Abstract
Background:
Abelmoschus esculentus (L.) Moench. fruit is a commonly consumed vegetable in many countries due to its rich medicinal value. However, till date, in vivo antioxidant property of A. esculentus has not been scientifically documented in animal models.
Objective:
The present investigation was aimed to evaluate the in vivo antioxidant property of A. esculentus (L.) Moench. peel and seed powder (AEPP and AESP) in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods:
In rats, acute toxicity assessment of AEPP and AESP at 2 g/kg did not show any toxicity. Diabetes was induced by STZ (60 mg/kg, i.p.) injection and diabetic rats received AEPP (100 and 200 mg/kg) as well as AESP (100 and 200 mg/ kg) orally up to 28 days. At the end of the 28 day, diabetic rats were killed and liver, kidney and pancreas were collected to determine superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), and lipid peroxidation level.
Results:
In diabetic rats, significant (P < 0.001) reduction of liver, kidney and pancreas SOD, CAT, GPx, GSH levels and increase in thiobarbituric acid reactive substances (TBARS) were observed as compared to normal control rats. Administration of both doses of AEPP and AESP significantly (P < 0.001 and P < 0.01) increased liver, kidney and pancreas SOD, CAT, GPx, GSH levels and decreased TBARS (P < 0.001) levels in diabetic rats compared to diabetic control rats.
Conclusion:
Our findings confirmed that A. esculentus peel and seed powder has significant in vivo antioxidant property in diabetic rats.
Keywords: Abelmoschus esculentus, antioxidant, diabetes, lipid peroxidation, streptozotocin
INTRODUCTION
Diabetes mellitus, a non-communicable disease with multiple etiologies, affects more than 100 million people worldwide and is considered as one of the five leading causes of death in the world.[1] It is a metabolic disorder affecting carbohydrate, fat, and protein metabolism. A worldwide survey reported that diabetes mellitus is affecting nearly 10% of the population every year.[2] Long-standing diabetes is prone to various complications which include cardiac, kidney, and eye problems. The oxidative stress is one of the important factors associated with various diseases such as cancer, cardiovascular diseases, neurodegenerative brain diseases, and diabetes.[3] In diabetes, increased generation of free radicals was observed due to hyperglycemia. The glucose oxidation, non-enzymatic glycation of proteins and subsequent oxidative degradation of glycated proteins are responsible for the formation of oxygen free radicals in diabetes.[4] The role of free radicals in the pathogenesis of diabetes mellitus is well established, and so is the fact that increased oxidative stress plays a major role in the development of diabetic complications.[5]
Antioxidants are natural substances which scavenge free radicals and prevent their deleterious effects in the body. Naturally, the human body has an abundant supply of antioxidants, such as superoxide dismutase, glutathione peroxidase, catalase, vitamin A, C and E which inhibit oxidation and scavenge the oxygen free radicals.[4] In traditional world, nutrition and health care are interconnected as many plants are consumed as food for health benefits.[6] The nutraceutical value and antioxidant property of wild, semi-cultivated or cultivated vegetables are considered worldwide as an important area for the nutritional and phytotherapic research.[7] Nowadays, people are giving great attention to natural medicine due to lesser side effects and multiple therapeutic benefits. Hence, plants are considered as useful dietary supplements in many diseases which include diabetes to control the blood glucose level and preventing its long-term complications.[8] Moreover, medicinal plants are recognized as a potential source for antioxidant compounds, and their therapeutic benefits are often attributed to their antioxidant properties.[9,10]
Abelmoschus esculentus [A. esculentus] (L.) Moench. (synonym-Okra) known in many English-speaking countries as lady′s fingers or gumbo is a flowering plant in the mallow family.[11] It is one of the most important vegetables widely grown in Nigeria for its tender fruits and young leaves. It is distributed throughout Africa, Asia, Southern Europe, and America.[12] In some regions, the leaves are also used for human consumption. Okra constitutes a combination of vitamins and mineral salts, including calcium, which are often lacking in the diet of people residing in developing countries.[13] The plant has a wide range of medicinal values and has been used to treat many diseases. Okra polysaccharide possesses anti-complementary and hypoglycemic activity in normal mice.[14] Our current literature search revealed that A. esculentus were reported for antidiabetic,[15–20] hypolipidemic,[21] neuroprotective,[22] and in vitro antioxidant properties.[12,23,24] Currently, there is no scientific data available for A. esculentus seed and peel powders to support its in vivo antioxidant property in disease conditions. Therefore, this study was undertaken to investigate in vivo antioxidant property of A. esculentus peel and seed powder in streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
Plant materials
A. esculentus (L.) Moench. was collected from the local farm in Coimbatore, Tamil Nadu, India. The plant material was identified and authenticated by Botanical Survey of India (BSI/SC/5/23/2010-11/Tech.1907), Coimbatore, and the certificate was deposited at our laboratory. The peel and seed were separated and dried under shade. The dried seed and peel materials were made into fine powders using a mixer, and these were then stored in an airtight containers until the completion of the study.
Chemicals
Streptozotocin and all other chemicals used in this study were of analytical grade and were procured from Himedia Laboratories, Mumbai, India. The glibenclamide had been received as a gift sample from Orchid Chemicals and Pharmaceuticals Ltd, Chennai, India.
Experimental animals
Male Wistar albino rats (160-180 g) were used to assess antidiabetic activity. The animals were kept and maintained under standard laboratory conditions [temperature (22 °C ± 2 °C) and humidity (45 °C ± 5 °C)] with 12:12 h day:night cycle. The animals were fed with standard laboratory diet and allowed to drink water ad libitum. Studies were carried out in accordance with institutional ethical guidelines for the care of laboratory animals of KMCH College of Pharmacy, Coimbatore, India, after the approval (KMCRET/Ph.D/02/2010).
Acute toxicity study
Acute oral toxicity of A. esculentus peel and seed powders (AEPP and AESP) was determined as per Organization for Economic Cooperation and Development guideline 423.[25] After the oral administration of AEPP (2 g/kg) and AESP (2 g/kg), animals were observed individually at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h, and thereafter for 14 days to check the residual toxicity of AEPP and AESP.
Induction of diabetes
Diabetes was induced in rats, made to fast overnight, by administration of STZ (60 mg/kg; i.p.) in 0.1 M cold citrate buffer (pH 4.5). STZ-induced hypoglycemia was prevented by feeding 10% dextrose solution after 6 h of STZ administration for the next 24 h to the rats. Induction of diabetes was verified after 72 h by measuring the blood glucose level with strips using a glucometer (Accu-ChekÒ Active, Roche Diagnostic Corporation, Mannheim, Germany) and the animals were allowed 14 days for the stabilization of the blood glucose level.[26] On day 14, animals having a blood glucose level higher than 250 mg/dL were considered as diabetic and selected for the experiment.
Experimental design for the assessment of antioxidant property
Animals were divided into seven groups and each group consisted of six rats. The grouping details are:
Group I served as normal control received 0.2% carboxy methyl cellulose (CMC) (5 mL/kg).
Group II served as diabetic control received 0.2% CMC (5 mL/kg).
Groups III and IV diabetic rats were treated with AEPP 100 and 200 mg/kg, respectively.
Groups V and VI diabetic rats were treated with AESP 100 and 200 mg/kg, respectively.
Group VII diabetic rats received standard drug, glibenclamide (5 mg/kg).
The vehicle (0.2% CMC), AEPP, AESP, and glibenclamide were administered orally to the respective group animals for 28 days. AEPP and AESP were triturated with distilled water and glibenclamide with the vehicle just before the oral administration.[26] The blood glucose level was determined using glucometer before and after the administration of AEPP and AESP. On day 28, overnight fasted animals of all groups received respective treatments and after 1 h, all animals were anesthetized with ketamine (100 mg/kg, i.p.) and killed by cervical dislocations to dissect out liver, kidney, and pancreas tissues. They were washed immediately with ice-cold saline to remove blood, kept at −70 °C for the estimation of antioxidants levels until the completion of study.
Tissue homogenate preparation, determination of lipid peroxidation and antioxidants levels
Liver, kidney, and pancreas tissues were weighed and 10% homogenate was prepared with 0.025 M Tris-HCl buffer, pH 7.5. After centrifugation at 10,000 rpm for 10 min, the supernatant was used to measure thiobarbituric acid reactive substances (TBARS). All three tissues were weighed and homogenized (10% w/v) with 0.1 M phosphate buffer (pH 7.0) and centrifuged for 10 min, the supernatant was used for the estimation of SOD, CAT, GPx, and GSH.[27]
Statistical analysis
All the data are expressed as mean ± SEM and were evaluated by one-way analysis of variance (ANOVA), followed by Dunnett′s test for multiple comparisons using prism Graphpad version 5.0 and values of P < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Oxidative stress has a considerable role in the pathogenesis and progression of diabetes due to the increased production of free radicals and reduction of antioxidant defenses. The reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radical are formed primarily by reduction of molecular oxygen from superoxide anions. The high levels of free radicals cause damage to cellular proteins, membrane lipids, and nucleic acids. Although many mechanisms have been reported for formation of ROS, glucose oxidation is believed to be the main source of free radicals. Further, hyperglycemia enhanced lipid peroxidation of low density lipoprotein (LDL) via a superoxide-dependent pathway leading to free radical formation.[4] The STZ-induced diabetic animal model is commonly used by researchers to assess antidiabetic and antioxidant potential of natural and synthetic products. Also, it was well documented that STZ-induced diabetes caused alkylation of DNA and transfer of the methyl group from STZ to the DNA molecule which results in fragmentation of β-cell DNA and defects its function. Our study data showed an increased blood glucose level after STZ administration which may be due to destruction of pancreatic β-cells and that the treatment of both the doses of AEPP and ASEP significantly decreases the blood glucose level towards normal [Figure 1]. Moreover, STZ has potential to act as an intracellular nitric oxide (NO) donor and generates ROS.[28] In a diabetic state, altered levels of non-enzymatic and enzymatic antioxidants were observed in various tissues and estimation of its levels are considered to be a biomarker to identify the oxidative stress status.[29] Therefore, in this study, in vivo antioxidants such as SOD, GPx, CAT, GSH, and lipid peroxidation (TBARS) levels in liver, kidney, and pancreas were determined after the administration of AEPP and AESP in diabetic rats.
Figure 1.

Blood glucose lowering effect of AEPP and AESP in diabetic rats. All data are expressed as mean ± SEM (n = 6).aP < 0.001, the diabetic control compared with the normal control. bP < 0.001, AEPP 100 and 200 mg/kg, AESP 100 and 200 mg/kg and glibenclamide 5 mg/ kg compared to the diabetic control
Normally, SOD converts superoxide anion radicals generated in the body to hydrogen peroxide, reducing interaction of superoxide anions with nitric oxide to form reactive peroxynitrite, thereby scavenging free radicals and preventing their deleterious actions. There are a number of studies reported that the liver, kidney, and pancreas SOD level were sharply reduced in hyperglycemic conditions.[4] It may be due to generation of more superoxide anion radicals in diabetes by glucose oxidation. In our study, STZ-induced diabetic rats showed significant (P < 0.001) reduction of the SOD level in the liver, kidney and pancreas when compared to normal control rats which may be due to above reasons [Tables 1–3]. However, administration of AEPP (100 and 200 mg/kg) and AESP (100 and 200 mg/kg) in diabetic rats significantly (P < 0.001) increased the SOD levels in all three tissues compared to diabetic control rats. Also, AEPP and AESP higher dose (200 mg/kg) showed significant (P < 0.001 and P < 0.05) efficacy than its lower dose (100 mg/kg). The above action may be due to blood glucose reduction ability of AEPP and AESP in diabetic rats and hence prevention of glucose oxidation, thereby reducing superoxide anion formation and restoration of the SOD level.
Table 1.
Effect of AEPP and AESP on liver antioxidant enzymes in STZ-induced diabetic rats
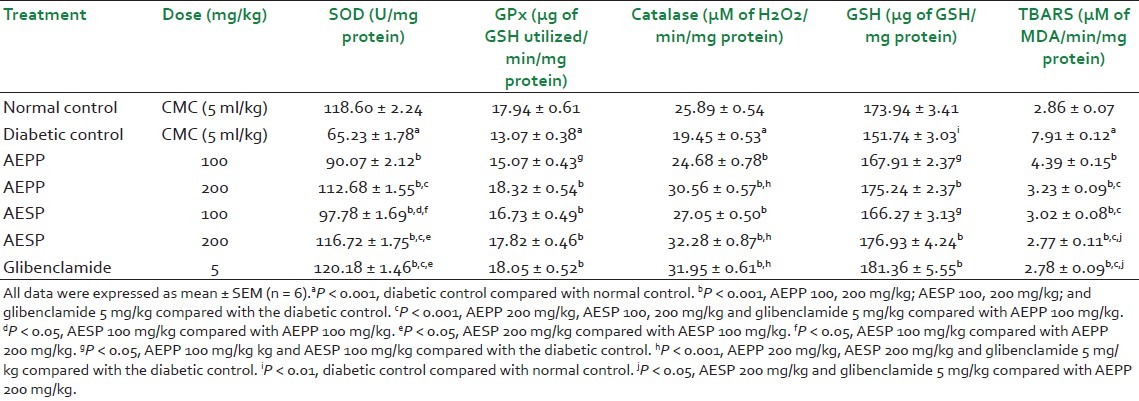
Table 3.
Effect of AEPP and AESP on pancreas antioxidant enzymes in STZ-induced diabetic rats
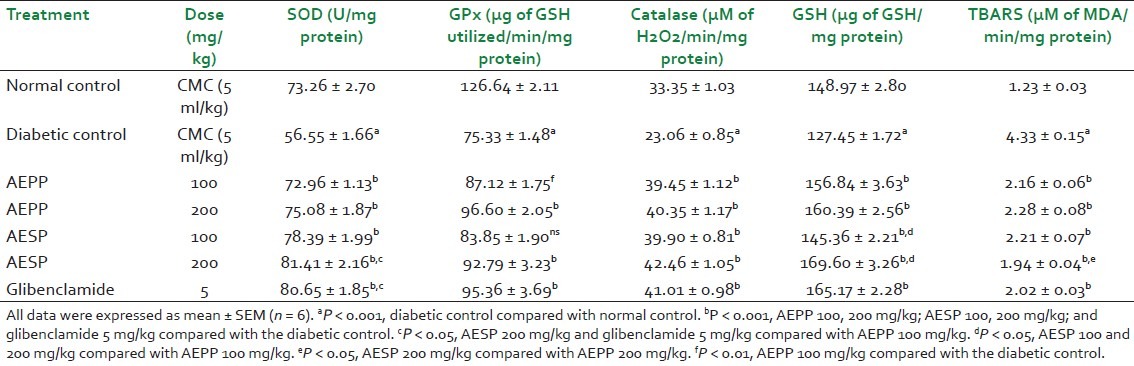
Table 2.
Effect of AEPP and AESP on kidney antioxidant enzymes in STZ-induced diabetic rats
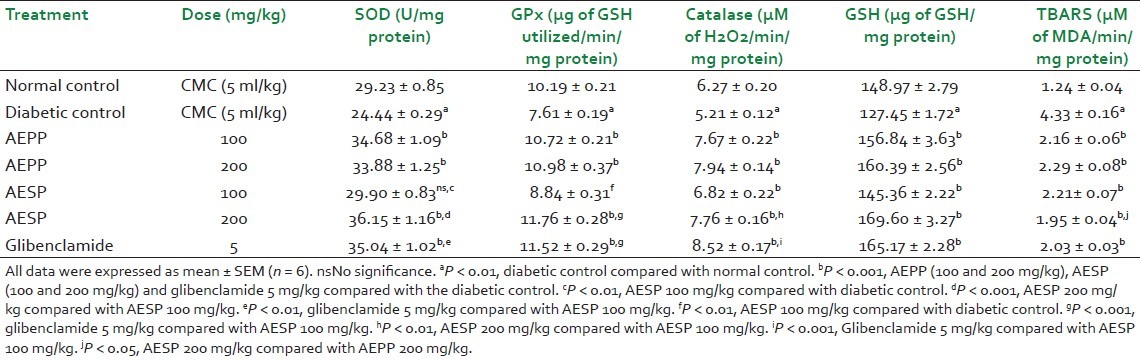
The GPx is found in the cytoplasm, mitochondria, and CAT present in peroxisomes. The role of both the enzymes is to metabolize hydrogen peroxide and peroxides to water and oxygen.[30] Hence, estimation of both these enzyme levels is considered to be a biomarker for the assessment of oxidative stress in diabetes. In this study, GPx and CAT levels in the liver, kidney and pancreas of diabetic rats were significantly (P < 0.001) reduced when compared to normal control rats which confirms the oxidative stress and free radicals generation after the induction of diabetes by STZ. The AEPP (100 and 200 mg/kg) and AESP (100 and 200 mg/kg) treatment to the diabetic rats showed significant (P < 0.001) increases in GPx and CAT levels. Administration of AEPP 200 mg/kg and AESP 200 mg/kg to the diabetic rats increased the GPx and CAT levels significantly (P < 0.001 and P < 0.05) compared to AEPP 100 mg/kg and ASEP 100 mg/kg. The restoration of GPx and CAT levels by AEPP and ASEP may be due to reduction of the fomation of hydrogen peroxide via prevention of glucose oxidation by control of the blood glucose level in diabetic rats.
Moreover, our study data revealed that the level of GSH was reduced significantly in three tissues, and it was supported by published reports. The reduced glutathione is a major intracellular redox buffer and it functions as a direct free-radical scavenger. It acts as a co-substrate for glutathione peroxidise activity, and as a cofactor for many enzymes. In diabetic conditions, the decreased level of GSH was reported in blood and other tissues.[4] The diabetic rats treated with both the doses of AEPP and ASEP significantly (P < 0.01 and P < 0.05) increased GSH levels as compared to the diabetic rats treated with the vehicle. This action supports the free radical scavenging property of AEPP and ASEP in diabetic rats.
In chronic hyperglycemic conditions, the increased level of lipid peroxidation was reported in many studies and it may be due to increased generation of oxygen free radicals. The increased lipid peroxidation level causes oxidative stress in the cell which leads to depletion of antioxidant enzymes. In both types of diabetes, lipid peroxide-mediated different tissue damages was reported.[31] In diabetes, the elevated level of TBARS was considered as a major indicator for the occurrence of lipid peroxidation. Also, diabetic complications such as atherogenesis, coronary heart failure, and nephropathy display causal relationship with oxidative stress and lipid peroxidation.[32] In this study, the TBARS level in the liver, kidney, and pancreas of diabetic rats were significantly (P < 0.001) increased than normal control rats which supported the occurrence of lipid peroxidation. Administration of AEPP and ASEP reduced the TBARS level in diabetic rats significantly (P < 0.001). This action supports anti-lipid peroxidation action of AEPP and ASEP in diabetic rats.
The antidiabetic, neuroprotective, hypolipidemic and in vitro antioxidant activities of A. esculentus fruits were studied well. Also, the high phenolic content (167.70 gallic acid equivalent mg/100 g) of A. esculentus was reported.[33] High-performance liquid chromatography (HPLC) analysis identified the presence of various phytoconstituents such as catechin, epicatechin, procyanidin B1 and B2, quercetin and rutin in the A. esculentus seed and pulp.[12] The okra seed contains high content of procyanidin B1 and B2 than catechin and epicatechin and this was documented using HPLC analysis. But, okra pulp contains high content of catechin and epicatechin than procyanidin B2. It is well-known that quercetin, rutin, catechin, and epicatechin have good antioxidant property and their therapeutic effects in many disease conditions are well established. Clinically, procyanidin oligomers were reported to possess greater antioxidant property than vitamins C and E. Moreover, procyanidin oligomers inhibited isolated human low-density lipoprotein oxidation greater than vitamins C and E and inhibited platelet aggregation, atherosclerosis, cancer, inflammatory mediators release, ulcer, and free radicals.[34,35] Based on published reports okra seed and pulp extracts contained more amount of catechin, epicatechin and procyanidin B1 and B2 than quercetin and rutin. Therefore, the above phytoconstituents might be responsible for the in vivo antioxidant property of A. esculentus seed and peel powders in diabetic rats. However, further studies are required to know the exact mechanism of antioxidant effects of A. esculentus fruit seed and peel powders.
CONCLUSION
The above study results confirmed that A. esculentus fruit seed and peel powders have equal and significant in vivo antioxidant property in diabetic conditions which has given scientific support to the published in vitro antioxidant studies.
ACKNOWLEDGMENTS
We thank to Mr. Mohammad Akbar, Department of Pharmacology, KMCH College of Pharmacy, Coimbatore, for his constant support throughout this research. We thank the management and Dr. A. Rajasekaran, Principal, KMCH College of Pharmacy, Coimbatore, for their support during the study period. Also, we thank Dr. K. Asokkumar, Professor, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, for the English verification and proof correction of this manuscript.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Zimmet PZ. Diabetes epidemiology as a tool to trigger diabetic research and care. Diabetologia. 1999;42:499–518. doi: 10.1007/s001250051188. [DOI] [PubMed] [Google Scholar]
- 2.Vetrichelvan T, Jagadeesan M, Uma Devi BA. Antidiabetic activity of alcohol of Celosia argentea Linn.seeds in rats. Biol Pharm Bull. 2002;25:526–8. doi: 10.1248/bpb.25.526. [DOI] [PubMed] [Google Scholar]
- 3.Gupta MM, Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J Physiol Pharmacol. 2005;49:187–92. [PubMed] [Google Scholar]
- 4.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants.A Review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Paolisso G, D′Amore A, Di Maro G, Galzerano D, Tesauro P, Varricchio M, et al. Evidence for a relationship between free radicals and insulin action in the elderly. Metabolism. 1993;42:659–63. doi: 10.1016/0026-0495(93)90228-g. [DOI] [PubMed] [Google Scholar]
- 6.Pieroni A, Price LL. New York: Haworth Press; 2006. Eating and Healing: Traditional Food as Medicine; pp. 65–99. [Google Scholar]
- 7.El SN, Karakaya S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int J Food Sci Nutr. 2004;55:67–74. doi: 10.1080/09637480310001642501. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher AM, Flatt PR, Duffy G, Abdel-Wahab YH. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr Res. 2003;23:413–24. [Google Scholar]
- 9.Zhang Z, Chang Q, Zhu M, Huang Y, Ho WK, Chen Z. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–52. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 10.Evans CR. Flavonoids and isoflavones: Absorption, metabolism and bioactivity. Free Radic Biol Med. 2004;36:827–8. doi: 10.1016/j.freeradbiomed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Chopra RN, Nayar SL, Chopra IC. New Delhi: 1956. Glossary of Indian medicinal Plants (Council of Industrial and Scientific Research) pp. 1–133. [Google Scholar]
- 12.Khomsug P, Thongjaroenbuangam W, Pakdeenarong N, Suttajit M, Chantiratikul P. Antioxidative activities and phenolic content from Okra (Abelmoschus esculentus L.) Res J Biol Sci. 2010;5:310–3. [Google Scholar]
- 13.IBPGR (International Board for Plant Genetic Resources) New Delhi, India: 1990. Report on International Workshop on Okra Genetic Resources held at the National Bureau for Plant Genetic Resources; pp. 8–12. [Google Scholar]
- 14.Tomoda M, Shimizu N, Gonda R, Kanari M, Yamada H, Hikino H. Anti-complementary and hypoglycemic activity of okra and hibiscus mucilage. Carbohydr Res. 1989;190:323–8. doi: 10.1016/0008-6215(89)84136-9. [DOI] [PubMed] [Google Scholar]
- 15.Subrahmanyam GV, Sushma M, Alekya A, Neeraja CH, Harsha HS, Ravindra J. Antidiabetic activity of Abelmoschus esculentus fruit extract. Int J Res Pharm Chem. 2011;1:17–20. [Google Scholar]
- 16.Uraku AJ, Onuoha SC, Offor CE, Ogbanshi ME, Ndidi US. The effects of Abelmoschus esculentus fruits on ALP, AST and ALT of diabetic albino rats. Int J Sci Nat. 2011;2:582–6. [Google Scholar]
- 17.Amin IM. Hypoglyclemic Effects in Response to Abelmoshus esculentus Treatment: A Research Framework using STZ-Induced Diabetic Rats. Int J Biosci Biochem Bioinform. 2011;1:63–7. [Google Scholar]
- 18.Uraku AJ, Ajah PM, Okaka AN, Ibiam UA, Onu PN. Effects of crude extracts of Abelmoschus esculentus on albumin and total bilirubin of diabetic albino rats. Int J Sci Nat. 2010;1:38–41. [Google Scholar]
- 19.Saha D, Jain B, Jain VK. Phytochemical evaluation and characterization of hypoglycemic activity of various extracts of Abelmoschus esculentus Linn.fruit. Int J Pharm Pharm Sci. 2011;3:183–5. [Google Scholar]
- 20.Ramachandran S, Sandeep VS, Srinivas NK, Dhanaraju MD. Anti-diabetic activity of Abelmoschus esculentus Linn on alloxan-induced diabetic rats. Res Rev BioSci. 2010:4. [Google Scholar]
- 21.Ngoc TH, Ngo QN, Van AT, Phung NV. Hypolipidemic effect of extracts from Abelmoschus esculentus L.(Malvaceae) on Tyloxapol-induced hyperlipidemia in mice. Warasan Phesatchasat. 2008;35:42–6. [Google Scholar]
- 22.Tongjaroenbuangam W, Ruksee N, Chantiratikul P, Pakdeenarong N, Kongbuntad W, Govitrapong P. Neuroprotective effects of quercetin, rutin and okra (Abelmoschus esculentus Linn.) in dexamethasone-treated mice. Neurochem Int. 2011;59:677–85. doi: 10.1016/j.neuint.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Adelakun OE, Oyelade OJ, Ade-Omowaye BI, Adeyemi IA, Van de Venter M. Chemical composition and the antioxidative properties of Nigerian Okra Seed (Abelmoschus esculentus Moench) Flour. Food Chem Toxicol. 2009;47:1123–6. doi: 10.1016/j.fct.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Reddy CV, Sreeramulu D, Ragjunath M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res Int. 2010;43:285–8. [Google Scholar]
- 25.OECD Guidelines for Testing of Chemicals 423. Acute oral toxicity - acute toxic class method. 2001 Dec [Google Scholar]
- 26.Ramachandran S, Asokkumar K, Uma Maheswari M, Ravi TK, Sivashanmugam AT, Saravanan S, et al. Investigation of antidiabetic, antihyperlipidemic and in vivo antioxidant properties of Sphaeranthus indicus Linn.in type 1 diabetic rats: An identification of possible biomarkers. Evid Based Complement Alternat Med 2011. 2011;pii:571721. doi: 10.1155/2011/571721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–8. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Lenzen S. The mechanism of alloxan and streptozotocin induced diabetes. Diabetologia. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 29.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:1–11. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szaleczky E, Prechl J, Fehér J, Somogyi A. Alterations in enzymatic antioxidant defence in diabetes mellitus - A rational approach. Postgrad Med J. 1999;75:13–7. doi: 10.1136/pgmj.75.879.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, et al. Lipid peroxidation and antioxidant status in experimental diabetes. Clin Chim Acta. 1999;284:31–43. doi: 10.1016/s0009-8981(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 32.Jakus V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratisl Lek Listy. 2000;101:541–51. [PubMed] [Google Scholar]
- 33.Sreeramulu D, Ragjunath M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res Int. 2010;43:1017–20. [Google Scholar]
- 34.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–9. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 35.Jeong WS, Kong AN. Biological Properties of Monomeric and Polymeric Catechins: Green Tea Catechins and Procyanidins. Pharm Biol. 2004;42:84–93. [Google Scholar]


