeLa cells were maintained in the same conditions as described for MCF7 cells (Figure 1). Cells were transfected with pCS2+β-catenin-2XFlag (or the empty vector) in 2.5 ml of optiMEM by the polyethylenimine (PEI, Euromedex) method (10 μl) in 100 mm diameter dishes with 2.5 μg of DNA for 6 h and then incubated for 48 h in 10 ml of fresh complete medium. One day later, cells were FCS-starved and reincubated with FCS for 1 h. The activity of the MAPK pathway was checked by evaluating phosphorylation of Erk. β-catenin was probed using the mouse monoclonal anti-Flag (M2 from Sigma) at a dilution of 1/5000. (left panel). Expression of cyclin D1 was also evaluated. β-catenin was immunopurified using the anti-FLAG antibody and the immunoprecipates were stained either with a mouse monoclonal anti-O-GlcNAc antibody (RL2, Ozyme, Saint-Quentin en Yvelines, France) at a dilution of 1/1000 or with the anti-FLAG antibody (right panel). β-catenin is heavily O-GlcNAcylated in exponentially HeLa growing cells (d). After transfection with pCS2+β-catenin-2XFlag, HeLa cells were cultured and collected either when in the exponential growth phase or when cell confluence was reached. Cells were cultured either without any drug or with 1 μℳ MG132 (N-carbobenzoxyl-Leu-Leu-leucinal, Sigma) or 20 mℳ glucosamine. β-catenin (anti-Flag) expression was measured by WB. Rabbit polyclonal anti-actin (I–19 from Santa Cruz Biotechnology) was used at a dilution of 1/10 000. β-catenin was also immunopurified using the anti-FLAG and immunoprecipates were stained either with anti-O-GlcNAc antibody (RL2) or with anti-FLAG antibody (M2). Light microscopy pictures (right) were taken just before cell collection. IP, immunoprecipitation; rIgG, rabbit non-immune immunoglobulin G; AS, asynchronous cells. Molecular mass markers are indicated at the left.
β-catenin is highly expressed and O-GlcNAcylated in response to FCS
Total levels of exogenous β-catenin and its O-GlcNAcylated fraction were evaluated by western blotting in β-catenin-2XFLAG overexpressing HeLa cells that were either FCS-starved or starved and reincubated with FCS for 1 h (Figure 2c, left panel). As expected, the total amount of β-catenin was found higher in FCS-stimulated cells. Moreover, and similar to MCF7 cells (Figure 1), an increased OGT expression was observed in response to FCS. In accordance, we also showed that modification of β-catenin by O-GlcNAc was remarkably enhanced concomitantly to OGT induction (Figure 2c, right panel). This is consistent with our previous report demonstrating that endogenous β-catenin was stabilized by O-GlcNAcylation in a model of oocyte maturation (X. laevis).13
Furthermore, β-catenin expression and O-GlcNAcylation levels were also compared in confluent and exponentially growing HeLa cells. Figure 2d shows that β-catenin protein was more abundant in proliferating cells than in quiescent cells. Strikingly, β-catenin was found O-GlcNAcylated in proliferating cells but not in quiescent one, suggesting that high level of β-catenin expression is dependent on O-GlcNAcylation.
Inhibition or downexpression of OGT impairs cell cycle entry
Because FCS stimulation induced OGT expression, we then tested the effect of OGT deficiency on cyclin D1 expression. To this end, we inhibited OGT either at the transferase activity level, using the recently described compound Ac-5SGlcNAc,24 or at the expression level, using RNA interference (siOGT). Treatment with Ac-5SGlcNAc resulted in a marked decrease of overall O-GlcNAcylation in response to FCS (Figure 3a). Furthermore, cyclin D1 did not accumulated following FCS addition as in control condition (Figure 3a). To know whether OGT controls the expression of other cyclins, and consequently is crucial for all steps of the cell cycle, we depleted asynchronous MCF7 of OGT through the use of siRNA (Figure 3b). We observed lower amounts of cyclin D1 and cyclin B1, corroborating previous findings,6, 7 in MCF7 transfected with siOGT when compared with siCtrl (universal negative control), whereas expression of cyclin E and cyclin A were unaffected. The same result was achieved by inhibiting OGT at the catalytic level (Supplementary Figure S1). Moreover, OGT silencing decreased the phosphorylated forms (active) of Akt and Erk1/2, the downstream effectors of the PI3K and MAPK pathways respectively, in asynchronous cells. In this way, longer time-course experiments showed that Ac-5SGlcNAc slightly delayed cell proliferation (Figure 3c). RNA interference of OGT also resulted in inhibition of FCS-induced cyclin D1 expression and in inhibition of PI3K/MAPK activation as observed above for asynchronous cells (Figure 3d). β-catenin expression remained similar in control and siOGT-treated cells. We next tested wortmannin, a PI3K inhibitor in FCS-stimulated cells (Figure 3e). We observed that reciprocally, inhibiting the PI3K pathway decreased FCS-induced OGT, and cyclin D1, expression, supposing a feedback loop between PI3K and OGT. To evaluate the impact of OGT silencing on the transactivation of genes responsive to the β-catenin/TCF/LEF complex, we performed a reporter gene analysis using the TOP/FOP Flash system in HEK293T cells (Figure 3f). Silencing OGT expression decreased by 50% the transcription activity of the β-catenin/TCF/LEF complex in this model. Analysis of β-catenin expression by western blot indicates that the transcriptional activity decrease correlated with a loss of β-catenin expression in FCS-induced HEK293T cells.
Figure 3.

Inhibiting OGT catalytic activity or interfering with its expression hinders FCS-stimulated cell cycle entry. (a) Following FCS starvation, MCF7 cells were incubated with FCS for the indicated time periods with or without the OGT inhibitor Ac-5SGlcNAc at a final concentration of 100 μℳ. Cell lysates were then analyzed by western blot (WB) according to their O-GlcNAc and cyclin D1 content. Equal loading was checked by using a rabbit polyclonal anti-GAPDH (Abcam, Paris, France) at a dilution of 1/5000. (b) Asynchronous MCF7 cells were reverse-transfected with Lipofectamine RNAiMax (Life Technologies, Carlsbad, CA, USA) according to manufacturer's instructions using 10 nℳ small interfering RNA targeting OGT25 or a control siRNA (MISSION siRNA universal negative control #1, Sigma). Cell lysates were analyzed by WB according to their cyclin D1, cyclin E, cyclin A, cyclin B1, phospho-Akt, Akt, phospho-Erk1/2, Erk1/2 and tubulin contents. (c) MCF7 cells (2 × 103) were cultured in 96-wells plates using Dulbecco's modified Eagle's medium with or without 100 μℳ Ac-5SGlcNAc over 5 days. Each day, cell growth was determined using the MTS reagent method (Promega, Madison, WI, USA) according to the manufacturer's directions at 490 nm (n=6). (d) Starved MCF7 cells were reverse-transfected with Lipofectamine using siRNA targeting OGT or a control siRNA as described in b. 24 h later, cells were FCS-deprived for 48 h and then stimulated for the indicated time periods. Cell lysates were analyzed by WB using anti-OGT, anti-O-GlcNAc, anti-β-catenin, anti-phospho-Akt, anti-Akt, anti-phospho-Erk1/2, anti-Erk1/2, anti-cyclin D1 and anti-tubulin antibodies. (e) Starved MCF7 cells were stimulated with FCS for the indicated time periods in conjunction with 10 nℳ wortmannin, an inhibitor of the PI3K pathway. Cell lysates were analyzed by WB according to their OGT and cyclin D1 content. Activation or inhibition of the PI3K pathway was assessed using an anti-P-Akt antibody. Equal loading was checked by using an anti-tubulin antibody. (f) HEK293T cells were cultured under the same conditions as described for MCF7 and HeLa cells (Figure 1). Following OGT silencing (see above for details), HEK293T cells were transfected with β-galactosidase, TOP Flash, FOP Flash and β-catenin2-XFlag vector or an empty vector by the Lipofectamine2000 reagent (Figure 2b) for 24 h to perform TOP/FOP Flash reporter assay. Histogram represents the relative luciferase activity (RLA). Results correspond to the mean value ±s.d. of three experiments (*P<0.05, ***P<0.001, respectively; NS not significant). OGT and β-catenin expression corresponding to the luciferase activity assay were measured by WB. GAPDH was used to attest equal loading.
All together, our results indicate that OGT is a potent regulator of the cell cycle entry essential for FCS-stimulated cells to activate mitogenic pathways and to express cyclin D1. Thus, we propose that abnormal increase of OGT activity, which may occur in a context of nutrient excess that directly modulates the levels of the OGT substrate UDP-GlcNAc, would affect the cell cycle, as recently discussed by others.12 In such a context, OGT could arguably exert some oncogenic effect, by allowing the cell cycle of the affected cells to bolt off.
Acknowledgments
This work has been supported by the ‘Ligue Contre le Cancer/Comité du Nord', the ‘Association pour la Recherche sur le Cancer', the University of Lille 1 and the ‘Centre National de la Recherche Scientifique'. SOVS is a recipient of a fellowship from the ‘Ministère de l′Enseignement Supérieur et de la Recherche. We are indebted to Dr David Vocadlo (Simon Fraser University), Dr Chunming Liu (University of Kentucky), Dr Randall T Moon (University of Washington) who provided us, respectively, Ac-5SGlcNAc, pCS2+β-catenin-2XFlag and TOPflash/FOPflash reporter plasmids. We also thank Pr. Ralph Schwarz (Marburg University) and Dr Sylvain Julien (King's College/Lille 1 University) for the final reading of the manuscript and for the grammatical corrections.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1996;261:8049–8057. [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284:547–555. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehennaut V, Hanoulle X, Bodart JF, Vilain JP, Michalski JC, et al. Microinjection of recombinant O-GlcNAc transferase potentiates Xenopus oocytes M-phase entry. Biochem Biophys Res Commun. 2008;369:539–546. doi: 10.1016/j.bbrc.2008.02.063. [DOI] [PubMed] [Google Scholar]
- Dehennaut V, Lefebvre T, Sellier C, Leroy Y, Gross B, et al. O-linked N-acetylglucosaminyltransferase inhibition prevents G2/M transition in Xenopus laevis oocytes. J Biol Chem. 2007;282:12527–12536. doi: 10.1074/jbc.M700444200. [DOI] [PubMed] [Google Scholar]
- Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–32956. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–4140. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YR, Song M, Lee H, Jeon Y, Choi EJ, et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11:439–448. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- Lefebvre T, Baert F, Bodart JF, Flament S, Michalski JC, Vilain JP. Modulation of O-GlcNAc glycosylation during Xenopus oocyte maturation. J Cell Biochem. 2004;93:999–1010. doi: 10.1002/jcb.20242. [DOI] [PubMed] [Google Scholar]
- Olivier-Van Stichelen S, Guinez C, Mir AM, Perez-Cervera Y, Liu C, et al. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of β-catenin and cell proliferation. Am J Physiol Endocrinol Metab. 2012;302:E417–E424. doi: 10.1152/ajpendo.00390.2011. [DOI] [PubMed] [Google Scholar]
- Zhu W, Leber B, Andrews DW. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 2001;20:5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayat R, Leber B, Grubac V, Wiltshire L, Persad S. O-GlcNAc-glycosylation of beta-catenin regulates its nuclear localization and transcriptional activity. Exp Cell Res. 2008;314:2774–2787. doi: 10.1016/j.yexcr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Hatsell S, Medina L, Merola J, Haltiwanger R, Cowin P. Plakoglobin is O-glycosylated close to the N-terminal destruction box. J Biol Chem. 2008;278:37745–37752. doi: 10.1074/jbc.M301346200. [DOI] [PubMed] [Google Scholar]
- Anagnostou SH, Shepherd PR. Glucose induces an autocrine activation of the Wnt/ β-catenin pathway in macrophage cell lines. Biochem J. 2008;416:211–218. doi: 10.1042/BJ20081426. [DOI] [PubMed] [Google Scholar]
- Vaira S, Friday E, Scott K, Conrad S, Turturro F. Wnt/β-catenin signaling pathway and thioredoxin-interacting protein (TXNIP) mediate the "glucose sensor" mechanism in metastatic breast cancer-derived cells MDA-MB-231. J Cell Physiol. 2012;227:578–586. doi: 10.1002/jcp.22757. [DOI] [PubMed] [Google Scholar]
- Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-GlcNAcylation in primary and metastatic colorectal cancer clones and effect of O-GlcNAcase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755–28769. doi: 10.1074/jbc.M112.345546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford K, Orford CC, Byers SW. Exogenous expression of beta-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855–868. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, Castel S, Vilaro S, Cano A. β-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell. 2003;14:2844–2860. doi: 10.1091/mbc.E03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20:453–460. doi: 10.1016/j.tcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol. 2011;7:174–181. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, et al. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22:2901–2911. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


