Abstract
Adipose-specific gene deletion in mice is crucial in determining gene function in adipocyte homeostasis and the development of obesity. We noted 100% mortality when the Hdac3 gene was conditionally deleted using Fabp4-Cre mice, the most commonly used model of adipose-targeted Cre recombinase. However, this surprising result was not reproduced using other models of adipose targeting of Cre, including a novel Retn-Cre mouse. These findings underscore the need for caution when interpreting data obtained using Fabp4-Cre mice and should encourage the use of additional or alternative adipose-targeting Cre mouse models before drawing conclusions about in vivo adipocyte-specific functions.
Spatial and temporal conditional gene targeting with the use of cell type-specific promoter sequence driving the expression of Cre recombinase is a powerful tool in the interrogation of gene function in mice (1). Adipose selective gene targeting with the use of a fragment of the Fabp4 promoter has been reported by multiple groups (2–4), and of these, the Fabp4-Cre mouse line originally developed and described by He et al. (2) is the most widely used tool to generate adipose-specific knockout mice (Table 1) (5–27).
Table 1.
A selection of floxed genes that have been targeted using Fabp4-Cre mice originally reported by He et al. (2) illustrating widespread implementation of this model in the current literature (the general phenotype reported is briefly summarized)
| Targeted gene | Reported phenotype | Reference |
|---|---|---|
| Acc1 | Growth retardation and reduced lipid accumulation | Mao et al., 2009 (5) |
| Acsl1 | Increased adipose mass and impaired cold tolerance | Ellis et al., 2010 (6) |
| Ar | Hyperleptinemia | Yu et al., 2008 (9) |
| Arfrp1 | Defective lipid droplet formation and lipodystrophy | Hommel et al., 2010 (10) |
| Atg7 | Decreased adipose mass and increased insulin sensitivity | Singh et al., 2009 (7) |
| Enpp2 | Increased fat mass associated with a high-fat diet | Dusaulcy et al., 2011 (11) |
| Fas | Increased insulin sensitivity and decreased adipose inflammation | Wueest et al., 2010 (12) |
| Lrp1 | Reduced lipid transport and increased insulin sensitivity | Hofmann et al., 2007 (13) |
| Mapk8 | Increased insulin sensitivity | Sabio et al., 2008 (14) |
| Ncor | Increased obesity, improved glucose tolerance, and decreased adipose inflammation | Li et al., 2011 (15) |
| Pnpla2 | Impaired thermogenesis, increased insulin sensitivity and defective lipolysis | Ahmadian et al., 2011 (16) and Wu et al., 2012 (17) |
| Ppard | Impaired cold tolerance | Pan et al., 2009 (8) |
| Pparg | Lipodystrophy and hepatic steatosis | He et al., 2003 (2) |
| Ppargc1a | Decreased body weight and loss of rosiglitazone mediated induction of UCP-1 in brown adipose | Pardo et al., 2011 (18) |
| Raptor | Less adipose and increased insulin sensitivity | Polak et al., 2008 (19) |
| Rictor | Increased body size and defective glucose and lipolysis | Cybulski et al., 2009 (20) and Kumar et al., 2010 (21) |
| Scl27a4 | Increased adipose mass | Lenz et al., 2011 (22) |
| Spry1 | Increased adipose mass and decreased bone mass | Urs et al., 2010 (23) |
| Stat3 | Increased adipose mass and impaired lipolysis | Cernkovich et al., 2008 (24) |
| Sufu | Lack of white adipose tissue | Pospisilik et al., 2010 (25) |
| Trp53 | Decreased adipose inflammation and increased insulin sensitivity | Minamino et al., 2009 (26) and Shimizu et al., 2012 (27) |
We have been systematically examining the function of class I histone deacetylase 3 (HDAC3), a component of a multiprotein transcriptional corepressor complex, in various tissues using the Cre-LoxP system (28–31). Here we report that Fabp4-Cre targeted deletion of HDAC3 leads to unexpected lethality in mice. We demonstrate that this phenotype cannot be due to adipose-specific loss of HDAC3 because we do not observe the same phenotype when we ablate HDAC3 to a similar degree in adipose tissue using two other independent models, including a novel Retn-Cre mouse line described for the first time here. The dramatically contrasting phenotypes obtained when using these three different adipose targeting Cre lines highlight the need for careful interpretation of the data obtained using adipose-specific Cre-LoxP models and should be considered when embarking on new studies of adipose physiology.
Materials and Methods
Mice
Floxed Hdac3 mice were generated previously (29). Fabp4-Cre and Rosa26 mice were purchased from Jackson Laboratories (Bar Harbor, ME) [B6.Cg-Tg(Fabp4-cre)1Rev/J, stock 005069 and B6.129S4-Gt(ROSA)26Sortm1Sor/J, stock 003474]. AdipoQ-Cre mice were a kind gift from Dr. Evan Rosen (Beth Israel Deaconess Medical Center, Boston, MA) and are now available from Jackson Laboratories [B6;FVB-Tg(Adipoq-cre)1Evdr/J, Stock 010803]. Retn-Cre mice were generated using bacterial artificial chromosome (BAC) modification methods. The structure of Retn was based on the annotated mouse genomic DNA sequences from the University of California, Santa Cruz, genome browser (http://genome.ucsc.edu/). An NLS-Cre-polyA fragment was introduced just in front of the start codon of Retn on a BAC clone using a method described by Gong et al. (32). Start sites of included neighboring genes were mutated to prevent aberrant transcription. A DNA fragment encompassing approximately 23 kb upstream and 10 kb downstream from the transcriptional start site of Retn was retrieved from the modified Retn-Cre BAC into a pBR322 vector (New England Biolabs, Beverly, MA) using a method described by Liu et al. (33). The construct was linearized by NotI digestion, separated in agarose gel and recovered by electroelution. The DNA fragment was purified using Elutip-d column (Whatman, Middlesex, UK), followed by dialysis, and microinjected into pronuclei of fertilized C57BL/6 embryos using standard method at the University Pennsylvania Transgenic Mouse Core Facility. Potential founders were identified by genomic PCR for Cre recombinase. Positive founders were then mated to C57BL/6 mice and offspring were tested for Cre expression (specificity) and used for subsequent experiments with ROSA26 mice (efficiency). The line that demonstrated the best specificity and efficiency is referred to herein as the Retn-Cre line.
Mice were genotyped by PCR using the following primer sequences: Cre, forward, 5′-GCA TTA CCG GTC GAT GCA ACG AGT GAT GAG-3′, reverse, 5′-GAG TGA ACG AAC CTG GTC GAA ATC AGT GCG-3′; Rosa26, oIMR8052, 5′-GCG AAG AGT TTG TCC TCA ACC-3′; oIMR8545, 5′-AAA GTC GCT CTG AGT TGT TAT-3′; and oIMR8546, 5′-GGA GCG GGA GAA ATG GAT ATG-3′ as described by Jackson Laboratories (jaxmice.jax.org); and HDAC3f/f, forward, 5′-GCA GTG GTG GTG AAT GGC TT-3′, reverse 5′-CCT GTG TAA CGG GAG CAG AAC TC-3′. Mice were maintained in a 12-h light, 12-h dark cycle and had free access to food and water. The Institutional Animal Care and Use Committee at the University of Pennsylvania approved all protocols for animal use and euthanasia.
Adipose fractionation
Adipose tissue was finely minced and then incubated in media containing 1.5 U/ml Collagenase D (Roche, Indianapolis, IN) and 2.4 U/ml of Dispase (Roche) while shaking at 37 C for 30 min. After centrifugation, the floating adipocyte fraction and the pelleted stomal-vascular fraction were separated, washed once in media, and then isolated for subsequent RNA isolation.
β-Galactosidase staining
Tissues were prefixed in 2% paraformaldehyde and 0.1% gluteraldehyde, washed and stained with 1 mg/ml x-gal, 4 mm potassium ferricyanide, and 4 mm potassium ferrocyanide at 37 C for 3–16 h. The reaction was stopped when background staining began to appear in tissues from control littermates without Cre. Stained tissues were then washed in PBS and postfixed in 4% paraformaldehyde overnight at 4 C before photographing.
Gene expression
RNA was isolated from tissue using the RNeasy kit (QIAGEN, Valenica, CA). cDNA was generated using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using Power SYBR Green PCR master mix on the 7900HT Fast real-time PCR system (Applied Biosystems). Data were analyzed using a standard curve and normalized to Arbp expression. Primer sequences used are as follows: Arbp, forward, 5′-GGC ACC GAG GCA ACA GTT-3′, reverse, 5′-TCA TCC AGC AGG TGT TTG ACA-3′; Cre, forward, 5′-GCA TTA CCG GTC GAT GCA ACG AGT GAT GAG-3′, reverse 5′-GAG TGA ACG AAC CTG GTC GAA ATC AGT GCG-3′; and Hdac3, forward, 5′-TTG GTA TCC TGG AGC TGC TT-3′, reverse, 5′-GAC CCG GTC AGT GAG GTA GA-3′.
Statistical analyses
Comparison of survival curves was performed using Graph Pad Prism (Graph Pad Inc., San Diego, CA), and statistically significant differences were determined using the Log-rank (Mantel-Cox) test.
Results
Fabp4-Cre-mediated deletion of HDAC3 is lethal
To determine the in vivo function of adipose HDAC3, we attempted to delete its expression in adipocytes by mating the commercially available Fabp4-Cre line originally generated by He et al. (2) with floxed Hdac3 mice (HD3f/f) (29). Surprisingly, no viable adult Fabp4-Cre+, HD3f/f mice were obtained. To determine whether the Fabp4-Cre+, HD3f/f mice were born normally, mice were genotyped at birth and monitored until weaning. Importantly, Fabp4-Cre+, HD3f/f mice were born at the expected Mendelian ratio (Fig. 1A). However, no Fabp4-Cre+, HD3f/f mice survived past 25 d of age (Fig. 1B). More than 50% of the mice died before postnatal d 5, and visual monitoring of the remaining mice revealed a failure to thrive and wasting phenotype before death (Fig. 1C). Importantly, adipose tissue developed and was morphologically normal at postnatal d 4 (Fig. 1D). To date, we are unaware of any conclusive reports of adipocyte-intrinsic defects leading to death. In fact, severely lipodystrophic mice, although metabolically abnormal, survive until adulthood (2, 34–38). Therefore, we rationalized that it was unlikely that the early death in the Fabp4-Cre+, HD3f/f mice was due to an adipose autonomous defect. Consistent with this hypothesis, there have been several reports of nonadipose Cre activity under the control of the 5.4-kb fragment of the Fabp4 promoter (39–41).
Fig. 1.
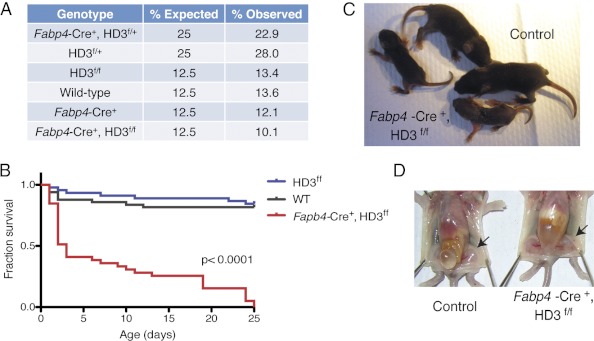
A Cre-LoxP recombination strategy to delete HDAC3 using Fabp4-Cre mice is lethal. Fabp4-Cre+, HD3f/f mice were born at the expected frequency. A total of 397 pups from Fabp4-Cre+, HD3f/+ x HD3f/+ breedings were genotyped between postnatal d 1 and 3 (A) and monitored for survival until weaning (B). C, Two representative Fabp4-Cre+, HD3f/f mice at postnatal d 7 are shown in comparison with littermate controls. D, Inguinal white adipose depots (arrows) at postnatal d 4.
Development of Retn-Cre mice for adipose selective targeting
To address the possibility that the early lethality in the Fabp4-Cre+, HD3f/f mice was due to loss of HDAC3 in a nonadipose tissue, we sought to delete floxed Hdac3 using an alternative Cre line selectively targeting adipose tissue. We generated a BAC transgenic mouse in which Cre recombinase was placed within a 33-kb fragment of Retn, the gene encoding resistin, an adipokine exclusively expressed in murine adipose tissue (Fig. 2A) (42). This construct contains a previously described adipocyte-specific enhancer (43).
Fig. 2.
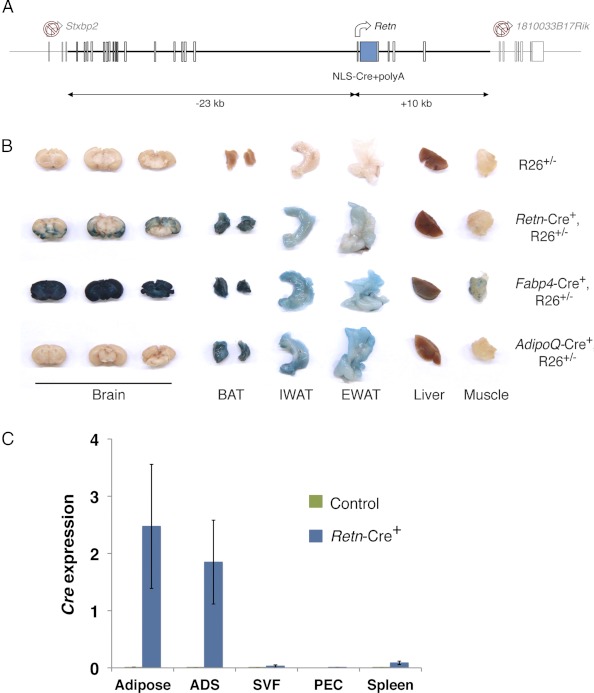
Development and validation of Retn-Cre mice for adipose-selective targeting. A, A BAC construct containing a 33-kb fragment surrounding the transcriptional start site of Retn was generated with NLS-Cre+polyA inserted in exon 2. Start sites of included neighboring genes were mutated to prevent aberrant transcription. B, Adult tissues from ROSA26 heterozygous (R26+/−) mice with and without Retn-Cre, Fabp4-Cre and AdipoQ-Cre were stained for β-galactosidase activity. BAT, Brown adipose tissue; IWAT, inguinal white adipose tissue; EWAT, epididymal white adipose tissue. C, Cre expression was examined by quantitative PCR in adipose, fractionated adipocytes (ADS), SVFs, PECs, and spleen isolated from Retn-Cre+ and control Cre− adult male mice and is shown normalized to the control gene arbp (n = 2–3).
To determine the efficiency and specificity of Cre expression, the Retn-Cre+ mice were crossed with a ROSA26 reporter strain in which recombinase activity leads to removal of a floxed stop codon and permanent expression of the lacZ gene (44). A comprehensive panel of tissues was harvested and stained for β-galactosidase activity (Fig. 2B and data not shown). Efficient recombination and removal of the floxed stop codon in the ROSA26 reporter mice was detected in all fat depots examined including epididymal and inguinal white adipose as well as intrascapular brown adipose (Fig. 2B). No activity was observed in other metabolic tissues including the liver, skeletal muscle, and pancreas, whereas scattered punctate staining was observed in some regions of the brain (Fig. 2B and data not shown). This was in stark contrast to the results obtained when crossing the Fabp4-Cre+ mice to the same reporter strain that revealed detectable Cre activity in all tissues examined (Fig. 2B and data not shown). Furthermore, Cre expression was negligible in the adipose stromal-vascular fraction (SVF), peritoneal exudate cells (PECs), and spleen, all of which contain a significant percentage of macrophages, an immune cell previously reported to have detectable Cre activity in Fabp4-Cre+ mice (Fig. 2C) (5). Of note, the specificity of Cre expression in the Retn-Cre+ mice was similar to that observed in mice previously generated expressing Cre under the control of the AdipoQ promoter (Fig. 2B) (45). Therefore, although expression of Cre from this construct is not identical with endogenous resistin expression (which is not detectable in brown adipose or brain), this Retn-Cre+ model does serve as an alternative tool for adipose-selective gene inactivation.
Adipose selective targeting of HDAC3 with Retn-Cre
We next generated Retn-Cre+, HD3f/f mice and assessed them for efficient deletion of Hdac3 and survival. Detailed phenotypic analyses of these mice will be described elsewhere. Importantly, Hdac3 expression was markedly reduced in epidydmal, inguinal, and brown adipose tissues but not in adipose, SVF, PECs, or spleen (Fig. 3A). The adipose depletion of Hdac3 in Retn-Cre+, HD3f/f mice was comparable with that in adipose tissue of Fabp4-Cre+, HD3f/f mice (Fig. 3B). The residual expression of Hdac3 is consistent with the fact that adipose tissue contains other cell types in addition to adipocytes, including preadipocytes, macrophages, and endothelial cells (46).
Fig. 3.
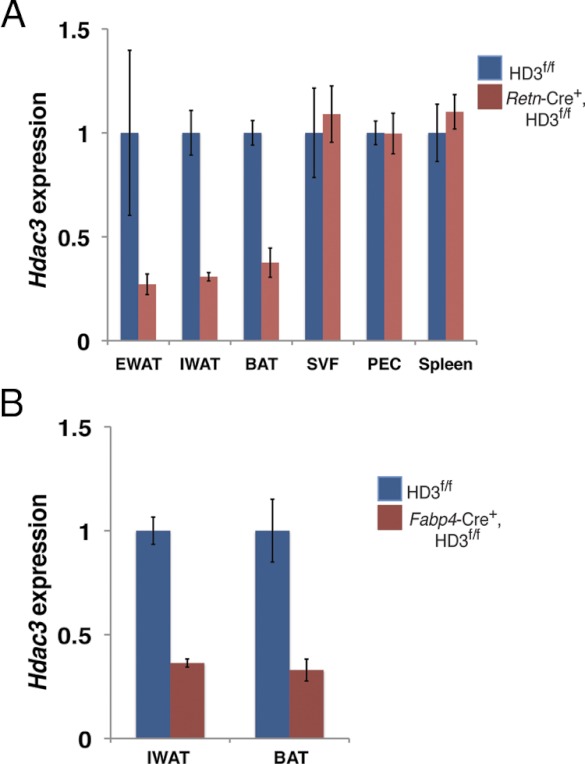
Depletion of HDAC3 in adipose tissue using Retn-Cre. A, Adipose Hdac3 expression determined by quantitative PCR is reduced in adult epididymal white adipose tissue (EWAT), inguinal white adipose tissue (IWAT), and brown adipose tissue (BAT) but not in the SVF of adipose, PECs, or spleen of Retn-Cre+, HD3f/f mice compared with littermate controls. B, A similar reduction of Hdac3 expression in adipose tissue of Fabp4-Cre+, HD3f/f mice is observed in comparison with littermate controls at postnatal d 4 (n = 2–4).
Depletion of HDAC3 in adipose tissue is not lethal
Adipose tissue selective Retn-Cre+, HD3f/f mice were born in an expected Mendelian ratio and, in remarkable contrast to the Fabp4-Cre+, HD3f/f mice, postnatal mice displayed no overt abnormalities and all survived until adulthood (Fig. 4A). The difference in survival between the Fabp4-Cre+, HD3f/f and Retn-Cre+, HD3f/f mice coupled with the widespread recombinase activity detected in the Fabp4-Cre+, ROSA26 mice suggests that the early lethality observed when Hdac3 is deleted under the control of the Fabp4 promoter is due to loss of HDAC3 in cells other than adipocytes. In support of this conclusion, crossing our floxed Hdac3 mice with those expressing Cre under the control of the AdipoQ promoter (45) led to similar results to those obtained from the Retn-Cre+, HD3f/f mice, that being no reduction in survival (Fig. 4B). In a longer follow-up, Retn-Cre+, HD3f/f and AdipoQ-Cre+, HD3f/f mice have shown no increase in mortality up to 6 months (data not shown).
Fig. 4.
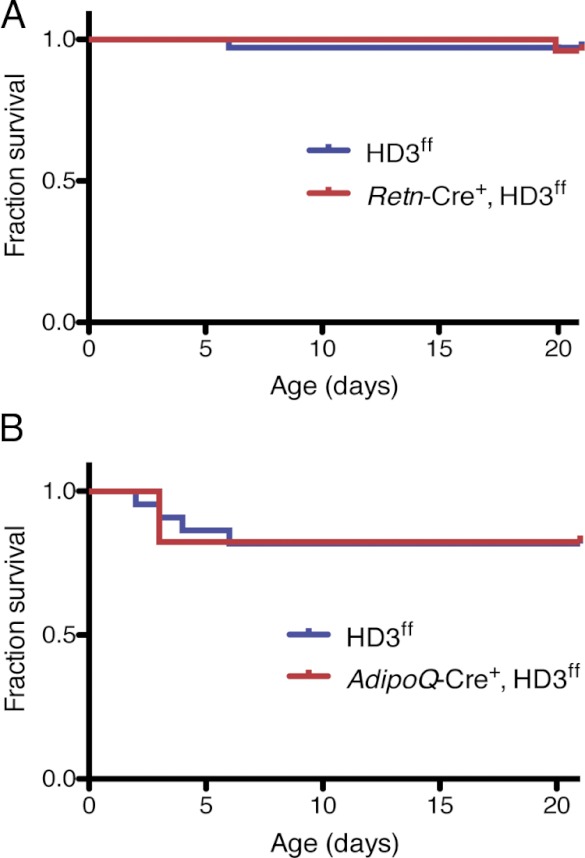
Deletion of adipose HDAC3 is not lethal. A, Normal early life expectancy of Retn-Cre+, HD3f/f mice. B, Normal early life expectancy of AdipoQ-Cre+, HD3f/f mice. Retn-Cre+, HD3f/f (A) and AdipoQ-Cre+ HD3f/f (B) pups and corresponding littermate controls (HD3f/f) were genotyped between postnatal d 1 and 3 and monitored for survival until weaning (n = 60 total pups in A and n = 39 total pups in B).
Discussion
Tissue-specific gene targeting in mice has become one of the most powerful tools in biomedical research in the last 2 decades (1). In contrast to global knockout models, it allows functions of gene products to be assigned to specific cell types. In particular, adipose specific gene deletion allows for a more detailed understanding of the peripheral effects of aberrant adipocyte lipid metabolism and storage and the endocrine actions of adipokines and hormones of which adipocytes can be both effector and/or target cells. Fabp4 is a gene highly expressed in adipocytes, and multiple groups have used a fragment of its promoter for transgenic expression of Cre recombinase in mouse adipose tissue (2–4). Despite several reports of nonadipose expression of Fabp4-Cre (39–41), the commercially available Fabp4-Cre mouse model originally described by He et al. (2) is the most widely used line in studies in which adipose-selective gene targeting is desired. Consistent with previous reports, we have detected extraadipose Cre activity under the control of the Fabp4 promoter, suggesting that it is not sufficient for adipose-selective gene targeting. More recently alternative adipose-targeting Cre lines have been generated using the regulatory sequences of Adipoq, another gene highly expressed in adipose tissue (45, 47). Unlike Fabp4-Cre and consistent with our findings, expression of Adipoq-Cre in cells other than adipocytes has not been reported.
Resistin is an adipokine whose production is limited to white adipose tissue in the mouse (42). In an attempt to express Cre recombinase in a similarly tissue-restricted manner, we generated a transgenic mouse with Cre inserted into a 33-kb fragment surround the Retn transcriptional start site that includes a previously reported adipocyte-specific enhancer (43). Using ROSA26 reporter mice, sufficient Cre activity was detected in white adipose tissue. However, unlike endogenous Retn expression, Retn-Cre activity was also detected in punctate regions of the brain and in brown adipose tissue. Therefore, although expression of Cre in this model is not white adipose restricted as anticipated, it is considerably more limited than that observed in the Fabp4-Cre model. Thus, this novel mouse line can serve as an additional resource for independent confirmation of results obtained with other adipose-selective systems, such as Adipoq-Cre.
Most importantly, we report here drastically different phenotypes upon deletion of floxed Hdac3 to similar levels with Fabp4-Cre, Retn-Cre, and Adipoq-Cre. Whereas deletion of Hdac3 with Fabp4-Cre results in unexpected postnatal lethality, Retn-Cre+, HD3f/f and Adipo-Cre+, HD3f/f mice exhibit survival rates comparable with their control littermates. These findings, in conjunction with the detection of nonadipose activity of Cre in Fabp4-Cre mice, clearly demonstrate that the observed dramatic phenotype in the Fabp4-Cre+, HD3f/f mice is not due to loss of HDAC3 in adipocytes.
The data presented here underscore the need for careful validation of the specificity of target gene deletion before drawing conclusions about adipose-specific function. In particular, great caution should be taken when interpreting data based on use of the commercially available Fabp4-Cre mouse line (2). Indeed, important conclusions already in the published literature ought to be verified using alternative models such as an AdipoQ-Cre line (45, 47) or the Retn-Cre mice introduced here. Moreover, given the imperfections of all of these models, we suggest that to avoid future errors of interpretation, conclusions about in vivo adipocyte-specific functions should be validated in at least two independent mouse models, particularly if the results are surprising and difficult to explain by a gene deletion that is restricted to adipose tissue.
Acknowledgments
We acknowledge the Transgenic Mouse Core of the Penn Diabetes Research Center (Grant P30DK19525) and Technical Director Dr. Jean Richa for assistance in generating the Retn-Cre mice. The NLS-Cre-polyA fragment was a gift from Dr. Klaus Kaestner at the University of Pennsylvania, and we thank Dr. Nathaniel Heintz at The Rockefeller University and Dr. Neal G. Copeland at the National Cancer Institute for providing materials for BAC modification.
This work was supported by National Institutes of Health Grants P01DK49210 and R37DK43806 and the Cox Institute for Medical Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAC
- Bacterial artificial chromosome
- HDAC3
- histone deacetylase 3
- PEC
- peritoneal exudate cell
- SVF
- stromal-vascular fraction.
References
- 1. Wang X. 2009. Cre transgenic mouse lines. Methods Mol Biol 561:265–273 [DOI] [PubMed] [Google Scholar]
- 2. He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. 2003. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100:15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. 2001. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409:729–733 [DOI] [PubMed] [Google Scholar]
- 4. Barlow C, Schroeder M, Lekstrom-Himes J, Kylefjord H, Deng CX, Wynshaw-Boris A, Spiegelman BM, Xanthopoulos KG. 1997. Targeted expression of Cre recombinase to adipose tissue of transgenic mice directs adipose-specific excision of loxP-flanked gene segments. Nucleic Acids Res 25:2543–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao J, Yang T, Gu Z, Heird WC, Finegold MJ, Lee B, Wakil SJ. 2009. aP2-Cre-mediated inactivation of acetyl-CoA carboxylase 1 causes growth retardation and reduced lipid accumulation in adipose tissues. Proc Natl Acad Sci USA 106:17576–17581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. 2010. Adipose acyl-CoA synthetase-1 (ACSL1) directs fatty acids towards β-oxidation and is required for cold thermogenesis. Cell Metab 12:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. 2009. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119:3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan D, Fujimoto M, Lopes A, Wang YX. 2009. Twist-1 is a PPARΔ-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell 137:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, Yeh S, Chang C. 2008. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology 149:2361–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hommel A, Hesse D, Völker W, Jaschke A, Moser M, Engel T, Blüher M, Zahn C, Chadt A, Ruschke K, Vogel H, Kluge R, Robenek H, Joost HG, Schürmann A. 2010. The ARF-like GTPase ARFRP1 is essential for lipid droplet growth and is involved in the regulation of lipolysis. Mol Cell Biol 30:1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dusaulcy R, Rancoule C, Grès S, Wanecq E, Colom A, Guigné C, van Meeteren LA, Moolenaar WH, Valet P, Saulnier-Blache JS. 2011. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res 52:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, Chervonsky AV, Rudich A, Schoenle EJ, Donath MY, Konrad D. 2010. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest 120:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschöp MH, Hui DY. 2007. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest 117:3271–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. 2008. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, Bandyopadhyay G, Scadeng M, Ofrecio JM, Nalbandian S, Olefsky JM. 2011. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell 147:815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. 2011. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu JW, Wang SP, Casavant S, Moreau A, Yang GS, Mitchell GA. 2012. Fasting energy homeostasis in mice with adipose deficiency of desnutrin/adipose triglyceride lipase. Endocrinology 153:2198–2207 [DOI] [PubMed] [Google Scholar]
- 18. Pardo R, Enguix N, Lasheras J, Feliu JE, Kralli A, Villena JA. 2011. Rosiglitazone-induced mitochondrial biogenesis in white adipose tissue is independent of peroxisome proliferator-activated receptor γ coactivator-1α. PLoS One 6:e26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN. 2008. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8:399–410 [DOI] [PubMed] [Google Scholar]
- 20. Cybulski N, Polak P, Auwerx J, Rüegg MA, Hall MN. 2009. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106:9902–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar A, Lawrence JC, Jr, Jung DY, Ko HJ, Keller SR, Kim JK, Magnuson MA, Harris TE. 2010. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes 59:1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lenz LS, Marx J, Chamulitrat W, Kaiser I, Gröne HJ, Liebisch G, Schmitz G, Elsing C, Straub BK, Füllekrug J, Stremmel W, Herrmann T. 2011. Adipocyte-specific inactivation of Acyl-CoA synthetase fatty acid transport protein 4 (Fatp4) in mice causes adipose hypertrophy and alterations in metabolism of complex lipids under high fat diet. J Biol Chem 286:35578–35587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urs S, Venkatesh D, Tang Y, Henderson T, Yang X, Friesel RE, Rosen CJ, Liaw L. 2010. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J 24:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cernkovich ER, Deng J, Bond MC, Combs TP, Harp JB. 2008. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology 149:1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Pennniger JM. 2010. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140:148–160 [DOI] [PubMed] [Google Scholar]
- 26. Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15:1082–1087 [DOI] [PubMed] [Google Scholar]
- 27. Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y, Minamino T. 2012. p53-Induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15:51–64 [DOI] [PubMed] [Google Scholar]
- 28. Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. 2011. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331:1315–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mullican SE, Gaddis CA, Alenghat T, Nair MG, Giacomin PR, Everett LJ, Feng D, Steger DJ, Schug J, Artis D, Lazar MA. 2011. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev 25:2480–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, Zhang D, Graham MJ, Unterman TG, Shulman GI, Sztalryd C, Bennett MJ, Ahima RS, Birnbaum MJ, Lazar MA. 2012. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med 18:934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun Z, Singh N, Mullican SE, Everett LJ, Li L, Yuan L, Liu X, Epstein JA, Lazar MA. 2011. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. J Biol Chem 286:33301–33309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong S, Yang XW, Li C, Heintz N. 2002. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kγ origin of replication. Genome Res 12:1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu P, Jenkins NA, Copeland NG. 2003. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. 2005. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 102:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. 1998. Life without white fat: a transgenic mouse. Genes Dev 12:3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross SR, Graves RA, Spiegelman BM. 1993. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev 7:1318–1324 [DOI] [PubMed] [Google Scholar]
- 37. Péterfy M, Phan J, Xu P, Reue K. 2001. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet 27:121–124 [DOI] [PubMed] [Google Scholar]
- 38. Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. 1998. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12:3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urs S, Harrington A, Liaw L, Small D. 2006. Selective expression of an aP2/fatty acid binding protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res 15:647–653 [DOI] [PubMed] [Google Scholar]
- 40. Martens K, Bottelbergs A, Baes M. 2010. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett 584:1054–1058 [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Wang Y, Gao Z, Yun Z, Ye J. 2012. Hypoxia-inducible factor 1 activation from adipose protein 2-cre mediated knockout of von Hippel-Lindau gene leads to embryonic lethality. Clin Exp Pharmacol Physiol 39:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. 2001. The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- 43. Tomaru T, Steger DJ, Lefterova MI, Schupp M, Lazar MA. 2009. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding proteins. J Biol Chem 284:6116–6125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- 45. Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. 2011. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cinti S. 2005. The adipose organ. Prostaglandins Leukot Essent Fatty Acids 73:9–15 [DOI] [PubMed] [Google Scholar]
- 47. Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. 2010. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151:2933–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]


