Abstract
In Thailand, the leaves of Aquilaria crassna have been used traditionally for the treatments of various disorders, but without any scientific analysis. In this study, the antipyretic, analgesic, anti-inflammatory and anti-oxidative properties of A. crassna leaves extract were investigated at a wide dose range in rodents. Experimental animals were treated orally with an aqueous extract of Aquilaria crassna leaves (ACE). They were tested for antipyretic (Baker's yeast-induced fever in rats), analgesic (hot plate test in mice) and anti-inflammatory (carrageenan-induced paw edema in rats) activities. An anti-oxidative effect of ACE was evaluated by using the DPPH anti-oxidant assay. The results showed that, after 5 hours of yeast injection, 400 and 800 mg/kg ACE significantly reduced the rectal temperature of rats. Mice were found significantly less sensitive to heat at an oral dose of 800 mg/kg ACE, after 60 and 90 min. No anti-inflammatory activity of ACE at an 800 mg/kg dose could be observed in the rat paw assay. An anti-oxidative activity of ACE was observed with an IC 50value of 47.18 μg/ ml. No behavioral or movement change could be observed in mice after oral administration of ACE (800 or 8,000 mg/kg) for seven consecutive days. Interestingly, from the second day of treatment, animals had a significant lower body weight at the 8,000 mg/kg dose of ACE compared to the control. No toxicity was identified and the results of this study state clearly that Aquilaria crassna leaves extracts possess antipyretic, analgesic and anti-oxidative properties without anti-inflammatory activity.
Keywords: Analgesic, antipyretic, anti-oxidative activity, Aquilaria crassna, rodents
INTRODUCTION
Herbal products and dietary supplements are now widely available and considered as complementary approaches for health promotion. They are intensively used to treat and prevent many diseases, such as neurodegenerative disorders, dementia, aging, cancer and cardiovascular disorders.[1,2] Many people believe in health benefits from these herbal preparations which are often linked with their antioxidant properties. Various substances, isolated from plants, were identified as active molecules in modern medicines and many plant extracts work as complex mixtures with synergistic effects.
Aquilaria is a genus of fifteen species of a tree in the Thymelaeaceae, which is native to Southeast Asia. They occur particularly in the rain forests of Thailand, Indonesia, Cambodia, Malaysia, Vietnam, Laos, Northern India, the Philippines and Borneo. The products of Aquilaria, including agarwood, leaves, etc., have been widely used in the treatment of various kinds of pain, cough and anaphylaxis for hundreds of years in Asia.[3,4] In general, many Aquilaria species, such as Aquilaria sinensis (Lour.) Gilg., Aquilaria agallocha Roxb. and Aquilaria crassna, are considered of similar medicinal use. Pharmacological studies showed that A. agallocha inhibited histamine release from mast cells and suppressed the immediate hypersensitivity reaction.[5] It may be potentially an anti-inflammatory and/or analgesic agent.[6,7] The leaves of A. sinensis were previously reported to have analgesic and anti-inflammatory activities.[8] Leaves of A. sinensis also contained eight α-glucosidase inhibitors,[9] which might be used as traditional medicine for the treatment of diabetes. Interestingly, many chemical ingredients with a similar structure have been isolated from both A. sinensis and A. agallocha.[10–12]
In Thailand, the leaves of A. crassna have been used traditionally for the treatments of various disorders and most recently they have been used as a healthy tea food additive. Although the effect of A. crassna on the lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha (TNF-α) production by human polymorphonuclear cells (hPBMC) and cell signaling response has been shown recently,[13] no other scientific study has been done to elucidate the pharmacological properties of the active ingredients of A. crassna in order to prove the claims.
MATERIALS AND METHODS
Plant material and extraction
Dried A. crassna leaves (the plant's voucher No. NAT001-002, Faculty of Pharmaceutical Sciences, Khon Kaen University) were powdered and macerated with methanol for 24 h. The filtrate was dried using a rotary vacuum evaporator giving the desired extract in 7.95% yield based on the dried leaves. The A. crassna leaves extract (ACE) was dissolved fresh in water, everytime before use.
Animals
Male ICR mice weighing 25-35 g, and male Sprague Dawley rats weighing 200-300 g were received from the Animal's House, Khon Kaen University, Khon Kaen, Thailand. They were maintained under standard environmental conditions and were fed with a standard pellet diet and water ad libitum.
Antipyretic activity
Baker′s yeast-induced fever
Antipyretic activity tests were performed as described.[14] Rats were divided into five groups of six rats, and they were trained to remain quiet in a restraint cage. The rectal temperature (TR) was recorded with 0.1°C precision by inserting a lubricated digital thermometer (external diameter 3 mm) into the rectum of each animal. After measuring basal TR, fever was induced by a subcutaneous injection of a pyrogenic dose of Baker's yeast (135 mg/kg), and subsequently TR was recorded every hour. Two hours after injection of yeast, either water (as the control), ACE (200, 400 or 800 mg/kg) or 150 mg/kg aspirin was administered orally to each rat, and the TRwas recorded every hour for another 4 hours. Results are expressed as the changes of the TRfrom basal TR. From our study, the effect of ACE was clearly seen at 800 mg/kg. Therefore, the 800 mg/kg dose was used to study analgesic and anti-inflammatory activities.
Analgesic activity
Hot plate test
Mice, six in each group, were tested for the reaction time by placing them on a hot plate that was thermostatically maintained at 50°C with a four-wall plexiglass container, confining the animals on the hot plate.[14] Time that each mouse spent on the hot plate (until it licked or jumped in response to pain) was recorded as the reaction time. The cut-off time of the test was set at 30 seconds in order to prevent tissue damage. The reaction time of each animal was recorded as the basal reaction time. After being orally fed with either water, ACE (800 mg/kg) or aspirin (150 mg/kg), the reaction time of each animal was determined at 30, 60 and 90 min after treatment.
Anti-inflammatory activity
Carrageenan-induced paw edema in rats
Rats were divided into three experimental groups ( n = 6). Different groups were orally treated with water (as the control), ACE (800 mg/kg) or aspirin (300 mg/kg). One hour after treatment, acute inflammation was produced by the sub plantar injection with 0.1 ml of 1% carrageenan in the right paw of the rats.[14] Rat's paw volume was measured at 0, 1, 2 and 3 hours after carrageenan injection using a plethysmometer. The results were expressed as the changes of paw volume from the basal value.
Anti-oxidative activity
DPPH antioxidant assay
Anti-oxidative activity of ACE was determined by DPPH (1,1-diphenyl-2-picrylhydrazyl) antioxidant assay as described earlier.[15] In brief, the extract was dissolved in methanol into various concentrations, including 500, 250, 125, 63, 31, 16, and 8 g/ml. Ascorbic acid in methanol at 5, 2.5, 1.25, 0.63, 0.31, 0.16, and 0.08 g/ml were used as reference standard. After adding 0.1 mm DPPH, the mixture was incubated in the dark for 30 min and the absorbance was measured at 490 nm. Concentration and % inhibition of the oxidation reaction of ascorbic acid and ACE was plotted and the 50% inhibition concentrations were determined as IC50.
Effects of repeated doses of ACE, initial toxicity
Water (as the control) or ACE at the doses of 800 mg/kg or 8,000 mg/kg (10 times higher than the effective dose) was administered orally to mice, once daily for seven days. There were 10 mice in each group. Animal behavioral changes were observed immediately after treatment up to 6 hours. Body weight of each animal and numbers of dead animals (if any) were recorded daily. At the end of the experiment, certain organs (heart, kidney, liver and stomach) were isolated, weighed and observed for any gross abnormality changes.
Statistical analysis
Results were expressed as the mean ± S.E.M. Data were assessed by one-way analysis of variance (ANOVA) and all pairwise multiple comparison procedures (Fisher LSD method). P value of <0.05 was considered as statistically significant.
RESULTS
Antipyretic activity
The results of the antipyretic activity (Baker's yeast-induced fever) are outlined in Figure 1. Animals that received Baker's yeast (135 mg/kg) had a significant increase in the rectal temperature 3 hours after the yeast injection, and the increase in TR was continued until the end of the experiment. The animals treated with 200 mg/kg ACE showed no antipyretic effect and the animal's TR during the observation period was comparable to the control. Animals treated with either 150 mg/kg aspirin or 400 or 800 mg/kg ACE showed a significant reduction of TR at 5 and 6 hours after the yeast injection when compared to the control at the same time point.
Figure 1.
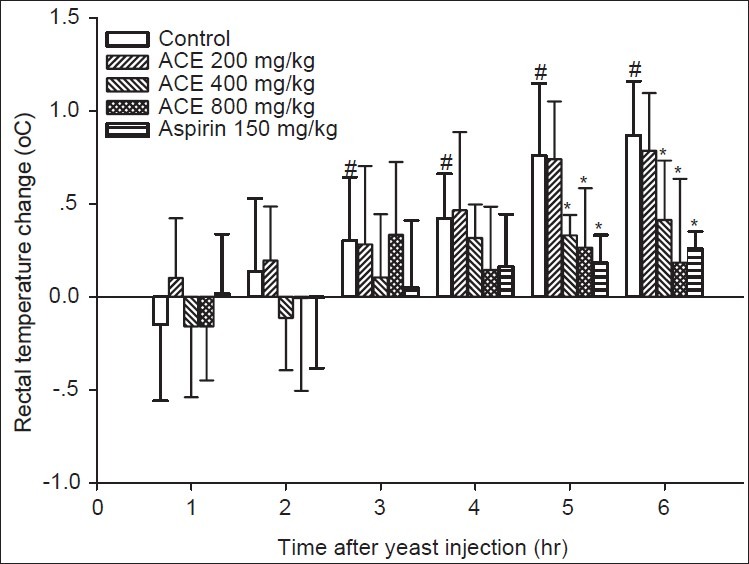
Effects of ACE (200, 400 or 800 mg/kg) or 150 mg/kg aspirin on rectal temperature of rats. * and # denoted P<0.05 when compared to the control at the same time point and basal TR, respectively
Hot plate reaction time in mice
The results showed that aspirin 150 mg/kg and ACE 800 mg/kg could significantly increase the thermal threshold when compared to the control [Figure 2]. The analgesic effect of 150 mg/kg aspirin could be seen at 30, 60 and 90 min after oral administration. The effect of 800 mg/kg ACE was clearly seen at 60 and 90 min after oral administration of the test extract. The magnitude of the effect of 800 mg/kg ACE was significantly less than 150 mg/kg aspirin at 60 min and identical at 90 min.
Figure 2.
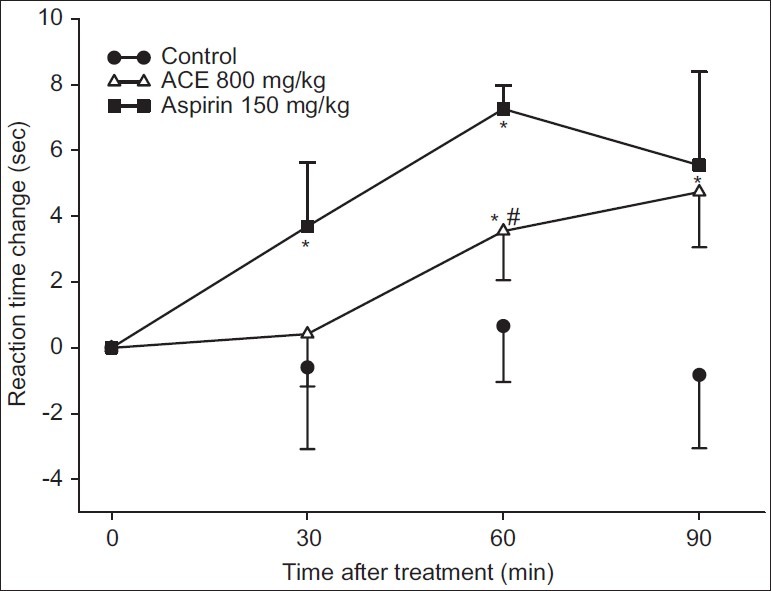
Analgesic effect of ACE. Mice were fed with water (control), 800 mg/kg ACE or 150 mg/kg aspirin. * and # denoted P<0.05 when compared to the control and aspirin-treated group at the same time point, respectively
Anti-inflammatory studies
Following carrageenan injection, the paw edema formation was gradually increased within the 1st hour for the control group, and the maximum effect was reached over the period of 3 hours [Figure 3]. Animals treated with 300 mg/kg aspirin showed fewer swollen paws, and the paw volumes were significantly less than the control group after 2 and 3 hours. No anti-inflammatory effect of 800 mg/kg ACE could be observed.
Figure 3.
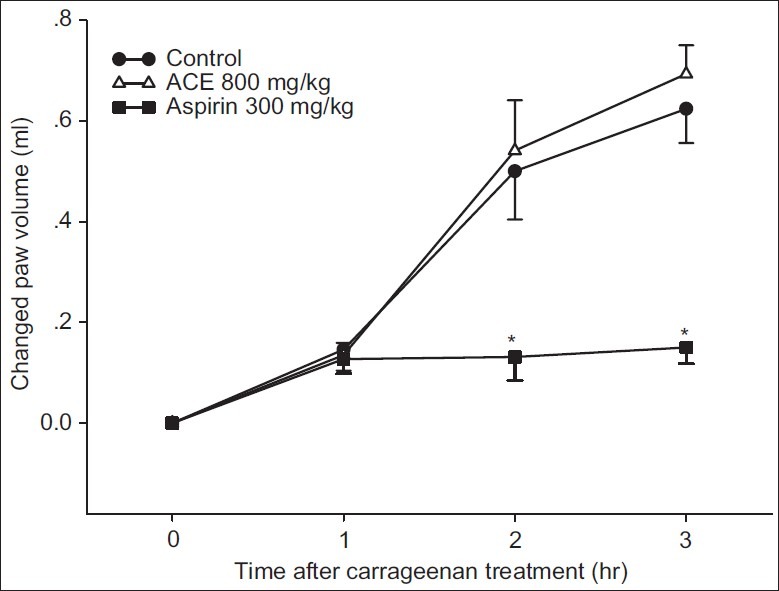
Carrageenan-induced paw edema test. Rats were fed with water (control), 800 mg/kg ACE or 300 mg/kg aspirin. * P<0.05 when compared to the control at the same time point
Anti-oxidative activity
DPPH antioxidant assay
As shown in Figure 4, ascorbic acid, 0.08-5 g/ml, inhibited DPPH oxidation by approximately 30-80%. From 8 to 500 g/ml ACE, the inhibition was determined in the range from 36% to 91%. The anti-oxidative activities of ascorbic acid and ACE were found to have IC50 equal to 2.19 g/ml and 47.18 g/ml, respectively.
Figure 4.
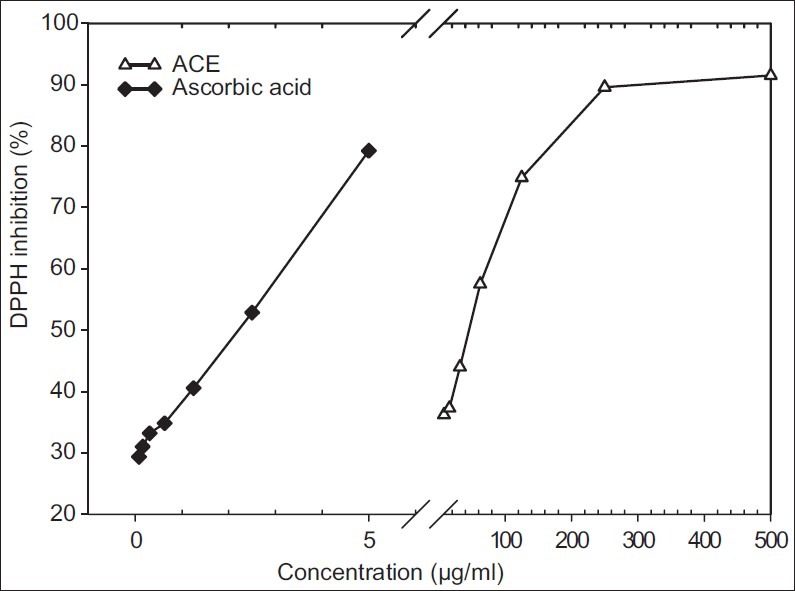
Comparison of DPPH radical scavenging capacity (%) of ascorbic acid and ACE
Effects of repeated doses of ACE
In general, animals treated with ACE at either 800 or 8,000 mg/kg showed no abnormal behavior. Interestingly, the repeated oral administration of 8,000 mg/kg ACE once daily for seven days, showed a significant reduction of the animal's body weight from day 2 until the end of the experiment when compared to the control [Figure 5]. No effect could be observed on weights or gross appearances of the heart, liver, kidney and stomach in animals treated with ACE at either 800 or 8,000 mg/kg for seven days (data not shown).
Figure 5.
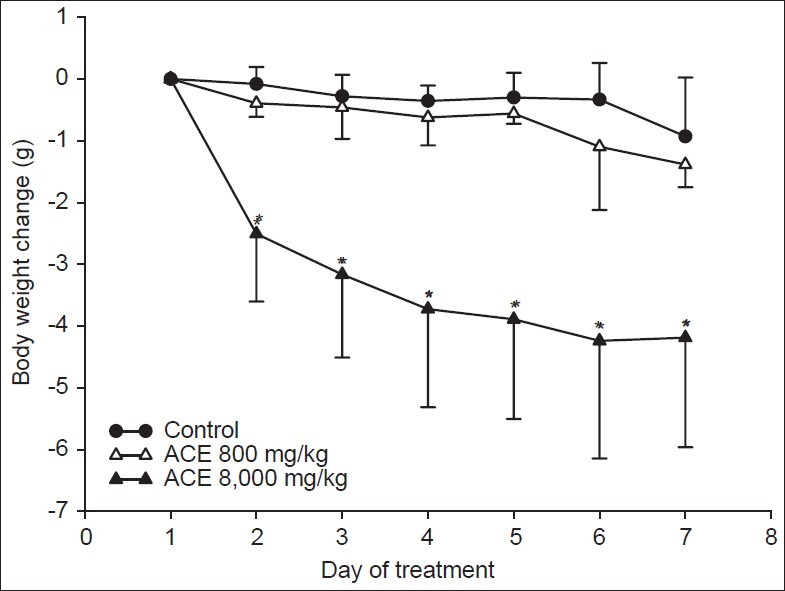
ACE treatment and animal′s body weight. Mice were fed once daily with water (control) or ACE. *P<0.05 when compared to the control at the same time point
DISCUSSION
In this study, it was clearly shown that the methanolic extract of A. crassna leaves (ACE) possesses antipyretic (Baker's yeast-induced fever in rats), analgesic (hot plate test in mice) and anti-oxidative (DPPH antioxidant assay) activities without anti-inflammatory activity (carrageenan-induced paw edema in rat). No effects on general behaviors of mice could be observed after repeated dose's treatment (seven consecutive days) of ACE, at either 800 mg/kg or 8,000 mg/kg. Most interestingly, from the second day of treatment, animals that received 8000mg/kg of ACE, displayed a significant lower body weight than the control.
Aspirin, an analgesic antipyretic and anti-inflammatory drug, was used as a positive control in this study. The mechanisms of actions of aspirin that are responsible for all those effects are believed to be involved with both central and peripheral mechanisms.[16] In this study, ACE, at 800 mg/kg, showed an antipyretic and analgesic effect, which is comparable to aspirin at 150 mg/kg, but without anti-inflammatory properties. A comparable drug, such as paracetamol, also displays analgesic and antipyretic effects without anti-inflammatory action, and it is proposed to act preferentially within the central nervous system through inhibition of a specific form of cyclooxygenase.[16] It might be possible that ACE, at the dose tested, acts in a similar pattern as paracetamol on the COX3 target, but not as aspirin or other NSAIDs, by centrally producing those effects. However, a higher dose of ACE might be able to show anti-inflammatory activity, since the ethanolic extract of A. sinensis displayed analgesic and anti-inflammatory at the dose of 848 but not 424 mg/kg,[8] and the heartwood of A. crassna inhibited LPS-induced tumor necrosis factor-alpha production by attenuating P38 MAPK activation.
The anti-oxidative activity of ACE possibly is one of the important properties, and although the extract was found less active than ascorbic acid, this activity may be linked with the phenolic part of the mixture of ACE. Oxidative mechanisms are proposed to be responsible for many neuronal disorders[2,17] and plant compounds are suggested to be neuroprotectants due to their anti-oxidative properties.[17] Many substances have been identified from fresh stem of A. sinensis such as aquilarin A, balanophonin and (+)-lariciresinol[18] and this part of the mixture might also be present in ACE and act as an antioxidant.
In this study, animals treated with the extract of A. crassna, 8,000 mg/kg, had a significant reduction of body weight. Recently, the laxative effect of A. sinensis has been reported and explained via acetylcholine receptor mediated acceleration of the gastrointestinal transit.[19,20] Laxative effect of A. sinensis was even observed in rats with low-fiber diet-induced constipation without causing any diarrhea and the authors suggested that A. sinensis may be useful as a therapeutic laxative agent in humans. In addition, α-glucosidase inhibitors were reported to be present in A. sinensis leaves which might interfere the absorption of carbohydrate food.[20,11] It might be the case that A. crassna leaves extract exerts its effect on body weight through both mechanisms and these need to be further elucidated.
In conclusion, this study suggests the possibility of using A. crassna leaves and a formulation thereof as an analgesic and antipyretic food supplement. Further, a medicinal application of the antioxidant and the possible α-glucosidase inhibiting property of the extract might have some roles in the treatment and prevention of metabolic disorders.
ACKNOWLEDGMENT
We thank Mr. Nuthawong Siriyodaroon for providing dried leaves of Aquilaria crassna.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar V. Potential medicinal plants for CNS disorders: An overview. Phytother Res. 2006;20:1023–35. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 2.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular Med. 2008;10:259–74. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. Effects of agarwood extracts on the central nervous system in mice. Planta Med. 1993;59:32–6. doi: 10.1055/s-2006-959599. [DOI] [PubMed] [Google Scholar]
- 4.Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull (Tokyo) 2003;51:560–4. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- 5.Kim YC, Lee EH, Lee YM, Kim HK, Song BK, Lee EJ, et al. Effect of the aqueous extract of Aquilaria agallocha stems on the immediate hypersensitivity reactions. J Ethnopharmacol. 1997;58:31–8. doi: 10.1016/s0378-8741(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 6.Peana AT, D'Aquila PS, Panin F, Serra G, Pippia P, Moretti MD. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–6. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 7.Peana AT, D'Aquila PS, Chessa ML, Moretti MD, Serra G, Pippia P. (–)-Linalool produces antinociception in two experimental models of pain. Eur J Pharmacol. 2003;460:37–41. doi: 10.1016/s0014-2999(02)02856-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou M, Wang H, Suolangjiba , Kou J, Yu B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg.Leaves extract. J Ethnopharmacol. 2008;117:345–50. doi: 10.1016/j.jep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Yang XW, Wang RF. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72:242–7. doi: 10.1016/j.phytochem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Yang JS, Chen YW. [Studies on the constituents of Aquilaria sinensis (Lour.) Gilg. I. Isolation and structure elucidation of two new sesquiterpenes, baimuxinic acid and baimuxinal] Yao Xue Xue Bao. 1983;18:191–8. [PubMed] [Google Scholar]
- 11.Yang JS, Chen YW. [Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. II. Isolation and structure of baimuxinol and dehydrobaimuxinol]. Yao Xue Xue Bao. 1986;21:516–20. [PubMed] [Google Scholar]
- 12.Yang JS, Wang YL, Su YL. [Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl) chromone derivatives]. Yao Xue Xue Bao. 1989;24:678–83. [PubMed] [Google Scholar]
- 13.Kumphune S, Prompunt E, Phaebuaw K, Sriudwong P, Pankla R, Thongyoo P. Anti-inflammatory effects of the ethyl acetate extract of Aquilaria crassna inhibits LPS-induced tumor necrosis factor-alpha production by attenuating P38 MAPK activation. Int J Green Pharm. 2011;5:43–8. [Google Scholar]
- 14.Suebsasana S, Pongnaratorn P, Sattayasai J, Arkaravichien T, Tiamkao S, Aromdee C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch Pharm Res. 2009;32:1191–200. doi: 10.1007/s12272-009-1902-x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–5. [Google Scholar]
- 16.Aronoff DM, Neilson EG. Antipyretics: Mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304–15. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 17.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang QH, Peng K, Tan LH, Dai HF. Aquilarin A, a new benzenoid derivative from the fresh stem of Aquilaria sinensis. Molecules. 2010;15:4011–6. doi: 10.3390/molecules15064011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakino M, Izuta H, Ito T, Tsuruma K, Araki Y, Shimazawa M, et al. Agarwood induced laxative effects via acetylcholine receptors on loperamide-induced constipation in mice. Biosci Biotechnol Biochem. 2010;74:1550–5. doi: 10.1271/bbb.100122. [DOI] [PubMed] [Google Scholar]
- 20.Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M, et al. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med. 2010;10:68. doi: 10.1186/1472-6882-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]


